Abstract
Background:
Systemic therapy options for salivary cancers are limited. MyPathway (NCT02091141), a phase IIa study, evaluates targeted therapies in non-indicated tumor types with actionable molecular alterations. Here, we present the efficacy and safety results for a subgroup of MyPathway patients with advanced salivary gland cancer (SGC) matched to targeted therapies based on tumor molecular characteristics.
Patients and methods:
MyPathway is an ongoing, multiple basket, open-label, non-randomized, multi-center study. Patients with advanced SGC received pertuzumab + trastuzumab (HER2 alteration), vismodegib (PTCH-1/SMO mutation), vemurafenib (BRAF V600 mutation), or atezolizumab [high tumor mutational burden (TMB)]. The primary endpoint is the objective response rate (ORR).
Results:
As of January 15, 2018, 19 patients with SGC were enrolled and treated in MyPathway (15 with HER2 amplification and/or overexpression and one each with a HER2 mutation without amplification or overexpression, PTCH-1 mutation, BRAF mutation, and high TMB). In the 15 patients with HER2 amplification/overexpression (with or without mutations) who were treated with pertuzumab + trastuzumab, 9 had an objective response (1 complete response, 8 partial responses) for an ORR of 60% (9.2 months median response duration). The clinical benefit rate (defined by patients with objective responses or stable disease >4 months) was 67% (10/15), median progression-free survival (PFS) was 8.6 months, and median overall survival was 20.4 months. Stable disease was observed in the patient with a HER2 mutation (pertuzumab + trastuzumab, n = 1/1, PFS 11.0 months), and partial responses in patients with the PTCH-1 mutation (vismodegib, n = 1/1, PFS 14.3 months), BRAF mutation (vemurafenib, n = 1/1, PFS 18.5 months), and high TMB (atezolizumab, n = 1/1, PFS 5.5+ months). No unexpected toxicity occurred.
Conclusions:
Overall, 12 of 19 patients (63%) with advanced SGC, treated with chemotherapy-free regimens matched to specific molecular alterations, experienced an objective response. Data from MyPathway suggest that matched targeted therapy for SGC has promising efficacy, supporting molecular profiling in treatment determination.
Keywords: advanced salivary gland carcinoma, targeted therapy, HER2-positive, pertuzumab, trastuzumab, molecular profiling
INTRODUCTION
The incidence of salivary gland cancer (SGC) has risen from 10.4 per 1 000 000 in 1973 to 16 per 1 000 000 in 2009.1 Advanced salivary gland carcinomas are particularly unresponsive to traditional chemotherapies.2 No standard of care for systemic therapy exists for metastatic disease,2 and no treatments have been approved by the USA Food and Drug Administration (FDA) specifically for SGC. As such, patients with advanced SGC have a clear unmet need for effective systemic treatment options.
SGCs have high heterogeneity in their molecular and genomic characteristics and commonly harbor potentially actionable alterations.3–6 Human epidermal growth factor receptor 2 (HER2) alterations, for example, are particularly prevalent in aggressive or high-grade tumor subtypes, including salivary duct carcinoma (SDC) and other salivary carcinomas.6,7 Other potentially targetable alterations include PIK3CA and BRAF mutations, tumor mutational burden (TMB) >10 mutations/Mb, and NTRK3 gene fusion, among others.6 Therapies targeting these molecular characteristics have demonstrated success in other cancer types, making SGC a prime candidate for existing targeted treatments.
Recent reports have suggested potential for targeted treatment of SGC. A phase II trial of patients with HER2-positive SDC reported a response rate of 70.2% following treatment with trastuzumab + docetaxel, although 60% of patients experienced grade 4 decreased neutrophil count.8 Encouraging results have also been observed anecdotally with targeted regimens free of traditional chemotherapy, including almost complete disease resolution in a heavily pretreated patient with HER2-amplified and overexpressing SDC treated with trastuzumab + lapatinib + bevacizumab,9 and a patient with HER2-overexpressing metastatic SDC treated with second-line trastuzumab emtansine (T-DM1) who experienced a prolonged response with low toxicity.10 Vemurafenib, a BRAF inhibitor approved for the treatment of patients with melanomas or Erdheime–Chester disease harboring BRAF V600 mutations, produced a complete response in a patient with BRAF V600-mutated SDC.11 Finally, treatment of TRK fusion-positive salivary tumors with the tyrosine receptor kinase inhibitors entrectinib and larotrectinib have resulted in complete and partial responses.12,13
MyPathway (NCT02091141), an ongoing, multiple basket, phase IIa study, evaluates the efficacy of established, chemotherapy-free targeted therapies for non-approved indications in patients with advanced solid tumors harboring potentially actionable genetic or molecular alterations.14 Eligible alterations include HER2 amplification, overexpression, and/or mutations (treated with pertuzumab + trastuzumab); Hedgehog pathway alterations (PTCH-1 or SMO mutations, treated with vismodegib); BRAF V600 mutations (treated with vemurafenib ± cobimetinib); high microsatellite instability, high TMB, or deficient mismatch repair (treated with atezolizumab); ALK genetic alterations (treated with alectinib); or EGFR-activating mutations (treated with erlotinib). This trial design allows MyPathway to address treatment efficacy in both common and rare tumor types, regardless of their site of origin. Here, we present the efficacy and safety results for the subgroup of MyPathway patients with advanced SGC treated on the basis of their molecular characteristics with pertuzumab + trastuzumab, vismodegib, vemurafenib, or atezolizumab.
METHODS
Study design and participants
MyPathway is a multiple basket, open-label, non-randomized, multi-center phase IIa trial of patients with treatment-refractory advanced solid tumors with potentially predictive molecular alterations (see supplementary Figure S1, available at Annals of Oncology online). Patients in this analysis were enrolled from 12 sites and had advanced SGC with HER2 amplification, overexpression, and/or mutations; a Hedgehog pathway alteration; a BRAF V600 mutation; or high TMB (by FoundationOne). Molecular alterations were locally assessed by Clinical Laboratory Improvement Amendments certified laboratory tests, as determined by the treating physician before enrollment, and reviewed by a study medical monitor for eligibility. Patients were aged ≥18 years, had measurable or evaluable lesions, and had an Eastern Cooperative Oncology Group (ECOG) performance status score of ≤2. Patients with active brain metastases, concurrent active anti-cancer therapy, pregnancy, or contraindications to study therapy were excluded.
