Abstract
The objective of this study was to understand the effects of processing on metabolites, flavonoids, black rice pigments and total antioxidant capacity of purple grains. The biochemical indicators and metabolites were determined before and after processing of purple grains. The results showed that the total antioxidant capacity, total phenol (TP), flavonoid (PD), oligomeric proanthocyanidin (OPC), ascorbic acid (AsA), cyanidin-3-O-glucoside (C3OG), peonidin 3-glucoside (P3G) contents of purple grains were greatly decreased after brown rice grains were processed into polished rice grains. The TP, PD, OPC, AsA, C3OG, and P3G of Yangzinuo No.1 brown rice (YZN1_B) or polished rice grains (YZN1_H) were higher than those of Yangzinuo No.2 brown rice (YZN2_B) or polished rice grains (YZN2_H). 154 differential metabolites (DMs) were identified between YZN1_B and YZN1_H. 52 DMs were identified between YZN2_B and YZN2_H. Citric acid and isocyanate are key metabolites affected during processing and have good correlations with various biochemical indicators.
Keywords: Purple rice, Metabolomic, Flavonoids, Total antioxidant capacity, Black rice pigment
Introduction
Rice is the largest staple food crop in China, and its cultivation area accounts for approximately 30 % of food crops (Bai et al., 2017). Among the rice germplasm resources in the Chinese genebank, black rice, purple rice, red rice, fragrant rice and other special rice germplasm resources with special traits, such as coloured seed coat and aroma, account for approximately 10 % (Bai et al., 2017). Jiangsu, Zhejiang, Hebei, Jiangxi, Sichuan and Guizhou provinces in China are the main provinces for planting colored rice. Purple brown rice contains some physiologically active substances that are beneficial to human health, such as flavonoids, phenols, vitamins, mineral elements, alkaloids, chancroid, and anthocyanins. After people eat purple rice or its active substances extracted by deep processing, they can balance the body nutrition, prevent disease occurrence and promote disease recovery (Hu and Hu, 2021, Yamuangmorn and Prom-u-Thai, 2021). The physiologically active components of high nutritional value in cereal crops are concentrated in the aleurone layer, and 20 % of the pure aleurone layer is equivalent to the nutrition of the whole grain (Zhang, 2021, Zhang et al., 2022). During rice processing, the aleurone layer of rice is damaged by human factors (Ma et al., 2022). Grinding is one of the important links in the processing of rice grains. Moderate grinding can help improve the taste and appearance of rice. As the degree of milling increased, the colour of black rice was significantly reduced, which also means that the anthocyanin content continuously decreased (Sapna et al., 2019). The anthocyanin content loss was not significant when the milling degree was 2 %. The content of total anthocyanins remained high when the milling degree was 2 %-4%, which had good taste quality. The loss rate of total anthocyanin content was 57 %-90 % when the milling degree was 4 %-7% (Ma et al., 2020). The loss rate of anthocyanin content was almost 100 % when the milling degree was 9 % (Paiva et al., 2014). Therefore, the milling of rice grains has caused the loss of nutrient components to varying degrees (Bagchi et al., 2021, Mohidem et al., 2022), especially in the milling of colored rice (Hu et al., 2022).
In summary, research has shown that during the milling and processing of brown rice grains into polished rice grains, the tissue structure and metabolic components of the rice grains undergo significant changes. The types and levels of metabolites are correlated with the nutrients of crops (Xu et al., 2019). At present, the large-scale detection of molecular metabolite species and quantitative changes using metabolomic technology has gradually increased (Xiong et al., 2022, Zhan et al., 2022). Based on metabolomic technology, previous studies explored the metabolic processes of nutrients in crops (wheat, rice, and maize) and fruits (grapes, bananas, and mangoes) and found complementary patterns of nutrients (Shi et al., 2022). In previous research, our team used metabolomic technology and found that the antioxidant capacity of purple rice was higher than that of white rice, and the flavonoids and phenol metabolites were closely related to the antioxidant capacity (Xiong et al., 2022b). The metabolites related to the nutritional components of purple rice were further analysed, and the regulatory roles of key metabolites in metabolic pathways were elucidated (Xiong et al., 2022c). The effects of processing on the nutritional and metabolic components of purple glutinous rice deserve further study.
The purpose of this study was to investigate the effects of milling on the grains black rice pigment content, flavonoid (PD) content, oligomeric proanthocyanidin (OPC) content, total phenol (TP) content, total antioxidant capacity and metabolites in the purple glutinous rice varieties. In this study, two purple glutinous rice varities (Yangzinuo No.1 and Yangzinuo No.2) independently selected by our team. The loss of nutrients during grain processing was further analysed, and the potential metabolic markers were identified during the process of purple glutinous brown rice grains milling into polished rice grains. This study provides a theoretical basis for cultivating special functional rice varieties with excellent nutritional quality.
Materials and methods
Plant materials and growth conditions
The experimental materials were Yangzinuo No.1 (YZN1) and Yangzinuo No.2 (YZN2), which were cultivated at the Agricultural College of Yangzhou University. The experimental materials were planted in Shatou Base, Guangling District, Yangzhou University. The seedlings were transplanted 20 days after rice sowing. Each variety was planted with three replicates.
