Highlights
-
•
Low-salt pickling increased the content of SFA, PUFA and MUFA in salted eggs.
-
•
Low-salt process can achieve the same effect as traditional processes and is healthy alternative.
-
•
Phospholipids may be responsible for the unique quality of salted egg yolk.
Keywords: Low-salt pickling, Salted duck egg, Fatty acids, Lipids, Lipidomics
Abstract
Lipids play an important role in the salting process of duck eggs. In this research, the low-salt pickling process was employed to salt duck eggs, and the quality parameters, fatty acids and lipidomics were analyzed. The low-salt pickling process maintained the quality of the salted duck egg yolk, and can be regard as a healthy alternative to the traditional curing process. After salting, levels of saturated fatty acid (SFA), monounsaturated fatty acid (MFA) and polyunsaturated fatty acid (PUFA) were increased by 57.77%, 41.59%, and 23.20%, respectively. The lipidomics data showed significant differences in 39 lipids in the phospholipid family during the low-salt pickling process of egg yolk (p < 0.05), in which lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE) and phosphatidylcholine (PC) were defined as differential lipid after low-salt pickling, and the levels of LPC and LPE were increased. Thus, phospholipids might be responsible for the unique quality of salted duck egg yolk.
Introduction
Eggs are highly nutritious as they are packed with multiple nutrients, especially n-3 fatty acids (FAs), vitamin E, selenium, and so on. These nutrients confer resistance against multiple chronic diseases (Venkatachalam & Nagarajan, 2019). Salting is a traditional method of egg preservation. It brings out a unique flavor in eggs, which is quite popular around China and Southeast Asia (Cheng et al., 2018). Compared to hen eggs, duck eggs are more suitable for making salted eggs as they possess more attractive sensory characteristics (Kaewmanee, Benjakul & Visessanguan, 2009a). In most case, salted duck eggs were prepared by adding saturated salt solution (25% w/v) or by coating in salted red soil for around 30 days (Kaewmanee, Benjakul & Visessanguan, 2009a). Salt affects the quality and flavor of salted duck eggs, and the osmotic pressure in salted eggs is altered due to the absorption of salt, which leads to water loss and decreased viscosity in egg white. It also alters the low-density protein structure of the egg yolk and increases the oil exudation (Cheng et al., 2018). During salting, salt content, oil exudate, and firmness of raw egg yolks increase with increasing time and salt concentration. However, the high salt concentration in food could result in high blood pressure and coronary heart disease. Besides, the used salt solution may lead to environmental pollution (Huang & Fang, 2018), therefore, the pickling method using low salt content is highly desirable. For the research on salt reduction technology in salted eggs, previous research has shown that different metal ions can partially replace sodium ions for the development of low-salt salted egg pickling agents, changing the form of salt or using ultra-high pressure and other processing methods can also reduce the amount of salt used (Guo et al., 2021, Du et al., 2022, Yu et al., 2022). According to the team's previous research, we observed that the salting process with low-salt content, chlorine dioxide pretreatment, and a two-stage immersion process decreased salt wastage and salt content in the salted duck egg white and regulated the oil exudation in the egg yolk (Zou et al., 2018).
Due to extreme salty taste and high health risks, salted duck egg white is less popular with consumers than salted duck egg yolk (Venkatachalam & Nagarajan, 2019). Salted duck egg yolk was matured in 20–25 days with balanced texture and rheological properties (Ai et al., 2017). These eggs were purchased based on the graininess of the egg yolk and the taste of the oil, which was formed by the exudation of the free lipids from the egg yolk during the dehydration process (Xu et al., 2019). Therefore, lipids are a determining factor for high quality salted duck egg yolk. Duck egg yolk lipids comprise unique fatty acids, including arachidonic acid (ARA) and docosahexaenoic acid (DHA) (Juneja & Kim, 1996). Harlina et al. (2019) compared the effects of duck eggs supplemented with galangal extract with salted duck eggs, found that galangal extract significantly affected the composition of fatty acids, and the major fatty acids found in salted duck eggs supplemented with galangal extract were oleic acid, palmitic acid and margaric acid.
A more detailed analysis is needed to understand changes in egg yolk lipids during the pickling period. Taking this into consideration, in this study, lipidomic analysis of salted duck egg yolk was performed on non-targeted lipidomics analysis platform based on ultra performance liquid chromatography (UPLC) – Orbitrap mass spectrometry system combined with Orbitrap™ mass spectrometer (MS). This technique have become the most popular methods for fat authentication in high-throughput quantitative lipidomic research and has been previously used in green tea (Li et al., 2021) and salted tilapia analysis (Wu et al., 2020). The salted duck egg yolk combined with clove extract showed significant differences in ten glycerolipid subclasses compared to the salted duck egg yolk group (Harlina, Ma & Shahzad, 2021). However, few studies have paid detailed attention to the role of lipid molecules during duck egg yolk pickling, especially in the low-salt process.
In this paper, quality parameters, fatty acid composition and lipidomics of the salted duck egg yolk were determined to elucidate the effects of low-salt processing on the quality of salted duck egg yolk lipids. The research data may better explain the role of lipids of salted duck egg yolk during low-salt pickling at the molecular level.
Materials and methods
Materials
Fresh duck eggs with a weight of 60–75 g were provided by Tonglu Fuhen Cooperative (Hangzhou, Zhejiang Province, China).
Sample preparation
Salted duck eggs (DE-S) were pickled by traditional process, fresh duck eggs were sterilized and placed into 18% (w/v) NaCl solution for 35 d. The salt content of egg white in DE-S group was 5.5–6.5%. Low-salted duck eggs (DE-L) were prepared by chlorine dioxide pretreatment and a two-stage immersion method (Zou et al., 2018). Fresh duck eggs were washed with tap water, sterilized in 40 mg/L chlorine dioxide solution for 3 min, and then air-dried naturally. In the first pickling stage, the dried eggs were put in a ceramic pot, 18% (w/v) NaCl solution was poured in until all the eggs were submerged in brine, and the duck eggs were cured at 20–25 °C for 10 d. 6% NaCl solution was applied in the second stage, in which the duck eggs were cured at 20–25 °C for another 25 d. The salt content of egg white in DE-L group was 3.0–4.0%. The fresh duck eggs (DE-F) group was stored at the same temperature (20–25 °C) for 35 d. After pickling, all groups were steamed at 105 °C for 25 min to simulate cooking conditions under daily consumption, the duck eggs were cooled and subjected to further analysis.