The primary endpoint is objective response rate (ORR); secondary endpoints include clinical benefit rate [CBR; defined as the percentage of patients who achieved an objective response or stable disease (SD) >4 months], duration of response, progression-free survival (PFS), overall survival (OS), and safety. Exploratory analyses include the molecular characterization of tumor biomarkers.
MyPathway is conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the institutional review board or ethics committee at each trial center. Patients provided written informed consent before screening.
Procedures and assessments
Patients were treated in accordance with the United States package inserts15–19 without systemic chemotherapy. Those with HER2 amplification, overexpression, and/or mutation received pertuzumab [840 mg intravenous infusion (i.v.) loading dose, followed by 420 mg i.v. every 3 weeks (q3w)] plus trastuzumab (8 mg/kg i.v. loading dose, followed by 6 mg/kg i.v. q3w). Patients with a Hedgehog pathway alteration (PTCH-1 or SMO mutation) were administered vismodegib (150 mg orally once daily in 28-day cycles). Patients with BRAF V600 mutations received vemurafenib (960 mg orally twice daily in 28-day cycles). Finally, patients with high TMB received atezolizumab (1200 mg i.v. q3w). Tumor burden was investigator-evaluated. Treatments were administered until disease progression, unacceptable toxicity, or other discontinuation criteria were met. Details regarding tumor assessments and molecular profiling methodology are available in the supplementary Methods, available at Annals of Oncology online.
Statistical analysis
The Cloppere–Pearson estimation method was used to calculate 95% confidence intervals (CIs) for ORR and CBR. Median PFS, OS, duration of response [in patients with complete response (CR) or partial response (PR)], and their 95% CIs were estimated using the Kaplane–Meier approach. Additional information on clinical outcome endpoints and planned sample size are provided in the supplementary Methods, available at Annals of Oncology online.
RESULTS
Patients
As of the data cutoff (15 January 2018), 19 patients with SGC were enrolled in MyPathway (supplementary Figure S2, available at Annals of Oncology online). Sixteen patients received pertuzumab + trastuzumab, of whom 15 had HER2 amplification and/or overexpression [with HER2 mutations in two patients (G776V and L755F plus D769H)] and one had a HER2 mutation (S310F) without amplification or overexpression (Table 1). The other three patients received vismodegib (n = 1, truncating PTCH-1 Q400* mutation), vemurafenib (n = 1, BRAF V600E mutation), or atezolizumab (n = 1, high TMB of 31 mut/Mb). All treated patients were evaluable for efficacy.
Table 1.
Baseline demographics and clinical characteristics by patient
| Pt | Sex | Age, years | Race | ECOG PS | Histology | Grade | Stage | Alteration | Testing platforma | Previous lines of therapyb | Sites of metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| HER2 amplification and/or overexpression: treated with pertuzumab + trastuzumab | |||||||||||
| 1 | M | 59 | White | 0 | Salivary duct adenocarcinoma | G3 | IV | HER2 amplification | NGS (copy number = 15) | 1 | Brain, lung, LN |
| 2 | M | 80 | White | 1 | Adenocarcinoma | G2 | IVA | HER2 overexpression | IHC (3+) | 1 | Bone, LN |
| 3 | M | 55 | Black/African American | 2 | Unspecified carcinoma | G3 | IVA | HER2 amplification + overexpression | FISH/CISH (ratio = 7.3), IHC (3+) | 2 | Bone, lung, LN |
| 4 | M | 70 | White | 1 | Invasive ductal carcinoma | G4 | IV | HER2 amplification + overexpression | FISH/CISH (ratio = 2.4), IHC (3+) | 1 | Bone, liver, LN |
| 5 | M | 73 | White | 1 | Adenocarcinoma | G3 | IV | HER2 amplification + overexpression | FISH/CISH (ratio = 9.9), IHC (3+) | 1 | Bone, LN, spleen |
| 6 | M | 47 | White | 1 | Adenocarcinoma | G3 | IVC | HER2 amplification, overexpression + mutation | NGS (copy number gain; L755F and D769H mutations), IHC (3+) | 0 | Bone, LN |
| 7 | M | 61 | White | 1 | Unspecified carcinoma | G3 | III | HER2 amplification + overexpression | NGS (copy number = 94); IHC (3+) | 0 | Liver, lung |
| 8 | F | 54 | White | 0 | Adenocarcinoma | G3 | IV | HER2 amplification + overexpression | NGS (copy number = 104), IHC (3+) | 0 | Liver, LN |
| 9 | M | 54 | Other | 1 | Unspecified carcinoma | G3 | III | HER2 amplification + mutation | FISH/CISH (ratio = 5.5), NGS (G776V mutation) | 0 | Bone, lung, LN |
| 10 | F | 75 | Asian | 0 | Adenocarcinoma | G3 | IVA | HER2 amplification | NGS (copy number gain) | 0 | Lung |
| 11 | M | 70 | White | 1 | Unspecified carcinoma | G1 | IVC | HER2 amplification | NGS (copy number = 60) | 2 | Bone, liver, lung, LN, intraorbital |
| 12 | M | 37 | White | 1 | Adenocarcinoma | GX | IV | HER2 overexpression | IHC (3+) | 1 | Bone, liver |
| 13 | M | 62 | American Indian or Alaska native | 1 | Mucoepidermoid carcinoma | G3 | III | HER2 amplification + overexpression | FISH/CISH (ratio = 7.8), NGS (copy number = 20), IHC (3+) | 3 | Adrenal gland, liver, lung, LN |
| 14 | M | 48 | Asian | 1 | Invasive ductal carcinoma | G4 | IVA | HER2 amplification + overexpression | FISH/CISH (ratio = 7.2), IHC (3+) | 1 | Brain, lung, LN |
| 15 | F | 44 | White | 2 | Adenocarcinoma | G3 | IV | HER2 amplification | NGS (copy number = 15) | 2 | Brain, chest wall, left eye, liver, LN, neck (subcutaneous tissue), parapharyngeal mucosa |
| HER2 mutation: treated with pertuzumab + trastuzumab | |||||||||||
| 16 | M | 68 | White | 0 | Adenocarcinoma | G3 | III | HER2 mutation | NGS (S310F mutation) | 0 | Lung, LN, mediastinum |
| Hh alteration: treated with vismodegib | |||||||||||
| 17 | M | 65 | White | 0 | Mucoepidermoid carcinoma | G3 | II | Hh alteration | NGS (PTCH-1 Q400* mutation) | 0 | Lung |
| BRAF V600 mutation: treated with vemurafenib | |||||||||||
| 18 | M | 51 | White | 1 | Mucoepidermoid carcinoma | G3 | IV | BRAF mutation | NGS (V600E mutation) | 1 | Liver, lung, LN |
| High TMB: treated with atezolizumab | |||||||||||
| 19 | M | 82 | White | 1 | Mucoepidermoid carcinoma | G3 | IVA | High TMB | NGS (31 mutations/Mb) | 0 | Adrenal gland, LN, skin |
ECOG PS, Eastern Cooperative Oncology Group performance status; F, female; FISH/CISH, fluorescence/chromogenic in situ hybridization; HER2, human epidermal growth factor receptor 2; Hh, Hedgehog; IHC, immunohistochemistry; LN, lymph node; M, male; NGS, next-generation sequencing; Pt, patient; PTCH-1, patched homolog-1; TMB, tumor mutational burden.