Sample collection
After the rice grains were mature and harvested, the YZN1 and YZN2 samples were processed into brown rice grains and polished rice grains, respectively (polished once). After harvesting, the two purple rice varieties were naturally dried to 14 % of the rice grain water content, and 3 biological replicates were set up. NP-4350 air separator (Jining, China) was used to screen plump grains. The rice grain sheller was used to hull the grains. The brown rice grains samples are collected (including 3 biological replicates), and then the LTJM-2099 rice miller (Changzhou, China) was used to grind the brown rice grains into polished rice grains (including 3 biological replicates). Brown rice grains and polished rice grains are the samples for the determination of indicators in this experiment. The brown rice grains and polished rice grains of Yangzinuo No.1 were labelled YZN1_B and YZN1_H, respectively, and the brown rice grains and polished rice grains of Yangzinuo No.2 were labelled YZN2_B and YZN2_H, respectively. Each variety was sampled for 3 biological replicates.
Determination of antioxidant capacity and flavonoid content
First, all rice grain samples were ground into powder. Weigh 0.1000 samples, add 1 mL of extraction solution for ice bath homogenization, then centrifuge at 4℃ for 10 min with 10000g. Take the supernatant and place it on ice for testing. Trolox was used as the control system to quantify the antioxidant capacity of antioxidant substances. ABTS (2 '- azinobis - (3-ethylbenzthiazoline-6-sulfonate) method was used to determine the total antioxidant capacity of rice grain samples (Zorzi et al., 2020). DPPH (2,2-diphenyl-1-picrylhydrazyl) method was used to determine the total antioxidant capacity of rice grain samples (Cömert et al., 2020). FRAP (ferric ion reducing antioxidant power) method was used to determine the total antioxidant capacity of rice grain samples (Abeyrathne et al., 2021). Refer to the method of Chu et al. (2019) to determine the oligomeric proanthocyanidin (OPC) content of rice grain samples. Weigh 0.0500g of dried samples, add 1 mL of extraction solution, and extract it by shaking at 60℃ for 2 h. Centrifuge 10000g at 25℃ for 10 min, and take the supernatant for testing. The OPC content can be calculated by measuring the absorbance value at 500 nm. Refer to the method of Njus et al. (2020) to determine the ascorbic acid (AsA) content of rice grain samples. The AsA content can be calculated by measuring the absorbance value at 534 nm. Refer to the method of Suleria et al. (2020) to determine the total phenol (TP) content of rice grain samples. The TP content can be calculated by measuring the absorbance value at 765 nm. Refer to the method of Ghasemzadeh et al. (2018) to determine the flavonoid (PD) content of rice grain samples. The PD content can be calculated by measuring the absorbance value at 510 nm. 2.4. Quantitative detection of C3OG and P3G.
Each 0.1000 g sample was weighed, 1.0 mL of 1 % hydrochloric acid methanol was added, and the sample was ground into a slurry by a grinder and ultrasonically extracted at 4 °C for 1 h. Then, the samples were centrifuged at 8000 g for 10 min, and the supernatant was collected, filtered and tested.Liquid chromatography-mass spectrometry (LC-MS) was performed on an Agilent 1100 high-performance liquid chromatograph with the following conditions: wavelength 530 nm; chromatography column, Compass C18 (2) reversed-phase chromatography column (250 mm*4.6 mm, 5 μm); column temperature, 30 °C; flow rate, 1 mL min−1; injection volume, 10 μL; mobile phase, 1 % aqueous formic acid:methanol = 80:20 (V/V).
Standard curve determination: 0.1 g of C3OG and P3G standards were dissolved in 1 % hydrochloric acid methanol, to prepared the 1 mg mL−1 stock solutions, and were diluted to 4 μg mL−1, 10 μg mL−1, 40 μg mL−1, 100 μg mL−1, 200 μg mL−1, and 500 μg mL−1, respectively. Standard solutions were analyzed by the method mentioned above, and the standard curves were acquired by fitting the serial concentrations and their corresponding peak areas. The standard curve, linear range and correlation coefficient of C3OG and P3G were calculated (Table S1).
Sample metabolite extraction and LC-MS analysis
First, all rice grain samples were crushed. and 50.00 mg samples were accurately weighed into a 2 mL centrifuge tube using metabolite extraction methods described by Xiong et al. (2022b). Chromatographic separation of the metabolites was performed on a Thermo UHPLC system equipped with an ACQUITY UPLC HSS T3 (100 mm × 2.1 mm i.d., 1.8 µm; Waters, Milford, USA). The mobile phases consisted of 0.1 % formic acid in water:acetonitrile (95:5, v/v) (solvent A) and 0.1 % formic acid in acetonitrile:isopropanol:water (47.5:47.5:5, v/v) (solvent B). The solvent gradient changed according to the following conditions: from 0 to 3.5 min, 0 % B to 24.5 % B (0.4 mL min−1); from 3.5 to 5 min, 24.5 % B to 65 % B (0.4 mL min−1); from 5 to 5.5 min, 65 % B to 100 % B (0.4 mL min−1); from 5.5 to 7.4 min, 100 % B to 100 % B (0.4 mL min−1 to 0.6 mL min−1); from 7.4 to 7.6 min, 100 % B to 51.5 % B (0.6 mL min−1); from 7.6 to 7.8 min, 51.5 % B to 0 % B (0.6 mL min−1 to 0.5 mL min−1); from 7.8 to 9 min, 0 % B to 0 % B (0.5 mL min−1 to 0.4 mL min−1); from 9 to 10 min, 0 % B to 0 % B (0.4 mL min−1) for equilibrating the systems. The sample injection volume was 2 µL, and the flow rate was set to 0.4 mL min−1. The column temperature was maintained at 40 °C during the period of analysis, all samples were stored at 4 °C.