Determination of viscosity, firmness, stickiness and oil exudation for DE-F, DE-L and DE-S
Egg white viscosity was determined using a viscometer (SNB-2, Jingtian Electronic Instrument Co., Ltd, Shanghai, China). The results were expressed as mPa·S. Firmness and stickiness of egg white were measured by texture analyzer (BROOKFIELD CT3, Middleboro, USA) equipped with TA44 probe, and the egg white was cut into 10 mm × 10 mm × 10 mm pieces by razor blade. The main parameters are as follows: test speed, 5.00 mm/s; target mode, strain (compression ratio, 75.00%; time, 5.00 s); trigger mode, button. Oil exudation of duck eggs in each group was analyzed by the method of Kaewmanee et al., (2009a) with some modifications. Accurately added 35 mL of n-hexane/2-propanol (3:2, v/v) to 3 g of cooked egg yolk, homogenized in a homogenizer at a speed of 5000 r/min for 10 min. After which the homogenate was filtered through a filter paper, and the filtrate was evaporated in a boiling water bath and dried to constant weight in an oven at 105 °C, the total lipid content could be obtained after weighing. To analyze the free lipid content, accurately weighed 5 g of egg yolk and mixed with 25 mL of distilled water, homogenized at 5000 r/min for 30 s, the homogenate was centrifuged at 5000 g and 25 °C for 30 min, the supernatant was collected and 25 mL of n-hexane/isopropanol (3:2, v/v) was added to dissolve the suspension to obtain the lipid layer. Evaporate the solvent of the lipid layer in a boiling water bath, and then dried to constant weight in an oven at 105 °C, and the free lipid content could be obtained after weighing. The formula for calculating the oil exudation of egg yolk is as follows:
Oil exudation (%) = × 100.
Determination of the microstructure of eggshell, shell membrane and yolk for DE-F, DE-L and DE-S
Microstructures of samples were analyzed by scanning electron microscope (HITACHI SU1510, Tokyo, Japan). The shell membrane was removed manually and dried with different concentrations of ethanol (50%, 70%, 80%, 90% and 100%). The samples were mounted on scanning electron microscope (SEM) stubs using a double backed cellophane tape, the stub and samples were coated with a thin gold layer.
The egg yolk was frozen, cut into 0.5 × 0.5 cm pieces and fixed in 2.5% glutaraldehyde and 0.2 M phosphate buffer (pH 7.2) at room temperature for 2 h. The yolk was rinsed with 0.2 M phosphate buffer (pH 7.2) for 15 min and fixed for another 2 h in 0.1% osmium solution at 25 °C, followed by dehydrating in different concentrations of ethanol (50%, 70%, 80%, 90% and 100%). The dehydrated sample was fixed on the stub of the scanning electron microscope (SEM) and a thin layer of gold was applied on the stub and the sample. (Kaewmanee, Benjakul & Visessanguan, 2009b).
FAs analysis for DE-F and DE-L
The extraction method of salted duck egg yolk lipid is as follows: 10 g sample was taken from 5 mixed salted duck egg yolks and was extracted with chloroform–methanol (2:1, v/v) solution. Under vacuum conditions, the extract was dried with a rotary evaporator to obtain lipid sample.
50 mg lipid sample was transferred to a 10 mL test tube, 1 mL 0.5 mol/L potassium hydroxide-methanol solution was added and mixed well, then the mixture was heated in a 60 °C water bath for 20 min until the oil droplets were completely dissolved. After cooling to 25 °C, 1 mL of 15% boron trifluoride-methanol solution was added in a 60 °C water bath for 20 min. After cooling, 3 mL of n-hexane solution was added to the solution and mixed well, stood for a while, the upper n-hexane solution was moved for filter, after which the filtrate was blown dry with nitrogen and constant volume with n-hexane. The fatty acids solution was stored in a refrigerator at −20 °C before further analysis.
FAs were determined by a GC-QQQ system (Agilent Technologies Inc., Palo Alto, CA) equipped with a TR-5MS capillary column (30 m × 0.2 mm inside diameter, 0.25 μm film thickness) by the method of Harlina et al. (2018). The injection volume was 1.0 μL. The temperature control procedure was as follows: The column oven was maintained at 40 °C for 1 min, increased to 150 °C at a rate of 10 °C/min, hold for 2 min, 10 °C/min to 220 °C, then 5 °C/min to 280 °C, hold for 3 min. Ultra-high purity helium was used as the carrier gas at a flow rate of 1.0 mL/min and a split ratio of 100:1. MS conditions were as follows: ion source, 230 °C; electron ionization source; electron energy, 70 eV; scan range, 35–450 mass units. Qualitative analysis was based on standard fatty acid methyl esters (FAME Mix C4-C24, catalog NO18919-1AMP, Sigma-Aldrich), and the content of fatty acid composition was calculated by peak area normalization method.
Lipidomics analysis for DE-F and DE-L
The salted duck yolk lipid extraction was conducted according to the method of Zhu et al., (2019) with some modifications. Briefly, after the samples were slowly thawed at 4 °C, 30 mg of each sample was weighed, and 200 µL of water, 240 µL of pre-cooled methanol and 800 µL of methyl tert-butyl ether were added in sequence, mixed by vortex, and then sonicated in a low-temperature water bath for 20 min, and placed at room temperature for 30 min. Centrifuge the mixture at 14,000g at 10 °C for 15 min, take the upper organic phase, dry with nitrogen, add 200 µL of 90% isopropanol/acetonitrile solution for mass spectrometry analysis, reconstitute, vortex, centrifuge at 14,000g at 10 °C for 15 min, and take the supernatant for injection analysis by using ultra-high-performance liquid chromatography (UHPLC) Nexera LC-30A equipped with Waters ACQUITY UPLC C·SH C18 column (100 mm × 2.1 mm, 1.7 µm) at 45 °C. Gradient elution using mobile phase A (acetonitrile:water = 6:4, v/v), mobile phase B (acetonitrile:isopropanol = 1:9, v/v), containing ammonium formate acetonitrile in water and ammonium formate acetonitrile, respectively, at a flow rate 300 µL/min and the injection volume was 3 µL.
After UHPLC separation, electrospray ionization (ESI) was used to acquire full scan data in positive and negative ion modes, respectively. 10 fragments were collected after each full scan (MS2 scan, HCD). MS1 had a resolution of 70,000 at M/Z 200, MS2 had a resolution of 17,500 at M/Z 200. Mix equal amounts of each group of samples to prepare quality control (QC) samples, which was used to balance the chromatography-mass spectrometry system and evaluate the stability of the system during the experiment by samples spectrum comparison.
