Graphical abstract

Keywords: Germination, Freezing, Thawing, Aglycones, Glucosides
Highlights
-
•
Freeze-thaw treatment destroyed the cell structure of germinated soybeans.
-
•
Isoflavone conjugate-hydrolyzing β-glucosidase activity was enhanced with freeze–thaw.
-
•
Freeze-thaw promoted the conversion of glucosides and malonyl-glucosides to aglycones.
-
•
Intercellular water of germinated soybeans increased with freeze–thaw.
Abstract
We studied the effect of freeze–thaw on isoflavone composition in germinated soybeans, particularly the conversion of aglycones, the isoflavone monomers with high biological activity. Germinated soybeans were frozen at −20 °C, −80 °C, and −196 °C respectively, and then the frozen samples (-20 °C) were thawed at 4 °C, 10 °C, and 25 °C respectively. Results showed total isoflavone increased after germination. Aglycones content increased most at −20 °C, which increased about 24 times. The effect of thaw temperature and time indicated there were approximately 89 % glucosides forms converted to aglycones during freeze–thaw. Isoflavone conjugate-hydrolyzing β-glucosidase (ICHG) activity increased by 65.78 % (25 °C) and 59.14 % (48 h) with freeze–thaw. The cells of germinated soybeans were broken, promoting ICHG contact with glucosides and malonyl-glucosides. These results indicated that freeze–thaw greatly changed the content and profile of isoflavones, resulting in a sharp increase in the content of aglycones.
1. Introduction
Germinated soybeans are a popular vegetable food consumed in Asian countries. In addition to the high nutritional value, germinated soybeans are low-cost. Most research is focused on the cultivation and breeding techniques, nutritional value, and preservation of fresh sprouts to improve the quality of germinated soybeans (Lee et al., 2007).
Germinated soybeans are rich in isoflavones, the main phenolic substances in soybeans. The main nine isoflavone monomers can be divided into three categories: aglycones (daidzein-DE, genistein-GE, and glycitein-GLE), glucosides (daidzin-DN, genistin-GN, and glycitin-GL), and malonyl-glucosides (malonyl-daidzin-MD, malonyl-genistin-MG, and malonyl-glycitin-MGL) (Quinhone & Ida, 2015). They can also be grouped into three categories on the basis of genus: Tdin (total content of daidzein, daidzin, and malonyl-daidzin), Tgin (totao content of genistin, genistein, and malonyl-genistin), and Tgly (total content of glycitein, glycitin, and malonyl-glycitin). Aglycones are produced by the phenylpropanoid synthesis pathway (Graham, 1991). Glucosides and malonyl-glucoside are derived from aglycones. Setchell et al. (2002) showed that glucoside and malonyl-glucoside cannot be digested and absorbed by humans, and glycosylation needs to be hydrolyzed to form aglycones that are easily absorbed by the small intestine. Aglycones play a major role in the pharmacological activities of isoflavones. Among the nine major isoflavone monomers, the antioxidant activity and biological function of daidzein and genistein are the highest.
In soybean cells, 7-O-glucosylated and 6″-O-malonylated (free isoflavones aglycones products) are produced through the combined effects of isoflavone 7-O-glucosyltransferase (IF7GT) and isoflavone 7-O-glucoside 6″-O-malonyltransferase (IF7MaT). The glucosides and malonyl-glucosides are more likely to dissolve in water than aglycones, and they often accumulate in vacuoles (Suzuki et al., 2006). These isoflavone conjugates are potential forms of isoflavonoids and must eventually be converted into aglycones to interact with symbiotic or pathogenic microorganisms. Furthermore, the enzymes participated in this conversion must be separated from those of IF7GT and IF7MaT as well as the vacuolar conjugate pools in space. The process during which aglycons in chickpeas are released includes two steps: the isoflavone 7-O-(6″-O-malonyl-β-d-glucosides) is hydrolyzed by malonylesterase, and then the β-glucosidic linkage is hydrolyzed by β-glucosidase. In comparison, soybean cells’ release of isoflavone aglycones from their conjugates seems to be caused by isoflavone conjugate-hydrolyzing β-glucosidase (ICHG) that plays a key effect in the turnover of conjugates for plant–microbe interactions (Suzuki et al., 2006).
Many studies have found that germination can increase the content of glucosides and total isoflavone in soybeans, while the content of aglycones remains unchanged or decreases. However, glucosides are not easy to be digested and absorbed by humans. Therefore, it is necessary to study methods that can promote the accumulation of aglycones that are easy to be digested and absorbed by humans. It is reported that fermentation (da Silva, Celeghini, & Chang, 2011) and pulsed electric field (Lu, Li, Yan, Mao, & Zhang, 2022) can convert glucosides into aglycones, so the content of aglycones increases. However, these methods are complex and energy consuming. The freeze–thaw treatment after germination is a simple and green method, which can greatly increase the content of aglycones through aglycone conversion. Therefore, this paper mainly studied the effect of freeze–thaw treatment after germination on the composition and content of isoflavones, ICHG activity, cell structure, and water distribution.