The mass spectrometric data were collected using a Thermo UHPLC-Q Exactive HF-X Mass Spectrometer equipped with an electrospray ionization (ESI) source operating in either positive or negative ion mode. The optimal conditions were set as follows: heater temperature, 425 °C; capillary temperature, 325 °C; sheath gas flow rate, 50 arb; aux gas flow rate, 13 arb; ion-spray voltage floating (ISVF), −3500 V in negative mode and 3500 V in positive mode; normalized collision energy, 20–40-60 V rolling for MS/MS. The full MS resolution was 60000, and the MS/MS resolution was 7500. Data acquisition was performed with the data-dependent acquisition (DDA) mode. The detection was carried out over a mass range of 70–1050 m/z.
Quality control sample
As a part of the system conditioning and quality control process, a pooled quality control (QC) sample was prepared by mixing equal volumes of all samples. The QC samples were disposed and tested in the same manner as the analytic samples. It is neccessary to represent the whole sample set, which would be injected at regular intervals (i.e., every 6 samples) to monitor the stability of the analysis.
Metabolome data analysis
After UPLC-MS analyses, the raw data were imported into Progenesis QI 2.3 (Nonlinear Dynamics, Waters, USA) for peak detection and alignment. The preprocessing results generated a data matrix that consisted of the retention time (RT), mass-to-charge ratio (m/z) values, and peak intensity. Differential metabolites (DMs) were screened based on variable importance for projection (VIP) values greater than 1 and P values<0.05. Multivariate statistical analysis was performed using the ropls (Version 1.6.2) R package from Bioconductor on the Majorbio Cloud Platform (https://cloud.majorbio.com). The DMs mapped into their biochemical pathways through metabolic enrichment and pathway analysis based on a database search (Kyoto Encyclopedia of Genes and Genomes, https://www.genome.jp/kegg/) (Ren et al., 2022).
Biochemical data analysis
WPS2021 was used to organize the raw data and plots of ABTS, DPPH, FRAP, TP, FD, OPC, AsA, C3OG, and P3G. ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, and P3G were analysed for significance and correlation using SPSS 18.0 software (Tukey’s test).
Results
Metabolic profiling
We first performed morphological observations of the grain samples (Fig. 1A, B, C, D). Through a principal component analysis (PCA) of the samples (including QC samples), the contribution rate of PC1 was 33.6 %, the contribution rate of PC2 was 18.1 %, and the sum of the contribution rates of the two principal components was 51.7 %, indicating that the two principal components reflected the main characteristic information of rice grain samples (Fig. 1E).
Fig. 1.
Overview of grain metabolite information. (A) YZN1 brown rice grains sample; (B) YZN1 polished rice grains sample; (C) YZN2 brown rice grains sample; (D) YZN2 polished rice grains sample; (E) PCA score; (E) PLS-DA score; (E) PLS-DA model overview; (F) PLS-DA permutation testing; (I) Comparison group DMs; (J) Comparison group Venn diagram.
Further PLS-DA analysis was performed on the data. The first principal component explanation degree of Component 1 was 27.9 %, and the second principal component explanation degree of Component 2 was 25.7 % (Fig. 1F). The larger the cumulative values of R2Y and Q2 were, the more stable and reliable the model (Fig. 1G). The R2 and Q2 decreased, and the regression line showed an upwards trend, indicating that the permutation test passed the test and that the model did not display overfitting (Fig. 1H). A total of 692 metabolites were detected in rice grains samples.154 DMs were identified in grains between YZN1_H and YZN2_H from 21 classes (Table S2). Among them, prenol lipids accounted for 9.62 %, glycerophospholipids accounted for 6.73 %, phenols accounted for 0.96 %, and flavonoids accounted for 0.96 % (Fig. S1A). Eighty-three DMs were identified in grains between YZN1_B and YZN1_H from 19 classes (Table S3). Fatty acyls accounted for 11.76 %, prenol lipids accounted for 9.80 %, 2-arylbenzofuran flavonoids accounted for 1.96 %, furans accounted for 1.96 %, and flavonoids accounted for 1.96 % (Fig. S1B). Fifty-two DMs were identified in grains between YZN2_B and YZN2_H from 15 classes (Table S4). Of these, glycerophospholipids accounted for 17.65 %, flavonoids accounted for 8.82 %, organooxygen compounds accounted for 5.88 %, prenol lipids accounted for 5.88 %, furans accounted for 2.94 %, and imidazopyrimidines accounted for 2.94 % (Fig. S1C). Forty-six DMs were identified in grains between YZN1_B and YZN2_B from 17 classes (Table S5). Prenol lipids accounted for 15.63 %, phenols accounted for 3.13 %, organooxygen compounds accounted for 3.13 %, and furans accounted for 3.13 % (Fig. S1D). In addition, we visually displayed the DMs in different comparison groups in the form of volcano plots (Fig. S2). A Venn diagram analysis of DMs in different comparison groups showed that there were specific DMs in each comparison group, indicating that there was a specific difference in the metabolic composition between different comparison groups (Fig. 1J).
