Highlights
-
•
Different metabolomics platforms and their prioritization in food are comparied.
-
•
NIRS is more suitable for food applications due to its non-destructive and fast advantages.
-
•
Combining metabolomic detection and chemometric analysis can quickly identify food adulteration.
-
•
Portable metabolomics analysis equipment and mobile terminal analysis software have been applied.
Keywords: Metabolomics, Analytical technologies, Food composition analysis, Food quality safety, Food traceability
Abstract
Metabolomics is a relatively new component in systems biology that focuses on the high-throughput characterization of small molecular metabolites in biological systems. It is widely used in several scientific fields, particularly in that of food. Due to its excellent detection and prediction capacities, metabolomics well suited to analyze such complex matrix. This review emphasizes the most commonly used food metabolomics analytical technologies with a focus on novel approaches that have emerged in recent years, highlighting their suitability for food samples analysis as aided by chemometric data visualization. A comparison is presented among different metabolomics platforms and their prioritization for which metabolite classes in food. Application of metabolomics are presented in the context of food composition analysis, food quality safety, and food traceability. Furthermore, the constraints and limitations of actual metabolomics applications are explored, bringing novel insights into metabolomics use in food science to maximize its application potential in that major industrial sector.
Introduction
Metabolomics (or metabonomics) was firstly proposed in 1999 (Nicholson, Lindon & Holmes, 1999), which has quickly emerged as a technology to explores the changes in the metabolic, response of organisms under the action of internal and external environments, it targets small molecule metabolites typically less than 1500 kDa (Lao et al., 2009, Kuehnbaum and Britz-McKibbin, 2013). Owing to some features of metabolomics, such as predictability and non-targeted attribute, metabolomics can qualitatively and quantitatively reflect the influence of internal and external factors on organisms’ metabolism from the overall level. It is a comprehensive and systematic analysis method that shows potential in elucidating the mechanism of action of bioactive components, dietary intervention, screening of new biomarkers, and evaluation of metabolic disease prescription interventions. In recent years, with the development and progress of technologies, such as ultra-performance liquid chromatography (UPLC), capillary electrophoresis (CE), and matrix-assisted laser desorption/ionization (MALDI) (Chen et al., 2021), metabolomics has gradually increased in applications, especially in the field of food owing to its excellent detection or structural elucidation capacity of such complex metabolome (Fig. 1). By comparing database information, such as Fiehn library, Golm Metabolome Database, and Wiley Database, mass spectrometry (MS) can quickly identify relevant metabolites (Putri et al., 2022). In food industry analysis, search engines such as Request and Mascot can be used to analyze peptide segments and then match corresponding proteins. The SPINE database in the nuclear magnetic resonance (NMR) community also facilitates NMR analysis, it builds to support the product sample production and structure determination efforts (Fraga et al., 2022). In addition, there are a series of publicly shared comprehensive database platforms like Mass Bank (Go, 2010).
Fig. 1.
Metabolomics research hotspots. Web of Science was used to search “All Fields” for “metabolomics” and “food”, 7267 paper information, use VOSviewer and Pajek software to extract keywords with a frequency greater than 10 in the paper information and draw a keyword cluster analysis diagram. The circular nodes of the same color in the graph represent the same type of keywords. The larger the node radius and font size, the higher the frequency of the keyword, and the connection between the nodes represents the relationship between the two keywords.
Food metabolites are typically affected by various factors such as species, environment, processing methods, and storage techniques. The changes of these metabolites will directly affect food safety and quality. The use of metabolomics technologies allows for the monitoring of metabolite changes during food processing, allowing for optimisation of processing procedures and better quality products (Farag et al., 2022). Potentially harmful components of food processing and production, such as food toxins and drug residues, can also be identified by metabolomics technology to ensure human health (Johanningsmeier, Harris, & Klevorn, 2016). With the improvement of science and technology in recent years, food counterfeiting through the addition of food additives or other substances has occurred frequently. There are also counterfeiting methods that use cheap ingredients of similar types to pass off as expensive foods. Traditional analytical methods usually have limitations such as long identification time and low identification accuracy, which make it difficult to solve these problems and bring impact to the food industry. By using advanced food metabolomics techniques, changes in food composition in terms of compositional analysis, food safety, food classification and traceability can be clearly shown, providing a new paradigm for answering questions in this field. This paper presents the main features of metabolomics-related technologies commonly used in food science in recent years, enumerating the applications of metabolomics technologies in the food industry, while highlighting future trends and areas for improvement.
Metabolomics technologies
Mass spectrometry (MS)
MS is currently-one of the most widely used platforms for metabolomics analyses and whose principle is to ionize molecules to generate charged ions with different mass-to-charge ratio (m/z), which form ion beam under the action of accelerating electric field and enter the analyzer for separation and further detection. MS is equivalent to a “highly sensitive balance”, which can directly weigh the atomic weight and molecular weight of substances, with high sensitivity, high resolution, fast analysis speed, and other advantages.
In recent years, different types of MS techniques have been constructed by improving ion sources and mass analyzers. Ion sources act as the heart of the mass spectrum, volatilizing and ionizing molecules into charged particle beams, determining the rate of m/z-based selective separation and identification, the extent of which determines the ability to detect and quantify the metabolome (Fraga-Corral et al., 2022). Ionization methods commonly used by ion sources include electron impact (EI), electrospray ionization (ESI), and atmospheric pressure chemical ionization (APCI) (Gowda & Djukovic, 2014). EI is a hard ionization method capable of destroying metabolites to produce sub-products, but can be detected with minimal matrix effects and is well suited for the detection of volatile and thermal stable compounds (Rigano et al., 2019). In contrast, ESI is a soft ionization technique in which ions are formed from liquids without significant fragmentation of their molecular ions. Therefore, ESI is more suited for analyzing ionic/polar compounds, refractory or thermally unstable compounds e.g., glycosides and alkaloids in foods. APCI, on the other hand, only forms single-charge ions and is generated in the gaseous state, which is appropriate to small molecular compounds with moderate or low polarity and certain volatility such as lipids.
With the development of the new generation mass analyzers, the quality resolution has been improved significantly. Currently, MS depending on its mass analyzers can be divided into Magnetic Sector MS, Quadrupole MS, Ion Trap MS, time of flight MS (TOF-MS), fourier transform Ion cyclotron resonance MS (FTICR-MS), with Orbitrap MS to emerge in recent years. Among these MS analyzers, TOF-MS, FTICR-MS, and Orbitrap MS are High Resolution Mass Spectrometer (HRMS) with resolution ≥ 10,000, which can be used for accurate qualitative and non-directional unknown screening in food samples (Kaufmann, 2012). In addition, it plays an unprecedented role in rapid detection of food composition because of its fast scanning time and positive/negative ion capability, as well as its ability to provide exact mass and possible elemental composition of precursor and fragment ions aiding in unknown compounds identification especially phytonutrients.
Due to the diversity of analyzed samples and the difference of analysis requirements, the ionization methods and ionization mechanisms are also different, which leads to the difference of ionization sources and mass analyzers. In order to meet the requirements of multi-directional analysis, many different arrangements and system combinations can be constructed through some engineering work. Therefore, MS and HRMS can work with gas chromatography (GC), CE, TOF, and FTICR, among others. For example, GC–MS combines features of GC and MS to identify different chemicals in samples (Castro-Puyana et al., 2017, Cevallos-Cevallos et al., 2011), which has strong separation ability, resolution and sensitivity, is suitable for qualitative and quantitative evaluation of volatile and semi-volatile organic compounds (such as esters, short-chain fatty acids, and flavonoid aglycans), but to some extent requires derivatization and lengthy pretreatment steps. During the use of GC–MS, avoid sample components with too high boiling points. In addition, substances with strong polarity and substances containing carboxylic acid cannot be directly analyzed by GC–MS. CE is a separation technology based on mobility and distribution behavior, CE-MS is commonly used for efficient analysis of polar and charged metabolites, such as amino acids, peptides, organic acids, and nucleic acids (Granados-Chinchilla and Rodríguez, 2017, Sugimoto et al., 2017, Sugimoto et al., 2020). TOF-MS combines the acquisition speed with the ability to collect complete spectral information. Meanwhile, with the development of spatial focusing, reflector, and vertical acceleration technology, TOF-MS has extremely fast scanning speed and high sensitivity, with a mass accuracy of 10-6 millimass units (mmu) and a resolution of more than 10,000 at present, allowing the detection of even minor peaks. Its quality accuracy can reach ppm level after strict correction, with the mass range of detected ions can reach hundreds of thousands of daltons. Currently, QMS combined with TOF-MS are widely used to analyze food samples (Farag et al., 2013). Compared with other types of MS, TOF-MS has a higher resolution and a wider range of ion quality detection, posing it as being suited for food profiling matrices. FTICR-MS is the highest resolution mass spectrometer among all HRMS, to reach 1,000,000 in accuracy while maintain acceptable sensitivity. Consequently, FTICR-MS is a powerful tool for molecular structure confirmation and molecular rearrangement reaction research in food compounds. However, its high price and complex operation limit its wide application in food testing especially in industry (Leavell et al., 2002). Linear ion trap electrostatic orbitrap combined MS (LTQ Orbitrap-MS) can parallel detection of ion trap and HRMS. The multi-stage MS of ion trap can obtain structural fragments and HRMS can obtain the molecular formula, providing comprehensive data for the identification of regioisomers and structural analogues (Eliuk & Makarov, 2015). In addition, UPLC quadrupole orbitrap MS (UPLC-Q-Orbitrap-MS) greatly improved both the sensitivity and specificity of analysis, achieving both accuracy, speed, and advantages of convenience (Zhang et al., 2018).