Not all patients were tested with all testing methods. Details from testing results are included, where available.
Previous regimens consisted of systemic chemotherapy in all patients.
All 19 patients had aggressive salivary gland carcinomas (salivary gland adenocarcinoma, n = 11; high-grade mucoepidermoid carcinoma, n = 4; and unspecified carcinoma, n = 4). The median age was 61 (range, 37–82) years and patients received a median of one (range, 1–3) previous systemic treatment regimen. Most patients were male [n = 16 (84%)], white [n = 14 (74%)], and had an ECOG performance status score of ≤1 [n = 17 (89%)] (Table 1).
Clinical outcomes
The median follow-up duration was 10.6 (range, 3–26) months. Nine of 15 patients with HER2 amplification and/or overexpression had objective responses to treatment with pertuzumab + trastuzumab (one CR, eight PRs) for an ORR of 60% (95% CI 32% to 84%) (Table 2). One additional patient had SD for 11.7 months [CBR 67% (n = 10), 95% CI 38% to 88%]. Treatment duration ranged from 0.7 to 26.1 [ongoing (+)] months and estimated median response duration in patients with an objective response was 9.2 (range, 1.4+ to 19.7+) months, with five out of nine responses (including the CR) ongoing at the time of data cutoff. Time on treatment and best change in target lesion size by patient are shown in Figure 1A,B. By the data cutoff, nine patients with HER2 amplification and/or overexpression had progressed or died (Table 2). Median PFS was 8.6 [95% CI 2.3 to not estimable (NE)] months (Figure 2A) and median OS was 20.4 (95% CI 8.2 to NE) months (Figure 2B).
Table 2.
Clinical outcomes by patient
| Pt | Alteration | Time on treatment, months | Best response | Duration of response, months | Duration of SD, months | Best change in target lesion size from baseline, % | PFS, months | OS, months |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HER2 amplification and/or overexpression: treated with pertuzumab + trastuzumab | ||||||||
| 1 | HER2 amplification | 16.5+ | CR | 15.2+ | – | −91.7a | 16.5+ | 16.5+ |
| 2 | HER2 overexpression | 26.1+ | PR | 19.7+ | – | −33.3 | 25.2+ | 26.1+ |
| 3 | HER2 amplification and overexpression | 12.6 | PR | 9.2 | – | −62.5 | 13.4 | 20.4 |
| 4 | HER2 amplification and overexpression | 8.3 | PR | 7.3 | – | −100.0b | 8.6 | 14.9+ |
| 5 | HER2 amplification and overexpression | 10.6+ | PR | 7.2+ | – | −66.7 | 8.5+ | 10.6+ |
| 6 | HER2 amplification, overexpression, and mutation (L755F and D769H) | 19.8 | PR | 4.2 | – | −85.7 | 5.6 | 21.2 |
| 7 | HER2 amplification and overexpression | 4.1+ | PR | 2.8+ | – | −73.0 | 4.0+ | 4.1+ |
| 8 | HER2 amplification and overexpression | 4.1c | PR | 2.7 | – | −68.2 | 9.1 | 9.1 |
| 9 | HER2 amplification and mutation (G776V) | 3.5+ | PR | 1.4+ | – | −55.7 | 2.8+ | 3.5+ |
| 10 | HER2 amplification | 11.2 | SD | – | 11.7 | −27.9 | 11.7 | 14.0+ |
| 11 | HER2 amplification | 3.5 | SD | – | 3.9 | −25.6 | 3.9 | 10.4 |
| 12 | HER2 overexpression | 2.9+ | SD | – | 2.9+ | −24.3 | 2.9+ | 2.9+ |
| 13 | HER2 amplification and overexpression | 2.1 | SD | – | 2.3 | 1.4 | 2.3 | 8.2 |
| 14 | HER2 amplification and overexpression | 0.7 | PD | – | – | 3.6 | 1.5 | 8.3 |
| 15 | HER2 amplification | 0.7 | PD | – | – | 22.5 | 1.4 | 3.1 |
| HER2 mutation: treated with pertuzumab + trastuzumab | ||||||||
| 16 | HER2 mutation (S310F) | 10.4 | SD | – | 11.0 | −12.8 | 11.0 | 13.7+ |
| Hh alteration: treated with vismodegib | ||||||||
| 17 | Hh alteration (PTCH-1 Q400*) | 14.4 | PR | 10.7 | −55.6 | 14.3 | 17.3+ | |
| BRAF V600 mutation: treated with vemurafenib | ||||||||
| 18 | BRAF mutation (V600E) | 16.8 | PR | 15.1 | – | −43.4 | 18.5 | 20.1 |
| High TMB: treated with atezolizumab | ||||||||
| 19 | High TMB (31 mutations/Mb) | 5.7+ | PR | 0.03+ | – | −56.0 | 5.5+ | 5.7+ |
CR, complete response; HER2, human epidermal growth factor receptor 2; Hh, Hedgehog; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; Pt, patient; PTCH-1, patched homolog-1; SD, stable disease; TMB, tumor mutational burden.
indicates measure was ongoing at the most recent assessment.
