Highlight
-
•
Anthocyanin extracts lowered pH better than anthocyanin standards.
-
•
Diglucoside of anthocyanins resulted in higher concentration of total SCFA than monoglucoside did.
-
•
Lactobacillus was promoted by monoglucoside anthocyanins, while Escherichia-Shigella was inhibited by diglucoside anthocyanins.
-
•
Anthocyanins may destroy the integrity of the bacterial membrane/wall to exhibit antimicrobial activity.
-
•
Anthocyanins might act as potential probiotics to promote intestinal health.
Keywords: Anthocyanins, Structures, Morphology, Gut microbiota, High-throughput sequencing, Incubation in vitro
Abstract
Yan 73 anthocyanin extracts and spine grape anthocyanin extracts, along with anthocyanin standards (malvidin-3-O-glucoside; malvidin-3,5-O-diglucoside), were used to investigate the role of anthocyanins and their structures (monoglucoside, diglucoside) on intestinal microbiota. The results showed that anthocyanins (especially monoglucoside) increased the alpha diversity and abundance of Bifidobacterium, Faecalibacterium, and Prevotella. Yan 73 anthocyanin extracts particularly promoted the growth of Lactobacillus and decreased Streptococcus abundance, while malvidin-3,5-O-diglucoside suppressed the proliferation of Escherichia-Shigella. The pH value was lower in anthocyanin extracts than in the standards. Short-chain fatty acid contents were higher in diglycososide anthocyanins than in monoglycoside. The results of scanning electron microscopic images suggested that anthocyanins might change bacterial cell morphology to exhibit antibacterial activity. Consequently, anthocyanins with different structures showed dynamic and multiple regulatory effects on intestinal microbiota for maintaining intestinal health through promoting short-chain fatty acids, lowering pH, and/or damaging bacterial cell morphology.
Introduction
Anthocyanins are water-soluble natural pigments belonging to the flavonoid family. They are widely found in nature and are responsible for the brilliant color of flowers and fruits of a great variety of plants. Structurally, anthocyanins are the glycosylated form of anthocyanidins that possess a C6-C3-C6 basic skeleton, a polyhydroxy or polymethoxy derivative of the 2-phenylbenzopyran cation, containing two benzoyl rings (A, B) and one oxygen-containing heterocycle (C) (Table S1) (Han et al., 2019). Based on the hydroxylation, methoxylation patterns on the B-ring and glycosylation with sugar units, six common monoglucoside anthocyanins in Vitis vinifera grape and wine are derived, namely, cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, malvidin-3-O-glucoside, pelargonidin-3-O-glucoside, peonidin-3-O-glucoside, and petunidin-3-O-glucoside, while anthocyanins in American grape, East Asian grape, and their red wines are mainly 3,5-O-diglucoside forms (Table S1).
Among the different foods, grape is one of the richest sources of anthocyanins. In Vitis vinifera L. grapes and red wine, the major anthocyanins include malvidin-3-O-glucoside, malvidin-3-O-(6-O-acetyl)-glucoside, and malvidin-3-O-(6-O-coumaryl)-glucoside. Yan 73 (Vitis vinifera L.) is a famous red grape variety grown in China that accumulates substantial amounts of anthocyanins in both berry skin and pulp. The total amount of anthocyanins in grape skin can even reach 20–30 g/kg dry weight, mainly malvidin-3-O-glucoside (Jin et al., 2009). Spine grape (Vitis davidii Foex) belongs to the category of East Asian Vitis spp. and is generally distributed in the mountains covered by the subtropical rainforest to the south of the Yangtze River in China. The anthocyanins in spine grape wine can reach more than 1400 mg/L, with malvidin-3,5-O-diglucoside being the main anthocyanin (Kong et al., 2019).
It has been reported that anthocyanins possess widespread biological functions, including antioxidant, anti-inflammatory, anticancer, anti-obesity, anti-diabetic, and antimicrobial effects, as well as the prevention of cardiovascular diseases (Alam et al., 2021). Despite the beneficial properties of anthocyanins, their bioavailability is very low. Only a small fraction of anthocyanins can be degraded and absorbed in the oral cavity or stomach, and most dietary anthocyanins will reach the colon intact, undergo biotransformation and metabolism by the gut microbiota, and absorbed by the intestinal epithelium (Han et al., 2019). Therefore, one of the possible reasons for anthocyanins to exert their beneficial activities is to regulate the gut microbiota.
The human gastrointestinal tract, especially the large intestine, is inhabited by a large number of bacteria, predominantly Firmicutes and Bacteroides (dominant phyla, accounting for more than 95 %), and other species are members of the phyla Actinobacteria, Proteobacteria, Verrumicrobia, and Fusobacteria (Erawijantari et al., 2020). There are nearly 100 trillion bacteria, possessing approximately 2–4 million genes, in the intestine of a healthy adult, and its genetic diversity is approximately 100–150 times larger than that of the human genome, which means that the intestinal flora has a huge metabolic capacity and plays a critical role in host physiology, such as digestion, absorption, and metabolism of dietary nutrients (de Vos et al., 2022).
Petunidin and its anthocyanin forms (mainly petunidin-3-O-glucoside) derived from Lycium ruthenicum and malvidin-3-O-glucoside from Cabernet Sauvignon could exert prebiotic activity by altering the structure of gut microbiota, such as promoting the growth of Ruminococcaceae and Akkermansia, as well as inhibiting the proliferation of Helicobacter and Desulfovibrionaceae ( Han et al., 2020, Tian et al., 2021). Dealcoholized red wine extract changed the gut microbiota composition of rats from a predominance of Bacteroides and Clostridium to a predominance of Lactobacillus and Bifidobacterium spp., implying that anthocyanins favor an increase in intestinal beneficial bacteria (Jamar, Estadella, & Pisani, 2017).
However, little information is available on the effects of monoglucoside and diglucoside anthocyanins from Vitis vinifera L. and Vitis davidii Foex on the gut microbiota. In addition, the mechanism by which anthocyanin regulates intestinal flora is not well understood. In the present study, therefore, the effect of anthocyanins from Yan 73 (Vitis Vinifera L.) and spine grape (Vitis davidii Foex) on gut microbiota was investigated by analyzing the short-chain fatty acid concentration, pH value, microbiota composition, and morphology. This research could provide a better understanding of the role of monoglucoside and diglucoside anthocyanins as prebiotics to regulate intestinal health and provide important theoretical support for the development of the grape and red wine of Vitis Vinifera L. and Vitis davidii Foex as functional food ingredients.
Materials and methods
Materials and chemicals
Methyl alcohol, formic acid, and acetonitrile were of HPLC grade and purchased from Tiancheng Chemical Reagent Co., Ltd. (Shaanxi, China), whereas standards of acetic, propionic, n-butyric, i-butyric, n-valeric, i-valeric, and 2-ethylbutyric acids were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Yan 73 and spine grape were picked from Shaanxi and Hunan Provinces in China, respectively. Reversed-phase C-18 silica gel was obtained from High Quality & Expert Co., Ltd. (Beijing, China).
Anthocyanin extraction and purification
The procedures for the extraction and purification of anthocyanins from grape skin were performed according to a reported method (Han et al., 2020). Briefly, grape skin (5 g) was mixed with 0.01 % HCl methanol (v/v) (20 mL). The mixture was carried out at 40 °C in a water bath for 30 min, followed by ultrasonic-assisted extraction for 30 min and centrifugation for 5 min at 8000 rpm. The supernatants were concentrated with a vacuum rotary evaporator at 40 °C. Afterward, the concentrated extracts were diluted with 0.01 % HCl distilled water (v/v) and purified by a C-18 silica gel column (2.6 × 30 cm) to remove the water-soluble compounds, including sugars and acids. The target constituents (anthocyanins) were eluted with 0.01 % HCl methanol (v/v) and concentrated. Other nonanthocyanin phenolic compounds were removed by ethyl acetate, subsequently removing ethyl acetate and redissolving target constituents. The anthocyanin extracts were obtained by vacuum freeze drying.