Ion mobility spectroscopy mass spectroscopy (IMS-MS) identifies ions by measuring their mobility. The constant electric field in IMS-MS drives ions against the inert gas to fly over the drift tube, and the ions with high mobility first pass through the drift tube. Because the flight speed of ions is affected by their own cross-sectional area, they can also identify isotopic ions with different structures. By adjusting the length of IMS-MS drift tube or the flow rate of reverse inert gas, IMS-MS can obtain different resolutions for different scenes (Delafield et al., 2022, Eldrid and Thalassinos, 2020). Since the IMS system does not need to operate in a vacuum environment, its entire device is relatively small and more convenient to use. In addition, IMS-MS can provide rapid analysis at the millisecond level. IMS-MS has become an attractive technology for separating and detecting metabolites with similar structures (Burnum-Johnson et al., 2019).
Mass spectrometry imaging (MSI)
MSI is a two-dimensional analysis method that allows MSI to determine and visualize the spatial distribution of intact molecules in a tissue or tissue section based on the molecular weight or m/z value of a specific chemical component without the need for extraction steps, purification, and separation (Fig. 2). In MSI, different layers of the sample surface are scraped off using laser beam irradiation on the instrument platform for analysis. By collecting multiple spectra at specific coordinates on the sample surface, and compiling all spectra into one data, their spatial distribution can be described as an image (Yoshimura et al., 2016). The ionization methods of MSI usually include MALDI, secondary ion mass spectrometry (SIMS), and desorption electrospray ionization (DESI) (Fig. 2). Both MALDI and SIMS are performed on samples placed in a vacuum, which provide analysis with a sub-micron resolution scale (Pacholski & Winograd, 1999). Both MALDI and SIMS imaging instruments are though expensive, with debris or matrix interference in the SIMS and MALDI process coupled with sample preparation that results in slow analysis. Therefore, it is reasonable to use them when high-resolution spatial resolution is required (Burrell, Earnshaw & Cranch, 2007). Conversely, the spatial resolution required in food imaging applications is often lower, so less expensive methods (such as immunostaining methods) are often used (Borges et al., 2006, Gorji et al., 2005). In recent years, the emerging (Desorption electrospray ionization MS imaging) DESI-MSI has solved the problems of analysis speed and sample pretreatment, and its appropriate resolution has gradually become the first choice in the analysis of food metabolites (Clendinen, Monge & Fernández, 2017).
Fig. 2.
MSI of food ingredients and ionization technique commonly used in MSI technology. (a) MSI enables a two-dimensional view of the distribution of ingredients in food. After the food is thinned, the components are analyzed by ionization methods, and then the mass spectral information of each area is integrated to form a two-dimensional image of the component distribution. (b) MALDI. For thermally sensitive samples, MALDI technology can be used for ionization, solid samples are covered with a matrix or liquid samples are proportionally diluted to fix the samples, under laser irradiation, the sample is instantly vaporized and ionized by the matrix, and then enters the detector. (c) SIMS. The sample is directly bombarded by the ion beam, and then the molecules on the surface of the sample are sputtered to become charged ions, which are detected and imaged by the detector. (d) DESI. DESI can directly process samples of various states under normal pressure. The sample is first dissolved in a solvent and then dripped onto the surface of the insulating material. After the solvent is volatilized, the surface of the sample is purged into the gas phase by using a nebulizer to accelerate the charged solvent with nitrogen. Afterwards, the solvent droplets surrounding the sample evaporate under the action of high-speed airflow, and the sample enters the detector for detection and imaging. In DESI, the height and angle of the nebulizer and detector from the sample can be adjusted for optimal imaging.
Matrix-assisted laser desorption/ionization MS imaging (MALDI-MSI) is a non-targeted two-dimensional analysis method under vacuum conditions, which can directly and widely detect all ionized intact molecules in tissue sections. The spatial resolution of MALDI-MS is between 5 and 200 μm, which is sufficient to obtain the molecular distribution of a single cell (Morisasa et al., 2019). MALDI-MSI can be performed on most food-related matrices, but fresh samples unaffected by chemicals should be preferred. Repeated freeze–thaw cycles should be avoided to prevent tissue degradation (Schwartz, Reyzer & Caprioli, 2003). Remarkably, the fixation of the sample should be avoided to cause ionization of the embedding material to affect the detection. MALDI-MSI usually requires tissue sections of 5–20 μm thickness. In the case of analyzing high molecular weight molecules (3–21 kDa), thinner tissue sections of 2–5 μm thickness are recommended (Goodwin, Pennington & Pitt, 2008). Plant tissue can be analyzed using thicker tissue sections of 20–50 μm (Peukert et al., 2012, Yoshimura et al., 2012). Sections require tissue washing to remove salts that affect the spectral quality (Andersson et al., 2008, Deutskens et al., 2011, Franck et al., 2010, Groseclose et al., 2007), followed by tissue digestion and derivatization, which increase metabolites detection and ionization rates (Aerni et al., 2006, Lemaire et al., 2007, Morita et al., 2010, Toue et al., 2014). In MALDI-MSI detection, the matrix ionized absorption laser beam energy uniformly applied to the tissue section is used to protect the intact molecules in the tissue from damage. The matrix used for MALDI-MSI usually includes sinapinic acid (SA) (3,5-dimethoxy-4-hydroxycinnamic acid), cyano-4-hydroxycinnamic acid (CHCA), DHB and 9-aminoacridine (9-AA) (Karas and Hillenkamp, 1988, Kaletaş et al., 2009).
SIMS imaging is an ultra-high vacuum technique that uses an ion beam to strike a detection target, generating and using secondary ions to image solid surfaces, during the bombardment of the ion beam, the chemical bonds and structures of some molecules may be destroyed, resulting in the introduction of impurity ions in the detection process to interfere with the accuracy (Hand, Ranasinghea, & Cooks, 1993). This detection is limited to the uppermost layer of the sample, the primary ion beam can be focused to a spot of tens of nanometers with a resolution of 5–200 μm, and its spectral characteristics are mainly used for the targeted detection of elemental ions and small fragment ions (Fletcher, 2009). In the later development, SIMS imaging has become the mainstream technology in detection through the combination of TOF. TOF-SIMS imaging with high ion utilization, high mass resolution, and good sensitivity can obtain the full spectrum of the mass range with one ion pulse, high ion utilization, high mass resolution, and good sensitivity. In principle, the detection mass range of TOF-SIMS imaging can be unlimited by controlling the reread frequency of the pulses (Amaral et al., 1998).
DESI-MSI is developed by Takáts et al. (2004) compared to technologies such as MALDI and SIMS, which requires more arbitrary environmental conditions, and does not require harsh vacuum conditions, and prior sample processing. DESI-MSI can analyze solid, liquid, frozen, and gaseous samples in multiple states, while MALDI and SIMS are limited to solid samples analysis (Demian et al., 2007). Since DESI-MSI does not require the addition of a matrix to the sample, there will be no matrix-related ions that are likely to interfere with sample detection. In DESI-MSI, to realize inhalation detection, pneumatically assisted electrospray sprays charged droplets onto the surface of the sample to be tested, then the spray droplets collide with a solvent film and splash secondary ions containing dissolved analytes into the air for detection (Cooks et al., 2006, Musah et al., 2012). The resolution of DESI-MSI is usually between 50 and 200 μm (Laskin et al., 2012).