2. Materials and methods
2.1. Materials
Thirteen varieties of soybean seeds, the conventional cultivars in North America, were grown in the 2018/2019 season and provided by the United States Department of Agriculture (USDA). Among these, three (PI378684B, PI424083A, and PI549032) were wild soybeans from Japan, South Korea, and China, respectively, one (Lincoln) was a major U.S. soybean ancestor, one (Essex) was a popular variety, and eight were the most diverse milestone soybean cultivars (Adams, Forrest, Harosoy, Hutcheson, Kent, Lee, Mercury, and Williams 82) which capture 75 % of the genetic diversity of 562 North American commercial cultivars in the USDA-ARS Soybean Germplasm Collection. The eight milestone soybean cultivars were selected per the following procedures: the pairwise distance among 562 North American commercial cultivars was calculated using the formular dij = 1-Sij, where Sij is the ratio of the number of identical SNP allele calls and the total number of SNPs for which allele calls for the pair ith and jth cultivars based on 42,509 SNP loci in their genomes (Song et al., 2015). The 562 cultivars were grouped into eight clusters which resulted in maximized diversity among clusters but minimized diversity within a cluster. A milestone soybean cultivar from each of the eight clusters of the 562 North American commercial soybean cultivars was selected. Song et al. (2015) genotyped entire USDA Soybean Germplasm Collection containing > 18,000 cultivated soybean and > 1100 wild soybean with SoySNP50K Beadchip Assay, which contained 42,509 single nucleotide polymorphism loci (Song et al., 2013), and the SoySNP50K dataset (Song et al., 2015) is publicly available at the Soybase (https://www.soybase.org/snps/). All the data for the above diversity analysis were extracted from the SoySNP50K dataset. The reagents used to measure isoflavone content were chromatographic grade, while other reagents were analytical grade.
2.2. Germination of soybeans
The soybean seeds (150 seeds) were soaked for about 15 min in a 1 % sodium hypochlorite solution for sterilization and washed in distilled water. The disinfected seeds were soaked for 7 h and germinated in a germination machine (DYJ-A03, Gamoy, China) at 30 °C in the dark. The germinated soybeans were harvested after 4 days and then immediately frozen at different temperatures (see step 2.3).
2.3. Freeze-thaw treatment
Germinated soybeans were frozen at −20 °C,-80 °C, and −196 °C (liquid nitrogen). Subsequently, they were thawed at 4 °C, 10 °C, and 25 °C for 2 h, 4 h, 8 h, 12 h, 24 h, and 48 h, respectively. All the samples were immediately frozen in liquid nitrogen and lyophilized in a lyophilizer.
2.4. Isoflavones analysis
The isoflavone content was determined using high-performance liquid chromatography (HPLC; UItiMate 3000, Thermo Fisher Scientific, Shanghai) equipped with an LC column (UItiMate®5μm AQ-C18 120 Å, 250 × 4.6 mm, Welch, China), as described by Ma, Wang, Yang, and Gu (2018). The lyophilized sample (0.2 g) was added with 6 mL 80 % methanol, and it was extracted under ultrasonic treatment at 40 °C for 60 min. The mixed solution was centrifuged at 13000 × g for 20 min (4 °C). The filtered solution (20 μL) was loaded onto the HPLC analyzer. Phase A was a 0.1 % acetic acid solution, while phase B was a 0.1 % acetic acid acetonitrile solution. During elution, the proportion of phase B was 13 %-35 %, 0–50 min; 35 %-13 %, 50–51 min. The flow rate was 1 mL/min. The wavelength of the UV detector was 260 nm. The standards of isoflavone were used for quantification, including DE, DN, GE, GN, GL, GLE, MD, MG, and MGL.
2.5. ICHG activity analysis
The ICHG activity was determined according to Falcão et al. (2018) with some modifications. The germinated soybean sample (1.0 g) was added to a small amount of a 50 mmol/L phosphate buffer (pH 7.0) to grind on a freezer grinder for a homogenate sample. The homogenate was transferred to a 50 mL centrifuge tube and diluted to 20 mL. It was then centrifuged at 10,000 g for 10 min at 4 °C, and the supernatant was stored at 4 °C for future use.
The enzyme solution (600 μL), phosphate buffer (200 μL, pH 7.0), and 4-nitrophenyl β-d-glucopyranoside (pNPG, 200 μL, 10 mmol/L) were added to a 5 mL tube. The solution and substrate were mixed and placed in a constant temperature water bath at 37 °C for 1 h. After the reaction, 1 mol/L Na2CO3 (2 mL) was added to terminate the reaction. The reaction solution was centrifuged at 4,000 g for 10 min, and the supernatant was taken for determination at a wavelength of 410 nm using the UV–vis (UV-5100, Shanghai Metash Instrument Co. ltd., China). The enzyme activity calculation formula is as follows:
A is the absorbance value of the enzymatic reaction, V2 is the total amount of the reaction solution (mL), V is the total amount of enzyme solution extracted (mL), k is the slope of the standard curve, V1 is the amount of enzyme solution in the reaction system (mL), M is the sample mass (g), and T is the reaction time (min). The unit of enzymatic activity is U/g.