The mixed anthocyanins were then purified by a Shimadzu preparative HPLC instrument (Shimadzu, Suzhou, China; pump: LC-6AD; diode array detector: SPD-M10-AVP; system controller: SCL-10AVP). An SP ODS-AQ C18 column (10 × 250 mm, 5 μm, High quality & Expert Co., ltd., Beijing, China) at 35 °C was used to separate the anthocyanins in the samples under a flow rate of 3 mL/min, and the detection wavelength was 520 nm. The solvents were (A) H2O/CH3CN/HCOOH (32/4/1) and (B) H2O/CH3CN/HCOOH (16/20/1). The gradient was programmed as follows: 0 to 15 min, 5 % to 20 % B; 15 to 20 min, 20 % to 25 % B; 20 to 35 min, 25 % to 30 % B; 35 to 40 min, 30 % to 40 % B; 40 to 42 min, 40 % to 100 % B; 42 to 56 min, 100 % B; 56 to 57 min, 100 % to 5 % B.
Analysis of anthocyanins by HPLC and HPLC-MS/MS
A Shimadzu LC-20AT HPLC (Shimadzu, Kyoto, Japan) equipped with a Synergi Hydro-RP C18 column (250 × 4.6 mm, 4 μm, Phenomenex, Beijing, China) was used to analyze the anthocyanin extracts under a flow rate of 1 mL/min. The gradient was as follows: 0 to 15 min, 0 % to 10 % B; 15 to 30 min, 10 % to 20 % B; 30 to 45 min, 20 % to 35 % B; 45 to 46 min, 35 % to 100 % B; 46 to 50 min, 100 % B; 50 to 51 min, 100 % to 0 % B. Other parameters for HPLC analysis, including solvents, detection wavelength, and oven temperature, were the same as mentioned above. Anthocyanins in the samples of Yan 73 and spine grape were quantified at 520 nm as equivalents of malvidin-3-O-glucoside and malvidin-3,5-O-diglucoside standards, respectively.
HPLC-MS/MS analyses were performed on a QTRAP 5500 system (AB SCIEX, USA) coupled with an Athena C18-WP column (4.6 × 150 mm, 5 μm, CNW, China). The column temperature was set at 30 °C, and the mobile phase consisted of (A) 0.1 % formic acid (v/v) in water and (B) acetonitrile under a 1 mL/min flow rate. The elution gradient was programmed as follows: 0 to 1 min, 0 to 5 % B; 1 to 15 min, 5 % to 95 % B; 15 to 18 min, 95 % B; 18 to 18.1 min, 95 % to 5 % B. MS parameters were performed according to a published method (Han et al., 2020). Mass spectra were scanned in positive ion mode between 100 and 1000 m/z.
Anaerobic incubation of intestinal microbiota in vitro
Fresh fecal materials were obtained from one healthy woman donor (24 years old) who did not have any intestinal disease or antibiotic treatment for the previous six months. The donor woman was told to avoid ingestion of foods rich in anthocyanins two weeks before fecal donation. Feces and phosphate buffer (PBS, pH 7.0) were homogenized at a ratio of 1 g:9 mL, filtered with sterile gauze, and centrifuged at 5000 rpm (37 °C for 5 min) to obtain fecal supernatant. Solutions of anthocyanins were prepared by dissolving in autoclaved basal medium (0.293 g/L K2HPO4, 0.176 g/L KH2PO4, 0.443 g/L NaCl, 0.45 g/L (NH4)2SO4, 0.045 g/L CaCl2, 0.50 g/L l-cysteine, 0.094 g/L MgSO4, 0.50 g/L vitamin C, 0.001 g/L resazurin, 4.0 g/L Na2CO3, 1.0 g/L peptone, 1.0 g/L agar, 2.0 g/L glucose). Afterward, 2.0 mL of fecal slurry supernatant was added to 18.0 mL of nutrient medium containing anthocyanins in a cell culture flask for anaerobic incubation in vitro. The concentrations of anthocyanins were set to 600 μmol/L, 300 μmol/L, and 100 μmol/L. Basal nutrient medium without any anthocyanins was used as a control. The triangular bottles were incubated at 37 °C for 24 h. Samples were removed at 0, 4, 12, and 24 h for further study.
Measurement of pH value
The incubated samples were removed at 0, 4, 12, and 24 h and centrifuged at 13000 rpm (4 °C for 10 min), and then the supernatants were transferred to 10 mL centrifuge tubes. The pH was determined using a pH meter (FiveEasy Plus, METTLER TOLEDO, Shanghai, China).
Determination of short-chain fatty acid concentrations
The contents of short-chain fatty acids, including acetic, propionic, n-butyric, i-butyric, n-valeric, and i-valeric acids, in incubated fluids were determined by gas chromatography (GC) equipped with a DB-FFAP capillary column (30 m × 0.25 mm × 0.5 μm, Agilent, Beijing, China). In brief, 20 μL 10 mol/L 2-ethylbutyric acid (internal standard) was added to 400 μL of sample or standard solution. The mixed solution was centrifuged (13000 r for 10 min), and the supernatant (1 μL) was loaded onto a GC (GC-2014C, Shimadzu, Kyoto, Japan) at a split ratio of 1:10. The oven temperature program was as follows: started with 50 °C and held on for 1 min, elevated to 150 °C at 10 °C/min and kept for 1 min, then rose to 240 °C at 20 °C/min, and further maintained 3.83 min. The operational temperature of the flame ionization detector and injection port was 250 °C, respectively.
Scanning electron microscopy (SEM) analysis
Bacteria were collected by centrifugation at 13,000 r for 10 min at 4 °C, fixed with glutaraldehyde (2.5 %, v/v) for 24 h, and then washed four times with 0.1 M PBS (pH 6.8). After centrifugation, bacteria were dehydrated in ethanol in a gradient (10, 30, 50, 70, 80, 90, and 100 %), coated with gold, and subsequently observed by SEM (Nano SEM-450, FEI Company, USA).
Analysis of intestinal microbiota
Total genomic DNA from the samples was extracted using the CTAB method. The concentration and purity of extracted DNA were monitored on 1 % agarose gels, and then the DNA concentration was diluted to 1 ng/µL with sterile water. The primers 341F (5́ CCTAYGGGRBGCASCAG 3́) and 806R (5́ GGACTACNNGGGTATCTAAT 3́) were used to amplify the V3 + V4 region of bacterial 16S ribosomal ribonucleic acid (rRNA) gene. Then, the polymerase chain reaction (PCR) products were measured by electrophoresis on a 2 % agarose gel and extracted with a Qiagen Gel Extraction Kit (Qiagen, Germany). The sequencing libraries were generated using a TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) following the manufacturer's recommendations. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform (Beijing, China).
The raw sequences were then analyzed on QIIME (V1.9.1, https://qiime.org/scripts/split_libraries_fastq.html). All effective tags were compared with the reference database (Silva database, https://www.arb-silva.de/) using the UCHIME algorithm (UCHIME, https://www.drive5.com/usearch/manual/uchime_algo.html). Sequence analysis was performed by UPARSE software (UPARSE v7.0.1001, https://drive5.com/uparse/). Sequences with ≥97 % similarity were assigned to the same operational taxonomic units. For each representative sequence, the Silva Database (https://www.arb-silva.de/) was used based on the Mothur algorithm to annotate taxonomic information. The alpha diversity indices in these samples were calculated with QIIME (Version 1.7.0) and displayed with R software (Version 2.15.3).
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Analysis of variance (ANOVA) was used to estimate statistically significant differences among the treatments. Differences were considered statistically significant at p < 0.05.
Results and discussion
Qualitative and quantitative analysis of anthocyanins by HPLC and HPLC-MS/MS
A total of 12 and 9 anthocyanins were identified by HPLC-MS/MS in Yan 73 and spine grape, respectively (Table 1). Quantification of anthocyanin by HPLC showed that the main anthocyanin present in the Yan 73 extracts was malvidin-3-O-glucoside (427.36 ± 0.14 mg/L) (Fig. S1A), while the major anthocyanin in the spine grape extracts was malvidin-3,5-O-diglucoside (134.74 ± 0.01 mg/L) (Fig. S1B). In addition, delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, peonidin-3-O-glucoside, and their coumarylated and acetylated compounds were detected in the Yan 73 anthocyanin extracts (Table 1). The diglucoside of the anthocyanins was identified in the spine grape anthocyanin extracts (Table 1). Yan 73 anthocyanin extracts and spine grape anthocyanin extracts, as well as their main anthocyanins (malvidin-3-O-glucoside and malvidin-3,5-O-diglucoside), were selected for further experiments.