Statistical analysis
Excel 2007 (Microsoft, USA) was used for analysis of variance. Duncan’s multiple range test was used to evaluate viscosity, firmness, stickiness and oil exudation differences between the DE-F, DE-L and DE-S groups. Statistical significance (p < 0.05) was considered significant. The LipidSearch software version 4.1 (Thermo Scientific™, MA, USA) was used to process lipidomics data for peak identification, lipid identification (secondary identification), peak extraction, peak alignment, and quantification. For the data extracted by LipidSearch, lipid molecules with >50% missing values in the group were deleted, and the data were normalized to the total peak area. The software SIMCA-P 14.1 (Umetrics, Umea, Sweden) was used for pattern recognition. After the data was preprocessed by Pareto-scaling, multidimensional statistical analysis was performed, including Principal Component Analysis (PCA), Partial Least Squares Discriminant Analysis (PLS-DA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA). Ri386 3.6.1 software map was employed for the heat map and hierarchical cluster analysis.
Results and discussion
Changes in quality parameters of DE-F, DE-L and DE-S
Changes in viscosity, firmness, stickiness and oil exudate of DE-F, DE-L and DE-S
The quality parameters in the DE-F, DE-L and DE-S were compared (Fig. 1). As shown in Fig. 1A, both the low-salt process and the traditional process significantly reduce the viscosity of salted egg whites, which were 4.1 and 3.9 mpa.s, respectively. This may be due to the fact that the bound water in the protein became free water. However, no difference was found between DE-L and DE-S (p > 0.05). Data for protein firmness and elasticity are shown in Fig. 1B and C, the firmness and elasticity of the three groups decreased with the increase of salinity, but did not reach statistical significance (p > 0.05). In this study, the oil exudate in the yolk of the DE-F group was extremely low (4.1 ± 0.3%), while in the pickled group (DE-L and DE-S), it was extremely high, which was 39.7% and 41.4%, respectively (p < 0.01). In addition, there was no significant between the low-salted and salted egg yolks. During the salting process, free lipids are released from egg yolk lipoprotein due to removal of moisture and increased salt concentration (Kaewmanee et al., 2009b). Kaewmanee et al. (2009a) reported that oil exudate from salted yolk increased with increasing salting time. These results suggested that although the low-salt process reduced the salt content, it still significantly increased the oil exudation from egg yolk.
Fig. 1.
Viscosity (A), Firmness (B), Stickiness (C) and Oil exudate (D) of DE-F, DE-L and DE-S.
Changes in the microstructure of DE-F, DE-L and DE-S
Microstructures of eggshell, inner membrane and egg yolk of DE-F, DE-L and DE-S were depicted in Fig. 2. Eggshell is mainly composed of calcareous hard shell and shell membrane. The eggshell contains small funnel-shaped holes on the surface of the shell for gas exchange known as pore canals, with the diameter ranges from 10 − 30 μm. An egg has about 7000–17,000 pore canals on the surface of each eggshell that allows the passage of salt and water into eggs during the salting process (Baker & Balch, 1962). No difference was observed in shell structures between DE-F and salted eggs (DE-L and DE-S), except for a crystalline particles layer on salted eggshells, which might be made up of salt particles (Fig. 2A, 2B and 2C).
Fig. 2.
Scanning electron microscopic photograph of shell (A–C), shell membrane (D–F) and yolk (G–I).
The inner membrane had a knitted net-like fibrous structure with a smooth and integrated surface (Fig. 2D), whereas the membranes of the cured duck eggs had a different fibrous matrix than fresh duck eggs (Fig. 2E and F). The inner membrane fibrous mesh structure scaffold of DE-L was partially fractured, with loose structure and uneven pore size (Fig. 2E), while DE-S showed a stronger degree of fracture and a looser structure compared to DE-L (Fig. 2F). Ovokeratin is the main component of the fibrous matrix (Hamilton, 1982). When sodium chloride enters the duck egg, proteins such as ovokeratin are denatured and hydrolyzed, which destroy the fibrous structures, disturb the fibrous threads and lose its smooth and complete structure. However, this altered fibrous structure is beneficial for the penetration and exchange of salt and water through egg whites during the salting process.
During the pickling process, the microstructure of the eggshell membrane loosened, making it conducive for the transportation of salt components into the egg yolk. This led to the disintegration of the lipoprotein structure due to dehydration. SEM analysis elucidated the polyhedral structure of fresh egg yolks granules ranged from 50 to 100 μm diameter (Fig. 2G–I). In a study by Yang & Cotterill, (1989) diameters of yolk granules of the salted duck egg yolks were found to be 23–127 μm, whereas Chi & Tseng (1998) reported it to be 90–100 μm. In this study, we observed that granules of salted duck egg yolk were denser than that of fresh egg yolk due to dehydration during the salting process (Fig. 2G–I), and DE-S showed a tighter state of protein particles compared with DE-L (Fig. 2H and I). These granules might have conferred gritty texture to egg yolks. Thus, we inferred that the stacking of granules is essential for gritty textured salted egg yolks. The polyhedral granules were formed by yolk spheres (Mineki & Kobayashi, 1997). The reason for the formation of a dense structure during egg curing is that the dehydration effect is more pronounced due to the closer position of the yolk particles. Moreover, the microstructure of the salted egg yolks showed many small bumps on the surface of the polyhedral due to lipid exudation, while these bumps were absent in fresh egg yolks.
In summary, the low-salt pickling process significantly changed the quality parameters of duck eggs, mainly manifested as a decrease in hardness, elasticity, and viscosity, an increase in the oil exudation the egg yolk, as well as the rupture of the inner membrane and denser egg yolk particles observed in the microstructure. Therefore, we conclude that the low-salt process can achieve the same effect as the traditional curing process, and reduce the intake of sodium salt, which is a healthy alternative. Therefore, the DE-L group will be studied in the remaining experiments.