Analysis of antioxidant capacity and flavonoid content
The total antioxidant capacity of purple glutinous brown rice grains was reduced to varying degrees after the rice was milled and processed into polished rice grains. The ABTS, DPPH and FRAP values of YZN1_H were 18.86 %, 27.21 %, and 37.5 % lower than those of YZN1_B (P < 0.05) (Table 1). The ABTS, DPPH, and FRAP values of YZN2_H were 12.14 %, 30.47 %, and 2 % lower than those of YZN2_B, respectively (P greater than 0.05) (Table 1). The ABTS and FRAP values of YZN1_B were 12.86 % and 34.4 % higher than those of YZN2_B, and the difference was significant (P < 0.05) (Table 1). The ABTS and DPPH values of YZN1_H were 4.76 % and 4.68 % higher than those of YZN2_H, respectively (P greater than 0.05) (Table 1). Overall, the total antioxidant capacity of YZN1_B was higher than that of YZN2_B, and the total antioxidant capacity of YZN1_H was higher than that of YZN2_H. The TP, PD, OPC, and AsA contents of YZN1_B were 126.01 %, 222.84 %, 124.51 %, and 69 % higher than those of YZN1_H (P < 0.05) (Table 1). The TP, PD, OPC, and AsA contents of YZN2_B were 22.82 %, 40.38 %, 23.33 %, and 17.83 % higher than those of YZN2_H, respectively. The TP, PD, OPC, and AsA contents of YZN1_H were 8.19 %, 11.15 %, 70 %, and 335.15 % higher than those of YZN2_H, respectively. The TP, FD, OPC, and AsA contents in YZN1_B were 99.45 %, 155.62 %, 209.46 %, and 524.14 % higher than those in YZN2_B (Table 1). The TP, PD, OPC and AsA of Yangzinuo No.1 brown rice or polished rice grains were higher than those of the corresponding Yangzinuo No.2 brown rice or polished rice grains. These results are explained by YZN1 having a higher nutrient content, and the nutrient content of brown rice grains was reduced by milling and processing. The black rice pigment was extracted by ultrasonication and separated by HPLC, and the separated components were identified by infrared spectroscopy and tandem mass spectrometry. Two anthocyanins were isolated and identified as C3OG and P3G by primary and secondary mass spectrometry. The black rice pigment contents of YZN1 brown rice grains and polished rice grains were quite different. The C3OG and P3G contents of YZN1_B were 102.04 % and 121.73 % higher than those of YZN1_H, respectively (P < 0.05) (Table 1). The C3OG and P3G contents of YZN2_B were 5.17 % and 6.68 % higher than those of YZN2_H, respectively (P greater than 0.05) (Table 1). In general, the black rice pigment content of YZN1_B was higher than that of YZN2_B, and the black rice pigment content of YZN1_H was higher than that of YZN2_H.
Table 1.
Differences in total antioxidant capacity, flavonoids and black rice pigment contents of purple glutinous brown rice grains and polished rice grains.
| Index | YZN1_H | YZN1_B | YZN2_H | YZN2_B |
|---|---|---|---|---|
| ABTS value (μmol Trolox g−1 FW) | 9.25 ± 0.04c | 11.40 ± 0.24a | 8.83 ± 0.2c | 10.05 ± 0.29b |
| DPPH value (μmol Trolox g−1 FW) | 3.13 ± 0.08b | 4.30 ± 0.2a | 2.99 ± 0.12b | 4.30 ± 0.2a |
| FRAP value (μmol Trolox g−1 FW) | 2.10 ± 0.01c | 3.36 ± 0.09a | 2.45 ± 0.2b | 2.50 ± 0.08b |
| TP content (mg g−1 FW) | 3.23 ± 0.15bc | 7.30 ± 0.41a | 2.98 ± 0.21c | 3.66 ± 0.18b |
| PD content (mg g−1 DW) | 2.89 ± 0.17c | 9.33 ± 0.39a | 2.60 ± 0.15c | 3.65 ± 0.21b |
| OPC content (mg g−1 FW) | 1.02 ± 0.06b | 2.29 ± 0.08a | 0.60 ± 0.02d | 0.74 ± 0.02c |
| AsA content (μg g−1 FW) | 289.94 ± 2.48b | 490.01 ± 12.6a | 66.63 ± 1.28c | 78.51 ± 2c |
| C3OG (mg g−1 FW) | 5.39 ± 0.54b | 10.89 ± 0.98a | 2.71 ± 0.09c | 2.85 ± 0.08c |
| P3G (mg g−1 FW) | 0.3690 ± 0.0069b | 0.8182 ± 0.0594a | 0.1377 ± 0.0029c | 0.1469 ± 0.0138c |
Different lowercase letters represent significance at the 0.05 level, the same as below.
Correlation analysis between the various biochemical indicators
To determine the relationships between the biochemical indices of YZN1 brown rice grains and polished rice grains, the correlations between the ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, and P3G indices were compared. In this study, we found that the correlation coefficients of each biochemical index of YZN1 brown rice grains and polished rice grains were all greater than 0.97, and they were all significantly correlated (Table 2). To determine the relationship between the biochemical indices of YZN2 brown rice grains and polished rice grains, the correlations between ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, and P3G indices were compared. In this study, we found that 19 of the biochemical indices of YZN2 brown rice grains and polished rice grains had pairwise correlation coefficients greater than 0.8, and they were all significantly correlated. ABTS was significantly positively correlated with DPPH, TP, PD, OPC and AsA. DPPH was significantly positively correlated with TP, PD, OPC and AsA. TP was significantly positively correlated with PD, OPC, AsA and C3OG. PD was significantly positively correlated with OPC, AsA and C3OG. OPC was significantly positively correlated with AsA and C3OG. C3OG was significantly positively correlated with P3G (Table 2).