Nuclear magnetic resonance (NMR)
NMR is a technology that uses radio frequency pulses to excite atomic nuclei with zero spin quantum number, causing the nuclear resonance to absorb energy and emit radio signals at a specific frequency, and then use a receiver to receive the signal and obtain data through computer processing, and is one of the most used analytical tools in metabolomics fingerprint and analysis (Marcone et al., 2013, Wishart, 2019, Yu et al., 2020). In the industrial production process of food, the producer needs to extract a certain percentage of products from each batch for food testing. For traditional invasive detection methods, such as probe or profile detection, food becomes incomplete after detection and is discarded. In order to ensure that the possibility of testing is appropriate, a considerable number of products need to be carried out. This type of invasive testing can be very wasteful when there are many production batches or when food ingredients are more expensive. NMR has intense penetration and is convenient for heterogeneous and complex food systems. It is also commonly used on solid and liquid substrates as a non-destructive approach well suited for the recovery of analyzed food samples compared MS techniques (Cao et al., 2018, Marcone et al., 2013). At the same time, it can monitor a variety of analytes/markers, providing unique structural information about metabolites, especially considering its strong structural elucidation power when coupled to two dimensional NMR (2D NMR) techniques even from crude matrix e.g., 2D 1H–1H correlation spectroscopy (COSY), Heteronuclear single Quantum Coherence (HSQC) and Heteronuclear Multiple Bond Correlations (HMBC) (Mahrous & Farag, 2015). Particularly, NMR based on isotopes is developed and applied to food analysis, mainly using hydrogen spectrum (1H NMR), carbon spectrum (13C NMR), or phosphorus spectrum (31P NMR), among which 1H NMR is the most widely used (Cao et al., 2021).
Hydrogen NMR spectrum (1H NMR) is a technology that uses magnetic nuclei with different energy levels in the static magnetic field to irradiate samples with electromagnetic waves of specific frequency. When electromagnetic wave energy is equal to energy level difference, the nuclei absorbs electromagnetic energy transition to generate resonance absorption signal and records the spectrum automatically using the recorder. 1H NMR spectroscopy is not only high throughput, of good reproducibility, easy to operate, and rich in structural information, but also can quantitatively identify and detect metabolic components. These advantages result in application like food composition analysis, adulteration test, and geographical traceability. However, NMR is relatively sophisticated and has many parameters, which requires personnel with relevant professional knowledge to operate and then greatly limits its application coverage in industry. In addition, NMR detection time is long and the price is relatively expensive, which also brings certain restrictions on use.
Indeed, quantitative NMR can provide quantification of all resolved peaks in spectra using one spiked standard and contrary to MS in which response factor limit the application of one standard for quantification of all peaks (Farag et al., 2019). Special acquisition parameters should be considered i.e., relaxation delay, to ensure full relaxation of targeted nuclei to be quantitative. Although sensitivity reasons make the 1H nucleus the most utilized nucleus, other nuclei such as 13C have also recently gained popularity for their ability to solve specific problems in food science. The 13C NMR is the same as that of 1H, with the chemical shift range of 13C spectrum is 20–30 times that of 1H spectrum. Consequently, 13C spectrum has more information than 1H spectrum, in addition to its the ability to resolve overlapped peaks in 1H NMR spectra along the carbon dimension (Truzzi et al., 2021). The low sensitivity towards 13C detection can be overcome by using two dimensional NMR experiments such as HSQC and HMBC in which carbon resonance is acquired from the proton channel (Mahrous & Farag, 2015). In recent years, 31P NMR has become a tool for analyzing food and studying factors that affect food quality because of its selectivity to phosphorus-containing compounds such as nucleotides, coupled with the increasing sensitivity and analytical power of modern NMR spectrometers. It is widely used to detect low levels of content of monoglycerides and diglycerides in milk and dairy products, meat, fruits, and vegetables, cereals, lipids and oils. Quantitative 31P NMR spectra can be extended to quantify the components of functional groups with unstable protons (Spyros & Dais, 2000).
Near infrared spectroscopy (NIR)
NIR is a convenient and fast non-destructive testing technique that requires little tedious sample preparation, and its detection range is usually in the 780–2500 nm electromagnetic spectrum (Nicolai et al., 2007). It scans the near-infrared spectrum of the sample to obtain the characteristic information of hydrogen-containing groups (OH, NH, CH) in the organic matter, and compares it with the established calibration model to predict the organic matter in the sample (Li et al., 2017, Pojić and Mastilović, 2013). However, the bands of these hydrogen-containing groups are usually very wide, and the chemical bonds are numerous and complex in structure, making it difficult to impart certain characteristics to specific chemical components, such as in case of NMR. The development of chemometric tools has further aided NIR technology to be more accurately applied towards the prediction and analysis of food, and has become an important part of auxiliary NIR (Qu et al., 2015). These methods usually include partial least squares (PLS), linear discriminant analysis (LDA), principal component analysis (PCA), etc. (Cen & He, 2007). By establishing standard data for model samples, and combining chemometrics models for comparative analysis. NIR can be used to evaluate phenols, terpenes, alkaloids, carbohydrates, sulfur compounds, and other secondary metabolites in food. And it can be widely used in food adulteration, food classification, and identification of food varieties and origins (Fig. 3). With the development and application of NIR technology, portable NIR was also developed and appears to be more suited for applications in the food industry. Such portable and the cheaper device is more popular for researchers than desktop NIR and to expand upon the application of NIR in food industry. For a comprehensive review on IR applications in food analysis, please refer to the study of Nagy, Wang & Farag (2022).
Fig. 3.
The principle of NIR variety identification. First, it is necessary to use NIR to take a certain number of samples of each variety (such as 10 grapes of three different varieties of S1, S2, and S3) for spectral identification, and then calculate the average of 10 pieces of data for each of the three varieties to establish The model data of each variety is used for feature location (feature peaks). After the model data is established, NIR is used to determine the spectral data of the unknown variety samples, and chemometric tool is compared with the model data to find the most similar model data, and then the variety of the sample to be tested can be determined with better accuracy.
Applications of metabolomics in food analysis
Traditional analysis techniques usually only analyse the macro-nutrients of food, such as sugars, lipids or proteins (Liang et al., 2022). The components within food products are complex and highly susceptible to transformation. The analysis of macro-nutrients is no longer sufficient to meet the needs of the current food industry. Metabolomics techniques enable the analysis of changes in composition during food processing and storage, this is highly beneficial for quality control in the processing or storage of food products (Fig. 4) (Cao et al., 2021, Zhao et al., 2019). In addition, MSI-like imaging techniques can provide a good insight into the spatial distribution of metabolites in food. This facilitates the analysis of the nutritional profile of different parts of the food and provides principles and mechanisms for determining the functionality of the food. The use of metabolomics techniques allows for the rapid quantification or localisation of certain types of substances in foods, which is a very convenient method in food composition studies. Based on this same principle, toxins, hormones, pesticides or drug residues in food can be identified for the purpose of food safety control (Yan et al., 2022). Flavour components of food products are also a popular research topic. Most flavour substances are usually secondary metabolites of food components and are characterised by their small molecular weight, low concentration and volatility. By using metabolomics methods, the composition and concentration of these volatile substances can be well analysed, so that the production and processing of food products can be optimised to obtain better flavoured products (Liu et al., 2019, Farghal et al., 2022). It is also possible to analyse the main volatile flavour substances in food products and provide ideas for the development of natural food additives.
Fig. 4.
Comparison of the application of major metabolomics techniques in the detection of food components highlighting their advantages and or limitations.
Falsification of origin and food adulteration is another major problem in the food industry. The high degree of consistency between substances often makes it difficult to distinguish such subtle differences using conventional analytical methods. Different substances have different metabolite compositions and concentrations from one another, and each variety of food can be fingerprinted independently using its own metabolite properties. By using this method, it is possible to directly compare the differences between the metabolite profiles of the food to be tested and the target food, for the purposes of adulteration identification, food classification and food traceability, and even to determine when the food was produced. The application of chemometric or statistical tools helps to compare and analyse the differences between the normal model and the substance under test to determine if differences exist. In addition, traditional food traceability tools often use blockchain technology, barcodes or traceable packaging to prove the origin of a food product, but with the growth of the transportation industry, the same batch of food ingredients may come from multiple regions of the world (Assis et al., 2022). The mixture of food properties from multiple regions makes it difficult to clearly trace multiple sources with traditional traceability tools, in which case the creation of individual source properties through the use of metabolomics of the database. In this case, the use of metabolomics to create a database of component characteristics for each source can be a good solution. In this process, it is also necessary to use chemometrics to visualise the data and avoid over-fitting models. More awareness of model validation has been reported in such studies, especially in supervised data analysis (e.g. OPLS and PLS). This study by Biancolillo & Marini (2018) can be as the guidelines on chemometric modelling of spectroscopic datasets. A recent update on metabolomics studies in Meat Quality Analysis and Authentication has been made (Zhang et al., 2021). Currently, with improved analytical methods, classification and traceability can be achieved with an accuracy of over 95 %. Metabolomics has been applied to the food industry as a powerful analytical tool. Some works have been done using metabolomics techniques for food composition analysis, food adulteration and food traceability (Table 1). It can be argued that the use of metabolomics tools optimises food processing and storage techniques and facilitates the development of the food industry. The introduction of food metabolite profiling has accelerated the identification of food adulteration and improved the accuracy of food traceability.
