Abstract
Here, we describe the molecular and immunological characterization of the bdr gene family of Borrelia turicatae, a relapsing-fever spirochete. Nine bdr alleles belonging to two different subfamilies were sequenced and localized to linear plasmids. Anti-Bdr antiserum was generated and used to analyze Bdr expression in pre- and postinfection isogenic populations. The analyses presented here provide a detailed characterization of the Bdr proteins in a relapsing-fever spirochete species, enhancing our understanding of these proteins at the genus-wide level.
Borrelia species are causative agents of several important diseases, including Lyme disease and relapsing fever (reviewed in references 2 and 10). Plasmid-encoded proteins are thought to be important in virulence and in adaptation as the Borrelia pass between mammals and arthropods (1, 3, 4, 8, 14, 16, 19, 21, 24, 26). The plasmid-carried bdr gene family of the Borrelia is a large group of genes of unknown function (6, 17, 18, 23, 25, 27). The Bdr proteins possess a large polymorphic repeat motif domain encoding putative Ser-Thr phosphorylation motifs and a potential transmembrane-spanning C-terminal domain. These features may indicate membrane anchoring and suggest a possible regulatory function. In this report, we have conducted an analysis of the bdr gene family of Borrelia turicatae to further characterize these proteins and to identify evolutionarily conserved domains.
B. turicatae OZ-1 (kindly provided by Alan Barbour) was cultivated in BSK-H medium (Sigma), supplemented to a 12% concentration with rabbit serum, at either 22, 32, or 37°C. The infectivity of B. turicatae OZ-1 was confirmed by the intradermal injection of ∼500 spirochetes between the shoulder blades of 6-week-old, male, C3H/HeJ mice and subsequent dark-field microscopic analysis of blood smears. Spirochetes were recovered by the cultivation of spirochetemic blood in BSK-H medium. Subsurface plating was performed as previously described (22). Only clonal populations were used in the following analyses. DNA was isolated from the spirochetes as previously described (12, 13).
bdr alleles were recovered either from a B. turicatae OZ-1 Lambda Zap II genomic library or by PCR amplification with a variety of primers targeting either the bdr genes directly or their flanking sequences. The library was constructed, packaged, and propagated as described by the manufacturer (Stratagene) with XbaI-EcoRI-digested genomic DNA serving as the starting material. The GigapackIII Gold system was used for packaging. Plaque lifts were performed with Hybond-N membranes (Amersham) according to procedures described within the Lambda Zap II library manual. The lifts were probed with a 666-bp bdr-targeting PCR probe generated by amplification of the B. turicatae bdrA1 gene (6). All probes and primers used in this study are described in Table 1. Probe labeling was accomplished with the Hi-Prime labeling kit (Boehringer Mannheim) and α-32P-dATP (3,000 Ci mmol−1; DuPont-NEN). The inserts carried by hybridization-positive plaques were excised from the recombinant phage with the ExAssist helper phage (Stratagene), and the resulting phagemids were prepared and amplified in Escherichia coli SOLR cells. Sequencing was performed with the Sequitherm Excel DNA sequencing kit (Epicentre Technologies). Additional bdr alleles were recovered by PCR with the bdrABF1-bdrA1R1 and the bdrABF1-BBG30hF2 primer sets. We previously demonstrated that the B. turicatae bdrA1 allele is 3′ flanked by an open reading frame (ORF) homologous to BBG30 of the Lyme disease spirochetes (6, 18). Use of the BBG30hF2 primer allowed for the recovery of bdr alleles not amplified with the bdrA1R1 reverse primer. Both primer sets yielded several PCR amplicons (data not shown) consistent with the detection of multiple bdr alleles in B. turicatae by hybridization (6). The amplicons were cloned into the pGEM-T Easy vector (Promega), and recombinants carrying the bdr hybridization-positive inserts were sequenced. In total, nine bdr alleles encoding acidic polypeptides ranging in size from 16.9 to 30.2 kDa were identified (Fig. 1). Analysis of the N-terminal domains, which could be unambiguously aligned, revealed that there are two Bdr subfamilies, BdrA (alleles bdrA1 through bdrA4) and BdrB (alleles bdrB1 through bdrB5). The nomenclature strategy used to name the bdr genes sequenced in this report has been previously described (7, 18). While the B. turicatae Bdr sequences are significantly divergent from those of the Lyme disease spirochetes (5, 9, 17, 25, 27), the repeat motif domain remains conserved (with the exception of a variable number of repeat elements). As with other Bdr protein sequences, the B. turicatae Bdr sequences possess a C-terminal variable domain that is predicted by TmPred analyses to be transmembrane spanning (scores ranged from 1,965 to 2,414; 500 is significant). Several of the bdr loci carry an oppositely oriented ORF on the opposing DNA strand (relative to the bdr genes) designated as the bdr (−) (previously referred to as the rep (−) [17]). The B. turicatae bdrA1 (−), bdrA2 (−), bdrA3 (−), and bdrA4 (−) loci encode putative basic Bdr− proteins of 10.5, 16, 15.9, and 13.6 kDa, respectively (data not shown). While expression of at least some bdr (−) alleles has been reported in Borrelia burgdorferi (17), the significance and biological role of the Bdr− proteins has not yet been addressed.