Table 1.
Anthocyanin profile identified by HPLC-MS/MS in Yan 73 and spine grape.
| Number | Anthocyanin | RTmin | M+ (m/z) | MS2/MS3 (m/z) | λmax |
|---|---|---|---|---|---|
| Yan 73 anthocyanin extracts | |||||
| 1 | Delphinidin-3-O-glucoside | 5.213 | 465 | 303 | 524 |
| 2 | Cyanidin-3-O-glucoside | 5.526 | 449 | 287 | 516 |
| 3 | Petunidin-3-O-glucoside | 5.690 | 479 | 317 | 524 |
| 4 | Peonidin-3-O-glucoside | 5.884 | 463 | 301 | 518 |
| 5 | Malvidin-3-O-glucoside | 6.014 | 493 | 331 | 528 |
| 6 | Peonidin-3-O-(6-O-acetyl)-glucoside | 6.707 | 505 | 301 | 522 |
| 7 | Malvidin-3-O-(6-O-acetyl)-glucoside | 6.748 | 535 | 331 | 528 |
| 8 | Delphinidin-3-O-(6-O-coumaroyl)-glucoside-glucoside | 6.815 | 611 | 303 | – |
| 9 | Malvidin-3-O-(6-O-caffeoyl)-glucoside | 6.953 | 655 | 331 | 532 |
| 10 | Petunidin-3-O-(6-O-coumaryl)-glucoside | 7.082 | 625 | 317 | 530 |
| 11 | Peonidin-3-O-(6-O-coumaryl)-glucoside | 7.285 | 609 | 301 | 524 |
| 12 | Malvidin-3-O-(6-O-coumaryl)-glucoside | 7.305 | 639 | 331 | 536 |
| Spine grape anthocyanin extracts | |||||
| 13 | Delphinidin-3,5-O-diglucoside | 5.333 | 627 | 465, 303 | 520 |
| 14 | Petunidin-3,5-O-diglucoside | 6.978 | 641 | 479, 317 | 523 |
| 15 | Peonidin-3,5-O-diglucoside | 5.358 | 625 | 463, 301 | 525 |
| 16 | Malvidin-3,5-O-diglucoside | 5.513 | 655 | 493, 331 | 523 |
| 17 | Malvidin-3-O-(6-O-acetyl)- glucoside −5-O-glucoside | 5.933 | 697 | 535, 493, 331 | 529 |
| 18 | Delphinidin-3-O-(6-O-coumaroyl)-glucoside-5-O-glucoside | 6.902 | 773 | 611, 465 | 527 |
| 19 | Malvidin-3-(6-O-caffeoyl)-glucoside-5-glucoside | 6.549 | 817 | 657, 493, 331 | 529 |
| 20 | Petunidin-3-O-(6-O-coumaroyl)-glucoside-5-O-glucoside | 6.594 | 787 | 625, 479, 317 | 529 |
| 21 | Malvidin-3-O-(6-O-coumaroyl)-glucoside-5-O-glucoside | 6.786 | 801 | 639, 493, 331 | 529 |
Note: ‘‘-’’ Not detected.
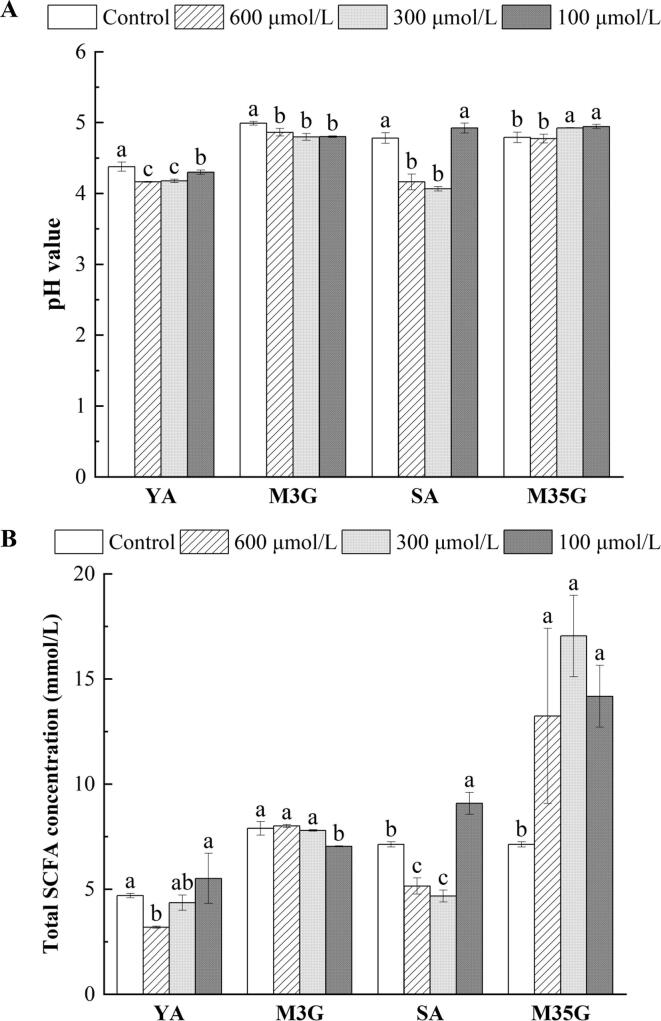
Fig. 1.
Ph value (a) and concentrations of total short-chain fatty acid (B) after incubation in vitro with Yan 73 anthocyanin extracts, malvidin-3-O-glucoside, spine grape anthocyanin extracts, and malvidin-3,5-O-diglucoside, respectively. YA, M3G, SA, and M35G represent Yan 73 anthocyanin extracts, malvidin-3-O-glucoside, spine grape anthocyanin extracts, and malvidin-3,5-O-diglucoside, respectively. Results are given as the mean ± SD in each group. Values with different letters (a-c) are significantly different (p < 0.05).
pH shift after incubation
Fecal pH, one of the vital parameters of colon health, is considered an indirect indicator of colon bacterial fermentation and metabolite production. Fig. 1A depicts the pH value after incubation in vitro with anthocyanins. Both Yan 73 anthocyanin extracts and malvidin-3-O-glucoside significantly decreased the pH value (p < 0.05) at 24 h with respect to the control (Fig. 1A). Similar results were observed in spine grape anthocyanin extracts (300 and 600 μmol/L) (p < 0.05) but not in malvidin-3,5-O-diglucoside (300 and 600 μmol/L) (Fig. 1A). These data showed that anthocyanins (except malvidin-3,5-O-diglucoside) can effectively decrease the intestinal pH. This is supported by the results of other findings, as feeding purple carrot anthocyanins (mainly cyanidin forms) produced a significant drop in the pH of the rat cecum (Żary-Sikorska et al., 2019).
The effect of anthocyanin extracts on lowering the pH value seemed to be more effective than that of standards. One possible reason is the presence of other anthocyanins in the extracts. Incubation of malvidin-3-O-glucoside with fecal bacteria mainly resulted in the formation of syringic acid, while other anthocyanins resulted in the formation of gallic, ferulic, and p-coumaric acids (Hidalgo et al., 2012). These phenolic acids produced by other anthocyanins contributed to reducing the pH of the medium (Kilua et al., 2020). Furthermore, glycoside structure also determines the stability and absorption efficiency of anthocyanins, which might influence the variations in fecal pH (Li et al., 2021). Diglucoside could enhance the stability of malvidin-3,5-O-diglucoside under physiological pH and temperature conditions, resulting in a lower degradation rate and phenolic acid production than malvidin-3-O-glucoside and eventually in a lower ability of malvidin-3,5-O-diglucoside to drop medium pH than malvidin-3-O-glucoside.
A decrease in colonic pH is desirable from a health point of view, as it can inhibit the growth of pathogens. When the pH value of the medium is lower than 5, the proliferation of Streptococcus can be strongly inhibited by damaging cell walls, reducing enzymes of glycolysis, and suppressing the expression of virulence factors (Seekatz et al., 2019). However, many probiotics, such as Bifidobacterium and Lactobacillus, are relatively tolerant of acidic pH and can survive easily when the pH of the medium decreases (Presti et al., 2015). Therefore, alterations to incubation environmental pH caused by anthocyanins (especially monoglucoside anthocyanins) may modulate the gut microbiota composition and function.