Difference in the FAs content of egg yolk
FAs are produced by the hydrolysis and degradation of yolk lipids, which play important roles in living organisms’ processes. FAs are the source of energy, structural elements in membrane’s polar lipids and bioactive particles (Huang & Fang, 2018). GC–MS based analysis of fatty acids in egg yolks of DE-F and DE-L led to the identification of 16 fatty acids, of which four were saturated fatty acid (SFA), three were monounsaturated fatty acids (MUFA), and nine were polyunsaturated fatty acids (PUFA) (Table 1, Table 2). Among them, palmitic acid (C16:0), oleic acid (C18:1n9) and linoleic acid (C18:2n6) were the main fatty acids in salted duck egg yolk. These findings were in line with the previous study (Kaewmanee et al., 2009a), which showed that the content of FAs significantly increased in Anas platyrhucus duck eggs after the pickling process. According to the study of Sinanoglou, Strati & Miniadis-Meimaroglou (2011), MUFA account for the highest percentage of fatty acids in egg yolk, followed by SFA and PUFA, whereas in the current study, SFA, MUFA, and PUFA accounted for 24.35%, 46.42%, and 29.23% of egg yolk, respectively. After salting, levels of SFA, MUFA, and PUFA increased by 57.77%, 41.59%, and 23.20%, respectively.
Table 1.
The composition and content of fatty acid in different egg yolks.
| Types | Retention time (min) | DE-F (mg/g) | DE-L (mg/g) |
|---|---|---|---|
| C14:0 | 21.42 | 1.17 ± 0.06b | 1.61 ± 0.06a |
| C16:1n7 | 25.98 | 5.98 ± 1.11b | 9.01 ± 0.26a |
| C16:0 | 26.65 | 23.47 ± 10.15b | 40.86 ± 2.32a |
| C17:0 | 28.86 | 0.49 ± 0.04a | 0.59 ± 0.08a |
| C18:3n6 | 30.00 | 0.59 ± 0.13a | 0.51 ± 0.03a |
| C18:2n6 | 30.48 | 17.40 ± 2.06 a | 23.38 ± 2.89a |
| C18:1n9 | 30.80 | 62.39 ± 6.24b | 87.83 ± 0.47a |
| C18:0 | 31.24 | 11.13 ± 1.10a | 14.13 ± 0.06a |
| C20:4n6 | 33.98 | 12.49 ± 2.33a | 14.42 ± 0.05a |
| C20:5n3 | 34.11 | 1.35 ± 0.16a | 1.67 ± 0.04a |
| C20:3n6 | 34.34 | 1.26 ± 0.08a | 1.48 ± 0.02a |
| C20:2n6 | 34.77 | 0.67 ± 0.15b | 1.034 ± 0.04a |
| C20:1n9 | 34.89 | 0.74 ± 0.12b | 1.01 ± 0.02a |
| C22:6n3 | 37.72 | 6.31 ± 0.89a | 7.25 ± 0.20a |
| C22:5n3 | 37.84 | 1.80 ± 0.25a | 1.65 ± 0.05a |
| C22:4n7 | 38.00 | 1.65 ± 0.03b | 2.22 ± 0.07a |
| Total FAs | 148.84 ± 16.87b | 208.61 ± 13.97a | |
| PUFA | 43.50 ± 0.86b | 53.59 ± 2.50a | |
| MUFA | 69.10 ± 5.01b | 97.84 ± 13.95a | |
| SFA | 36.25 ± 11.00b | 57.19 ± 2.60a |
Note: Different letters in the same line indicate significant difference from fresh duck eggs (p < 0.05).
Table 2.
Significantly different lipids in DE-F and DE-L groups.
| Lipid Group | Class | Fatty Acid | RT-(min) | VIP | P value |
|---|---|---|---|---|---|
| LPE(16:0) + H | LPE | (16:0) | 2.71 | 1.86153 | 0.008 |
| LPE(18:1) + H | LPE | (18:1) | 2.87 | 1.03624 | 0.002 |
| LPE(18:0) + H | LPE | (18:0) | 3.87 | 1.84414 | 0.036 |
| LPC(16:1) + H | LPC | (16:1) | 2.06 | 1.0893 | 0.003 |
| LPC(16:0) + H | LPC | (16:0) | 2.59 | 6.10154 | 0.001 |
| LPC(18:2) + H | LPC | (18:2) | 2.19 | 1.94747 | 0.003 |
| LPC(18:1) + H | LPC | (18:1) | 2.74 | 3.74652 | 0.001 |
| LPC(18:0) + H | LPC | (18:0) | 3.67 | 3.0546 | 0.022 |
| LPE(22:5) + H | LPE | (22:5) | 2.49 | 1.22017 | 0.000 |
| LPC(18:2) + Na | LPC | (18:2) | 2.19 | 1.1994 | 0.000 |
| LPC(20:4) + H | LPC | (20:4) | 2.11 | 1.82073 | 0.006 |
| LPC(20:3) + H | LPC | (20:3) | 3.69 | 1.33263 | 0.001 |
| LPC(20:4) + Na | LPC | (20:4) | 2.11 | 1.19202 | 0.001 |
| LPC(22:5) + H | LPC | (22:5) | 2.39 | 1.38921 | 0.001 |
| PC(36:5) + H | PC | (16:1/20:4) | 9.23 | 1.38895 | 0.015 |
| PC(36:4) + H | PC | (22:3/14:1) | 11.61 | 1.11463 | 0.015 |
| PC(36:4) + H | PC | (16:0/20:4) | 10.59 | 3.06373 | 0.002 |
| PC(36:2) + H | PC | (36:2) | 12.19 | 2.3184 | 0.046 |
| PC(40:4) + H | PC | (18:1/22:3) | 11.77 | 1.2129 | 0.034 |
| TG(52:5) + NH4 | TG | (16:0/18:2/18:3) | 18.29 | 3.84935 | 0.031 |
| TG(52:2) + NH4 | TG | (16:0/18:1/18:1) | 21.86 | 3.18747 | 0.000 |
| TG(54:5) + NH4 | TG | (18:1/18:2/18:2) | 19.24 | 2.23518 | 0.011 |
| FA(22:5)-H | FA | (22:5) | 4.91 | 1.05757 | 0.004 |
| LPE(16:1)-H | LPE | (16:1) | 2.12 | 1.1889 | 0.000 |
| LPE(16:0)-H | LPE | (16:0) | 2.73 | 6.92187 | 0.010 |
| LPE(18:2)-H | LPE | (18:2) | 2.27 | 3.47219 | 0.000 |
| LPE(18:1)-H | LPE | (18:1) | 2.87 | 5.40403 | 0.002 |
| LPE(18:0)-H | LPE | (18:0) | 3.86 | 8.71497 | 0.015 |
| LPG(16:0)-H | LPG | (16:0) | 2.16 | 1.85184 | 0.000 |
| LPE(20:4)-H | LPE | (20:4) | 2.18 | 3.01481 | 0.004 |
| LPG(18:0)-H | LPG | (18:0) | 2.95 | 1.61539 | 0.000 |
| LPE(22:6)-H | LPE | (22:6) | 2.08 | 2.91207 | 0.000 |
| LPE(22:5)-H | LPE | (22:5) | 2.47 | 5.36971 | 0.000 |