Patient’s target lesions were both lymph nodes with a measurement of <10 mm and absent/normal status of non-target lesions following treatment cycle 12, considered a CR per RECIST v1.1.
Patient had a 100% reduction in the target lesion but had a remaining non-target lesion and therefore did not qualify for a CR per RECIST v1.1.
Patient had an ongoing tumor response but withdrew from treatment and later died.
Figure 1. Time on treatment and (B) best change in target lesion size by patient.
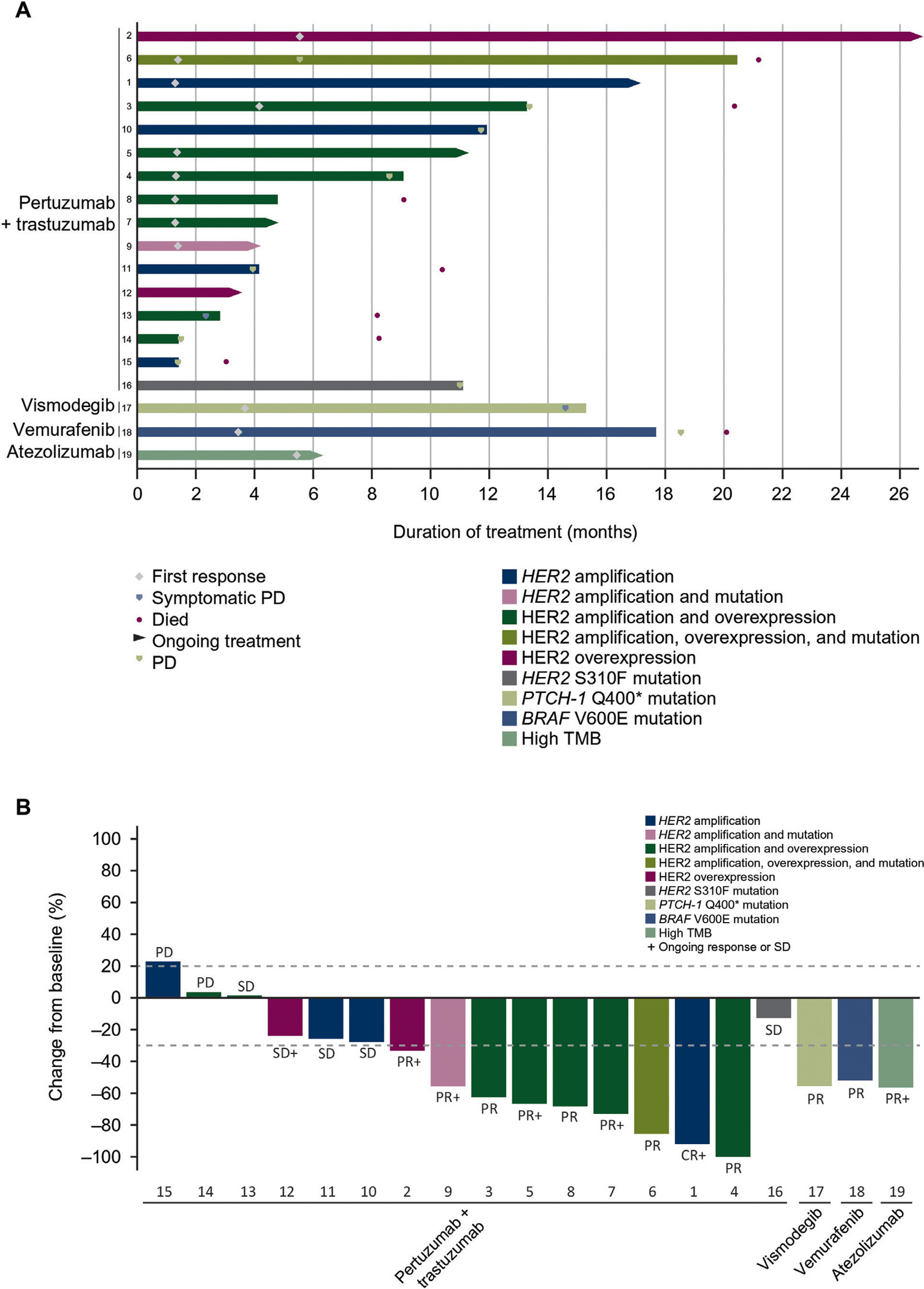
(A) (n = 19).a CR, complete response; HER2, human epidermal growth factor receptor 2; Hh, Hedgehog; PD, progressive disease; PR, partial response; PTCH-1, patched homolog-1; SD, stable disease; TMB, tumor mutational burden.
a Lanes represent individual patients.
Figure 2. Progression-free survival and (B) overall survival in patients with HER2 amplification and/or overexpression (n = 15).