Short-chain fatty acid analysis
Short-chain fatty acids are major end products of gut microbiota activity that have a positive impact on host health, serving as an energy source for the colonic epithelium, having anticancer and anti-inflammatory functions, and regulating the nervous system and host metabolism (van der Hee & Wells, 2021). Therefore, we assessed the potential impact of anthocyanins on short-chain fatty acid production for 24 h. At the end of incubation, high short-chain fatty acid concentrations were observed in Yan 73 anthocyanin extracts at 100 μmol/L and malvidin-3-O-glucoside at 600 μmol/L relative to the control, but there was no obvious change (Fig. 1B). However, the total short-chain fatty acid content (9.09 ± 0.52 mmol/L) in the spine grape anthocyanin extract 100 μmol/L group was significantly higher than that in the control group (p < 0.05) (Fig. 1B). Meanwhile, each group of malvidin-3,5-O-diglucoside also remarkably elevated the content of total short-chain fatty acids (p < 0.05) (Fig. 1B). Incubation of malvidin-3,5-O-diglucoside at 300 μmol/L gave the highest production of short-chain fatty acids (17.05 ± 1.94 mmol/L) among treatments. The short-chain fatty acid content for spine grape anthocyanin extracts was lower than that of malvidin-3,5-O-diglucoside, which might be associated with the matrix effects.
Compared with malvidin-3,5-O-diglucoside, the presence of other anthocyanins in the spine grape anthocyanin extracts probably shifted the metabolic pathways of microorganisms and eventually affected the production of short-chain fatty acids (Liu et al., 2021). Additionally, diglucoside of anthocyanins also helps the generation of short-chain fatty acids, since diglucoside can be hydrolyzed by β-glucosidase from the gut microbiota to release two molecules of glucose, which could be directly used as substrates for bacterial metabolism to produce more short-chain fatty acids (Cheng et al., 2016). This contributes to explaining the higher amount of total short-chain fatty acids in diglucoside anthocyanin treatments than in the monoglucoside.
Acetic, propionic, and n-butyric acids were the three main fermentation products (Table 2). After incubation, Yan 73 anthocyanin extracts at 100 μmol/L increased acetic, propionic, and n-butyric acids with respect to the control, without significant differences. Acetic acid remained unaffected with malvidin-3-O-glucoside at 600 μmol/L, while propionic and n-butyric acids were significantly increased by malvidin-3-O-glucoside at 300 and 600 μmol/L after 24 h, respectively (p < 0.05) (Table 2). Wu et al. (2020) found that supplementation with malvidin-3-O-glucoside extracted from blueberry also significantly increased the content of propionic and n-butyric acids during in vitro 24 h colonic fermentation. These three organic acids were strongly promoted by the spine grape anthocyanin extracts at 100 μmol/L compared with the control at 24 h (p < 0.05). Notably, malvidin-3,5-O-diglucoside (300 μmol/L) induced the highest concentrations of acetic (7.61 ± 1.37 mmol/L), propionic (2.85 ± 0.23 mmol/L), and n-butyric acids (6.49 ± 0.32 mmol/L) among the groups after anaerobic incubation. In addition, the n-valeric, i-valeric, and i-butyric acids were also dramatically increased by malvidin-3,5-O-diglucoside relative to the control at 24 h (p < 0.05) (Fig. S2).
Table 2.
Contents of acetic, propionic and n-butyric acids after incubation in vitro (mmol/L).
| Treatment | Acetic acid | Propionic acid | n-Butyric acid |
|---|---|---|---|
| Yan 73 anthocyanin extracts | |||
| Control | 1.94 ± 0.12a | 0.82 ± 0.01a | 1.83 ± 0.23ab |
| 600 μmol/L | 1.71 ± 0.01a | 0.76 ± 0.02a | 0.68 ± 0.06c |
| 300 μmol/L | 1.93 ± 0.29a | 0.77 ± 0.05a | 1.61 ± 0.02b |
| 100 μmol/L | 2.48 ± 0.80a | 0.93 ± 0.17a | 2.03 ± 0.22a |
| Malvidin-3-O-glucoside | |||
| Control | 3.56 ± 0.20a | 1.92 ± 0.06b | 2.35 ± 0.06b |
| 600 μmol/L | 3.56 ± 0.06a | 1.87 ± 0.04b | 2.50 ± 0.03a |
| 300 μmol/L | 3.51 ± 0.05a | 2.08 ± 0.15a | 2.15 ± 0.06c |
| 100 μmol/L | 3.18 ± 0.04b | 1.79 ± 0.04b | 2.02 ± 0.06d |
| Spine grape anthocyanin extracts | |||
| Control | 2.33 ± 0.08b | 1.43 ± 0.01b | 3.31 ± 0.19b |
| 600 μmol/L | 2.11 ± 0.16b | 1.09 ± 0.09c | 1.90 ± 0.46c |
| 300 μmol/L | 2.36 ± 0.20b | 1.10 ± 0.04c | 1.17 ± 0.53d |
| 100 μmol/L | 3.21 ± 0.27a | 1.64 ± 0.04a | 4.17 ± 0.21a |
| Malvidin-3,5-O-diglucoside | |||
| Control | 2.33 ± 0.08b | 1.43 ± 0.01b | 3.31 ± 0.19b |
| 600 μmol/L | 5.71 ± 2.53a | 2.28 ± 0.53a | 5.17 ± 1.09a |
| 300 μmol/L | 7.61 ± 1.37a | 2.85 ± 0.23a | 6.49 ± 0.32a |
| 100 μmol/L | 6.23 ± 0.25a | 2.25 ± 0.25a | 5.61 ± 0.96a |
Results are expressed as mean ± SD in each group. Different letters (a-d) within the same column indicate significant differences (p < 0.05).
The data above indicated that the generation of short-chain fatty acids was strengthened by anthocyanin diglucoside at a lower concentration during anaerobic incubation, in line with the role of anthocyanins from Chilean currants (Ribes spp.) (Burgos-Edwards et al., 2018), and the increase in short-chain fatty acids can promote positive microbiota proliferation and decrease the growth of pathogenic bacterial species. The mechanism of short-chain fatty acids against pathogens is postulated as intracellular acidification caused by the uptake of these free acids at acidic pH. Acidified bacterial cells have reduced transmembrane potentials and disrupted cellular biological activities (such as DNA replication and catabolism), thereby exhibiting a low growth phenotype (Zhang et al., 2020). For instance, acetic acid exerts an antimicrobial effect by accumulating in Escherichia coli O157:H7 (Diez-Gonzalez & Russell, 1997). On the other hand, the toxicity of short-chain fatty acids can be further enhanced by a lower pH value, which strongly inhibits the growth of pathogenic bacteria by downregulating virulence gene expression and suppressing the production of factors required for commensal colonization (Zhang et al., 2020). Thus, anthocyanins, particularly diglucoside anthocyanins, can maintain intestinal health by increasing the concentration of short-chain fatty acids in synergy with lowering pH to inhibit the growth of pathogens.
Intestinal microbiota diversity and composition analysis
To further understand the effect of anthocyanins on gut microbiota, the alpha diversity (Chao richness and Shannon diversity) and composition of gut microbiota were determined using high throughput 16S rDNA gene sequencing (Table S2). Compared with the control, Yan 73 anthocyanin extracts significantly increased Chao richness at 300 and 600 μmol/L after 24 h (p < 0.05) but had no significant effect on Shannon diversity. Malvidin-3-O-glucoside was more efficient in promoting alpha diversity than Yan 73 anthocyanin extracts, especially at 100 μmol/L. Species richness did not show a significant change in spine grape anthocyanin extracts at 300 μmol/L; however, a larger extent of bacterial diversity was determined in malvidin-3,5-O-diglucoside (100 μmol/L) (p < 0.05) than in the control after incubation in vitro.