| LPE(22:4)-H | LPE | (22:4) | 2.78 | 2.47059 | 0.002 |
| LPC(16:1) + HCOO | LPC | (16:1) | 2.04 | 1.79075 | 0.002 |
| LPC(16:0) + HCOO | LPC | (16:0) | 2.59 | 9.24107 | 0.001 |
| LPC(18:2) + HCOO | LPC | (18:2) | 2.20 | 2.53296 | 0.003 |
| LPC(18:1) + HCOO | LPC | (18:1) | 2.74 | 4.2849 | 0.000 |
| LPC(18:0) + HCOO | LPC | (18:0) | 3.68 | 5.0018 | 0.006 |
| LPC(20:4) + HCOO | LPC | (20:4) | 2.10 | 2.10542 | 0.010 |
| LPC(22:5) + HCOO | LPC | (22:5) | 2.39 | 1.76682 | 0.002 |
| LPI(20:4)-H | LPI | (20:4) | 1.73 | 1.0812 | 0.000 |
| Cer(d40:1) + HCOO | Cer | (d18:1/22:0) | 14.23 | 2.22993 | 0.046 |
| Cer(d42:3) + HCOO | Cer | (d18:1/24:2) | 13.23 | 1.44861 | 0.000 |
| Cer(d42:2) + HCOO | Cer | (d18:1/24:1) | 14.15 | 1.46441 | 0.000 |
| Cer(d42:1) + HCOO | Cer | (d18:1/24:0) | 15.39 | 2.1403 | 0.016 |
| PI(36:4)-H | PI | (16:0/20:4) | 8.85 | 1.27834 | 0.028 |
| SQDG(40:2) + HCOO | SQDG | (40:2) | 2.64 | 1.26151 | 0.007 |
| CerG2GNAc1(d32:1)-H | CerG2GNAc1 | (d32:1) | 2.59 | 2.1144 | 0.002 |
| CerG2GNAc1(d34:2)-H | CerG2GNAc1 | (d34:2) | 2.65 | 1.0147 | 0.001 |
C16:0 and stearic acid (C18:0) were the most abundant SFAs with the content of 23.47 ± 10.15 mg/g and 11.13 ± 1.10 mg/g of egg yolk before salting and 40.86 ± 2.32 mg/g and 14.13 ± 0.06 mg/g of egg yolk after salting, respectively, of which the content of C16:0 increased most significantly. Besides, C18:1n9 and C16:1n7 were the most abundant MUFAs, which accounted for 62.39 ± 6.24 mg/g and 5.98 ± 1.11 mg/g before salting, and 87.83 ± 0.47 mg/g and 9.01 ± 0.26 mg/g after salting, respectively.
Nine PUFA, including essential FAs n-3 and n-6, were also detected in GC–MS-based analysis of yolk lipid. After salting, the content of FAs n3, such as docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA), was increased from 9.46 ± 0.98 mg/g to 10.56 ± 0.28 mg/g, but the difference between them was not significant (p > 0.05), while the content of n6 FAs, such as linoleic acid (LA, C18:2n6) and arachidonic acid (ARA), was increased significantly from 32.40 ± 0.09 mg/g to 40.81 ± 2.86 mg/g (p < 0.05). Petrović et al. (2012) reported that LA and alpha linolenic acid (ALA) (C18:3n3) are essential fatty acids as the human body could not synthesize them. LA and ALA are converted into n6 and n3 long-chain PUFAs and eicosanoids that participate in multiple physiological functions by undergoing desaturation and elongation. LA is considered to be the main n6 PUFA, which undergoes desaturation and elongation enzymatic reactions similar to ALA. Therefore, it can be transformed into ARA (an important n6 PUFA in brain cells) and DPA n6 (Domenichiello et al., 2016).
DHA (C22:6n3) is also an essential PUFA that cannot be synthesized by human body. Duck egg yolk is a good source of DHA (6.31 ± 0.89 mg/g). After 30 days of curing, the DHA concentration in duck egg yolk increased to 7.25 ± 0.20 mg/g. In general, the PUFA level in egg yolk was higher than MUFA and SFA, and the n6 PUFA level was higher than n3 PUFA. PUFA and MUFA have a beneficial effect on cardiovascular diseases as they can increase levels of high-density lipoprotein (Huang & Fang, 2018). After salting, the contents of PUFA and MUFA increased (Table 1), thus increasing the nutritional value.
Analysis of differences in lipid properties of egg yolk
The permutation test of the PLS-DA model in the DE-F and DE-L groups was depicted in Fig. S1A, in which the R2Y and Q2 indicated the reliability of the model. The PLS-DA score of DE-F and DE-L groups was shown in Fig. S1B. The principal component t[1] could be used to distinguish the lipid molecules of DE-F and DE-L, which were distributed on the left and right sides of the principal component t[1]. The results demonstrated that the validity of the PLS-DA model in this data.
A comprehensive landscape of lipid species in duck egg yolk was established using lipidomics profiling to analyze the individual lipid members. In our study, a total of 29 lipid classes were identified by positive and negative ion models, including 1343 lipid species. In the identified lipids, 43.93% were phospholipids, which accounted for 590 lipid species in 12 lipid classes, 38.79% were glycerides, which accounted for 521 lipid species, and 2 others belonged to different lipid classes. Sphingolipids, sugar esters, and fatty acids accounted for 17.97%, 0.89% and 0.45%, respectively (Fig. S2). In a study by Mi, Shang, Zhang & Fan (2019), 1633 lipid species (43.78% glycerophospholipids, 25.66% glycerolipids, 16.66% fatty acyls, 6.86% sphingolipids and so on) in three types of egg yolks were identified based on LC-MS/MS lipidomics method.