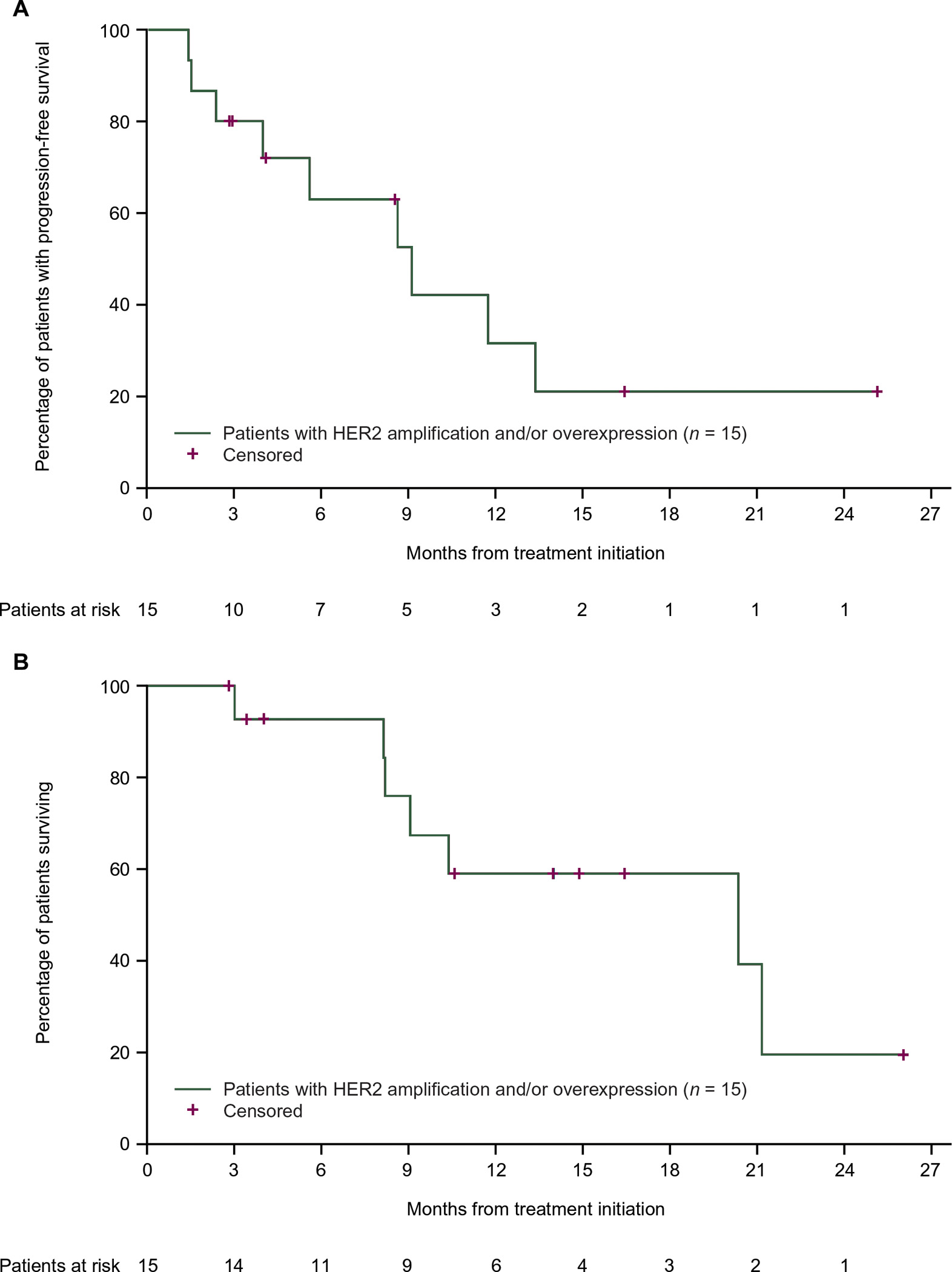
(A) HER2, human epidermal growth factor receptor 2.
One patient with a HER2 S310F mutation without amplification or overexpression had SD lasting 11.0 months as the best response following treatment with pertuzumab + trastuzumab (Table 2). The patients with a PTCH-1 Q400* mutation (treated with vismodegib) and BRAF V600E mutation (treated with vemurafenib) experienced PRs lasting 10.7 and 15.1 months but had discontinued treatment due to clinical progression and progressive disease by the data cutoff, respectively. Finally, following SD of 5.4 months, a PR was reported in the atezolizumab-treated patient with high TMB (31 mut/Mb) at the last tumor assessment before the data cutoff; the patient remained on treatment.
Overall, 12 of 19 patients (63%) achieved an objective response when matched to therapy based on the genomic characteristics of their tumors. Including patients with SD >4 months, 14 of 19 patients (74%) experienced clinical benefit. Median PFS for all 19 patients was 11.7 months (95% CI 5.6% to 18.5%) and median OS was 20.4 months (95% CI 9.1 to NE). Case reports for one patient in each treatment arm are presented in the supplementary Results and Figures S3–S6, available at Annals of Oncology online.
Tumor molecular profiles
Genomic profiling data were available for 11 of 15 patients with HER2 amplification and/or overexpression, and all patients in the remaining cohorts (see supplementary Table S1, available at Annals of Oncology online). Mutational profiles are shown in Figure 3 and described in detail in the supplementary Results, available at Annals of Oncology online.
Figure 3. Mutational profile of selected oncogenic alterations in patients with advanced salivary gland carcinoma (n =19).