Gut microbiota alpha diversity has been linked to human health, with lower levels of diversity associated with several acute and chronic diseases (Manor et al., 2020). The diversity and richness of the gut microbiota were improved after anthocyanin intervention. Monoglucoside anthocyanins did better in increasing alpha diversity than diglucoside after 24 h of incubation. This may indicate the selective nature of the anthocyanins with different structures toward particular species since it represented the difference in fermentable material during the incubation. A similar impact on microbial diversity was observed for Mankai® anthocyanins (Diotallevi et al., 2021), showing that fermentation of anthocyanins by gut microbiota can select for particular bacteria able to use them as a growth substrate at the expense of other species that cannot derive energy or carbon sources from the test anthocyanins and ultimately lead to higher alpha diversity of monoglucoside anthocyanins with respect to diglucoside.
Gut microbiota profile at the phylum level
The relative abundance of the bacteria at the phylum level is presented in Fig. S3. Firmicutes and Bacteroidetes were the main phyla, with a total proportion exceeding 90 %, followed by small proportions of Proteobacteria and Actinobacteria. The ratio of Firmicutes/Bacteroidetes (F/B) is an important marker representing the status of gut microbiota. A high F/B ratio is usually associated with dysbiosis of the gut microbiome, as well as related to a high-fat diet and metabolic diseases (Grigor'eva, 2020). Malvidin-3-O-glucoside induced a significant decrease in the F/B ratio at 300 μmol/L over the control after incubation (p < 0.05) (Fig. 2A), suggesting that malvidin-3-O-glucoside might have a potential anti-obesity effect. Contrary to the results observed in malvidin-3-O-glucoside, there was a trend toward an increased F/B ratio in Yan 73 anthocyanin extracts compared with its control, although the difference was not significant (Fig. 2A). Consistent with this finding, the F/B value was also increased in mice fed the monoglucoside anthocyanin extracts (Han et al., 2020). The decline in the F/B ratio at 24 h was observed both in spine grape anthocyanin extracts (100 μmol/L) and malvidin-3,5-O-diglucoside (100 and 300 μmol/L) (Fig. 2A), even without significance. Anthocyanin standards were more conducive to repressing the F/B ratio than extracts. Compared with malvidin-3,5-O-diglucoside (300 μmol/L), malvidin-3-O-glucoside (300 μmol/L) had a strong ability to reduce F/B values. This different result may be attributed partially to the high short-chain fatty acid concentration in malvidin-3,5-O-diglucoside because short-chain fatty acids negatively affect the proliferation of Bacteroides at mild acid pH, ultimately increasing the F/B value (Firrman et al., 2022).
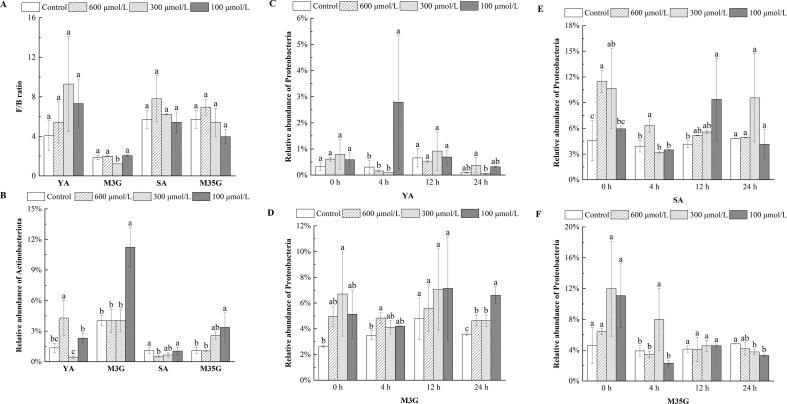
Fig. 2.
F/B ratio (A), the relative abundance of Actinobacteria (B) and Proteobacteria (C-F) during incubation in vitro with Yan 73 anthocyanin extracts(C), malvidin-3-O-glucoside(D), spine grape anthocyanin extracts(E), and malvidin-3,5-O-diglucoside(F), respectively. YA, M3G, SA, and M35G represent Yan 73 anthocyanin extracts, malvidin-3-O-glucoside, spine grape anthocyanin extracts, and malvidin-3,5-O-diglucoside, respectively. Results are given as the mean ± SD in each group. Values with different letters (a-c) are significantly different (p < 0.05).
As one of the four major phyla, Actinobacteria has many carbohydrate-active enzymes that humans lack, and these enzymes are required to digest most of the complex dietary polysaccharides in humans (Jiao et al., 2021). Complex polysaccharides will eventually be hydrolyzed into short-chain fatty acids and H2O. Yan 73 anthocyanin extracts (600 μmol/L), malvidin-3-O-glucoside (100 μmol/L), and malvidin-3,5-O-diglucoside (100 μmol/L) significantly amplified the Actinobacteria population compared with the control (p < 0.05) (Fig. 2B). Furthermore, a slight expansion of Actinobacteria in spine grape anthocyanin extracts at 100 μmol/L was determined (Fig. 2B). Notably, malvidin-3-O-glucoside at 100 μmol/L had the highest Actinobacteria abundance (11.23 %) among all samples. Moreover, there was a similar concentration effect on the promotion of Actinobacteria between spine grape anthocyanin extracts, and malvidin-3,5-O-diglucoside, which means that malvidin-3,5-O-diglucoside may be a key functional component of spine grape anthocyanin extracts in the regulation of Actinobacteria in vitro. In this experiment, anthocyanins from Yan 73 and spine grape may act as prebiotics and support the growth of Actinobacteria.
Another bacterium monitored at the phylum level is Proteobacteria, which is a facultative anaerobe, unlike the other bacteria. An increased prevalence of Proteobacteria is a marker for an unstable microbial community (dysbiosis) and a potential diagnostic criterion for disease (Guo et al., 2020). Proteobacteria abundance was downregulated effectively in malvidin-3,5-O-diglucoside treatments (p < 0.05) after 24 h (Fig. 2F). Both Yan 73 anthocyanin extracts (300 μmol/L) and spine grape anthocyanin extracts (100 μmol/L) slightly decreased the proportion of Proteobacteria, although without significance (Fig. 2C, E). In contrast, malvidin-3-O-glucoside notably promoted the growth of Proteobacteria at 24 h (p < 0.05) (Fig. 2D).
According to these data, anthocyanins inhibited the growth of Proteobacteria, and diglucoside anthocyanins, especially malvidin-3,5-O-diglucoside, seemed to possess a better role in decreasing Proteobacteria abundance than monoglycoside anthocyanins. A possible reason is that diglucoside anthocyanins remarkably inhibited the growth of Escherichia-Shigella in this experiment (Fig. 3 H), which is one of the major members of Proteobacteria. The decrease in the relative abundance of Escherichia-Shigella contributed to lowering the level of Proteobacteria at the end of the culture. Furthermore, the antioxidant ability of anthocyanins may decrease the oxygen pressure in the matrix and bacterial cells, resulting in the promotion of anaerobic bacteria and the inhibition of aerobic bacteria.