2.6. Cell structure analysis
According to the method of Yang et al. (2020), the germinated soybeans were cut into slices and placed on glass slides and then observed with an optical microscope (BK-POL, Chongqing Optec Instrument Co., ltd) to obtain the microstructure cell structure image.
2.7. Low-field nuclear magnetic resonance measurements
Following the method described by Li et al., 2015, Zhao et al., 2022, the low-field nuclear magnetic resonance (LF NMR) measurements were obtained using a 22.4-MHz nuclear magnetic resonance (NMR) analyzer (NMI20-040 V-I, Suzhou NIUMAG Analytical Instrument Corporation, China). One soybean sprout was placed in a 25-mm NMR glass tube, which was placed in the NMR probe. Then, T2 relaxation times were tested. The measurement was carried out at 32 °C.
2.8. Statistical analysis
For each treatment, data were obtained from at least three independent replicate trials. The results were recorded as the mean ± standard deviation of the replicates. The statistical analysis was conducted with SPSS 19.0 (SPSS Inc., Chicago, USA) using a one-way analysis of the variance test and Duncan’s test to determine statistically significant differences (p < 0.05).
3. Results and discussion
3.1. Isoflavone content of different cultivar soybeans seeds after germination
The total isoflavone was high in Forrest, Lee, and Williams 82 soybean seeds, and it increased 35 %, 52 %, and 36 %, respectively, after germination (Table S1). Although the total isoflavone was low in Adams, Harosoy, Kent, and Mercury, the levels increased post-germination by 55 %, 46 %, 11 %, and 72 %, respectively. The aglycone, glucosides, and malonyl-glucosides levels changed in different seeds during germination, which showed that the amount of isoflavones in soybean differed significantly between varieties and cultivars. An intensive investigation is still being conducted to identify the most valuable source of these compounds. Aglycones are absorbed and metabolized more rapidly and influence the human organism more effectively than bonded forms (Coward et al., 1993, Salces et al., 2019, Szymczak et al., 2017). Aglycones also have a minor nutritional significance because their amount is relatively low. Glucosides and malonyl-glucosides were the dominant isoflavones present in all soybean seeds. This shows that the Lee cultivar proved to be the most beneficial. The Lee cultivar was the richest source of total isoflavones after germination, and although the aglycone in the seeds was low, it increased 20 times after germination. Lee is the best cultivar to accumulate total isoflavones and aglycones by biophysical methods among the eight cultivars.
3.2. Isoflavone content of different cultivar germinated soybeans after freeze–thaw
The liquid phase diagrams of the standards and samples can be observed in Fig. 1. We selected the cultivars Forrest, Lee, Williams 82, Adams, and Mercury to continue research on freeze–thaw conditions. These cultivars were selected due to their high content and high increase rate (Table S2). Interestingly, the glucosides (DN, GL, GN) and malonyl-glucosides (MD, MGL, MG) were almost absent after freeze–thaw (AF), and though there were no aglycones (DE, GLE, GE) before freeze–thaw (BF), they increased largely after the freeze–thaw treatment. The aglycone content increased from 0 to 3705 μg/g, 95 to 4409 μg/g, 71 to 3885 μg/g, 115 to 3910 μg/g, and 79 to 3187 μg/g in Adams, Forrest, Lee, Mercury, and William, respectively. The total isoflavone content remained stable in Adams and Mercury and decreased by 41 %, 41 %, and 52 % in Forrest, Lee, and William, respectively. This suggested that glucosides and malonyl-glucosides may be hydrolyzed to aglycones by ICHG. The increased degree of hydrolysis might be a result of damage caused by ice crystals during freeze–thaw to bring glucoside and malonyl-glucosides into contact with ICHG, and then hydrolysis occurred (Guo, Yang, Wang, & Gu, 2015).
Fig. 1.
HPLC chromatograms of standards and a typical sample. DN: daidzin; GL: glycitin; GN: genistin; MD: malonyl-daidzin; MGL: malonyl-glycitin; MG: malonyl-genistin; DE: daidzein; GLE: glycitein; GE: genistein.