Table 2.
Simple correlation analysis of the biochemical indicators of brown rice grains and polished rice grains. The lower part is YZN1, and the upper part is YZN2.
| Index | ABTS | DPPH | FRAP | TP | PD | OPC | AsA | C3OG | P3G |
|---|---|---|---|---|---|---|---|---|---|
| ABTS | 0.971** | 0.387 | 0.853* | 0.930** | 0.894* | 0.896* | 0.634 | 0.512 | |
| DPPH | 0.991** | 0.416 | 0.928** | 0.965** | 0.960** | 0.925** | 0.768 | 0.55 | |
| FRAP | 0.987** | 0.984** | 0.487 | 0.498 | 0.533 | 0.507 | 0.65 | 0.803 | |
| TP | 0.983** | 0.985** | 0.999** | 0.978** | 0.965** | 0.817* | 0.901* | 0.72 | |
| PD | 0.992** | 0.986** | 0.999** | 0.998** | 0.980** | 0.891* | 0.820* | 0.669 | |
| OPC | 0.983** | 0.976** | 0.997** | 0.994** | 0.994** | 0.938** | 0.844* | 0.618 | |
| AsA | 0.987** | 0.981** | 1.000** | 0.998** | 0.999** | 0.997** | 0.659 | 0.413 | |
| C3OG | 0.989** | 0.998** | 0.977** | 0.980** | 0.982** | 0.970** | 0.974** | 0.827* | |
| P3G | 0.992** | 0.994** | 0.994** | 0.994** | 0.996** | 0.985** | 0.993** | 0.989** |
*P < 0.05; **P < 0.01.
Correlation analysis between biochemical indicators and metabolites
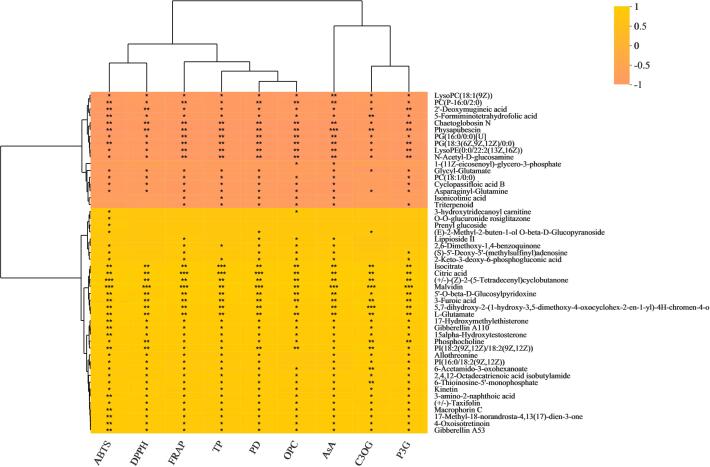
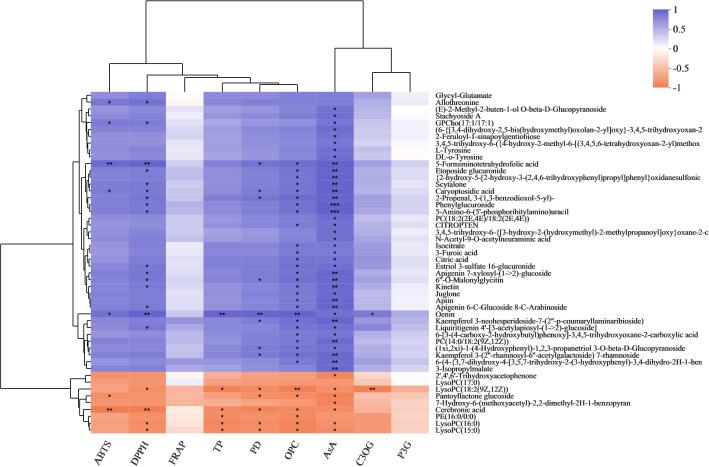
To understand the relationship between various biochemical indicators and metabolites during the processing of purple glutinous rice from brown rice grains to polished rice grains, we performed a correlation analysis between the ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, and P3G indicators and metabolites. The identified metabolites kinetin, citric acid, 5-formiminotetrahydrofolic acid, 3-furoic acid, and isocitrate are metabolites shared by YZN1 and YZN2 processed from brown rice grains to polished rice grains. In the YZN1_B and YZN1_H comparison groups, kinetin, citric acid, 3-furoic acid, and isocitrate were significantly positively correlated with ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, and P3G. 5-Formiminotetrahydrofolic acid was significantly negatively correlated with ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, and P3G (Fig. 2). In the YZN2_B and YZN2_H comparison groups, kinetin was significantly positively correlated with DPPH. 5-Formiminotetrahydrofolic acid was significantly positively correlated with ABTS, DPPH, PD, OPC, and AsA. Isocitrate, 3-furoic acid, kinetin, and citric acid were significantly positively correlated with OPC and AsA (Fig. 3).
Fig. 2.
Correlation analysis of ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, P3G with DMs between YZN1_B and YZN1_H. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 3.