Table 1.
Application of metabolomics analysis techniques in food composition analysis, classification, adulteration, and traceability.
| Platform | Sample | Result/Objective | References |
|---|---|---|---|
| LC-MS | Legumes | Be able to differentiate between lentils, white beans and chickpeas. | Llorach et al. (2019) |
| LC-MS/MS | Almond | It can simultaneously determine various aflatoxin in almond. Without purification, high sensitivity (0.34–0.5 μg/kg) | Ouakhssase et al. (2021) |
| GC–MS | Black tea | To monitor the dynamic changes of metabolites during the processing. | Wu et al. (2019) |
| GC–MS | Saffron | Ketoisophorone and safranal identified as freshness versus and ageing marker. Safranal was identified as a marker to identify saffron adulteration. | Farag et al. (2020) |
| GC/GC-TOF-MS | Rice | Non targeted analysis of volatile metabolic compounds released during rice cooking. | Daygon et al. (2016) |
| HPLC-QTOF-MS/MS | Plantago depressa | Effective exploration of polyphenol spectrum of complex natural products. | Xu et al. (2020) |
| UPLC-Q/TOF-MS | Fish sauce | 46 metabolites were identified as the key chemical components of fish sauce flavor. | Wang et al. (2019) |
| UPLC-QTOF/MS | Pomegranate juice | It can detect 1 % apple juice and grape juice mixed in pomegranate juice. | Dasenaki et al. (2019) |
| UPLC-ESI-MS/MS | Pork | Determine the source of pork by using more than 100 lipid metabolites. | Mi et al. (2019) |
| DESI-MSI 3D imaging | Beef | Beef tissue can be directly tested for steroid ester injections. | De Rijke et al. (2013) |
| DESI-MSI | Potato | Clarification of the distribution of the potato toxins α-chaconine and α-lonokinin, based on m/z 852 and m/z 868. | Cabral et al. (2013) |
| ESI-MS/MS | Coffee bean | Simultaneous determination of pesticides and mycotoxins in green coffee beans | Reichert et al. (2018) |
| TOF-SIMS imaging | Chicken | Higher concentrations of vitamin E were found in the fat of chickens fed soybean oil and flaxseed oil. | Marzec et al. (2016) |
| MALDI-MSI | Chocolate | Differentiate between different chocolate producers and cocoa varieties. | De Oliveira et al. (2018) |
| MALDI-MSI | Strawberry | The distribution of flavan-3-ols, organic acids, anthocyanins and ellagic glycosides in strawberry was found. | Enomoto et al. (2020); Enomoto (2021) |
| MALDI-MSI | Pork | Phosphatidylcarnosine is most widely distributed in the spine and lumbar muscles. | Enomoto et al. (2021) |
| MALDI-MSI | Persimmon epidermis | During the drying, the concentration of vitamin A1 increased, the vitamins B1 and B6 unchanged. | Shikano et al. (2020) |
| MALDI-MSI | Rice | The molecular types of lysophosphatidylcholine and the distribution of unsaturated fatty acids in rice were explored, and it was found that the content of lysophosphatidylcholine would affect the flavor of rice wine. | Zaima et al. (2014) |
| NMR | Chicken breast | Differentiation of Korean Chicken Breast with Free Amino Acids. | Kim, Ko & Jo (2021) |
| 1H NMR | Celery | Identification of the origin of celery using amino acids, organic acids and mannitol. | Lau et al. (2020) |
| 1H NMR | Milk powder | Use of low molecular weight metabolites to differentiate between milk powder types. | Zhao et al. (2017) |
| 1H NMR | Olive oils | Differentiation of olive oils from different regions by fatty acyl. | Ün & Ok (2018) |
| 1H NMR | Rice | Comparison of multiple metabolite levels to distinguish the origin of Chinese rice. | Huo et al. (2017) |
| 1H NMR | Olive oil | Fatty acyl is an important metabolite marker that can aid to determine shelf life of olive oil. | Ün & Ok (2018) |
| 1H NMR | Duck breast | Anserine, aspartic acid, and carnosine were correlated with quality, and nicotinamide with cooking degree. | Wang et al. (2020) |
| 13C NMR | Essential oils | Identification of impurities such as vegetable oil. | Truzzi et al. (2021) |
| FT-IR and NMR | Saffron | Proposed some metabolomics markers for product shelf life, authenticity and quality of saffron. | Consonni et al. (2016) |
| NIR | Honey | Determination of hydroxybenzoic acid in honey. | Tahir et al. (2020) |
| NIR | Fruits | Qualitative and quantitative analysis of anthocyanins. | Teng et al. (2020) |
| NIR | Cantaloupe | Distinguish different varieties of cantaloupe with 100 % accuracy. | Németh et al. (2019) |
| NIR | Truffle | Identification of adulteration of cheap truffle raw materials. | Segelke et al. (2020) |
| NIR | Hungarian honey | Identifying the botanical origin of Hungarian honey with 99 % accuracy. | Bodor et al. (2021) |
| NIR | Beef | 100 % probability of detecting the presence of adulterants such as pork, fat and offal. | Morsy & Sun (2013) |
| NIR | Rice | Capable of detecting more than 5 % of other rice. | Liu et al. (2020) |
PCA, principal component analysis; PLS-DA, partial least squares-discriminant analysis; ANOVA, analysis of variance; LDA, linear discriminant analysis; SVM, support vector machines.
Future trends and applications
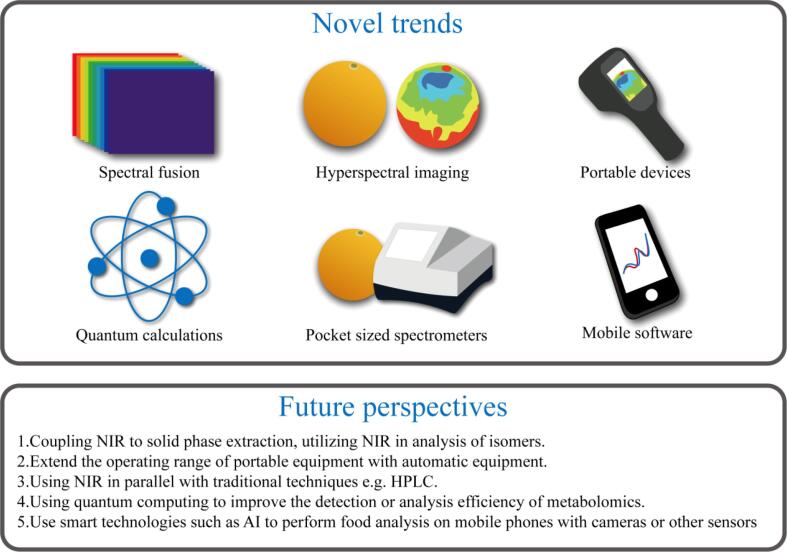
Metabolomics has become a powerful tool in food research such as food analysis, food safety testing, and food traceability. A summary of metabolomics new applications in food analysis with future trends is presented in Fig. 5. GC/MS is a powerful used platform to unravel volatiles behind unique food aroma, which are mainly generated during processing such as roasting in coffee, or fermentation in dairy products. Other analytical platforms, including NMR and LC/MS appears more suited for the profiling of non-volatile polar phytonutrients such as phenolics, glycosides to more contribute to food health benefits warranting for the employment of comparative metabolomics approaches to assess food metabolome more holistically. Compared to hyphenated techniques, direct spectroscopic measurement i.e., NIR appears more suited for these industrial food applications especially for monitoring consistency among food batches as routinely performed in drug analysis aided by chemometric tools. In recent years, with the development and popularization of MSI, portable NIR and other related analysis and detection technologies and instruments has facilitated metabolomics applications as industrial level. However, the accuracy and sensitivity of metabolomics-related technologies need to be improved. Many technologies are difficult to distinguish substances of the same masses or isomers, in addition to difficulty in detection of some metabolites with low content as in contaminants e.g., aflatoxins and small molecular weight. The database of food-related metabolites is not comprehensive enough, and the data processing technology and statistical methods have not been unified and standard initiatives can be developed to allow for comparison among different experimental setups and or devices. In addition, metabolomics research can typically only analyze the output chemical, and it is difficult to characterize the production pathways and action mechanisms of metabolites by metabolomics-related technologies. For such goal, it can be combined with other omics technologies such as genomics, proteomics and other omics technologies for joint analysis.