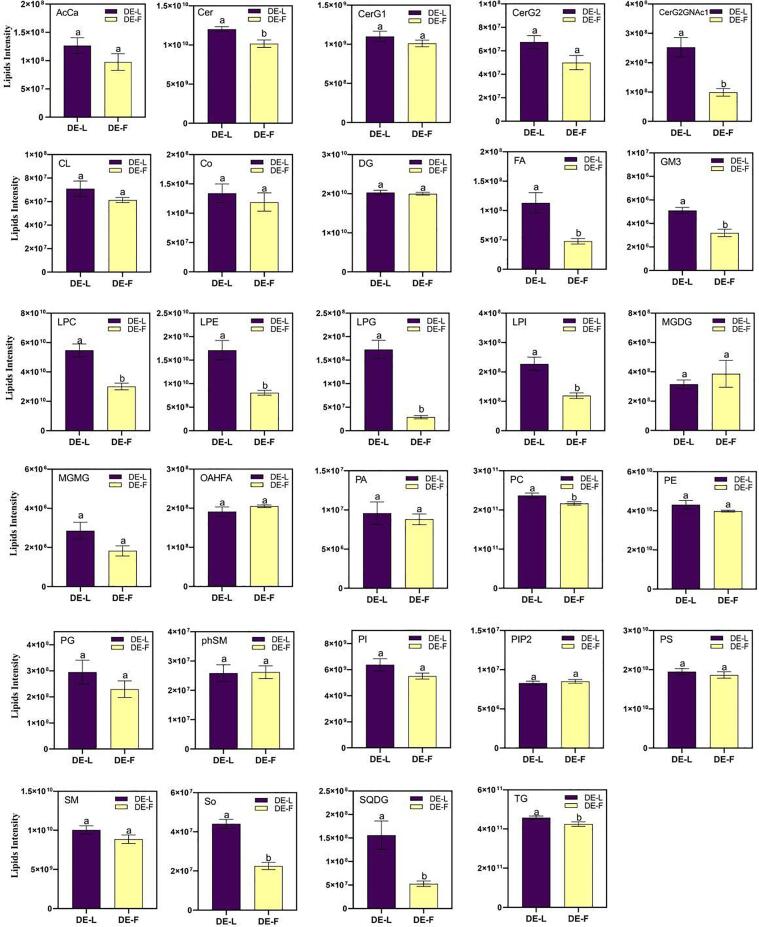
The relative content of 29 lipid classes differed in low-salted and fresh duck eggs (Fig. 3). Twelve lipid classes between DE-F and DE-L lipids were observed to have significant differences (p < 0.05). In these 12 lipid classes, 5 classes belonged to phospholipids (phosphatidylcholine, PC; lysophosphatidylcholine, LPC; lysophosphatidylethanolamine, LPE; lysophosphatidylinositol, LPI; lysophosphatidylglycerol, LPG), 1 to glycerides (triglycerides, TG), 3 to sphingolipids (Cer So, GM3), 1 to glycolipids (thioisorhamnose diglyceride, SQDG), 1 to fatty acids (fatty acid, FA), and 1 to other lipid classes (CerG2GNAc1). Phospholipids are the basic components of the cell membrane, nuclear membrane and plastid membrane in plants and animals. As per a previous report, phospholipids account for 31% of egg yolk lipids, 73% of which is PC, followed by phosphatidylethanolamine (PE, 15%) and LPC (5.8%) (Mine & Kovacs-Nolan, 2004). Sphingolipids are a class of complex compounds with sphingosine as the skeleton, which could be categorized into Cer, GM3 and ceramide (Wang et al., 2021). Cer has been detected in dairy products, such as cow and donkey milk, and has important nutritional value in the development and utilization of dairy products (Potočki, 2016, Qu et al., 2016). In this work, the low-salt pickling method significantly affected the lipid composition of duck egg yolk, especially phospholipids and sphingolipids.
Fig. 3.
The changes of 29 lipid classes between DE-F and DE-L groups. Note: Different letters indicate significant difference from fresh duck eggs (p < 0.05).
The Variable Importance in Projection (VIP) values based on the OPLS-DA model (Fig. S3) can be used to estimate the influence of lipid expression patterns in the classification and discrimination of each group of samples, and to discover different lipids with biological significance, in which the standard of the differential lipids is VIP value >1 and p value <0.05. As shown in Table 2, a total of 50 lipid species were defined as differentially expressed lipids. It indicated that pickling increased the level of differential lipids in duck eggs, which was in line with the level of oil yield of the egg yolks. Hierarchical Cluster Analysis was used to more comprehensively visualize the relationship between the two groups and the differences in lipid expression patterns among the groups. The lipid Hierarchical Clustering results showed significant differences between fresh and low-salted duck eggs (Fig. 4). Fold Change Analysis (FC Analysis) was used to analyze the differences between DE-F and DE-L groups (Fig. S4). Apart from PC (22:3/14:1), PC (36:2) and TG (16:0/18:1/18:1), DE-L showed higher intensity on the other significant difference lipid than DE-F.
Fig. 4.
The lipid hierarchical clustering heat map depicting significant differences in DE-F and DE-L groups.
Lipids, such as phospholipids and some neutral lipids, are exudated from duck eggs during the salting process (Xu et al., 2019). Cheng et al. (2018) reported that salt-induced lipid exudation from egg yolk and the water exudation from egg white might be due to the increased lipid and water freedom. In this research, we also found that most differential lipid species showed a higher intensity compared to DE-F after pickling. Among them, the 39 lipid species significantly affected by low-salt processing were phospholipids, which were categorized as differential lipids. This may explain the increase in the proportion of SFA in FA after pickling, because most phospholipids are saturated fatty acids (Bernardo, Videira, Valentão, Veiga & Andrade, 2019). PC, an important phospholipid component in egg yolk, provides nourishment and protection in humans. Chen et al. (2019) reported that egg yolk PC could protect nerves as it entails a high level of unsaturated fatty acids. LPE could be synthesized from phosphatidylethanolamine (PE) via a phospholipase A-type reaction (Makide et al., 2009). LPE affect the growth, development, and post-harvest life of exogenously treated horticultural crops (Amaro & Almeida, 2013). LPC (C16:0) is the most abundant phospholipids in egg yolk of duck, hen and quail egg (Ali et al., 2017). In a study by Wang et al., (2017) LPC supplemented diet was found to be beneficial for increasing the level of omega-6 PUFA in the hen’s egg yolk. The addition of NaCl resulted in an increase in the total phospholipid content from 20.95 μg mg−1 to 28.28 μg mg−1 during the curing of low-salt salmon (Wang et al., 2021). Zhang, Xiujuan, Xiaoliang, Hui & Yanping (2021) reported that the increase of PC may be due to the destruction of cell membrane, and the change trend of LPC and LPE was similar to PC and PE during flaxseed oil roasting. In this study, the lipidomics data of the DE-L group and the DE-F group were significantly different, especially the phospholipids data of LPC, LPE and PC classes after low-salt pickling and the levels of LPC and LPE were increased. This may be due to the rupture of cell membranes due to lipid oxidation during low-salt solidification, resulting in an increase in LPC and LPE. Thus, this study revealed that phospholipids played an important role during the pickling period, which might lead to the formation of the unique quality of salted duck egg yolk.