Correlation analysis of ABTS, DPPH, FRAP, TP, PD, OPC, AsA, C3OG, P3G with DMs between YZN2_B and YZN2_H. *P < 0.05; **P < 0.01; ***P < 0.001.
Analysis of metabolic markers
The expression patterns of metabolites in each differential group and the P values of metabolites in the VIP and single-dimensional statistics of multivariate statistical analysis were displayed using clustering heatmaps and VIP bar charts. In this way, the importance values and expression trends of differential metabolites can be observed intuitively. We arranged the VIP values of metabolites in each comparison group from large to small, and we considered that these metabolites make a greater contribution to the difference between the two comparison groups. The VIP values of 5-formiminotetrahydrofolic acid were significantly different in the YZN1_B and YZN1_H comparison groups (Fig. S3). In the YZN2_B and YZN2_H comparison groups, the VIP values of 5-formiminotetrahydrofolic acid, citric acid, kinetin, isocitrate, and 3-furoic acid were significantly different (Fig. 4). It is clear that 5-formiminotetrahydrofolic acid, citric acid, kinetin, isocitrate, and 3-furoic acid metabolites are more important for the processing of purple glutinous brown rice grains into polished rice grains and can be used as candidate metabolic markers during processing.
Fig. 4.
VIP value analysis between YZN2_B and YZN2_H. The left panel is the metabolite clustering dendrogram. The closer the branches are, the closer the expression patterns of all metabolites in the sample. Each column represents a sample, with the sample name below. Each row represents a metabolite, and the colour represents the relative expression level of the metabolite in this group of samples. The corresponding relationship between the colour gradient and the value is shown in the gradient colour block. The right side is the metabolite VIP bar chart. The length of the bar represents the contribution value of the metabolite to the difference between the two groups. The default value is not<1. The larger the value is, the greater the difference between the two groups. The colour of the bar indicates the significance of the difference between the two groups of metabolites; that is, the P_value; the smaller the P_value, the larger the -log10(P value) and the darker the colour. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
Rice is the staple food of more than half of China's population. The contents of nutrients and active substances in rice often vary with the variety (Gong et al., 2020, Chen et al., 2022). Purple rice grains are rich in vitamins, a large amount of trace elements, and polyphenols such as flavonoids and anthocyanins, which have specific health care functions (Zhang, 2021, Xiong et al., 2022). The rich nutrients in purple rice are primarily distributed in the aleurone layer, and the nutrients in the outer layer of purple rice are easily lost as byproducts when brown rice grains are processed into polished rice grains (Zhang et al., 2022, Hu et al., 2022). In this study, the contents of TP, PD, OPC, AsA, C3OG, and P3G in YZN1_B were significantly higher than those in YZN1_H during the processing of YZN1 brown rice grains into polished rice grains (Table 1). Moreover, the correlation degree of each biochemical index of YZN1 brown rice grains and polished rice grains was very high, and all of the differences were extremely significant (Table 2). The TP, PD and OPC contents of YZN2_B were significantly higher than those of YZN2_H during this processing phase (Table 1). However, the TP, PD, OPC, AsA, C3OG, and P3G of YZN1 brown rice grains or polished rice grains were higher than those of the corresponding YZN2 brown rice or polished rice grains (Table 1). These results indicate that YZN1 is also richer in nutrients. In addition, purple glutinous brown rice grains has been milled to reduce the active nutrient substances in brown rice grains, resulting in the loss of rice nutrients. Rice grains are rich in metabolites, with 173 metabolites found in 30 rice varieties (Feng et al., 2019). In this study, 84 differential metabolites were found in the YZN1_B and YZN1_H comparison groups (Fig. 1I), including 19 types of substances such as fatty acyls, prenol lipids, 2-arylbenzofuran flavonoids, and flavonoids (Fig. S1B). Fifty-two differential metabolites were found in the YZN2_B and YZN2_H comparison groups, including 15 types of substances such as prenol lipids, fatty acyls, and flavonoids (Fig. S1C). During the processing of purple glutinous rice from brown rice grains to polished rice grains, the metabolite components, such as nutrients and antioxidant properties, of purple rice were dramatically changed (Table 1). We investigated the differences in small-molecule metabolites between different purple glutinous rice varieties during processing and found that the metabolite abundances of the two purple glutinous rice varieties changed significantly when they were processed from brown rice grains to polished rice grains (Fig. 1I) and even showed that the metabolites were completely lost. The abundances and types of metabolites (including nutrients) of different purple glutinous rice varieties were significantly affected by human factors during the processing of two purple glutinous brown rice grains into polished rice grains. Varieties with higher contents of active nutritive substances in purple glutinous rice were more seriously affected, which may be related to the loss of tissues such as the embryo and aleurone layer during rice processing. These findings further demonstrated that the processing byproducts of purple rice varieties with higher nutrient contents were more valuable. In addition, the numbers and types of differential metabolites (Fig. S1) were significantly changed during the processing of brown rice grains into polished rice grains. Furthermore, we verified that anthropogenic rice grain processing factors significantly affected the metabolite components of rice varieties. Previous studies have shown that citric acid has antioxidant and cholesterol-lowering functions, which are helpful in preventing arteriosclerosis, softening blood vessels and lowering blood pressure (Xu et al., 2020, Zhang et al., 2020, Lo Scalzo, 2021, Fikry et al., 2021). In this study, the identified metabolite citric acid was found to be a candidate metabolic marker during the processing of purple glutinous rice. The identified metabolite isocitrate is also a candidate metabolic marker during this rice processing step. The results showed that the processing process affected (decreased or increased) the metabolite, flavonoids, black rice pigment and total antioxidant capacity of purple glutinous rice grains. This study provides a theoretical basis for further improving the processing process of special rice and maximizing the added value of grain nutrition.