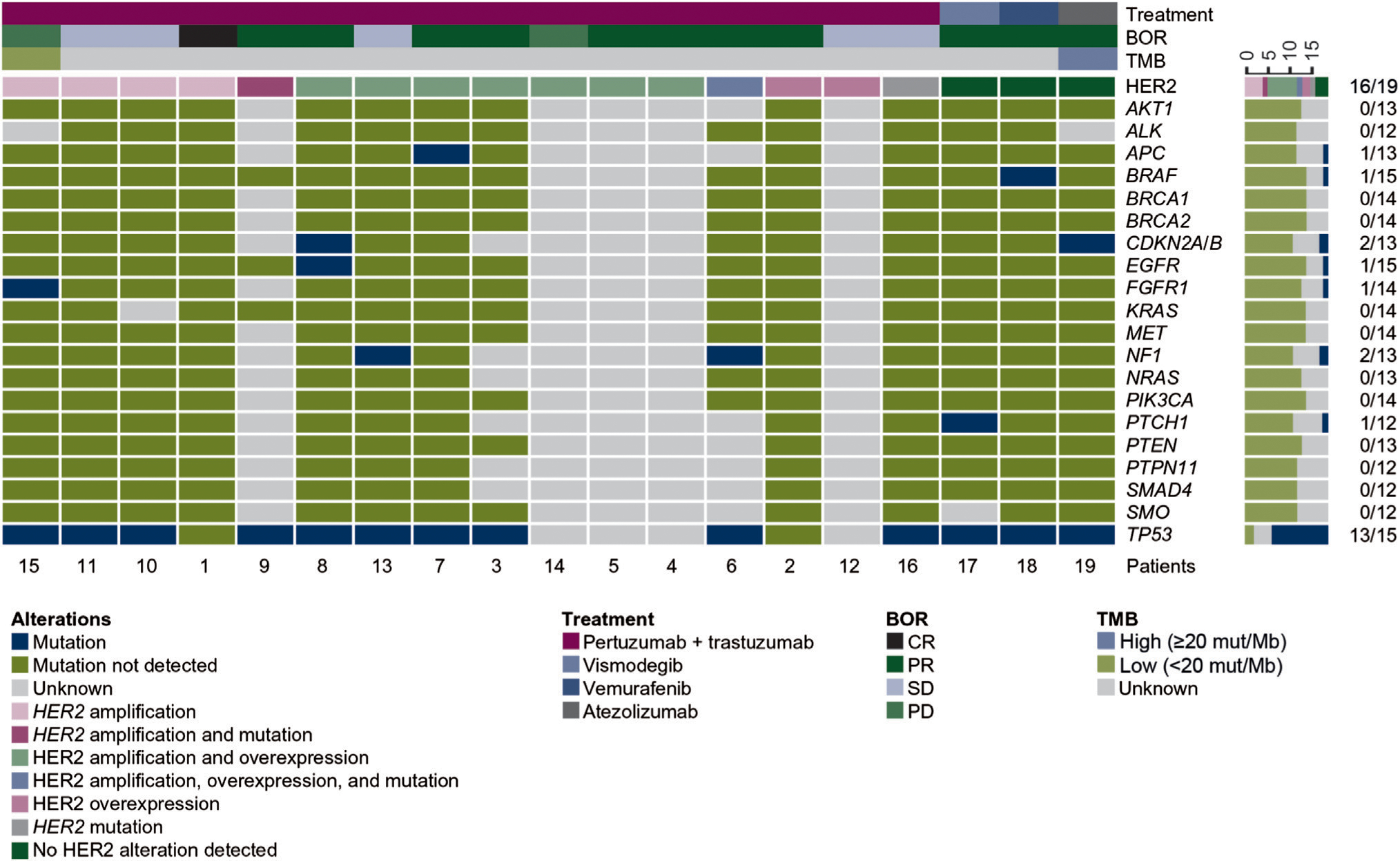
BOR, best overall response; CR, complete response; HER2, human epidermal growth factor receptor 2; PD, progressive disease; PR, partial response; SD, stable disease; TMB, tumor mutational burden.
Safety
Of 16 patients treated with pertuzumab + trastuzumab, treatment-emergent adverse events (TEAEs) and TEAEs thought to be related to one or both study drugs were reported in 94% (n = 15) and 88% (n = 14) of patients, respectively. Serious TEAEs and grade 3–4 TEAEs were observed in 13% (n = 2) and 25% (n = 4) of patients, respectively, with no serious or grade 3–4 TEAEs experienced by more than one patient. There were no deaths due to a TEAE. The most common drug-related TEAEs were diarrhea [50% (8/16)], pruritus [31% (5/16)], and chills [25% (4/16)] (see supplementary Table S2, available at Annals of Oncology online). One patient had grade 3 peripheral neuropathy considered related to the study drugs (supplementary Table S3, available at Annals of Oncology online). Of the three reported grade 4 TEAEs, none were considered related to the study drugs.
Each of the patients treated with vismodegib, vemurafenib, and atezolizumab experienced TEAEs, including at least one grade 3 TEAE. TEAEs thought to be related to the study drugs and serious TEAEs were reported in the vismodegib and vemurafenib-treated patients. Grade 3 drug-related TEAEs included diarrhea, decreased weight, and hypokalemia in the vismodegib-treated patient, and maculopapular rash in the vemurafenib-treated patient. No TEAEs higher than grade 3 were observed.
DISCUSSION
SGCs are refractory to many common chemotherapeutic treatments, but few studies have been conducted to identify effective systemic therapies due to the rarity of the disease. Data from MyPathway support the potential of chemotherapy-free targeted therapy for patients with advanced SGC. We observed promising efficacy in the subgroup of patients with HER2-amplified and/or overexpressing salivary gland carcinomas treated with pertuzumab + trastuzumab, the largest cohort assessed in this analysis [ORR, 60% (95% CI 32% to 84%); CBR, 67% (95% CI 38% to 88%); median PFS, 8.6 (95% CI 2.3 to NE) months; median OS, 20.4 (95% CI 8.2 to NE) months]. Responses were durable (median 9.2 months), and five of nine were ongoing at data cut off, including the CR. Furthermore, we observed promising results in individual patients with tumors harboring a HER2 S310F mutation (pertuzumab + trastuzumab; SD, 11.0 months); a PTCH-1 Q400* mutation (vismodegib; PR, 10.7 months); a BRAF V600E mutation (vemurafenib; PR, 15.1 months); and high TMB (atezolizumab; PR as of the data cutoff date, following a prolonged SD of 5.4 months).