TABLE 1.
Oligonucleotide probe and primer sequencesa
| Oligonucleotide probe designation | Oligonucleotide sequence (5′ to 3′) | Probe binding site |
|---|---|---|
| bdrABF1 | AAGGAGATTTTTATGGGAC | Bases −12 to +7 of bdrA and bdrB alleles |
| bdrA1R1 | CTCATAGCTCATAGATGC | Bases 637 to 654 of bdrA1; binds to the 3′ end of other bdrA alleles |
| bdrAsfR1 | GTTATCAAGTTCGGTTTTAACAG | Bases 201 to 225 of bdrA alleles |
| bdrBsfR1 | GGTATCGAGTTCAGTTTTGAGAG | Bases 201 to 225 of bdrB alleles |
| BBG30hF2 | GGCAATAATGATTATTATTGTG | Binds near the 3′ end of the pBt2.2 insert within the BBG30h gene |
| bdrAB-PCR | PCR-generated probe | 12 bp upstream of the coding sequence of bdrA1 through base 654; obtained with the bdrABF1-bdrA1R1 primer set and pBt2.2 as template |
| BA60 | TTTGATATCATGAAACCAGCACTACCAAATATTGC | Targets the 5′ end of the bdrF1 gene of B. afzelii |
| BA61 | TCAGATATCTTTAAGGATTATTTGTAATTTCTATT | Targets the 3′ end of the bdrF1 gene of B. afzelii |
The F and R designations included in the primer names indicate that the primers target either the positive or negative strand of DNA of the bdr locus and are therefore forward or reverse primers, respectively. The underlined sequences in the BA60 and BA61 primers indicate the positions of the introduced EcoRV restriction sites that were incorporated for cloning purposes.
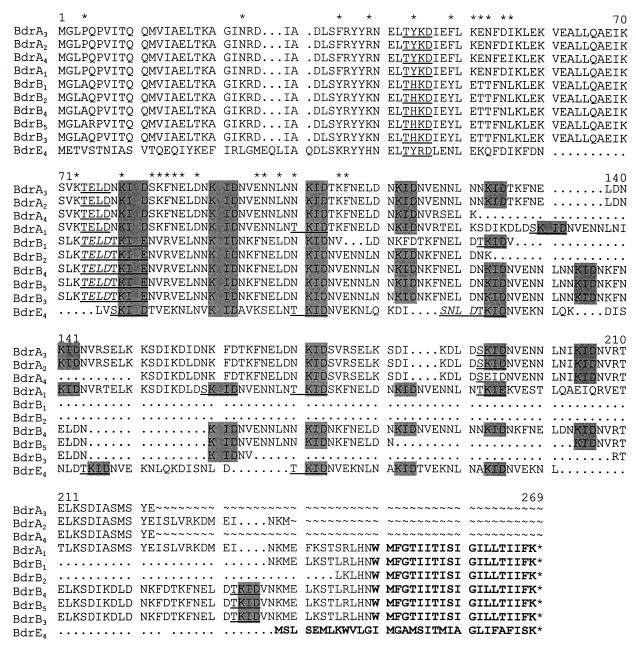
FIG. 1.
Alignment of deduced Bdr amino acid sequences. The determined sequences were translated and aligned using the Genetics Computer Group software package. Gaps in the sequence introduced by alignment are indicated by a dot (.). Some sequences are partial and regions that were not determined are indicated by ∼. The repeated KID/E motif is indicated by boxing in gray, and potential Ser-Thr phosphorylation motifs are indicated by underlining. In cases where two such motifs exist directly after one another, the first of the Ser-Thr phosphorylation motifs is also indicated by italics. Positions where Bdr subfamilies A and B) differ within the phylogenetically informative N-terminal domain are indicated by an asterisk above the alignment. The putative transmembrane domain of each sequence is indicated by boldface lettering. A representative B. burgdorferi bdr sequence was included for comparative purposes.