Fig. 5.
A layout of metabolomics novel trends and future perspective in food analysis.
In the future, as metabolomics develops more efficiently and comprehensive analysis coverage of food-related metabolites in the database, it will be more conducive to elucidate the functions and mechanisms of related metabolites in food either raw or in response to different processing methods such as fermentation, roasting, sprouting etc. Application of metabolomics to optimize for such food processes can help ensure best parameters leading to desired chemical output using such untargeted analyses. Compared to the extensive reports on the application of metabolomics in food of plant origin classification and origin traceability, animal based products especially fish and sea type are much less explored and needs future attention especially considering their different matrix type from that of plant based food. It seems that it is difficult for a single food metabolism analysis technology to provide completely reliable analysis results. Spectral fusion technology can combine multiple data sources, and comprehensively analyze the changes of trace components in food through a combination of multiple data changes and calculations, which can make the analysis results more reliable. In addition, the computing power and efficiency of traditional computers based on electronic computing are relatively low, and the mature application of quantum computing in the future may speed up the data processing and analysis efficiency of food metabolomics. Application of metabolomics to assess uniformity in pre-prepared or convenience food mixtures has also yet to be reported to ensure consistency and quality.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The project was funded by Key Project of the Natural Science Foundation of Fujian Province (2020J02032) and Fujian ‘Young Eagle Program’ Youth Top Talent Program.
Data availability
Data will be made available on request.
References
- Aerni H.R., Cornett D.S., Caprioli R.M. Automated acoustic matrix deposition for MALDI sample preparation. Analytical Chemistry. 2006;78(3):827–834. doi: 10.1021/ac051534r. [DOI] [PubMed] [Google Scholar]
- Amaral A., Galle P., Escraig F., Cossonnet C., Henge-Napoli M.H., Ansoborio E., Zhang L. The use of SIMS for uranium localization in biological research. Journal of Alloys and Compounds. 1998;271:19–24. doi: 10.1016/S0925-8388(98)00016-4. [DOI] [Google Scholar]
- Andersson M., Groseclose M.R., Deutch A.Y., Caprioli R.M. Imaging mass spectrometry of proteins and peptides: 3D volume reconstruction. Nature Methods. 2008;5(1):101–108. doi: 10.1038/nmeth1145. [DOI] [PubMed] [Google Scholar]
- Assis M.T.Q.M., Lucas M.R., Rainho M.J.M. A meta-analysis on the trust in agrifood supply chains. Food Frontiers. 2022;3:413–427. doi: 10.1002/fft2.137. [DOI] [Google Scholar]
- Biancolillo A., Marini F. Chemometric methods for spectroscopy-based pharmaceutical analysis. Frontiers in Chemistry. 2018;6:576. doi: 10.3389/fchem.2018.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor Z., Kovacs Z., Benedek C., Hitka G., Behling H. Origin identification of hungarian honey using melissopalynology, physicochemical analysis, and near infrared spectroscopy. Molecules. 2021;26(23):7274. doi: 10.3390/molecules26237274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J.P., Jauneau A., Brulé C., Culerrier R., Barre A., Didier A., Rougé P. The lipid transfer proteins (LTP) essentially concentrate in the skin of Rosaceae fruits as cell surface exposed allergens. Plant Physiology and Biochemistry. 2006;44(10):535–542. doi: 10.1016/j.plaphy.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Burnum-Johnson K.E., Zheng X., Dodds J.N., Ash J., Fourches D., Nicora C.D., Wendler J.P., Metz T.O., Waters K.M., Jansson J.K., Smith R.D., Baker E.S. Ion mobility spectrometry and the omics: Distinguishing isomers, molecular classes and contaminant ions in complex samples. Trends in Analytical Chemistry. 2019;116:292–299. doi: 10.1016/j.trac.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell M., Earnshaw C., Clench M. Imaging matrix assisted laser desorption ionization mass spectrometry: A technique to map plant metabolites within tissues at high spatial resolution. Journal of Experimental Botany. 2007;58(4):757–763. doi: 10.1093/jxb/erl139. [DOI] [PubMed] [Google Scholar]
- Cabral E.C., Mirabelli M.F., Perez C.J., Ifa D.R. Blotting assisted by heating and solvent extraction for DESI-MS imaging. Journal of the American Society for Mass Spectrometry. 2013;24(6):956–965. doi: 10.1007/s13361-013-0616-y. [DOI] [PubMed] [Google Scholar]
- Cao H., Saroglu O., Karadag A., Diaconeasa Z., Zoccatelli G., Conte-Junior C.A., Gonzalez-Aguilar G.A., Ou J., Bai W., Zamarioli C.M., Freitas L.A.P., Shpigelman A., Campelo P.H., Capanoglu E., Hii C.L., Jafari S.M., Qi Y., Liao P., Wang M., Zou L., Bourke P., Simal-Gandara J., Xiao J. Available technologies on improving the stability of polyphenols in food processing. Food Frontiers. 2021;2:109–139. doi: 10.1002/fft2.65. [DOI] [Google Scholar]
- Cao R., Liu X., Liu Y., Zhai X., Cao T., Wang A., Qiu J. Applications of nuclear magnetic resonance spectroscopy to the evaluation of complex food constituents. Food Chemistry. 2021;342 doi: 10.1016/j.foodchem.2020.128258. [DOI] [PubMed] [Google Scholar]
- Cao R., Zhao Y., Zhou Z., Zhao X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. Journal of the Science of Food and Agriculture. 2018;98(6):2422–2427. doi: 10.1002/jsfa.8734. [DOI] [PubMed] [Google Scholar]
- Castro-Puyana M., Pérez-Míguez R., Montero L., Herrero M. Reprint of: Application of mass spectrometry-based metabolomics approaches for food safety, quality and traceability. TrAC Trends in Analytical Chemistry. 2017;96:62–78. doi: 10.1016/j.trac.2017.08.007. [DOI] [Google Scholar]
- Cen H., He Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends in Food Science & Technology. 2007;18(2):72–83. doi: 10.1016/j.tifs.2006.09.003. [DOI] [Google Scholar]
- Cevallos-Cevallos J.M., Danyluk M.D., Reyes-De-Corcuera J.I. GC-MS based metabolomics for rapid simultaneous detection of Escherichia coli O157: H7, Salmonella typhimurium, Salmonella muenchen, and Salmonella hartford in ground beef and chicken. Journal of Food Science. 2011;76(4):M238–M246. doi: 10.1111/j.1750-3841.2011.02132.x. [DOI] [PubMed] [Google Scholar]
- Chen H., Wang Z., Fan F., Shi P., Xu X., Du M., Wang C. Analysis method of lactoferrin based on uncoated capillary electrophoresis. eFood. 2021;2(3):147–153. [Google Scholar]
- Clendinen C.S., Monge M.E., Fernández F.M. Ambient mass spectrometry in metabolomics. The Analyst. 2017;142(17):3101–3117. doi: 10.1039/c7an00700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni R., Ordoudi S.A., Cagliani L.R., Tsiangali M., Tsimidou M.Z. On the traceability of commercial saffron samples using 1H-NMR and FT-IR metabolomics. Molecules. 2016;21(3):286. doi: 10.3390/molecules21030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooks R.G., Ouyang Z., Takats Z., Wiseman J.M. Detection technologies. Ambient mass spectrometry. Science. 2006;311(5767):1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- Dasenaki M.E., Drakopoulou S.K., Aalizadeh R., Thomaidis N.S. Targeted and untargeted metabolomics as an enhanced tool for the detection of pomegranate juice adulteration. Foods. 2019;8(6):212. doi: 10.3390/foods8060212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daygon V.D., Prakash S., Calingacion M., Riedel A., Ovenden B., Snell P., Fitzgerald M. Understanding the Jasmine phenotype of rice through metabolite profiling and sensory evaluation. Metabolomics. 2016;12(4):1–15. doi: 10.1007/s11306-016-0989-6. [DOI] [Google Scholar]
- De Oliveira D.N., Camargo A., Melo C., Catharino R.R. A fast semi-quantitative screening for cocoa content in chocolates using MALDI-MSI. Food Research International. 2018;103:8–11. doi: 10.1016/j.foodres.2017.10.035. [DOI] [PubMed] [Google Scholar]