3.3. Isoflavone content of germinated soybeans at different freeze temperature
Due to the high isoflavone content and aglycone conversion, we selected the cultivar Lee for further research. The effect of the freeze temperature on the isoflavone content of germinated soybeans is shown in Table S3, which also shows that the content of different isoflavone monomers varies with freeze temperatures. Tdin content decreased at all the freeze temperatures during the freeze–thaw treatment. It decreased by 34 % (-20 °C), 34 % (-80 °C), and 41 % (-196 °C), respectively. Tgin content also decreased by 17 % (-20 °C), 13 % (-80 °C), and 21 % (-196 °C), respectively. In contrast, Tgly content significantly increased at all the freeze temperatures during the freeze–thaw treatment. It increased by 449 % (-20 °C), 194 % (-80 °C), and 292 % (-196 °C), respectively. The aglycone content significantly increased at all the freeze temperatures during the freeze–thaw treatment. It had the highest content at −20 °C, which increased from 110 μg/g (0.42 μmol/g) in the control to 2623 μg/g (9.41 μmol/g). The transfer ratio of aglycones is 45.4 % (-20 °C), 29.7 % (-80 °C) and 40.1 % (-196 °C) respectively, which shows that the maximum conversion of glucosides and malonyl-glucosides to aglycones is at −20 °C. The glucoside and malonyl-glucoside content both decreased at all the freeze temperatures during the freeze–thaw treatment. This may be due to the breakage of germinated soybean cells caused by freezing, which leads to ICHG contacting with glucoside and malonyl-glucoside and converting them into aglycones. The content of total isoflavones changes little, while glucosides decrease significantly, and aglycones increase significantly. Therefore, it is speculated that the increase of aglycones under freeze–thaw treatment may be mainly due to the conversion of glucosides.
3.4. Isoflavone content of germinated soybeans at different thaw temperature
As shown in Table S4, the content of different isoflavone monomers varies with thaw temperature. Tdin content decreased after freeze–thaw treatment, and the lower the thaw temperature, the lower the Tdin content, decreasing from 4305 (control) to 1256 (4 °C) μg/g. Tgin content also decreased after the freeze–thaw treatment and was lowest at 10 °C. It decreased from 3242 μg/g (control) to 1211 μg/g (10 °C). Tgly content significantly increased during the freeze–thaw treatment. It increased by 153 % (4 °C), 268 % (10 °C), and 248 % (25 °C), respectively. This may be due to damage on cells caused by ice crystals that promote the contact of specific isoflavone isomerase with substrates, such as Tdin and Tgin. The total aglycone content also significantly increased during the freeze–thaw treatment. It increased from 134 μg/g (0.51 μmol/g) in the control to 2642 μg/g (9.66 μmol/g) at 4 °C and then increased more to 3595 μg/g (13.07 μmol/g) and 3526 μg/g (12.85 μmol/g) at 10 °C and 25 °C, respectively. The transfer ratio of aglycones is 68.9 % (4 °C), 79.6 % (10 °C) and 73.5 % (25 °C), respectively. The total glucosides and malonyl-glucosides both decreased after the freeze–thaw treatment. They decreased from 7166 μg/g (16.89 μmol/g) and 857 μg/g (1.67 μmol/g) in the control to 1161 μg/g (2.74 μmol/g) and 42 μg/g (0.08 μmol/g) at 10 °C. This indicated that there was approximately 89 % of the overall glycosidic forms converted to aglycones during the freeze–thaw treatment. It could be hypothesized that germinated soybean cells ruptured with increasing thaw temperature, resulting in ICHG making contact with glucosides and malonyl-glucosides, which were converted to aglycones. The total isoflavone content decreased, which may be due to the loss of the malonyl and glucosyl groups during conversion. Hsiao and Hsieh (2018) reported the increase of aglycones in soybean during soaking is due to the conversion of glucosides under the action of ICHG. In addition, the ICHG activity increased in this study, and the content of total isoflavones changes slightly, while aglycones increase significantly, and glucosides decrease significantly. Therefore, it is very reasonable to speculate that the increase of aglycones under freeze–thaw treatment may be completely caused by the conversion of glucosides.