Conclusion
Our results showed that DE-L and DE-S have similar quality characteristics, so the low-salt process is a relatively healthy alternative. After salting, the contents of SFA, MUFA and PUFA increased by 57.77%, 41.59%, and 23.20%, respectively. The lipidomics data demonstrated that PC, LPC and LPE were the main differential lipids during the low-salt pickling process of egg yolks. Also, as per the lipidomics data, phospholipids could alter the quality of salted duck egg yolks during the pickling process, especially PC, LPC and LPE lipid classes. Thus, we speculate that phospholipids were responsible for the unique quality of salted duck egg yolk. However, the underlying mechanism is unclear and requires further investigation.
CRediT authorship contribution statement
Zou Ligen: Conceptualization, Data curation, Investigation, Writing – review & editing. Wang Qian: . Weng Liping: Data curation, Validation. Wang Tenghao: Investigation. Qiu Jing: Formal analysis, Data curation. Liu Junbo: Formal analysis. Jiang Huiyan: Investigation, Resources. Wu Yuanfeng: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors gratefully acknowledge the financial support of Hangzhou Social Development Research Program (20160533B89), Major science and technology projects of Hangzhou Academy of Agricultural Sciences (2022HNCT-12).
Availability of Data and Material
All data can be shared if required.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100502.
Contributor Information
Zou Ligen, Email: jgszlg@163.com.
Wang Qian, Email: 1310259949@qq.com.
Weng Liping, Email: lipingweng@163.com.
Wang Tenghao, Email: wangth85@126.com.
Qiu Jing, Email: scqiujing@126.com.
Liu Junbo, Email: junbliu@126.com.
Jiang Huiyan, Email: jgsjhy@163.com.
Wu Yuanfeng, Email: wuyuanfeng@zju.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
Data availability
Data will be made available on request.
References
- Ai M., Guo S., Zhou Q., Wu W., Jiang A. The investigation of the changes in physicochemical, texture and rheological characteristics of salted duck egg yolk during salting. Lwt-Food Science and Technology. 2017;88:119–125. doi: 10.1016/j.lwt.2017.10.013. [DOI] [Google Scholar]
- Ali A.H., Zou X., Lu J., Abed S.M., Yao Y., Tao G.…Wang X. Identification of phospholipids classes and molecular species in different types of egg yolk by using UPLC-Q-TOF-MS. Food Chemistry. 2017;221:58–66. doi: 10.1016/j.foodchem.2016.10.043. [DOI] [PubMed] [Google Scholar]
- Amaro A.L., Almeida D.P.F. Lysophosphatidylethanolamine effects on horticultural commodities: A review. Postharvest Biology and Technology. 2013;78:92–102. doi: 10.1016/j.postharvbio.2012.12.011. [DOI] [Google Scholar]
- Baker J.R., Balch D.A. A study of the organic material of hen's-Egg shell. Biochemical Journal. 1962;82(2):352–361. doi: 10.1042/bj0820352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo J., Videira R.A., Valentão P., Veiga F., Andrade P.B. Extraction of phospholipid-rich fractions from egg yolk and development of liposomes entrapping a dietary polyphenol with neuroactive potential. Food and Chemical Toxicology. 2019;133 doi: 10.1016/j.fct.2019.110749. [DOI] [PubMed] [Google Scholar]
- Chen J., Lin S., Sun N., Bao Z., Shen J., Lu X. Egg yolk phosphatidylcholine: Extraction, purification and its potential neuroprotective effect on PC12 cells. Journal of Functional Foods. 2019;56:372–383. doi: 10.1016/j.jff.2019.03.037. [DOI] [Google Scholar]
- Cheng S., Zhang T., Wang X., Song Y., Wang H., Wang H.…Tan M. Influence of salting processes on water and lipid dynamics, physicochemical and microstructure of duck egg. Lwt-Food Science and Technology. 2018;95:143–149. doi: 10.1016/j.lwt.2018.04.074. [DOI] [Google Scholar]
- Chi S., Tseng K. Physicochemical properties of salted pickled yolks from duck and chicken eggs. Journal of Food Science. 1998;63(1):27–30. doi: 10.1111/j.1365-2621.1998.tb15668.x. [DOI] [Google Scholar]
- Domenichiello A.F., Kitson A.P., Chen C.T., Trépanier M., Stavro P.M., Bazinet R.P. The effect of linoleic acid on the whole body synthesis rates of polyunsaturated fatty acids from α-linolenic acid and linoleic acid in free-living rats. The Journal of Nutritional Biochemistry. 2016;30:167–176. doi: 10.1016/j.jnutbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Du M., Sun Z., Liu Z., Yang Y., Liu Z., Wang Y.…Liu C. High efficiency desalination of wasted salted duck egg white and processing into food-grade pickering emulsion stabilizer. LWT. 2022;161 doi: 10.1016/j.lwt.2022.113337. [DOI] [Google Scholar]
- Guo X., Di W., Zhou B., Chen Z., Li B., Wang S.…Liang H. Reinforced pickering emulsions stabilized by desalted duck egg white nanogels with Ca2+ as binding agents. Food Hydrocolloids. 2021;121 doi: 10.1016/j.foodhyd.2021.106974. [DOI] [Google Scholar]
- Hamilton R.M.G. Methods and factors that affect the measurement of egg shell quality. Poultry Science. 1982;61(10):2022–2039. doi: 10.3382/ps.0612022. [DOI] [Google Scholar]
- Harlina P.W., Ma M., Shahzad R., Gouda M.M., Qiu N. Effect of clove extract on lipid oxidation, antioxidant activity, volatile compounds and fatty acid composition of salted duck eggs. Journal of Food Science and Technology. 2018;55(12):4719–4734. doi: 10.1002/ejlt.202000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlina P.W., Ma M., Shahzad R. Quantification of lipidomics profiling using UPLC-QE-HESI- lipid analysis on the salted duck egg incorporated with clove extract. European Journal of Lipid Science and Technology. 2021;123(4):2000284. [Google Scholar]
- Harlina P.W., Shahzad R., Ma M., Wang N., Qiu N. Effects of galangal extract on lipid oxidation, antioxidant activity and fatty acid profiles of salted duck eggs. Journal of Food Measurement and Characterization. 2019;13(3):1820–1830. doi: 10.1007/s11694-019-00100-z. [DOI] [Google Scholar]
