Pulmonary fibrosis is an incurable progressive lung disease associated with the formation of scar tissue and impaired lung function. Lung fibrosis develops in a number of diseases, including interstitial lung diseases, idiopathic interstitial pneumonias, and childhood interstitial lung disease syndromes, and in response to many types of lung injury, including radiation and some chemotherapeutic drugs (1, 2). Idiopathic pulmonary fibrosis (IPF) is a severe fibrotic lung disease with a poor prognosis (3). Male sex and environmental exposure to toxicants, including smoke and asbestos, are also strongly associated with IPF (4). Features of IPF include interstitial pneumonia, excess collagen deposition, fibroblast expansion, progressive dyspnea, and cough. The mechanisms responsible for IPF are not clear, but repetitive lung injury that results in the release of several inflammatory and profibrotic mediators in fibrotic niches are believed to be critical for the initiation and maintenance of fibrotic lesions (5). IL-1β is a well-studied proinflammatory cytokine that has been shown to be upregulated in several fibrotic lung diseases, including IPF, and is implicated to play a critical role in both wound repair and pulmonary fibrosis (2). In the puzzle of lung fibrogenesis, identifying innovative ways to inhibit IL-1β production or its profibrotic functions would be an important step toward new treatment opportunities against severe fibrotic lung diseases.
In this issue of the Journal, Itano and colleagues (pp. 654–665) now suggest that neuropeptides are critical effectors of fibrosis progression in the lungs (6). The team focused on NPY (neuropeptide Y), a small peptide normally synthesized in the central and peripheral nervous system and implicated in inflammatory airway diseases, including asthma and chronic obstructive pulmonary disease. They considered evidence that NPY can decrease IL-1β (7, 8), thus leading them to form the hypothesis that NPY signaling might modulate progression of fibrosis in IPF lungs.
To test this hypothesis, the authors studied genetically engineered mice lacking NPY (NPY−/−) and exposed them to bleomycin. Bleomycin causes pulmonary fibrosis in both humans (9) and animals (10). The authors first examined the extent to which bleomycin induced inflammation and fibrosis in mice lacking NPY and found greater collagen deposition and fibrosis in NPY−/− than in wild-type control mice. Furthermore, NPY−/− mice administered bleomycin exhibited greater numbers of inflammatory cells and concentrations of IL-1β in the airways than wild-type control mice administered bleomycin. These data strongly supported the idea that NPY played a protective role in bleomycin-induced fibrosis by limiting inflammation.
To expand on this finding, the authors measured NPY concentrations in the BAL fluid and found increased concentrations in wild-type mice 2 days after bleomycin administration. However, 21 days later, bleomycin-exposed wild-type mice had significantly decreased NPY concentrations compared with vehicle control mice. These data highlighted a temporal regulation of NPY after bleomycin exposure. To investigate the source of NPY in the airways, the authors examined airway cross-sections and found ectopic NPY expression in the bronchial epithelia and in some inflammatory cells. Combined, these data suggested that local NPY production occurred within the airway and that production was augmented acutely in response to bleomycin administration, followed by an overall reduction later.
The authors next tested the prediction that both prophylactic and therapeutic NPY administration would ameliorate bleomycin-induced airway inflammation and remodeling. To do this, they administered NPY to mice either 24 hours before bleomycin exposure or 7 days after bleomycin exposure. Both interventions reduced the fibrotic changes associated with bleomycin in the airways. Furthermore, NPY administration reduced the concentrations of IL-1β.
This observation led the authors to hypothesize that unchecked IL-1β signaling was responsible for the enhanced fibrotic and inflammatory response of NPY−/− mice to bleomycin. To test this, they neutralized IL-1β with antibodies and found that doing so mitigated the fibrosis associated with bleomycin in the NPY−/− mice. Furthermore, they found that activation of the NPY-Y1 receptor inhibited release of IL-1β in human A549 cells exposed to bleomycin in vitro. Blocking NPY-Y1 receptor pharmacologically prevented the antiinflammatory effect of NPY.