3.5. Isoflavone content of germinated soybeans at different thaw time
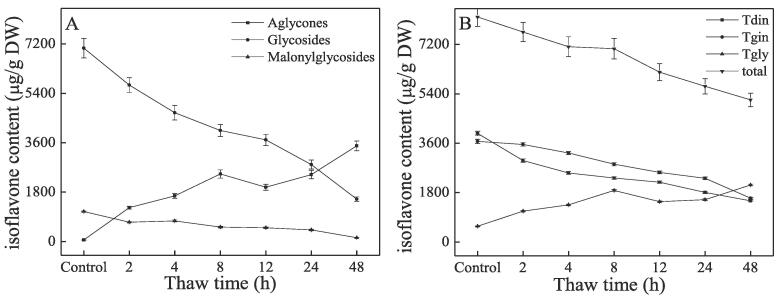
Table S5 shows that the content of different isoflavone monomers varies based on thaw time. The longer the thaw time, the lower the DN content. It decreased from 3601 μg/g (control) to 727 μg/g (48 h). GL decreased from 502 μg/g in the control to 161 μg/g at 2 h and disappeared after 4 h. GN decreased from 2945 μg/g in the control to 819 μg/g at 48 h. MD content decreased from 355 μg/g in the control to 33 μg/g at 48 h. MGL content was 73 μg/g and could not be found after the thaw treatment. MG content also decreased with thaw time. It decreased from 666 μg/g in the control to 110 μg/g at 48 h. DE and GLE were not detected in the control, and the GE content in the control was low (58 μg/g). However, the content of DE, GLE and GE all increased largely with thaw time, and they were 740, 2079, and 669 μg/g at 48 h, respectively. Tdin and Tgin both significantly decreased (Fig. 2) from 3957 μg/g and 3668 μg/g in the control to 1500 μg/g and 1598 μg/g, respectively. Tgly significantly increased from 575 μg/g in the control to 2079 μg/g at 48 h. This indicated that the isoflavone isomerase was significantly activated with thaw time. The total aglycone content also significantly increased with thaw treatment time from 58 μg/g (0.21 μmol/g) in the control to 3488 μg/g (12.71 μmol/g) at 48 h. The transfer ratio of aglycones is 21.9 % (2 h), 31.1 % (4 h), 44.6 % (8 h), 41.2 % (12 h), 53.3 % (24 h) and 75.3 % (48 h) respectively, which shows that the maximum conversion of glucosides and malonyl-glucosides to aglycones is at 48 h. The glucoside and malonyl-glucoside content significantly decreased from 7048 μg/g (16.6 μmol/g) and 1094 μg/g (2.13 μmol/g) in the control to 1546 μg/g (3.64 μmol/g) and 143 μg/g (0.28 μmol/g) at 48 h, respectively. It was also hypothesized that cells were destroyed with thaw time and that ICHG made contact with glucosides and malonyl-glucosides. The total isoflavone content decreased from 8200 μg/g (18.94 μmol/g) in the control to 5177 μg/g (16.63 μmol/g) at 48 h due to the hydrolysis of the malonyl and glucosyl groups, as mentioned.
Fig. 2.
Isoflavone content of germinated soybeans at different thaw time. A, Aglycones, Glycosides, and Malonyl-glycosides; B, Tdin, Tgin, Tgly, and total isoflavones.
3.6. ICHG activity of germinated soybeans at different thaw temperature and time
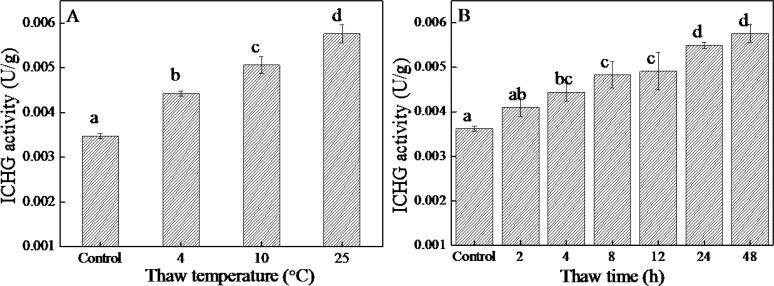
Sanches de Lima and Ida (2014) have shown that ICHG can convert glucosides into the corresponding aglycones. Yeom, Kim, Kim, and Oh (2012) also found that the content of glucosides (genistin, diadzin, and glycitin) decreased and the content of aglycones (genistein, daidzein, and glycitein) increased after heat treatment. ICHG was thermally stable, and heating did not destroy its activity (Yadav, Pandey, & Dubey, 2021). Thus, it could convert glucosides into aglycones. Fig. 3 shows the effects of thaw temperature and thaw time on ICHG activity. It can be observed that the activity of ICHG increased with the increase in thaw temperature and time, which can explain the results provided in Table S4 and Table S5. The content of aglycones increased, and the content of glucosides and malonyl-glucosides decreased with thaw temperature and thaw time.
Fig. 3.
ICHG activity of germinated soybeans at different thaw temperature and time. A, Thaw temperature; B, Thaw time. (Different letters indicate the results of Duncan’s multiple range test at 5 % level of significant difference).