- De Rijke E., Hooijerink D., Sterk S.S., Nielen M.W. Confirmation and 3D profiling of anabolic steroid esters in injection sites using imaging desorption electrospray ionisation (DESI) mass spectrometry. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment. 2013;30(6):1012–1019. doi: 10.1080/19440049.2013.794307. [DOI] [PubMed] [Google Scholar]
- Delafield D.G., Lu G., Kaminsky C.J., Li L. High-end ion mobility mass spectrometry: A current review of analytical capacity in omics applications and structural investigations. Trends in Analytical Chemistry. 2022;157 doi: 10.1016/j.trac.2022.116761. [DOI] [Google Scholar]
- Demian R.I., Justin M.W., Qingyu S., Graham R.C. Development of capabilities for imaging mass spectrometry under ambient conditions with desorption electrospray ionization (DESI) International Journal of Mass Spectrometry. 2007;259(1–3):8–15. doi: 10.1016/j.ijms.2006.08.003. [DOI] [Google Scholar]
- Deutskens F., Yang J., Caprioli R.M. High spatial resolution imaging mass spectrometry and classical histology on a single tissue section. Journal of Mass Spectrometry. 2011;46(6):568–571. doi: 10.1002/jms.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldrid C., Thalassinos K. Developments in tandem ion mobility mass spectrometry. Biochemical Society Transactions. 2020;48(6):2457–2466. doi: 10.1042/BST20190788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliuk S., Makarov A. Evolution of Orbitrap mass spectrometry instrumentation. Annual Review of Analytical Chemistry. 2015;8:61–80. doi: 10.1146/annurev-anchem-071114-040325. [DOI] [PubMed] [Google Scholar]
- Enomoto H. Unique distribution of ellagitannins in ripe strawberry fruit revealed by mass spectrometry imaging. Current Research in Food Science. 2021;4:821–828. doi: 10.1016/j.crfs.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H., Kotani M., Ohmura T. Novel blotting method for mass spectrometry imaging of metabolites in strawberry fruit by desorption/ionization using through hole alumina membrane. Foods. 2020;9(4):408. doi: 10.3390/foods9040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H., Takeda S., Hatta H. Spatial analysis of phosphatidylinositol molecular species in pork chop tissues using matrix-assisted laser desorption/ionization-mass spectrometry imaging. Journal of Oleo Science. 2021;70(7):979–987. doi: 10.5650/jos.ess21042. [DOI] [PubMed] [Google Scholar]
- Farag M.A., El Senousy A.S., El-Ahmady S.H., Porzel A., Wessjohann L.A. Comparative metabolome-based classification of Senna drugs: A prospect for phyto-equivalency of its different commercial products. Metabolomics. 2019;15(5):80. doi: 10.1007/s11306-019-1538-x. [DOI] [PubMed] [Google Scholar]
- Farag M.A., Hegazi N., Dokhalahy E., Khattab A.R. Chemometrics based GC-MS aroma profiling for revealing freshness, origin and roasting indices in saffron spice and its adulteration. Food Chemistry. 2020;331 doi: 10.1016/j.foodchem.2020.127358. [DOI] [PubMed] [Google Scholar]
- Farag M.A., Sheashea M., Zhao C., Maamoun A.A. UV fingerprinting approaches for quality control analyses of food and functional food coupled to chemometrics: A comprehensive analysis of novel trends and applications. Foods. 2022;11(18):2867. doi: 10.3390/foods11182867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M.A., Weigend M., Luebert F., Brokamp G., Wessjohann L.A. Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC-Q-TOF-MS metabolomic profiles. Phytochemistry. 2013;96:170–183. doi: 10.1016/j.phytochem.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Farghal H.H., Mansour S.T., Khattab S., Zhao C., Farag M.A. A comprehensive insight on modern green analyses for quality control determination and processing monitoring in coffee and cocoa seeds. Food Chemistry. 2022;394 doi: 10.1016/j.foodchem.2022.133529. [DOI] [PubMed] [Google Scholar]
- Fletcher J.S. Cellular imaging with secondary ion mass spectrometry. The Analyst. 2009;134(11):2204–2215. doi: 10.1039/b913575h. [DOI] [PubMed] [Google Scholar]
- Fraga-Corral M., Carpena M., Garcia-Oliveira P., Pereira A.G., Prieto M.A., Simal-Gandara J. Analytical metabolomics and applications in health, environmental and food science. Critical Reviews in Analytical Chemistry. 2022;52(4):712–734. doi: 10.1080/10408347.2020.1823811. [DOI] [PubMed] [Google Scholar]
- Fraga K.J., Huang Y.J., Ramelot T.A., Swapna G., Kendary L.A., Li E., Korf I., Montelione G.T. SpecDB: A relational database for archiving biomolecular NMR spectral data. Journal of Magnetic Resonance. 2022;342 doi: 10.1016/j.jmr.2022.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck J., Ayed M.E., Wisztorski M., Salzet M., Fournier I. On tissue protein identification improvement by N-terminal peptide derivatization. Methods in Molecular Biology. 2010;656:323–338. doi: 10.1007/978-1-60761-746-4_19. [DOI] [PubMed] [Google Scholar]
- Go E.P. Database resources in metabolomics: An overview. Journal of Neuroimmune Pharmacology. 2010;5(1):18–30. doi: 10.1007/s11481-009-9157-3. [DOI] [PubMed] [Google Scholar]
- Goodwin R.J., Pennington S.R., Pitt A.R. Protein and peptides in pictures: Imaging with MALDI mass spectrometry. Proteomics. 2008;8(18):3785–3800. doi: 10.1002/pmic.200800320. [DOI] [PubMed] [Google Scholar]
- Gorji M., Helene P., Rickmer D., Sabine B., Yan M., Alessio M., Marzio Z., Erik, van der W., Zora H., Daniel K., Friedrich A., Margit L. Localisation and distribution of the major allergens in apple fruits. Plant Science. 2005;169(2):387–394. doi: 10.1016/j.plantsci.2005.03.027. [DOI] [Google Scholar]
- Gowda G.A., Djukovic D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods in Molecular Biology. 2014;1198:3–12. doi: 10.1007/978-1-4939-1258-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Chinchilla F., Rodríguez C. Tetracyclines in food and feedingstuffs: From regulation to analytical methods, bacterial resistance, and environmental and health implications. Journal of Analytical Methods in Chemistry. 2017;2017:1315497. doi: 10.1155/2017/1315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose M.R., Andersson M., Hardesty W.M., Caprioli R.M. Identification of proteins directly from tissue: In situ tryptic digestions coupled with imaging mass spectrometry. Journal of Mass Spectrometry. 2007;42(2):254–262. doi: 10.1002/jms.1177. [DOI] [PubMed] [Google Scholar]
- Hand O.W., Ranasinghea A., Cooks R.G. Chemical reactions at interfaces-hydrogen deuterium exchange using a solid ND4Cl matrix in molecular secondary ion massspectrometry. Organic Mass Spectrometry. 1993;28:176–184. doi: 10.1002/oms.1210280308. [DOI] [Google Scholar]
- Huo Y., Kamal G.M., Wang J., Liu H., Zhang G., Hu Z.…Du H. 1H NMR-based metabolomics for discrimination of rice from different geographical origins of China. Journal of Cereal Science. 2017;76:243–252. doi: 10.1016/j.jcs.2017.07.002. [DOI] [Google Scholar]
- Johanningsmeier S.D., Harris G.K., Klevorn C.M. Metabolomic technologies for improving the quality of food: Practice and promise. Annual Review of Food Science and Technology. 2016;7:413–438. doi: 10.1146/annurev-food-022814-015721. [DOI] [PubMed] [Google Scholar]
- Kaletaş B.K., van der Wiel I.M., Stauber J., Dekker L.J., Güzel C., Kros J.M., Luider T.M., Heeren R.M. Sample preparation issues for tissue imaging by imaging MS. Proteomics. 2009;9(10):2622–2633. doi: 10.1002/pmic.200800364. [DOI] [PubMed] [Google Scholar]
- Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Analytical Chemistry. 1988;60(20):2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Kaufmann A. The current role of high-resolution mass spectrometry in food analysis. Analytical and Bioanalytical Chemistry. 2012;403(5):1233–1249. doi: 10.1007/s00216-011-5629-4. [DOI] [PubMed] [Google Scholar]
- Kim H.C., Ko Y.J., Jo C. Potential of 2D qNMR spectroscopy for distinguishing chicken breeds based on the metabolic differences. Food Chemistry. 2021;342 doi: 10.1016/j.foodchem.2020.128316. [DOI] [PubMed] [Google Scholar]