Management of early SGC typically involves surgery or radiotherapy, with no standard of care for the systemic treatment of advanced cancer.2 Previously investigated chemotherapeutic regimens were associated with low response rates and high toxicity.20 Although the recent phase II study of trastuzumab + docetaxel in patients with HER2-positive SDC produced a high response rate, more than half of the patients experienced grade 4 decreased neutrophil count.8 In MyPathway, we observed a response rate of 60% with pertuzumab + trastuzumab in patients with HER2-amplified and/or overexpressing advanced SGCs. This chemotherapy-free regimen was well tolerated, with only one grade 3 TEAE considered to be related to the study drugs, and no grade 4 related TEAEs. The median 20.4-month OS in this cohort compares favorably with the 15-month OS previously observed after the development of distant metastases in patients with salivary gland carcinomas unselected for HER2 status.21 Finally, while the rarity of SGC limited the assessment of vismodegib, vemurafenib, and atezolizumab in only one patient each, the preliminary observations of extended PRs or SDs in each of these patients, all of whom had recurrent disease after previous treatment of salivary cancer, suggest that patients can be successfully matched to a variety of targeted therapies based on the genomic characteristics of the tumors.
All 19 patients with SGC enrolled in the MyPathway study had high-grade carcinomas. While genomic and molecular alterations associated with adverse outcomes are common in such tumors,6 some of the reported alterations are potentially targetable with currently available agents, providing new opportunities for the treatment of these challenging salivary tumor subtypes. HER2 alterations, in particular, are common in aggressive tumor subtypes, such as SDCs.6,7 In the largest study of comprehensive molecular profiling in salivary gland cancers, HER2 alterations occurred in 32% of SDCs and 17% of other adenocarcinomas.6 The high incidence of HER2 alterations and the efficacy of HER2-targeted therapy suggest similarities between SDC and breast intraductal carcinomas. Salivary and mammary glands share similar histogenetic origins; in addition to frequent HER2 alterations, carcinomas arising from these tissues share similar histology, morphology, and gene expression profiles.22–25 These similarities, in addition to the high response rate we observed in patients with HER2 amplified and/or overexpressing salivary gland carcinomas, suggest that the success of HER2-targeted therapy in breast cancer may extend to patients with HER2-positive salivary gland carcinomas.
Limitations of this analysis include small patient cohorts and non-blinded investigator assessments. In addition, local molecular alteration testing was conducted at the investigator’s discretion using site-determined methodology, with central genomic re-testing limited to two patients with available and sufficient archival samples for next-generation sequencing. Because of the limited sample size, the impact of co-mutations on efficacy could not be determined; this merits study in future investigations. Although data from MyPathway suggest that targeted therapy has potential in the treatment of SGC, the small number of patients with this rare cancer may necessitate additional study to assess efficacy. Preliminary data from other recent or ongoing basket trials have indicated positive results for the targeted treatment of SGC with trastuzumab emtansine (T-DM1), neratinib, vemurafenib, entrectinib, and larotrectinib11–13,26–28; additional results from these and other studies are anticipated.
Results from MyPathway provide a proof-of-principle for matching patients to effective, chemotherapy-free treatment of tumors based on molecular characteristics rather than site of origin, a particularly valuable opportunity for cancer patients with limited treatment options or who are unable to tolerate traditional chemotherapies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to the patients, families, and study teams who participated in MyPathway. We would also like to thank Ben George (Medical College of Wisconsin) and Petros Nikolinakos (University Cancer & Blood Center) for their assistance with the patient case reports, and Yong Wang (Genentech) for her programming support. Third-party writing assistance was provided by Sabrina Hom, PhD, of CodonMedical, an Ashfield Company, part of UDG Healthcare plc, and was funded by F. Hoffmann-La Roche/Genentech.
DISCLOSURES
RK received research funding from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, Konica Minolta, Debiopharm, Boehringer Ingelheim, and OmniSeq; served as a consultant/advisor for LOXO, X-Biotech, Actuate Therapeutics, Roche, NeoMed, Gaido, and Solventis; served on a speakers bureau for Roche; received honoraria from Roche, NeoGenomics, Sysmex, NeoMed Therapeutics, Advanced Therapy Program, LEK Consulting, Chugai, CME Education, Avera, Wiley, and LOXO AACR; has an ownership interest/stock in IDbyDNA CureMatch Inc. and Soluventis; and has a leadership role in CureMatch, Inc. DWB has an ownership interest/stock in Bristol-Myers Squibb. HK served as a consultant for and received honoraria from Loxo and Ignyta; his institution received research funding from Immunogen, VentiRx, Plexxikon, Merck, AstraZeneca, Advaxis, and Bristol-Myers Squibb. FM-B received honoraria from Sumitomo Dainippon Pharma and Dialectica; served as a consultant/advisor for Aduro, Aileron, AstraZeneca, Bayer, Calithera, Curis, CytomX, Darwin Health, Debiopharm Group, Genentech, Inflection, Jackson Lab, Kolon Life, Mersana, Novartis, OrigiMed, Parexel, Pfizer, Pieris, Puma, Samsung Bioepis, Seattle Genetics, Spectrum, Taiho, Xencor, and Zymeworks; received research funding from AbbVie, Aileron, AstraZeneca, Bayer, Calithera, Curis, CytomX, eFFECTOR, Guardant Health, Daiichi-Sankyo, Debiopharm Group, Genentech, GlaxoSmithKline, Novartis, Pfizer, Puma, Taiho, and Zymeworks; and received travel reimbursement from Debiopharm Group, Genentech, Pfizer, and Taiho. JH received research funding paid to his institution from Astellas Pharma, AstraZeneca, Genentech, and Novartis and served as a consultant/advisor for Roche. DRS has a leadership role with Centennial Medical Center (HCA; BOT-chair); served as a consultant for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Evelo Therapeutics, Foundation Medicine, Roche/Genentech, GlaxoSmithKline, Illumina, Lilly, Merck, Moderna Therapeutics, Nektar, Novartis, Pfizer, PharmaMar, Precision Oncology, Takeda, and TRM Oncology; received