3.7. Cell structure of germinated soybeans at different thaw temperature and time
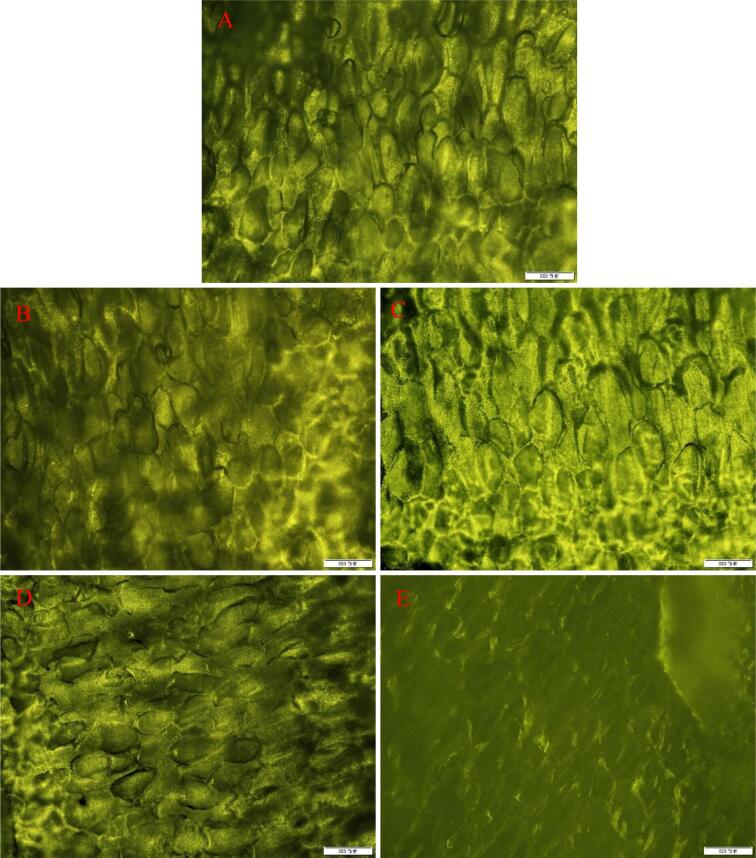
Fig. 4 shows the effect of thaw temperature and time on the cell structure of germinated soybeans. The cells were broken with an increase in thaw temperature and time and severely ruptured at 48 h, and the outlines of the cells disappeared. Phothiset and Charoenrein (2014) found that papaya cells are irregular in shape and rupture with a thaw temperature of 4 °C. Yang et al. (2020) found that after quick freezing in liquid nitrogen, the cells were relatively complete, and the outlines of the cells were clear before thaw; However, after thaw treatments, the cells began to be destroyed, and the cells were severely damaged as the thaw time increased. This confirmed the hypothesis for the results of Table S4 and Table S5, indicating that the decrease in the content of glucosides and malonyl-glucosides and the increase in the content of aglycones after freeze–thaw treatment might be because cell ruptures caused ICHG contact with glucosides and malonyl-glucosides, increasing the probability of being converted into aglycones.
Fig. 4.
Cell structure of germinated soybeans at different thaw temperature and time. A, control; B, 4 °C; C, 10 °C; D, 24 h; E, 48 h.
3.8. The water status distribution of germinated soybeans after freeze–thaw
The physical change in soybeans during germination has been studied (Quinhone et al., 2015). However, further studies are required to gain insight into the proton dynamics, water distribution, and interactions between water and macromolecules in germinated soybeans (Li et al., 2015). The effect of freeze–thaw on the water status of germinated soybeans can be indicated by water–solid interactions, so performing the LF NMR analysis would be highly beneficial (Ma et al., 2018).
Fig. 5 shows the relaxation time distribution curves of germinated soybeans. There were three or four different regions in this distribution curve, which indicated multiple water regions presented in the germination soybeans. The peak within the range of 0.1–10 ms represented the combined water or structural water that integrated with protein molecules (Wu, Li, & Gao, 2016), namely T2b and T21. Ritota, Gianferri, Bucci, and Brosio (2008) connected the peak with water molecules in macromolecules. A peak within the range of 10–100 ms was considered to be fixed water between cells, meaning the T22 peak (Wu et al., 2016). The peak within the range of 100–1000 ms, called the T23 peak, indicated free water (Wu et al., 2016).
Fig. 5.
The water status of germinated soybeans after freeze–thaw. The relaxation time distribution curve. A, Freeze temperature; B, Thaw temperature; C, Thaw time.
As shown in Fig. 5A, the signal amplitude of the T22 fraction increased, which indicated that more water existed in the intercellular space (Li et al., 2015). In addition, the T22 population (M22) of samples after freezing was higher than that of the control. This may be due to damage to cell membranes caused by ice, resulting in the increase of cell permeability and the release of water into the intercellular space. T23 also increased, indicating that the T23 water in soybeans was in a more fluid environment after freeze–thaw. Moreover, the proportions of T23 (M23) significantly increased after freeze–thaw. Fig. 5B shows that T22 increased after thaw treatment, and M22 was at its maximum at a thaw temperature of 25 °C. Fig. 5C shows that T22 increased as the thaw time increased. M22 was the largest when thawing for 48 h, which indicated that intercellular water increased with thaw time. Nieto, Salvatori, Castro, and Alzamora (1998) confirmed that the freeze–thaw treatment could enhance the moisture diffusion by destroying the cellular structure. This is consistent with the freeze–thaw treatment that could destroy the cell structure of germinated soybeans. These results again confirmed that the increase in aglycone content was caused by the contact of ICHG with glucosides and malonyl-glucosides due to cell rupture.