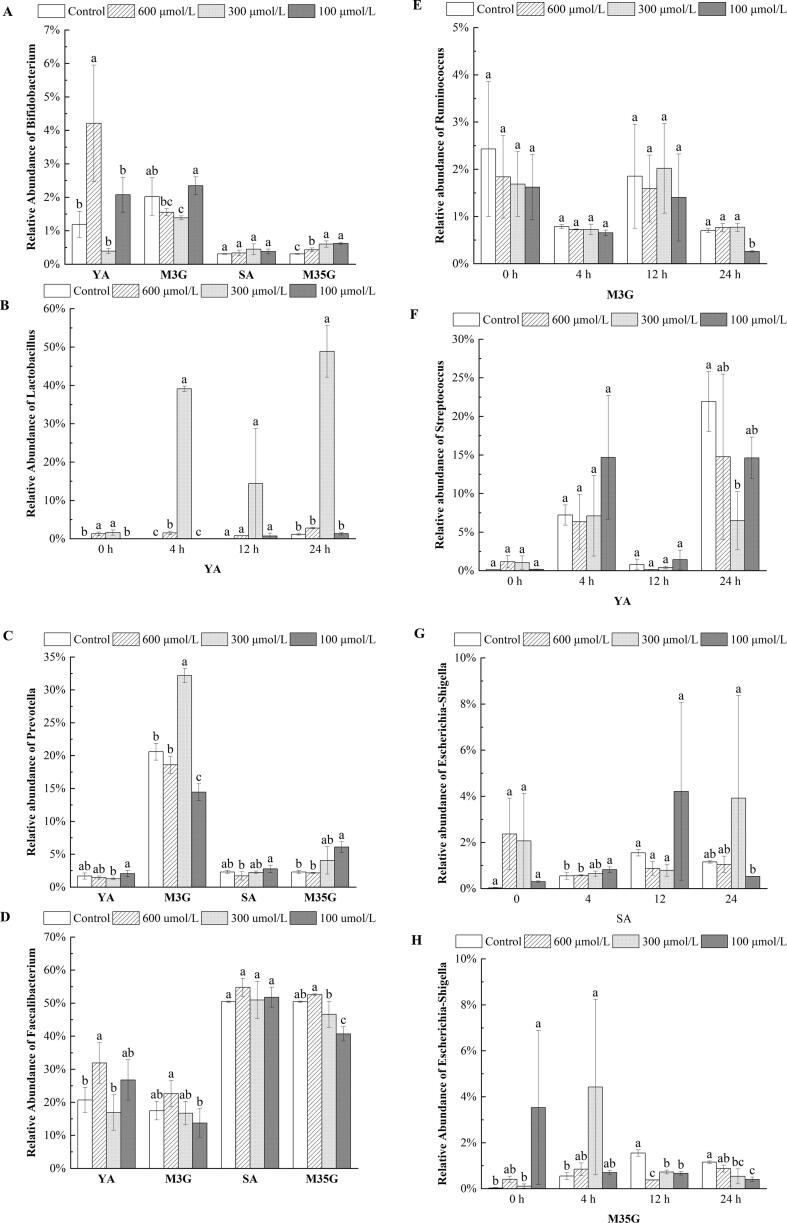
Fig. 3.
Relative abundance of Bifidobacterium (A), Lactobacillus (B), Prevotella (C), Faecalibacterium (D), Ruminococcus (E), Streptococcus (F), Escherichia-Shigella (G, H) after incubation in vitro. (B, F) treated with Yan 73 anthocyanin extracts; (E) treated with malvidin-3-O-glucoside; (G) treated with spine grape anthocyanin extracts; (H) treated with malvidin-3,5-O-diglucoside. YA, M3G, SA, and M35G represent Yan 73 anthocyanin extracts, malvidin-3-O-glucoside, spine grape anthocyanin extracts, and malvidin-3,5-O-diglucoside, respectively. Results are given as the mean ± SD in each group. Values with different letters (a-c) are significantly different (p < 0.05).
Gut microbiota structure at the genus level
At the genus level, apart from Yan 73 anthocyanin extracts, which were mainly composed of Lactobacillus, Faecalibacterium, and Agathobacter, the other samples were primarily composed of Faecalibacterium, Bacteroides, and Prevotella (Fig. S4). All anthocyanins had the potential effect of improving Bifidobacterium growth, which has been observed in many similar studies (Han et al., 2020). Surprisingly, the highest relative abundance of Bifidobacterium (4.21 %) in Yan 73 anthocyanin extracts (600 μmol/L) was observed compared with other groups (p < 0.05) (Fig. 3A). The promotion of Bifidobacterium in malvidin-3,5-O-diglucoside was obviously higher than that in the control (p < 0.05). Malvidin-3-O-glucoside (100 μmol/L) and spine grape anthocyanin extracts also promoted the expansion of Bifidobacterium relative to the control (Fig. 3A), but the difference was not significant. Moreover, Lactobacillus was particularly facilitated by Yan 73 anthocyanin extracts (300 μmol/L), and the proportion reached 48.87 % at 24 h (Fig. 3B). Bifidobacterium and Lactobacillus are well-known health-promoting bacteria involved in the global maintenance of gut function and are vital producers of short-chain fatty acids. It has been reported that Bifidobacterium is positively correlated with acetic acid content (Alcon-Giner et al., 2020), which may be one of the important acetic acid contributors in spine grape anthocyanin extracts and malvidin-3,5-O-diglucoside groups, as the variations in Bifidobacterium abundance during incubation were consistent with the changes in acetic acid concentration. Meanwhile, Lactobacillus belongs to the phylum Firmicutes, which probably explains the high F/B ratio in Yan 73 anthocyanin extracts at 300 μmol/L after 24 h of incubation in vitro.
Prevotella is a dominant bacterial genus within the human gut. Multiple Prevotella coexist in some individuals, specifically those consuming plant-based diets. Prevotella exhibit variability in the utilization of diverse and complex carbohydrates through polysaccharide utilization loci and are beneficial for maintaining glucose homeostasis and modulating host metabolism (Galvez et al., 2020). Malvidin-3-O-glucoside (300 μmol/L) and malvidin-3,5-O-diglucoside (100 μmol/L) markedly promoted the growth of Prevotella at 24 h with respect to the control (p < 0.05), and the former showed the highest Prevotella abundance (32.18 %) (Fig. 3C). Yan 73 anthocyanin extracts and spine grape anthocyanin extracts were weaker at stimulating the expansion of Prevotella than malvidin-3-O-glucoside and malvidin-3,5-O-diglucoside (Fig. 3C), respectively. As the proficient producer of propionate, the possible contribution of Prevotella to the production of propionic acid in malvidin-3-O-glucoside at 300 μmol/L should be considered, since this dosage group yielded the highest content of propionate among the malvidin-3-O-glucoside treatments. Recent studies suggest that gut microbiota-derived propionate exerts multiple beneficial effects on host health, not only by enhancing lipolysis in the liver and thus reducing total cholesterol and triglycerides in blood serum but also by regulating cell proliferation, differentiation, and inflammation (Abdelli et al., 2019, Żary-Sikorska et al., 2019). Therefore, as a bioactive ingredient, malvidin-3-O-glucoside from Vitis Vinifera L. may exert its prebiotic function to maintain host health by supporting the survival of Prevotella in the gut, which is similar to previous reports (Han et al., 2020).
Faecalibacterium, a member of the Clostridium leptum group, was promoted after exposure to anthocyanins. It is sensitive to oxygen and represents one of the most prevalent species in the gut microbiome of healthy human adults (De Filippis et al., 2020). Faecalibacterium has received increasing interest in recent years as a biomarker of gut health. Our results were consistent with previous studies that showed increased levels of Faecalibacterium after in vitro incubation (Tian et al., 2021). The bacteria were significantly boosted by Yan 73 anthocyanin extracts at 600 μmol/L compared with the control after incubation (p < 0.05) (Fig. 3D). Malvidin-3-O-glucoside (600 μmol/L), spine grape anthocyanin extracts, and malvidin-3,5-O-diglucoside (600 μmol/L) exhibited similar growth-promotion effects on Faecalibacterium at 24 h, but no significant differences were observed over the control (Fig. 3D). In particular, malvidin-3-O-glucoside attenuated the decrease in Ruminococcus during incubation, which is a vital anaerobic cellulolytic bacterium containing cellulosomes that degrades complex carbohydrates and produces butyric acid (Artzi et al., 2017). The relative abundance of Ruminococcus in malvidin-3-O-glucoside (300 μmol/L) was only reduced by 54.18 %, lower than that of the control (71.06 %) (Fig. 3E), implying the beneficial effect of malvidin-3-O-glucoside on promoting Ruminococcus. Both Faecalibacterium and Ruminococcus are producers of butyric acid; thus, the increased relative abundances of these two bacteria in malvidin-3-O-glucoside might be the reason for the increase in butyric acid.