- Kuehnbaum N.L., Britz-McKibbin P. New advances in separation science for metabolomics: Resolving chemical diversity in a post-genomic era. Chemical Reviews. 2013;113(4):2437–2468. doi: 10.1021/cr300484s. [DOI] [PubMed] [Google Scholar]
- Lao Y.M., Jiang J.G., Yan L. Application of metabonomic analytical techniques in the modernization and toxicology research of traditional Chinese medicine. British Journal of Pharmacology. 2009;157(7):1128–1141. doi: 10.1111/j.1476-5381.2009.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin J., Heath B.S., Roach P.J., Cazares L., Semmes O.J. Tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Analytical Chemistry. 2012;84(1):141–148. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H., Laserna A., Li S. 1H NMR-based metabolomics for the discrimination of celery (Apium graveolens L. var. dulce) from different geographical origins. Food Chemistry. 2020;332 doi: 10.1016/j.foodchem.2020.127424. [DOI] [PubMed] [Google Scholar]
- Leavell M.D., Leary J.A., Yamasaki R. Mass spectrometric strategy for the characterization of lipooligosaccharides from Neisseria gonorrhoeae 302 using FTICR. Journal of the American Society for Mass Spectrometry. 2002;13(5):571–576. doi: 10.1016/S1044-0305(02)00360-4. [DOI] [PubMed] [Google Scholar]
- Lemaire R., Desmons A., Tabet J.C., Day R., Salzet M., Fournier I. Direct analysis and MALDI imaging of formalin-fixed, paraffin-embedded tissue sections. Journal of Proteome Research. 2007;6(4):1295–1305. doi: 10.1021/pr060549i. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo Y., Liu C., Wang W., Rao P., Fu C., Wang S. SPA combined with swarm intelligence optimization algorithms for wavelength variable selection to rapidly discriminate the adulteration of apple juice. Food Analytical Methods. 2017;10(6):1965–1971. doi: 10.1007/s12161-016-0772-3. [DOI] [Google Scholar]
- Liang L., Duan W., Zhao C., Zhang Y.Y. Recent development of two-dimensional liquid chromatography in food analysis. Food Analytical Methods. 2022;15:1214–1225. doi: 10.1007/s12161-021-02190-2. [DOI] [Google Scholar]
- Liu D., Chen Y.Q., Xiao X.W., Zhong R.T., Yang C.F., Liu B., Zhao C. Nutrient properties and nuclear magnetic resonance-based metabonomic analysis of macrofungi. Foods. 2019;8(9):397. doi: 10.3390/foods8090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li Y., Peng Y., Yang Y., Wang Q. Detection of fraud in high-quality rice by near-infrared spectroscopy. Journal of Food Science. 2020;85(9):2773–2782. doi: 10.1111/1750-3841.15314. [DOI] [PubMed] [Google Scholar]
- Llorach R., Favari C., Alonso D., Garcia-Aloy M., Andres-Lacueva C., Urpi-Sarda M. Comparative metabolite fingerprinting of legumes using LC-MS-based untargeted metabolomics. Food Research International. 2019;126 doi: 10.1016/j.foodres.2019.108666. [DOI] [PubMed] [Google Scholar]
- Mahrous E.A., Farag M.A. Two dimensional NMR spectroscopic approaches for exploring plant metabolome: A review. Journal of Advanced Research. 2015;6(1):3–15. doi: 10.1016/j.jare.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcone M.F., Wang S., Albabish W., Nie S., Somnarain D., Hill A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Research International. 2013;51(2):729–747. doi: 10.1016/j.foodres.2012.12.046. [DOI] [Google Scholar]
- Marzec M.E., Wojtysiak D., Połtowicz K., Nowak J., Pedrys R. Study of cholesterol and vitamin E levels in broiler meat from different feeding regimens by TOF-SIMS. Biointerphases. 2016;11(2):02A326. doi: 10.1116/1.4943619. [DOI] [PubMed] [Google Scholar]
- Mi S., Shang K., Li X., Zhang C.H., Liu J.Q., Huang D.Q. Characterization and discrimination of selected china's domestic pork using an lc-ms-based lipidomics approach. Food Control. 2019;100:305–314. doi: 10.1016/j.foodcont.2019.02.001. [DOI] [Google Scholar]
- Morisasa M., Sato T., Kimura K., Mori T., Goto-Inoue N. Application of matrix-assisted laser desorption/ionization mass spectrometry imaging for food analysis. Foods. 2019;8(12):633. doi: 10.3390/foods8120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Ikegami K., Goto-Inoue N., Hayasaka T., Zaima N., Tanaka H., Uehara T., Setoguchi T., Sakaguchi T., Igarashi H., Sugimura H., Setou M., Konno H. Imaging mass spectrometry of gastric carcinoma in formalin-fixed paraffin-embedded tissue microarray. Cancer Science. 2010;101(1):267–273. doi: 10.1111/j.1349-7006.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy N., Sun D.W. Robust linear and non-linear models of NIR spectroscopy for detection and quantification of adulterants in fresh and frozen-thawed minced beef. Meat Science. 2013;93(2):292–302. doi: 10.1016/j.meatsci.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Musah R.A., Domin M.A., Walling M.A., Shepard J.R. Rapid identification of synthetic cannabinoids in herbal samples via direct analysis in real time mass spectrometry. Rapid Communications in Mass Spectrometry. 2012;26(9):1109–1114. doi: 10.1002/rcm.6205. [DOI] [PubMed] [Google Scholar]
- Nagy M.M., Wang S.P., Farag M.A. Quality analysis and authentication of nutraceuticals using near IR (NIR) spectroscopy: A comprehensive review of novel trends and applications. Trends in Food Science & Technology. 2022 doi: 10.1016/j.tifs.2022.03.005. [DOI] [Google Scholar]
- Németh D., Balazs G., Daood H.G., Kovacs Z., Bodor Z., Zaukuu J.Z., Szentpeteri V., Kokai Z., Kappel N. Standard analytical methods, sensory evaluation, NIRS and electronic tongue for sensing taste attributes of different melon varieties. Sensors. 2019;19(22):5010. doi: 10.3390/s19225010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J.K., Lindon J.C., Holmes E. 'Metabonomics': Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- Nicolai B.M., Beullens K., Bobelyn E., Peirs A., Saeys W., Theron K.I., Lammertyn J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biology and Technology. 2007;46(2):99–118. doi: 10.1016/j.postharvbio.2007.06.024. [DOI] [Google Scholar]
- Ouakhssase A., Fatini N., Ait Addi E. A simple extraction method with no lipid removal for the determination of aflatoxins in almonds by liquid chromatography tandem-mass spectrometry (LC-MS/MS) Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment. 2021;38(9):1561–1570. doi: 10.1080/19440049.2021.1925167. [DOI] [PubMed] [Google Scholar]
- Pacholski M.L., Winograd N. Imaging with mass spectrometry. Chemical Reviews. 1999;99(10):2977–3006. doi: 10.1021/cr980137w. [DOI] [PubMed] [Google Scholar]
- Peukert M., Matros A., Lattanzio G., Kaspar S., Abadía J., Mock H.P. Spatially resolved analysis of small molecules by matrix-assisted laser desorption/ionization mass spectrometric imaging (MALDI-MSI) The New Phytologist. 2012;193(3):806–815. doi: 10.1111/j.1469-8137.2011.03970.x. [DOI] [PubMed] [Google Scholar]
- Pojić M.M., Mastilović J.S. Near infrared spectroscopy—advanced analytical tool in wheat breeding, trade, and processing. Food Bioprocess Technol. 2013;6(2):330–352. doi: 10.1007/s11947-012-0917-3. [DOI] [Google Scholar]
- Putri S.P., Ikram M., Sato A., Dahlan H.A., Rahmawati D., Ohto Y., Fukusaki E. Application of gas chromatography-mass spectrometry-based metabolomics in food science and technology. Journal of Bioscience and Bioengineering. 2022;133(5):425–435. doi: 10.1016/j.jbiosc.2022.01.011. [DOI] [PubMed] [Google Scholar]
- Qu J., Liu D., Cheng J., Sun D., Ma J., Pu H., Zeng X. Applications of near-infrared spectroscopy in food safety evaluation and control: A review of recent research advances. Critical Reviews in Food Science and Nutrition. 2015;55(13):1939–1954. doi: 10.1080/10408398.2013.871693. [DOI] [PubMed] [Google Scholar]
- Reichert B., de Kok A., Pizzutti I.R., Scholten J., Cardoso C.D., Spanjer M. Simultaneous determination of 117 pesticides and 30 mycotoxins in raw coffee, without clean-up, by LC-ESI-MS/MS analysis. Analytica Chimica Acta. 2018;1004:40–50. doi: 10.1016/j.aca.2017.11.077. [DOI] [PubMed] [Google Scholar]