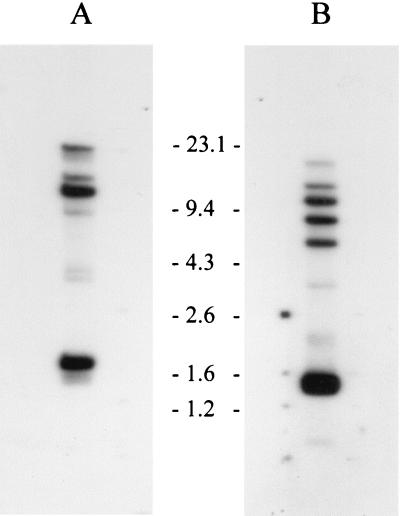
Hybridization analyses of the B. turicatae bdr genes using subfamily-specific probes were conducted to provide an indication of the minimum number of members of each bdr subfamily carried by B. turicatae (Fig. 2). XbaI-digested genomic DNA was electrophoresed, blotted, and hybridized with end-labeled oligonucleotides as previously described (6). The subfamily A probe (bdrAsfR1) hybridized with five bands (two strong and three weak), while the subfamily B probe (bdrBsfR1) hybridized with seven bands (five strong and two weak) indicating that there are 12 or more bdr alleles in B. turicatae OZ-1. B. burgdorferi B31 carries 18 bdr alleles (9) that are organized into three distinct subfamilies (18).
FIG. 2.
Subfamily-specific hybridization analyses of the bdr gene family. DNA from B. turicatae OZ-1 was digested with XbaI and fractionated in a 0.8% GTG-agarose gel in Tris-acetate-EDTA buffer. The blots in panels A and B were probed with the bdrAsfR1 and bdrBsfR1 probes, respectively. Size standards are indicated.
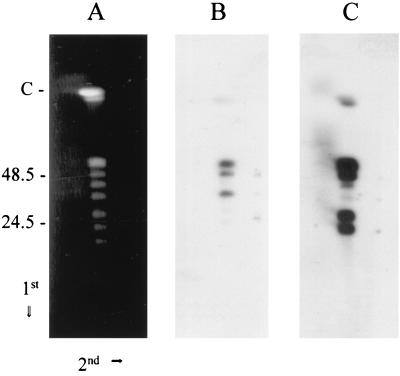
The identity and conformation of the plasmids carrying the B. turicatae bdr genes were determined by two-dimensional contour-clamped homogenous electric field (CHEF)–pulsed-field gel electrophoresis (2D-CHEF-PFGE) and Southern blotting with subfamily-specific probes as previously described (11). In this 2D-CHEF-PFGE electrophoresis system, the circular plasmids exhibit diffuse banding, migrating behind the axis of migration of the linear plasmids. The parameters for PFGE were as follows: run time, 19 h and 18 min; buffer, 0.5× TBE (Tris-borate-EDTA [pH 8.0]); temperature, 14°C; ramping constant, −1.400; initial switch time, 0.47 s; final switch time, 4.48 s; angle, 120°; gradient, 6 V cm−1. After electrophoresis in the first dimension, the gels were rotated 90° and electrophoresed for 3 h in 0.5× TBE at 80 V (constant field). The fractionated DNA was blotted and hybridized with subfamily-specific probes. Probes specific for the bdrA and bdrB subfamilies hybridized exclusively with linear plasmids (Fig. 3). Four linear plasmids of ∼56, 50, 30, and 25 kb hybridized strongly with the bdrBsfR1 probe (plasmids of ∼220 and 42 kb hybridized faintly). With the bdrAsfR1 probe, three linear plasmids of 56, 50, and 39 kb hybridized strongly (plasmids of 220 and 25 kb hybridized faintly). The detection of multiple hybridizing linear plasmids is consistent with the detection of multiple hybridizing restriction fragments in the analyses described above. The hybridization of both probes to plasmids of the same size (e.g., 220, 56, and 50 kb) may indicate that some plasmids carry both bdrA and bdrB subfamily members. Alternatively, there may be comigrating bdr-carrying plasmids. From these analyses, it can be concluded that in contrast to B. burgdorferi, which carries its bdrD and bdrE genes on circular plasmids and its bdrF genes on linear plasmids (reviewed in reference 18), the B. turicatae bdr genes are carried exclusively by linear plasmids.
FIG. 3.