Some pathogenic bacteria also changed after treatment with anthocyanins. Streptococcus, a potential pathogen prevailing in patients with liver cirrhosis or alcoholic liver disease (Behary et al., 2021), was significantly inhibited in Yan 73 anthocyanin extracts at 300 μmol/L (6.49 %) compared with the control (21.92 %) after incubation (p < 0.05) (Fig. 3F). Lactobacillus, along with its products of short-chain fatty acids in Yan 73 anthocyanin extracts, may help to suppress the prevalence of Streptococcus by competing for nutrients or inhibiting the expression of genes encoding catabolic enzymes and amino acid transport systems in bacterial cells (Yang et al., 2021). Escherichia-Shigella is another common intestinal pathogen that can cause moderate-to-severe diarrhea in children and increase the risk of stunting and death due to other infectious diseases (Anderson et al., 2019). Malvidin-3,5-O-diglucoside (100 and 300 μmol/L) significantly suppressed Escherichia-Shigella at 24 h in comparison with the control (p < 0.05) (Fig. 3H). Malvidin-3,5-O-diglucoside at 600 μmol/L, as well as spine grape anthocyanin extracts at 600 and 100 μmol/L, produced a tendency to decrease Escherichia-Shigella after 24 h relative to the control, but there were no significant differences (Fig. 3G and H). High counts of Faecalibacterium in spine grape anthocyanin extracts and malvidin-3,5-O-diglucoside, a bacterium that is negatively related to Escherichia-Shigella, may contribute to repressing the growth of Escherichia-Shigella (Zhang et al., 2020). On the other hand, the higher concentration of short-chain fatty acids in malvidin-3,5-O-diglucoside probably decreased the intracellular pH and disrupted the cellular biological activities of Escherichia-Shigella, ultimately suppressing the proliferation of this pathogen. The decrease in Escherichia-Shigella and Streptococcus in anthocyanin treatments may have health-promoting properties.
Effect of anthocyanins on bacterial morphology
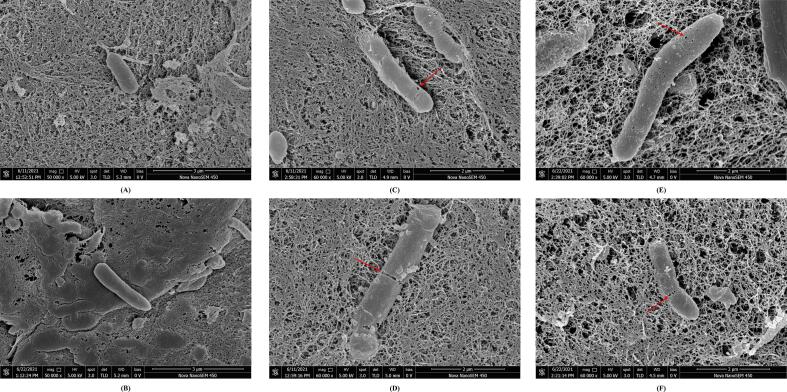
The above data suggested that a lower concentration of anthocyanins was more conducive to the production of short-chain fatty acids, promoting the growth of probiotics (Bifidobacterium) and inhibiting activity of pathogens (Escherichia-Shigella) than a higher dosage. Considering the bioavailability of anthocyanins, a concentration of 100 μmol/L is easily achieved in the human colon (Brown et al., 2014). Thus, 100 μmol/L Yan 73 anthocyanin extracts, spine grape anthocyanin extracts, malvidin-3-O-glucoside, and malvidin-3,5-O-diglucoside were selected for scanning electron microscopic (SEM) observation to further explore the influence of anthocyanins on the morphology of intestinal flora.
The SEM images showed that the control bacteria displayed regular morphology, with a smooth, rounded, and intact surface without ruptures or pores (Fig. 4A, B and Fig. S5A, B). However, bacterial cells treated with anthocyanins for 24 h were severely damaged. In the anthocyanin groups, the rod bacterial cell membrane/wall was recessed, with obvious holes or even broken (Fig. 4C-F), and increasing particles were found on the surface of the coccus strains, with a shriveled shape and disordered flagella (Fig. S5C-F). Apparently, the significant external damage of strain cells treated with anthocyanins could cause cell membrane dysfunction, and this may be one of the reasons for the decrease in abundance of Escherichia-Shigella and Streptococcus. This result was consistent with previous studies showing that anthocyanins exhibit antimicrobial activity by destroying biofilm formation and are intact in Staphylococcus aureus and Escherichia coli (Xu et al., 2014, Tian et al., 2021).
Fig. 4.
SEM images of gut microbiota after incubation in vitro. (A, B) control; (C) treated with Yan 73 anthocyanin extracts; (D) treated with malvidin-3-O-glucoside; (E) treated with spine grape anthocyanin extracts; (F) treated with malvidin-3,5-O-diglucoside.
In this study, anthocyanins exhibit prebiotic potential that selectively favors probiotic microbiota, improving its survival in the intestine, similar to inulin as prebiotics. Meanwhile, the biotransformation of small molecule phenolic acids from anthocyanins may synergistically enhance the beneficial effects of prebiotics. In this context, foods rich in anthocyanins might be able to improve gut health by inhibiting the proliferation of pathogens and improving the probiotic microbiota. Although the fraction of anthocyanins that reaches the human intestine in one intake may be less than the amount in in vitro studies, it can be continuously ingested for a long time, presumably inducing beneficial effects similar to those of prebiotics.
Conclusion
Anthocyanins can inhibit the proliferation of harmful bacteria by reducing the pH and increasing the content of short-chain fatty acids, as well as directly promoting the death of harmful bacteria by destroying the integrity of the cell wall/membrane. Anthocyanin extracts play a better role in reducing the pH value than standards, which may reveal the advantages of the synergistic effect of other anthocyanins in the complex environment of gut microbiota. In addition, the effects of Yan 73 and spine grape anthocyanins on the intestinal flora may be due to malvidin-3-O-glucoside and malvidin-3,5-O-diglucoside, respectively. Monoglucoside anthocyanins particularly increased the alpha diversity and abundance of Lactobacillus, while diglucoside anthocyanins remarkably promoted the production of short-chain fatty acids and inhibited Escherichia-Shigella. In summary, different structures of anthocyanins from Yan 73 and spine grape showed dynamic and multivariate regulatory effects on gut microbiota in vitro, which is favorable for maintaining intestinal homeostasis. Further studies are needed to assess the biological activity potential of anthocyanins as prebiotics in vivo.
CRediT authorship contribution statement
Wenxiu Yue: Investigation, Methodology, Writing – original draft. Fuliang Han: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by “The National Science and Technology Reform and Development Special Project (106001000000150012)” and “The National Key R&D Program of China (Grant No. 2016YFD0400500)”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100501.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- Abdelli L.S., Samsam A., Naser S.A. Propionic acid induces gliosis and neuro-inflammation through modulation of PTEN/AKT pathway in autism spectrum disorder. Scientific Reports. 2019;9(1):8824. doi: 10.1038/s41598-019-45348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.A., Islam P., Subhan N., Rahman M.M., Khan F., Burrows G.E.…Sarker S.D. Potential health benefits of anthocyanins in oxidative stress related disorders. Phytochemistry reviews. 2021;20(4):705–749. doi: 10.1007/s11101-021-09757-1. [DOI] [Google Scholar]
- Alcon-Giner C., Dalby M.J., Caim S., Ketskemety J., Shaw A., Sim K.…Hall L.J. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: An observational study. Cell Reports Medicine. 2020;1(5) doi: 10.1016/j.xcrm.2020.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.D., Bagamian K.H., Muhib F., Amaya M.P., Laytner L.A., Wierzba T., Rheingans R. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: A modelling analysis. The Lancet Global Health. 2019;7(3):e321–e330. doi: 10.1016/s2214-109x(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzi L., Bayer E.A., Morais S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nature Reviews Microbiology. 2017;15(2):83–95. doi: 10.1038/nrmicro.2016.164. [DOI] [PubMed] [Google Scholar]
- Behary J., Amorim N., Jiang X.T., Raposo A., Gong L., McGovern E.…Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nature Communitations. 2021;12(1):187. doi: 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.M., Nitecki S., Pereira-Caro G., McDougall G.J., Stewart D., Rowland I.…Gill C.I. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: Potential impact on colonic health. BioFactors. 2014;40(6):611–623. doi: 10.1002/biof.1173. [DOI] [PubMed] [Google Scholar]