- Huang J., Fang T.J. Use of recycled mustard pickle brine in the production of salted duck eggs. International Journal of Food Engineering. 2018;14(3):1–9. doi: 10.1515/ijfe-2017-0278. [DOI] [Google Scholar]
- Juneja L.R., Kim M. CRC Press; Boca Raton: 1996. Egg yolk proteins. [Google Scholar]
- Kaewmanee T., Benjakul S., Visessanguan W. Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chemistry. 2009;112(3):560–569. doi: 10.1016/j.foodchem.2008.06.011. [DOI] [Google Scholar]
- Kaewmanee T., Benjakul S., Visessanguan W. Effect of salting processes on chemical composition, textural properties and microstructure of duck egg. Journal of the Science of Food and Agriculture. 2009;89(4):625–633. doi: 10.1002/jsfa.3492. [DOI] [PubMed] [Google Scholar]
- Li J., Hua J., Yuan H., Deng Y., Zhou Q., Yang Y.…Jiang Y. Investigation on green tea lipids and their metabolic variations during manufacturing by nontargeted lipidomics. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.128114. [DOI] [PubMed] [Google Scholar]
- Makide K., Kitamura H., Sato Y., Okutani M., Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins & Other Lipid Mediators. 2009;89(3–4):135–139. doi: 10.1016/j.prostaglandins.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Mi S., Shang K., Zhang C., Fan Y. Characterization and discrimination of selected chicken eggs in China’s retail market based on multi-element and lipidomics analysis. Food Research International. 2019;126 doi: 10.1016/j.foodres.2019.108668. [DOI] [PubMed] [Google Scholar]
- Mine Y., Kovacs-Nolan J. Biologically Active Hen Egg Components in Human Health and Disease. Journal of Poultry Science. 2004;41:1–29. doi: 10.2141/jpsa.41.1. [DOI] [Google Scholar]
- Mineki M., Kobayashi M. Microstructure of yolk from fresh eggs by improved method. Journal of Food Science. 1997;62(4):757–761. [Google Scholar]
- Petrović M., Gačić M., Karačić V., Gottstein Ž., Mazija H., Medić H. Enrichment of eggs in n-3 polyunsaturated fatty acids by feeding hens with different amount of linseed oil in diet. Food Chemistry. 2012;135(3):1563–1568. doi: 10.1016/j.foodchem.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Potočki S. Potential health benefits of sphingolipids in milk and dairy products. Mljekarstvo. 2016:251–261. doi: 10.15567/mljekarstvo.2016.0401. [DOI] [Google Scholar]
- Qu S., Barrett-Wilt G., Fonseca L.M., Rankin S.A. A profile of sphingolipids and related compounds tentatively identified in yak milk. Journal of Dairy Science. 2016;99(7):5083–5092. doi: 10.3168/jds.2015-10431. [DOI] [PubMed] [Google Scholar]
- Sinanoglou V.J., Strati I.F., Miniadis-Meimaroglou S. Lipid, fatty acid and carotenoid content of edible egg yolks from avian species: A comparative study. Food Chemistry. 2011;124(3):971–977. doi: 10.1016/j.foodchem.2010.07.037. [DOI] [Google Scholar]
- Venkatachalam K., Nagarajan M. Assessment of different proteases on degree of hydrolysis, functional properties and radical scavenging activities of salted duck egg white hydrolysate. Journal of Food Science and Technology. 2019;56(6):3137–3144. doi: 10.1007/s13197-019-03645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang H.J., Wang X.C., Wu S.G., Wang J., Xu L., Qi G.H. Dietary choline and phospholipid supplementation enhanced docosahexaenoic acid enrichment in egg yolk of laying hens fed a 2% Schizochytrium powder-added diet. Poultry Science. 2017;96(8):2786–2794. doi: 10.3382/ps/pex095. [DOI] [PubMed] [Google Scholar]
- Wang J., Honghai W., Weibo L., Min Z., Jing X., Xina Y.…Haixing W. Low-salted salmon: Effects of salt reduction on physicochemical, lipidomic, and sensory characteristics. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112311. [DOI] [Google Scholar]
- Wu T., Guo H., Lu Z., Zhang T., Zhao R., Tao N.…Zhong J. Reliability of LipidSearch software identification and its application to assess the effect of dry salting on the long-chain free fatty acid profile of tilapia muscles. Food Research International. 2020;138(Part:B), 109791. doi: 10.1016/j.foodres.2020.109791. [DOI] [PubMed] [Google Scholar]
- Xu L., Zhao Y., Xu M., Yao Y., Wu N., Du H., Tu Y. Changes in physico-chemical properties, microstructure, protein structures and intermolecular force of egg yolk, plasma and granule gels during salting. Food Chemistry. 2019;275:600–609. doi: 10.1016/j.foodchem.2018.09.078. [DOI] [PubMed] [Google Scholar]
- Yang S.S., Cotterill O.J. Physical and functional properties of 10% salted egg yolk in mayonnaise. Journal of Food Science. 1989;54(1):210–213. doi: 10.1111/j.1365-2621.1989.tb08603.x. [DOI] [Google Scholar]
- Yu Z., Guo H., Liu C., Wang R., Zhang L., Zhang X., Chen Y. Ultrasound accelerates pickling of reduced-sodium salted duck eggs: An insight into the effect on physicochemical, textural and structural properties. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111318. [DOI] [PubMed] [Google Scholar]
- Zhang D., Xiujuan L., Xiaoliang D., Hui S., Yanping C. Lipidomics reveals the changes in lipid profile of flaxseed oil affected by roasting. Food Chemistry. 2021;364 doi: 10.1016/j.foodchem.2021.130431. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wang K., Wu C., Zhao Y., Yin X., Zhang B.…Xu C. Effect of Ethylene on Cell Wall and Lipid Metabolism during Alleviation of Postharvest Chilling Injury in Peach. Cells. 2019;8(12):1612. doi: 10.3390/cells8121612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Zhao Y., Qiu J., Weng L., Liu J., Jiang H. Optimization of process parameters in two-stage brining of salted eggs with low NaCl content. International Journal of Food Engineering. 2018;4(3):200–205. doi: 10.18178/ijfe.4.3.200-205. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.
All data can be shared if required.