Conclusion
In this study, metabolites were detected in brown rice grains and polished rice grains from the YZN1 and YZN2 varieties as well as the contents of black rice pigment, flavonoids, and AsA, the total antioxidant capacity and other indicators. The changes in metabolite abundance before and after the processing of the two types of purple glutinous rice grains and the relationship between each biochemical index and metabolite were clarified. The ABTS, DPPH, and FRAP values of YZN1_H were 18.86 %, 27.21 %, and 37.5 % lower than those of YZN1_B, respectively. The ABTS, DPPH, and FRAP values of YZN2_H were 12.14 %, 30.47 %, and 2 % lower than those of YZN2_B, respectively. The total antioxidant capacity of YZN1_B was higher than that of YZN2_B, and the total antioxidant capacity of YZN1_H was higher than that of YZN2_H. Compared with the corresponding YZN1_H and YZN2_H, the TP, PD, OPC, AsA, C3OG and P3G of YZN1_B and YZN2_B increased to varying degrees. The nutrient content of YZN1 was higher. The processing the two types of purple glutinous rice grains reduced the nutrients in brown rice grains. The DMs YZN1_B and YZN1_H and YZN2_B and YZN2_H were primarily involved in the citrate cycle, amino acid metabolism, anthocyanin biosynthesis, flavone and flavonol biosynthesis and other pathways. Citric acid and isocitrate are key metabolites in metabolic pathways. The candidate metabolic markers of purple glutinous rice before and after processing were determined by correlation analysis between metabolites and various biochemical indicators and metabolite VIP values, which included 5-formiminotetrahydrofolic acid, citric acid, kinetin, isocitrate, and 3-furoic acid.
CRediT authorship contribution statement
Jinyan Zhu: Investigation, Writing – original draft. Qiang Shi: Data curation. Changhui Sun: Investigation, Formal analysis. Jinlong Hu: Software. Nianbing Zhou: Resources. Haiyan Wei: Writing – review & editing. Haohua He: Writing – review & editing. Dahu Zhou: Writing – review & editing. Hongcheng Zhang: Validation. Qiangqiang Xiong: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32101816), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22_1782), Jiangsu Province Seed Industry Revitalization Project [JBGS(2021)036], Key R & D projects in Jiangsu Province (Modern Agriculture) (BE2019342), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100492.
Contributor Information
Dahu Zhou, Email: zhoudahu2008@163.com.
Qiangqiang Xiong, Email: qqxiong@yzu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abeyrathne E.D.N.S., Nam K., Ahn D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants. 2021;10:1587. doi: 10.3390/antiox10101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi T.B., Chattopadhyay K., Sivashankari M., Roy S., Kumar A., Biswas T., et al. Effect of different processing technologies on phenolic acids, flavonoids and other antioxidants content in pigmented rice. Journal of Cereal Science. 2021;100 [Google Scholar]
- Bai H., Ma X., Cao G., Liu X., Han L. The differences of nutritional and functional components content in different types of special rice. Journal of Plant Genetic Resources. 2017;18:1013–1022. [Google Scholar]
- Chen Y., Wang Z., Wang C., Li H., Huang D., Zhou D., et al. Comparisons of metabolic profiles for carbohydrates, amino acids, lipids, fragrance and flavones during grain development in indica rice Cultivars. Rice Science. 2022;29:155–165. [Google Scholar]
- Chu M.J., Du Y.M., Liu X.M., Yan N., Wang F.Z., Zhang Z.F. Extraction of proanthocyanidins from Chinese wild rice (Zizania latifolia) and analyses of structural composition and potential bioactivities of different fractions. Molecules. 2019;24:1681. doi: 10.3390/molecules24091681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cömert E.D., Mogol B.A., Gökmen V. Relationship between color and antioxidant capacity of fruits and vegetables. Current Research in Food Science. 2020;2:1–10. doi: 10.1016/j.crfs.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Wang C., Li X., Fu T., Zhang L. Effect of geographical origin on rice metabolites as analyzed by gas chromatography-mass spectrometry. Food Science. 2019;40:208–214. [Google Scholar]
- Fikry A.M., Attia A.I., Ismail I.E., Alagawany M., Reda F.M. Dietary citric acid enhances growth performance, nutrient digestibility, intestinal microbiota, antioxidant status, and immunity of Japanese quails. Poultry Science. 2021;100 doi: 10.1016/j.psj.2021.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh, A., Baghdadi, A., ZE Jaafar, H., Swamy, M.K., & Megat Wahab, P.E. (2018). Optimization of flavonoid extraction from red and brown rice bran and evaluation of the antioxidant properties. Molecules, 23, 1863. [DOI] [PMC free article] [PubMed]
- Gong R., Huang D., Chen Y., Li H., Wang Z., Zhou D., et al. Comparative metabolomics analysis reveals the variations of eating quality among three high-quality rice cultivars. Molecular Breeding. 2020;40:112. [Google Scholar]
- Hu K., Chen D., Sun Z. Structures, physicochemical properties, and hypoglycemic activities of soluble dietary fibers from white and black glutinous rice bran: A comparative study. Food Research International. 2022;159 doi: 10.1016/j.foodres.2022.111423. [DOI] [PubMed] [Google Scholar]