4. Conclusion
Among these varieties, which account for 75 % of the genetic diversity of the 562 North American commercial varieties of the USDA-ARS Soybean Germplasm Collection, the Lee variety has the highest post-germination total isoflavone content and the most increased aglycone content after freeze–thaw treatment. Freeze-thaw treatments destroyed the cell structure of germinated soybeans, increased water to penetrate the intercellular space, and enhanced the mobile microenvironment. It enhanced the enzyme activity of ICHG and the interaction of ICHG with substrates, such as glucosides and malonyl-glucosides, to thereby promote the conversion of glucosides and malonyl-glucosides into aglycones. The freeze–thaw treatment is an effective method used to convert glucosides and malonyl-glucosides to aglycones. The freeze-thawed germinated soybeans can be used as raw material for extracting aglycones, and also as raw material for making food rich in aglycones.
CRediT authorship contribution statement
Wenmin Ji: Investigation, Methodology, Data curation, Writing – original draft. Tianbao Yang: Validation, Formal analysis, Methodology, Writing – review & editing. Qijian Song: Resources, Software, Supervision. Meng Ma: Conceptualization, Data curation, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We thank Irma Ortiz and Ellen Turner for their comments and edits.
Funding sources
This project was funded by Natural Science Foundation of China (grant number 32001614); China Scholarship Council (grant number 201806850075); the Open Project Program of China-Canada Joint Lab of Food Nutrition and Health, Beijing Technology and Business University (grant number KFKT-ZJ-2106); Advanced Talents Foundation of QAU (grant number 1120038); USDA-ARS (8042-43000-016-00D); and USDA NE-1836.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100493.
Contributor Information
Tianbao Yang, Email: tianbao.yang@usda.gov.
Meng Ma, Email: mamengss@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Coward L., Barnes N.C., Setchell K., Barnes S. Genistein, daidzein, and their.beta.-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. Journal of Agriculture and Food Chemistry. 1993;41(11):1961–1967. doi: 10.1021/jf00035a027. [DOI] [Google Scholar]
- da Silva L.H., Celeghini R.M.S., Chang Y.K. Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chemistry. 2011;128(3):640–644. doi: 10.1016/j.foodchem.2011.03.079. [DOI] [Google Scholar]
- Falcão H.G., Handa C.L., Silva M.B.R., de Camargo A.C., Shahidi F., Kurozawa L.E., Ida E.I. Soybean ultrasound pre-treatment prior to soaking affects β-glucosidase activity, isoflavone profile and soaking time. Food Chemistry. 2018;269:404–412. doi: 10.1016/j.foodchem.2018.07.028. [DOI] [PubMed] [Google Scholar]
- Graham T.L. Flavonoid and Isoflavonoid Distribution in Developing Soybean Seedling Tissues and in Seed and Root Exudates. Plant Physiology. 1991;95(2):594–603. doi: 10.1104/pp.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Yang R., Wang Z., Gu Z. Effect of freezing methods on sulforaphane formation in broccoli sprouts. [10.1039/C5RA03403E] RSC Advances. 2015;5(41):32290–32297. doi: 10.1039/C5RA03403E. [DOI] [Google Scholar]
- Hsiao Y.-H., Hsieh J.-F. The conversion and deglycosylation of isoflavones and anthocyanins in black soymilk process. Food Chemistry. 2018;261:8–14. doi: 10.1016/j.foodchem.2018.03.152. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Ahn J.K., Khanh T.D., Chun S.C., Kim S.L., Ro H.M.…Chung I.M. Comparison of isoflavone concentrations in soybean (Glycine max (L.) Merrill) sprouts grown under two different light conditions. Journal of Agriculture and Food Chemistry. 2007;55(23):9415–9421. doi: 10.1021/jf071861v. [DOI] [PubMed] [Google Scholar]
- Li T., Tu C., Rui X., Gao Y., Li W., Wang K.…Dong M. Study of water dynamics in the soaking, steaming, and solid-state fermentation of glutinous rice by LF-NMR: A novel monitoring approach. Journal of Agriculture and Food Chemistry. 2015;63(12):3261–3270. doi: 10.1021/acs.jafc.5b00769. [DOI] [PubMed] [Google Scholar]
- Lu C., Li F., Yan X., Mao S., Zhang T. Effect of pulsed electric field on soybean isoflavone glycosides hydrolysis by β-glucosidase: Investigation on enzyme characteristics and assisted reaction. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2021.132032. [DOI] [PubMed] [Google Scholar]
- Ma M., Wang P., Yang R., Gu Z. Effects of UV-B radiation on the isoflavone accumulation and physiological-biochemical changes of soybean during germination. Food Chemistry. 2018;250:259–267. doi: 10.1016/j.foodchem.2018.01.051. [DOI] [PubMed] [Google Scholar]