Hybridization analysis of B. turicatae OZ-1 DNA fractionated by 2D-CHEF-PFGE. Agarose plugs containing B. turicatae DNA were prepared, and 2D-CHEF-PFGE was conducted as described in the text. The direction of migration in each dimension is indicated with arrows, and the migration position of the size standards are shown. The C designation above the size standards indicates the migration position of the 910-kb linear chromosome. Note that under the electrophoresis conditions employed, the 220-kb linear plasmid migrates just ahead of the chromosome. Panel A shows the ethidium bromide-stained gel. The blots in panels B and C were probed with the bdrAsf1R1 and bdrBsfR1 oligonucleotides, respectively.
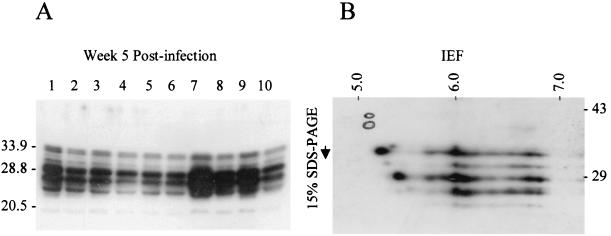
To analyze the expression of the B. turicatae Bdr proteins, anti-Bdr antiserum was generated with a recombinant Bdr protein derived from Borrelia afzelii DK1. The bdrF1 gene was amplified by PCR with the BA60 and BA61 primers, the amplicon was cut with EcoRV, ligated into pMST24 digested with SmaI (yielding a hexahistidyl-tagged fusion), cloned, expressed, and purified as previously described (23). Anti-Bdr antiserum was generated in rabbits as previously described (23). The goals of the immunological analyses were to determine if (i) there are differences in expression patterns in clonal populations, (ii) temperature influences expression, (iii) expression patterns change as a result of passage through mice, (iv) expression patterns change after 6 weeks of cultivation, and (v) molecular changes occur in the evolutionarily unstable repeat motif region during infection or after in vitro cultivation (as inferred from changes in the molecular weights [MW] of the expressed proteins). To accomplish these goals, immunoblot analyses were performed as previously described (18). The set of expressed Bdr proteins (both in terms of the number of alleles expressed and their MW) was the same in all postinfection clonal populations (Fig. 4). The expression patterns were the same as that seen in the preinfection parental strain, were not influenced by temperature (data not shown), and did not change as a result of 6 weeks of in vitro cultivation. The stability of the MW of the expressed Bdr proteins indicates that polymorphisms in the repeat motif domain did not arise under the conditions employed in this study. Lastly, to analyze the pI of the Bdr proteins, cell lysate from a clonal population was subjected to isoelectric focusing (IEF), two-dimensional electrophoresis according to the method of O'Farrell (15) (Kendrick Labs, Inc., Madison, Wis.), and immunoblotting. These analyses revealed that at least six different Bdr species were expressed (based on MW differences). Several of the expressed proteins exhibited pI's in the range predicted by their primary sequences; however, numerous isomorphs of each species were observed, indicating that there may be variable modifications of the Bdr proteins that alter the isoelectric point.
FIG. 4.
Immunoblot analysis of B. turicatae Bdr expression in postinfection clonal populations. Panel A shows the immunoblot of cell lysates of clonal populations (clone designations are indicated above each lane) cultivated for 5 weeks after infection. The lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 15% gel. In panel B, a cell lysate of a clonal population was fractionated by IEF and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The pH gradient of the first-dimension IEF tube gel is indicated across the top and was measured with a surface pH probe. One microgram of a tropomysin pI standard (a doublet, 33 and 34 kDa; pI, ∼5.2) was added to each sample and its migration position, as determined by Coomassie staining of the polyvinylidene difluoride blot prior to immunoblot analyses, was circled for reference. Molecular standards are indicated for each panel in kilodaltons. The immunoblots were screened with anti-Bdr antiserum generated in rabbits with recombinant B. afzelii DK1 BdrF1.
In conclusion, we have identified and characterized several linear plasmid-carried bdr genes that form two subfamilies in B. turicatae. The putative functional domains of these proteins, specifically the Ser-Thr phosphorylation motifs and transmembrane-spanning domains, were demonstrated to be conserved in their primary sequence and/or physical properties at the interspecies level, implying an important role in Bdr function and Borrelia biology. Expression analyses demonstrated that a subset of bdr alleles are expressed during in vitro cultivation and that overall expression patterns remained consistent among clonal populations and were not effected by passage through mice, in vitro cultivation, or changes in the temperature of cultivation. IEF and two-dimensional electrophoresis experimentally confirmed the predicted acidic pI for most Bdr proteins but also demonstrated that there are isomorphs of each species, which may indicate protein modifications. It has been postulated that the Bdr proteins may be anchored to the inner membrane via their C-terminal hydrophobic domain and undergo Ser-Thr phosphorylation within the repeat motif domain in response to changing environmental conditions (18). Sequencing of the B. burgdorferi genome and subsequent analysis of putative active site motifs revealed the presence of ORFs encoding a putative Ser-Thr kinase designated BB0648 and a member of the PPM family of eucaryotic protein Ser-Thr phosphatases (BB0836) (9, 20). Regarding the multiallelic nature of the bdr genes, Bdr variation and redundancy could provide functional or regulatory flexibility in the diverse environments that the Borrelia encounter in the course of cycling between mammals and arthropods. The demonstration of the conservation of specific putative Bdr functional domains and the existence of distinct subfamilies can now serve as a foundation for the design of experiments to test their putative functions.