- Burgos-Edwards A., Jimenez-Aspee F., Theoduloz C., Schmeda-Hirschmann G. Colonic fermentation of polyphenols from Chilean currants (Ribes spp.) and its effect on antioxidant capacity and metabolic syndrome-associated enzymes. Food Chemistry. 2018;258:144–155. doi: 10.1016/j.foodchem.2018.03.053. [DOI] [PubMed] [Google Scholar]
- Cheng J.R., Liu X.M., Chen Z.Y., Zhang Y.S., Zhang Y.H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chemistry. 2016;213:721–727. doi: 10.1016/j.foodchem.2016.07.032. [DOI] [PubMed] [Google Scholar]
- De Filippis F., Pasolli E., Ercolini D. Newly explored Faecalibacterium diversity is connected to age, lifestyle, geography, and disease. Current Biology. 2020;30(24):4932–4943 e4934. doi: 10.1016/j.cub.2020.09.063. [DOI] [PubMed] [Google Scholar]
- de Vos W.M., Tilg H., Van Hul M., Cani P.D. Gut microbiome and health: Mechanistic insights. Gut. 2022;71(5):1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Gonzalez F., Russell J. The ability of Escherichia coli 0157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology-UK. 1997;143:1175–1180. doi: 10.1099/00221287-143-4-1175. [DOI] [PubMed] [Google Scholar]
- Diotallevi C., Gaudioso G., Fava F., Angeli A., Lotti C., Vrhovsek U.…Tuohy K. Measuring the effect of Mankai® (Wolffia globosa) on the gut microbiota and its metabolic output using an in vitro colon model. Journal of Functional Foods. 2021;84 doi: 10.1016/j.jff.2021.104597. [DOI] [Google Scholar]
- Erawijantari P.P., Mizutani S., Shiroma H., Shiba S., Nakajima T., Sakamoto T.…Yamada T. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut. 2020;69(8):1404–1415. doi: 10.1136/gutjnl-2019-319188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firrman J., Liu L., Mahalak K., Tanes C., Bittinger K., Tu V.…Van den Abbeele P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. FEMS Microbiology Ecology. 2022;98(5) doi: 10.1093/femsec/fiac038. [DOI] [PubMed] [Google Scholar]
- Galvez E.J.C., Iljazovic A., Amend L., Lesker T.R., Renault T., Thiemann S.…Strowig T. Distinct polysaccharide utilization determines interspecies competition between intestinal Prevotella spp. Cell Host Microbe. 2020;28(6):838–852 e836. doi: 10.1016/j.chom.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Grigor'eva I.N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. Journal of Personalized Medicine. 2020;11(1) doi: 10.3390/jpm11010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Yin M., Han X., You Y., Huang W., Zhan J. The influence of oxygen on the metabolites of phenolic blueberry extract and the mouse microflora during in vitro fermentation. Food Research International. 2020;136 doi: 10.1016/j.foodres.2020.109610. [DOI] [PubMed] [Google Scholar]
- Han F., Oliveira H., Bras N.F., Fernandes I., Cruz L., De Freitas V., Mateus N. In vitro gastrointestinal absorption of red wine anthocyanins - Impact of structural complexity and phase II metabolization. Food Chemistry. 2020;317 doi: 10.1016/j.foodchem.2020.126398. [DOI] [PubMed] [Google Scholar]
- Han F., Yang P., Wang H., Fernandes I., Mateus N., Liu Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends in Food Science & Technology. 2019;83:211–224. doi: 10.1016/j.tifs.2018.11.025. [DOI] [Google Scholar]
- Han X., Guo J., Yin M., Liu Y., You Y., Zhan J., Huang W. Grape extract activates brown adipose tissue through pathway involving the regulation of gut microbiota and bile acid. Molecular Nutrition & Food Research. 2020;64(10):e2000149. doi: 10.1002/mnfr.202000149. [DOI] [PubMed] [Google Scholar]
- Hidalgo M., Oruna-Concha M.J., Kolida S., Walton G.E., Kallithraka S., Spencer J.P., de Pascual-Teresa S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. Journal of Agricultural and Food Chemistry. 2012;60(15):3882–3890. doi: 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- Jamar G., Estadella D., Pisani L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BIOFACTORS. 2017;43(4):507–516. doi: 10.1002/biof.1365. [DOI] [PubMed] [Google Scholar]
- Jiao J.Y., Fu L., Hua Z.S., Liu L., Salam N., Liu P.F.…Li W.J. Insight into the function and evolution of the Wood-Ljungdahl pathway in Actinobacteria. The ISME Journal. 2021;15(10):3005–3018. doi: 10.1038/s41396-021-00935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.M., He J.J., Bi H.Q., Cui X.Y., Duan C.Q. Phenolic compound profiles in berry skins from nine red wine grape cultivars in northwest China. Molecules. 2009;14(12):4922–4935. doi: 10.3390/molecules14124922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilua A., Han K.H., Fukushima M. Effect of polyphenols isolated from purple sweet potato (Ipomoea batatas cv. Ayamurasaki) on the microbiota and the biomarker of colonic fermentation in rats fed with cellulose or inulin. Food & Function. 2020;11(11):10182–10192. doi: 10.1039/d0fo02111c. [DOI] [PubMed] [Google Scholar]
- Kong C.-L., Li A.-H., Su J., Wang X.-C., Chen C.-Q., Tao Y.-S. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT - Food Science and Technology. 2019;109:83–92. doi: 10.1016/j.lwt.2019.03.101. [DOI] [Google Scholar]
- Li W., Gu M., Gong P., Wang J., Hu Y., Hu Y.…Yang H. Glycosides changed the stability and antioxidant activity of pelargonidin. Lwt. 2021;147 doi: 10.1016/j.lwt.2021.111581. [DOI] [Google Scholar]
- Liu X., Martin D.A., Valdez J.C., Sudakaran S., Rey F., Bolling B.W. Aronia berry polyphenols have matrix-dependent effects on the gut microbiota. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129831. [DOI] [PubMed] [Google Scholar]
- Manor O., Dai C.L., Kornilov S.A., Smith B., Price N.D., Lovejoy J.C.…Magis A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nature Communications. 2020;11(1):5206. doi: 10.1038/s41467-020-18871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti I., D’Orazio G., Labra M., La Ferla B., Mezzasalma V., Bizzaro G.…Di Gennaro P. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Applied Microbiology and Biotechnology. 2015;99(13):5613–5626. doi: 10.1007/s00253-015-6482-8. [DOI] [PubMed] [Google Scholar]
- Seekatz A.M., Schnizlein M.K., Koenigsknecht M.J., Baker J.R., Hasler W.L., Bleske B.E.…Sun D. Spatial and Temporal Analysis of the stomach and small-intestinal microbiota in fasted healthy humans. mSphere. 2019;4(2) doi: 10.1128/mSphere.00126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Zhao J., Zhang M., Chen Z., Ma Q., Liu H.…Li J. Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Molecular Nutrition & Food Research. 2021;65(8):e2000745. doi: 10.1002/mnfr.202000745. [DOI] [PubMed] [Google Scholar]
- van der Hee B., Wells J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends in Microbiology. 2021;29(8):700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Wu Y., Han Y., Tao Y., Li D., Xie G., Show P.L., Lee S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Research International. 2020;132 doi: 10.1016/j.foodres.2020.109098. [DOI] [PubMed] [Google Scholar]
- Xu C., Yagiz Y., Hsu W.Y., Simonne A., Lu J., Marshall M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. Journal of Agricultural and Food Chemistry. 2014;62(28):6640–6649. doi: 10.1021/jf501073q. [DOI] [PubMed] [Google Scholar]
- Yang K.M., Kim J.S., Kim H.S., Kim Y.Y., Oh J.K., Jung H.W.…Bae K.H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Scientific Reports. 2021;11(1):1631. doi: 10.1038/s41598-020-80921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żary-Sikorska E., Fotschki B., Fotschki J., Wiczkowski W., Juśkiewicz J. Preparations from purple carrots containing anthocyanins improved intestine microbial activity, serum lipid profile and antioxidant status in rats. Journal of Functional Foods. 2019;60 doi: 10.1016/j.jff.2019.103442. [DOI] [Google Scholar]
- Zhang S., Dogan B., Guo C., Herlekar D., Stewart K., Scherl E.J., Simpson K.W. Short chain fatty acids modulate the growth and virulence of pathosymbiont Escherichia coli and host response. Antibiotics (Basel) 2020;9(8) doi: 10.3390/antibiotics9080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Taylor L., Shommu N., Ghosh S., Reimer R., Panaccione R.…Raman M. A diversified dietary pattern is associated with a balanced gut microbial composition of Faecalibacterium and Escherichia/Shigella in patients with Crohn's disease in remission. Journal of Crohns Colitis. 2020;14(11):1547–1557. doi: 10.1093/ecco-jcc/jjaa084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.