- Rigano F., Tranchida P.Q., Dugo P., Mondello L. High-performance liquid chromatography combined with electron ionization mass spectrometry: A review. TrAC Trends in Analytical Chemistry. 2019;118:112–122. doi: 10.1016/j.trac.2019.05.032. [DOI] [Google Scholar]
- Schwartz S.A., Reyzer M.L., Caprioli R.M. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: Practical aspects of sample preparation. Journal of Mass Spectrometry. 2003;38(7):699–708. doi: 10.1002/jms.505. [DOI] [PubMed] [Google Scholar]
- Segelke T., Schelm S., Ahlers C., Fischer M. Food authentication: Truffle (Tuber spp.) species differentiation by FT-NIR and chemometrics. Foods. 2020;9(7):922. doi: 10.3390/foods9070922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikano H., Miyama Y., Ikeda R., Takeshi H., Suda J., Yoshinaga K., Taira S. Localization analysis of multiple vitamins in dried persimmon (Diospyros kaki) using matrix-assisted laser desorption/ionization mass spectrometry imaging. Journal of Oleo Science. 2020;69(8):959–964. doi: 10.5650/jos.ess20143. [DOI] [PubMed] [Google Scholar]
- Spyros A., Dais P. Application of (31)P NMR spectroscopy in food analysis. 1. Quantitative determination of the mono- and diglyceride composition of olive oils. Journal of Agricultural and Food Chemistry. 2000;48(3):802–805. doi: 10.1021/jf9910990. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Obiya S., Kaneko M., Enomoto A., Honma M., Wakayama M., Soga T., Tomita M. Metabolomic profiling as a possible reverse engineering tool for estimating processing conditions of dry-cured hams. Journal of Agricultural and Food Chemistry. 2017;65(2):402–410. doi: 10.1021/acs.jafc.6b03844. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Sugawara T., Obiya S., Enomoto A., Kaneko M., Ota S., Soga T., Tomita M. Sensory properties and metabolomic profiles of dry-cured ham during the ripening process. Food Research International. 2020;129 doi: 10.1016/j.foodres.2019.108850. [DOI] [PubMed] [Google Scholar]
- Tahir H.E., Arslan M., Mahunu G.K., Shi J., Zou X., Gasmalla M.A.A., Mariod A.A. Data fusion approach improves the prediction of single phenolic compounds in honey: A study of NIR and Raman Spectroscopies. eFood. 2020;1:173–180. doi: 10.2991/efood.k.191018.001. [DOI] [Google Scholar]
- Takáts Z., Wiseman J.M., Gologan B., Cooks R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- Teng Z., Jiang X., He F., Bai W. Qualitative and quantitative methods to evaluate anthocyanins. eFood. 2020;1:339–346. doi: 10.2991/efood.k.200909.001. [DOI] [Google Scholar]
- Toue S., Sugiura Y., Kubo A., Ohmura M., Karakawa S., Mizukoshi T., Yoneda J., Miyano H., Noguchi Y., Kobayashi T., Kabe Y., Suematsu M. Microscopic imaging mass spectrometry assisted by on-tissue chemical derivatization for visualizing multiple amino acids in human colon cancer xenografts. Proteomics. 2014;14(7–8):810–819. doi: 10.1002/pmic.201300041. [DOI] [PubMed] [Google Scholar]
- Truzzi E., Marchetti L., Benvenuti S., Ferroni A., Rossi M.C., Bertelli D. Novel strategy for the recognition of adulterant vegetable oils in essential oils commonly used in food industries by applying 13C NMR spectroscopy. Journal of Agricultural and Food Chemistry. 2021;69(29):8276–8286. doi: 10.1021/acs.jafc.1c02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ün İ., Ok S. Analysis of olive oil for authentication and shelf life determination. Journal of Food Science and Technology. 2018;55(7):2476–2487. doi: 10.1007/s13197-018-3165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Jiang G., Kebreab E., Li J., Feng X., Li C., Zhang X., Huang X., Fang C., Fang R., Dai Q. 1H NMR-based metabolomics study of breast meat from Pekin and Linwu duck of different ages and relation to meat quality. Food Research International. 2020;133 doi: 10.1016/j.foodres.2020.109126. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li C., Li L., Yang X., Chen S., Wu Y., Zhao Y., Wang J., Wei Y., Yang D. Application of UHPLC-Q/TOF-MS-based metabolomics in the evaluation of metabolites and taste quality of Chinese fish sauce (Yu-lu) during fermentation. Food Chemistry. 2019;296:132–141. doi: 10.1016/j.foodchem.2019.05.043. [DOI] [PubMed] [Google Scholar]
- Wishart D.S. NMR metabolomics: A look ahead. Journal of Magnetic Resonance. 2019;306:155–161. doi: 10.1016/j.jmr.2019.07.013. [DOI] [PubMed] [Google Scholar]
- Wu H., Huang W., Chen Z., Chen Z., Shi J., Kong Q., Sun S., Jiang X., Chen D., Yan S. GC-MS-based metabolomic study reveals dynamic changes of chemical compositions during black tea processing. Food Research International. 2019;120:330–338. doi: 10.1016/j.foodres.2019.02.039. [DOI] [PubMed] [Google Scholar]
- Xu J., Tong C., Fu Q., Guo K., Shi S., Xiao Y. Comprehensive polyphenolic profile of plantago depressa using high-speed countercurrent chromatography off-line with high-performance liquid chromatography-diode array detector-quadrupole time-of-flight tandem mass spectrometry. eFood. 2020;1:94–105. doi: 10.2991/efood.k.191101.001. [DOI] [Google Scholar]
- Yan X., Chen H., Du G., Guo Q., Yuan Y., Yue T. Recent trends in fluorescent aptasensors for mycotoxin detection in food: Principles, constituted elements, types, and applications. Food Frontiers. 2022;3:428–452. doi: 10.1002/fft2.144. [DOI] [Google Scholar]
- Yoshimura Y., Enomoto H., Moriyama T., Kawamura Y., Setou M., Zaima N. Visualization of anthocyanin species in rabbiteye blueberry Vaccinium ashei by matrix-assisted laser desorption/ionization imaging mass spectrometry. Analytical and Bioanalytical Chemistry. 2012;403(7):1885–1895. doi: 10.1007/s00216-012-5876-z. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Goto-Inoue N., Moriyama T., Zaima N. Significant advancement of mass spectrometry imaging for food chemistry. Food Chemistry. 2016;210:200–211. doi: 10.1016/j.foodchem.2016.04.096. [DOI] [PubMed] [Google Scholar]
- Yu L., Liao Z., Zhao Y., Zeng X., Yang B., Bai W. Metabolomic analyses of dry lemon slice during storage by NMR. Food Frontiers. 2020;1:180–191. [Google Scholar]
- Zhao C., Liu Y.Y., Lai S.S., Cao H., Guan Y., Cheang W.S., Liu B., Zhao K.W., Miao S., Riviere C., Capanoglu E., Xiao J.B. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends in Food Science & Technology. 2019;85:55–66. doi: 10.1016/j.tifs.2019.01.004. [DOI] [Google Scholar]
- Zaima N., Yoshimura Y., Kawamura Y., Moriyama T. Distribution of lysophosphatidylcholine in the endosperm of Oryza sativa rice. Rapid Communications in Mass Spectrometry. 2014;28(13):1515–1520. doi: 10.1002/rcm.6927. [DOI] [PubMed] [Google Scholar]
- Zhang G., Chen S., Zhou W., Meng J., Deng K., Zhou H., Hu N., Suo Y. Rapid qualitative and quantitative analyses of eighteen phenolic compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and their antioxidant activity. Food Chemistry. 2018;269:150–156. doi: 10.1016/j.foodchem.2018.06.132. [DOI] [PubMed] [Google Scholar]
- Zhang T., Chen C., Xie K., Wang J., Pan Z. Current state of metabolomics research in meat quality analysis and authentication. Foods. 2021;10(10):2388. doi: 10.3390/foods10102388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen H., Feng J., Chen Z., Cai S. 1H NMR-based compositional identification of different powdered infant formulas. Food Chemistry. 2017;230:164–173. doi: 10.1016/j.foodchem.2017.03.020. [DOI] [PubMed] [Google Scholar]
Further reading
- Campos M.I., Debán L., Antolín G., Pardo R. Evaluation by NIRS technology of curing process of ham with low sodium content. Meat Science. 2020;163 doi: 10.1016/j.meatsci.2020.108075. [DOI] [PubMed] [Google Scholar]
- Jin G., Wang Y.J., Li M., Li T., Huang W.J., Li L., Deng W.W., Ning J. Rapid and real-time detection of black tea fermentation quality by using an inexpensive data fusion system. Food Chemistry. 2021;358 doi: 10.1016/j.foodchem.2021.129815. [DOI] [PubMed] [Google Scholar]
- Kaufmann K.C., Favero F.F., de Vasconcelos M., Godoy H.T., Sampaio K.A., Barbin D.F. Portable NIR spectrometer for prediction of palm oil acidity. Journal of Food Science. 2019;84(3):406–411. doi: 10.1111/1750-3841.14467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.