- Nieto A., Salvatori D., Castro M.A., Alzamora S.M. Air drying behaviour of apples as affected by blanching and glucose impregnation. Journal of Food Engineering. 1998;36(1):63–79. doi: 10.1016/S0260-8774(98)00043-0. [DOI] [Google Scholar]
- Phothiset S., Charoenrein S. Effects of freezing and thawing on texture, microstructure and cell wall composition changes in papaya tissues. Journal of the Science of Food and Agriculture. 2014;94(2):189–196. doi: 10.1002/jsfa.6226. [DOI] [PubMed] [Google Scholar]
- Quinhone A., Ida E.I. Profile of the contents of different forms of soybean isoflavones and the effect of germination time on these compounds and the physical parameters in soybean sprouts. Food Chemistry. 2015;166:173–178. doi: 10.1016/j.foodchem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Ritota M., Gianferri R., Bucci R., Brosio E. Proton NMR relaxation study of swelling and gelatinisation process in rice starch–water samples. Food Chemistry. 2008;110(1):14–22. doi: 10.1016/j.foodchem.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Salces F.R., Rostagno M.A., Amaya-Farfan J. Novel process of hydration, followed by incubation and thermal processing, for high isoflavone bioconversion in soybeans. Food Research International. 2019;121:691–696. doi: 10.1016/j.foodres.2018.12.040. [DOI] [PubMed] [Google Scholar]
- Sanches de Lima F., Ida E.I. Optimisation of soybean hydrothermal treatment for the conversion of β-glucoside isoflavones to aglycones. LWT - Food Science and Technology. 2014;56(2):232–239. doi: 10.1016/j.lwt.2013.12.006. [DOI] [Google Scholar]
- Setchell K.D., Brown N.M., Zimmer-Nechemias L., Brashear W.T., Wolfe B.E., Kirschner A.S., Heubi J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. The American Journal of Clinical Nutrition. 2002;76(2):447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- Song Q., Hyten D.L., Jia G., Quigley C.V., Fickus E.W., Nelson R.L., Cregan P.B. Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS ONE. 2013;8(1):e54985. doi: 10.1371/journal.pone.0054985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Hyten D.L., Jia G., Quigley C.V., Fickus E.W., Nelson R.L., Cregan P.B. Fingerprinting Soybean Germplasm and Its Utility in Genomic Research. G3 Genes|Genomes|Genetics. 2015;5(10):1999–2006. doi: 10.1534/g3.115.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Takahashi S., Watanabe R., Fukushima Y., Fujita N., Noguchi A.…Nakayama T. An Isoflavone Conjugate-hydrolyzing β-Glucosidase from the Roots of Soybean (Glycine max) Seedlings: PURIFICATION, GENE CLONING, PHYLOGENETICS, AND CELLULAR LOCALIZATION*. Journal of Biological Chemistry. 2006;281(40):30251–30259. doi: 10.1074/jbc.M605726200. [DOI] [PubMed] [Google Scholar]
- Szymczak G., Wójciak-Kosior M., Sowa I., Zapała K., Strzemski M., Kocjan R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. Journal of Food Composition and Analysis. 2017;57:40–48. doi: 10.1016/j.jfca.2016.12.015. [DOI] [Google Scholar]
- Wu J., Li Y., Gao X. Simultaneous determination of oil and water in soybean by LF-NMR relaxometry and chemometrics. Chemical Research in Chinese Universities. 2016;32(5):731–735. doi: 10.1007/s40242-016-6096-4. [DOI] [Google Scholar]
- Yadav S., Pandey A.K., Dubey S.K. Molecular modeling, docking and simulation dynamics of β-glucosidase reveals high-efficiency, thermo-stable, glucose tolerant enzyme in Paenibacillus lautus BHU3 strain. International Journal of Biological Macromolecules. 2021;168:371–382. doi: 10.1016/j.ijbiomac.2020.12.059. [DOI] [PubMed] [Google Scholar]
- Yang R., Hui Q., Feng X., Feng L., Gu Z., Wang P. The mechanism of freeze-thawing induced accumulation of γ-aminobutyric acid in germinated soybean. Journal of the Science of Food and Agriculture. 2020;100(3):1099–1105. doi: 10.1002/jsfa.10118. [DOI] [PubMed] [Google Scholar]
- Yeom S.-J., Kim B.-N., Kim Y.-S., Oh D.-K. Hydrolysis of Isoflavone Glycosides by a Thermostable β-Glucosidase from Pyrococcus furiosus. Journal of Agricultural and Food Chemistry. 2012;60(6):1535–1541. doi: 10.1021/jf204432g. [DOI] [PubMed] [Google Scholar]
- Zhao A., Shi P., Yang R., Gu Z., Jiang D., Wang P. Isolation of novel wheat bran antifreeze polysaccharides and the cryoprotective effect on frozen dough quality. Food Hydrocolloids. 2022;125 doi: 10.1016/j.foodhyd.2021.107446. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.