- Hu S., Hu P. Research progress and prospect of functional rice. Chinese Journal of Rice Science. 2021;35:311–325. [Google Scholar]
- Lo Scalzo R. EPR free radical scavenging activity on superoxide, hydroxyl and tert–butyl hydroperoxide radicals by common hydrophilic antioxidants: Effect of mixing and influence of glucose and citric acid. European Food Research and Technology. 2021;247:2253–2265. [Google Scholar]
- Ma Z.Q., Yi C., Wu N.N., Tan B. Steaming retains more phenolics, dietary fiber and antioxidant activities than cooking for rice with different milling processes. Cereal Chemistry. 2022;99:664–667. [Google Scholar]
- Ma Z.Q., Yi C.P., Wu N.N., Tan B. Reduction of phenolic profiles, dietary fiber, and antioxidant activities of rice after treatment with different milling processes. Cereal Chemistry. 2020;97:1158–1171. [Google Scholar]
- Mohidem N.A., Hashim N., Shamsudin R., Che Man H. Rice for food security: Revisiting its production, diversity, rice milling process and nutrient content. Agriculture. 2022;12:741. [Google Scholar]
- Njus D., Kelley P.M., Tu Y.J., Schlegel H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radical Biology and Medicine. 2020;159:37–43. doi: 10.1016/j.freeradbiomed.2020.07.013. [DOI] [PubMed] [Google Scholar]
- Paiva F.F., Vanier N.L., Berrios J.D.J., Pan J., de Almeida Villanova F., Takeoka G., et al. Physicochemical and nutritional properties of pigmented rice subjected to different degrees of milling. Journal of Food Composition and Analysis. 2014;35:10–17. [Google Scholar]
- Ren, Y., Yu, G., Shi, C., Liu, L., Guo, Q., Han, C., et al. (2022). Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta, 1, e12. [DOI] [PMC free article] [PubMed]
- Sapna I., Kamaljit M., Priya R., Jayadeep P.A. Milling and thermal treatment induced changes on phenolic components and antioxidant activities of pigmented rice flours. Journal of Food Science and Technology. 2019;56:273–280. doi: 10.1007/s13197-018-3487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Guo Y., Wang Y., Li M., Li K., Liu X., et al. Metabolomic analysis reveals nutritional diversity among three staple crops and three fruits. Foods. 2022;11:550. doi: 10.3390/foods11040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleria H.A., Barrow C.J., Dunshea F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods. 2020;9:1206. doi: 10.3390/foods9091206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q., Sun C., Li A., Zhang J., Shi Q., Zhang Y., et al. Metabolomics and biochemical analyses revealed metabolites important for the antioxidant properties of purple glutinous rice. Food Chemistry. 2022;389 doi: 10.1016/j.foodchem.2022.133080. [DOI] [PubMed] [Google Scholar]
- Xiong Q., Sun C., Shi H., Cai S., Xie H., Liu F., et al. Analysis of related metabolites affecting taste values in rice under different nitrogen fertilizer amounts and planting densities. Foods. 2022;11:1508. doi: 10.3390/foods11101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q., Zhang J., Shi Q., Zhang Y., Sun C., Li A., et al. The key metabolites associated with nutritional components in purple glutinous rice. Food Research International. 2022;160 doi: 10.1016/j.foodres.2022.111686. [DOI] [PubMed] [Google Scholar]
- Xu Y., Huang J., Li W., Zheng Y., Jiang J., Ding Z. Dietary supplementation of vitamin E and citric acid could significantly promote the relative expression of PPARα and aconitase genes, concentration of polyunsaturated fatty acids, antioxidant enzyme activities, and growth of juvenile cobia. Aquaculture. 2020;518 [Google Scholar]
- Xu Y., Yao G., Liu P., Zhao J., Wang X., Sun J., et al. Review on the application of metabolomic approaches to investigate and analysis the nutrition and quality of agro-products. Scientia Agricultura Sinica. 2019;52:3163–3176. [Google Scholar]
- Yamuangmorn S., Prom-u-Thai C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants. 2021;10:833. doi: 10.3390/antiox10060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C., Shen S., Yang C., Liu Z., Fernie A.R., Graham I.A., et al. Plant metabolic gene clusters in the multi-omics era. Trends in Plant Science. 2022 doi: 10.1016/j.tplants.2022.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang P., Xia C., Cheng Y., Guo X., Li Y. Effects of malic acid and citric acid on growth performance, antioxidant capacity, haematology and immune response of Carassius auratus gibelio. Aquaculture Research. 2020;51:2766–2776. [Google Scholar]
- Zhang, Q. (2021). Purple tomatoes, black rice and food security. Nature Reviews Genetics, 22, 414-414. [DOI] [PubMed]
- Zhang S., Ma Q., Dong L., Jia X., Liu L., Huang F., et al. Phenolic profiles and bioactivities of different milling fractions of rice bran from black rice. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2021.132035. [DOI] [PubMed] [Google Scholar]
- Zorzi M., Gai F., Medana C., Aigotti R., Morello S., Peiretti P.G. Bioactive compounds and antioxidant capacity of small berries. Foods. 2020;9:623. doi: 10.3390/foods9050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.