travel reimbursement from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, EMD Serono, Genentech, Genzyme, Intuitive Surgical, Lilly, Merck, Perdue Pharma, Pfizer, Spectrum Pharmaceuticals, and Sysmex; and his institution received research funding from AbbVie, Acerta Pharma, Aeglea BioTherapeutics, Amgen, ARMO Bio-Sciences, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celldex, Clovis Oncology, Daiichi-Sankyo, EMD Serono, Foundation Medicine, GlaxoSmithKline, G1 Therapeutics, Roche/Genentech, GRAIL, Ipsen, Lilly, Merck, Millennium, Nektar, Novartis, Neon Therapeutics, Pfizer, Takeda, Tesaro, Transgene, and University of Texas Southwestern Medical Center – Simmons Cancer Center. RB served as a consultant for Roche/Genentech; received honoraria from Roche/Genentech, Foundation Medicine, and Novartis; and received research funding from Puma Biotechnology. HB reports employment, leadership, and an ownership interest/stock in HCA Healthcare/Sarah Cannon; has been paid for expert testimony by Novartis; served as consultant/advisor with fees paid to his institution for AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Eisai, FORMA Therapeutics, Incyte, Janssen, MedImmune, Mersana, Novartis, Roche/Genentech, and Tolero Pharmaceuticals; and received research funding paid to his institution from Agios, Arch, Arvinas, AstraZeneca, BioAtla, BioMed Valley Discoveries, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, CicloMed, CytomX Therapeutics, eFFECTOR Therapeutics, Gilead Sciences, GlaxoSmithKline, Harpoon Therapeutics, Immunocore, Incyte, Janssen, Jiangsu Hengrui Medicine, Jounce Therapeutics, Kyocera, Lilly, Loxo, MacroGenics, MedImmune, Merck, Millennium, Moderna Therapeutics, Novartis, Revolution Medicines, Roche/Genentech, Seattle Genetics, Takeda, Tessaro, TG Therapeutics, Verastem, and Vertex. CJS served as a consultant for Amgen, Astellas Pharma, AstraZeneca, Bayer, Tolmar, Roche/Genentech, Janssen Biotech, and Sanofi; owns stock in Leuchemix; and has patents with Leuchemix and Exelixis; his institution received research funding from Astellas Pharma, Exelixis, and Janssen Biotech. MSB, SB, KS, and VC are employed by and own stock in Roche/Genentech. CS received grant support from Pfizer, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and Roche-Ventana; served as a consultant for Pfizer, Novartis, GlaxoSmithKline, MLD, Bristol-Myers Squibb, Celgene, AstraZeneca, Genentech, Roche-Ventana, GRAIL, Medicxi, Sarah Cannon Research Institute, and Illumina; and has an ownership interest/stock in Apogen Biotech, Epic Biosciences, GRAIL, and Achilles Therapeutics (co-founder). All authors received non-financial support from Roche in the form of medical writing support for this manuscript.
FUNDING
This work was supported by F. Hoffmann-La Roche/Genentech (no grant number).
REFERENCES
- 1.Del Signore AG, Megwalu UC. The rising incidence of major salivary gland cancer in the United States. Ear Nose Throat J. 2017;96:E13–E16. [DOI] [PubMed] [Google Scholar]
- 2.Chintakuntlawar AV, Okuno SH, Price KA. Systemic therapy for recurrent or metastatic salivary gland malignancies. Cancers Head Neck. 2016;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato S, Elkin SK, Schwaederle M, et al. Genomic landscape of salivary gland tumors. Oncotarget. 2015;6:25631–25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, McDermott JD, Schrock AB, et al. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequent BAP1, PIK3CA, and other actionable genomic alterations. Ann Oncol. 2017;28:748–753. [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Russell JS, McDermott JD, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. 2016;22:6061–6068. [DOI] [PubMed] [Google Scholar]
- 6.Ross JS, Gay LM, Wang K, et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann Oncol. 2017;28:2539–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10:944–946. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, Tada Y, Saotome T, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. 2019;37:125–134. [DOI] [PubMed] [Google Scholar]
- 9.Falchook GS, Lippman SM, Bastida CC, et al. Human epidermal receptor 2-amplified salivary duct carcinoma: regression with dual human epidermal receptor 2 inhibition and anti-vascular endothelial growth factor combination treatment. Head Neck. 2014;36:E25–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrêa TS, Matos GDR, Segura M, et al. Second-line treatment of HER2-positive salivary gland tumor: ado-trastuzumab emtansine (T-DM1) after progression on trastuzumab. Case Rep Oncol. 2018;11:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016;27:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36:536–542. [DOI] [PubMed] [Google Scholar]
- 15.Erivedge (vismodegib). [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2017. [Google Scholar]
- 16.Herceptin (trastuzumab). [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2018. [Google Scholar]
- 17.Perjeta (pertuzumab). [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2018. [Google Scholar]
- 18.Tecentriq (atezolizumab). [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2018. [Google Scholar]
- 19.Zelboraf (vemurafenib). [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2017. [Google Scholar]
- 20.Wang X, Luo Y, Li M, et al. Management of salivary gland carcinomas – a review. Oncotarget. 2017;8:3946–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam SJ, Roh JL, Cho KJ, et al. Risk factors and survival associated with distant metastasis in patients with carcinoma of the salivary gland. Ann Surg Oncol. 2016;23:4376–4383. [DOI] [PubMed] [Google Scholar]
- 22.Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838–E1847. [DOI] [PubMed] [Google Scholar]
- 23.Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142–151. [DOI] [PubMed] [Google Scholar]
- 25.Boecker W, Stenman G, Loening T, et al. K5/K14-positive cells contribute to salivary gland-like breast tumors with myoepithelial differentiation. Mod Pathol. 2013;26:1086–1100. [DOI] [PubMed] [Google Scholar]
- 26.Jhaveri KL, Makker V, Wang XV, et al. Ado-trastuzumab emtansine (T-DM1) in patients (pts) with HER2 amplified (amp) tumors excluding breast and gastric/gastro-esophageal junction (GEJ) adenocarcinomas: results from the National Cancer Institute (NCI) Molecular Analysis for Therapy Choice (MATCH) trial. J Clin Oncol. 2018;36(suppl):abstr 100. [Google Scholar]
- 27.Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li BT, Makker V, Buonocore DJ, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol. 2018;36(suppl):abstr 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