Nucleotide sequence accession numbers.
The B. turicatae bdr genes have been assigned the accession numbers AF128445 through AF128452, respectively.
Acknowledgments
We wish to acknowledge the input of the Molecular Pathogenesis Group at Virginia Commonwealth University.
This work was supported by a grant from the Jeffress Trust.
REFERENCES
- 1.Akins D, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadavid D, Pennington P M, Kerentseva T A, Bergsrom S, Barbour A G. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadavid D, Thomas D, Crawley R, Barbour A. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caimano M J, Yang X, Popova T G, Clawson M L, Akins D R, Norgard M V, Radolf J D. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect Immun. 2000;68:1574–1586. doi: 10.1128/iai.68.3.1574-1586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlyon J A, Marconi R T. Cloning and molecular characterization of a multicopy, linear plasmid-carried, repeat motif-containing gene from Borrelia turicatae, a causative agent of relapsing fever. J Bacteriol. 1998;180:4974–4981. doi: 10.1128/jb.180.18.4974-4981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlyon J A, Roberts D M, Marconi R T. Evolutionary and molecular analyses of the Borrelia bdr super gene family: delineation of distinct sub-families and demonstration of the genus wide conservation of putative functional domains, structural properties and repeat motifs. Microb Pathog. 2000;28:89–105. doi: 10.1006/mpat.1999.0326. [DOI] [PubMed] [Google Scholar]
- 8.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0 kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser C, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischman R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 10.Johnson R C, Schmid G P, Hyde F W, Steigerwalt A G, Brenner D J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 11.Marconi R T, Casjens S, Munderloh U G, Samuels D S. Analysis of linear plasmid dimers in Borrelia burgdorferi sensu lato isolates: implications concerning the potential mechanism of linear plasmid replication. J Bacteriol. 1996;178:3357–3361. doi: 10.1128/jb.178.11.3357-3361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marconi R T, Samuels D S, Landry R K, Garon C F. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi R T, Wigboldus J, Weissbach H, Brot N. Transcriptional start and MetR binding sites on the Escherichia coli metH gene. Biochem Biophys Res Commun. 1991;175:1057–1063. doi: 10.1016/0006-291x(91)91672-y. [DOI] [PubMed] [Google Scholar]
- 14.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 16.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porcella S F, Popova T G, Akins D R, Li M, Radolf J R, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts, D. M., J. A. Carlyon, M. Theisen, and R. T. Marconi. The bdr gene families of the Lyme disease and relapsing fever spirochetes: potential influence on biology, pathogenesis and evolution. Emerg. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 19.Sadziene A, Barbour A G, Rosa P A, Thomas D D. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993;61:3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Potts M, Kennelly P J. The serine, threonine and/or tyrosine protein kinases and protein phosphatases of procaryotic organisms: a family portrait. FEMS Microbiol Rev. 1998;22:229–253. doi: 10.1111/j.1574-6976.1998.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 21.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung S Y, McDowell J, Carlyon J A, Marconi R T. Mutation and recombination in the UHB-flanked ospE-related genes of the Lyme disease spirochetes results in the development of new antigenic variants during infection. Infect Immun. 2000;68:1319–1327. doi: 10.1128/iai.68.3.1319-1327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theisen M. Molecular cloning and characterization of nlpH, encoding a novel, surface-exposed, polymorphic, plasmid-encoded 33-kilodalton lipoprotein of Borrelia afzelii. J Bacteriol. 1996;178:6435–6442. doi: 10.1128/jb.178.22.6435-6442.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Popova T G, Hagman K E, Wikel S K, Schoeler G B, Caimano M J, Radolf J D, Norgard M. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun. 1999;67:6008–6018. doi: 10.1128/iai.67.11.6008-6018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J-R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 27.Zückert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]