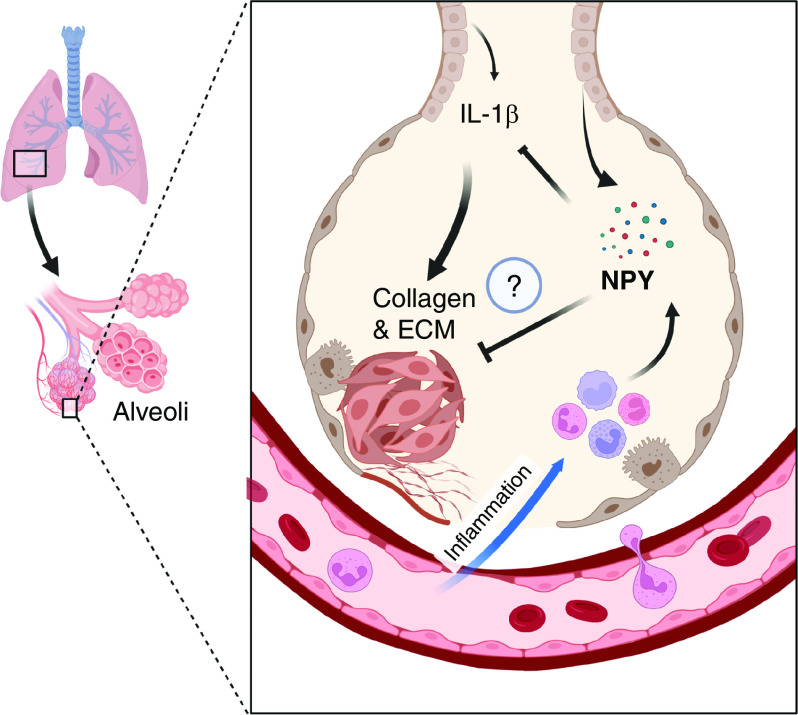
Last, the authors examined serum concentrations of NPY in patients with IPF and healthy control subjects. They discovered a reduction in NPY serum concentrations in patients with IPF. They also examined tissue sections from human donor lungs and found NPY expression in the bronchial epithelia, mirroring their findings in mice. Interestingly, the number of NPY cells in the lungs of patients with IPF was smaller, also mirroring observations in mice, in which NPY concentrations showed a temporal decrease after bleomycin exposure. Combined, these data directly linked NPY signaling to IPF and suggested that NPY limits pulmonary fibrosis by dampening immune responses mediated by IL-1β (Figure 1).
Figure 1.
Schema showing the protective effects of NPY involve inhibition of IL-1β–driven fibroblast activation and pulmonary fibrosis. ECM = extracellular matrix proteins; NPY = neuropeptide Y.
Although the results generated by Itano and colleagues highlight a regulatory role of NPY in the establishment and progression of IPF, there are questions that remain. For example, the authors found that NPY expression was mainly observed in the bronchial epithelium, although expression in nerves innervating the airway has been repeatedly described. Therefore, it is unknown whether bronchial epithelial expression of NPY per se exerts an antiinflammatory role or whether NPY from nerves also contributes. Moreover, it is not clear whether NPY expression is only reduced in bronchial epithelial cells in animal models of IPF and humans with pulmonary fibrosis or if other cells, including the nerves, are also involved. Furthermore, it is unclear whether NPY administration or NPY agonists would have a beneficial impact if administered during established and ongoing pulmonary fibrosis in mice and humans. Therefore, establishing the preclinical efficacy of NPY using severe fibrotic lung disease models with repetitive injury in aged mice is essential to transition toward clinical testing of NPY against IPF or other fibrotic lung diseases. To date, however, no U.S. Food and Drug Administration–approved NPY agonists exist.
In summary, the results by Itano and colleagues open new doors for the development of potential antifibrotic therapeutics. It is possible that with more investigation, NPY could be the unsung hero to prevent or reverse ongoing fibrosis in the lungs.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2022-0375ED on September 29, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med . 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med . 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 4. Gulati M, Redlich CA. Asbestosis and environmental causes of usual interstitial pneumonia. Curr Opin Pulm Med . 2015;21:193–200. doi: 10.1097/MCP.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest . 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itano J, Taniguchi A, Senoo S, Asada N, Gion Y, Egusa Y, et al. Antagonizes development of pulmonary fibrosis through IL-1β inhibition. Am J Respir Cell Mol Biol . 2022;67:654–665. doi: 10.1165/rcmb.2021-0542OC. [DOI] [PubMed] [Google Scholar]
- 7. Ferreira R, Santos T, Viegas M, Cortes L, Bernardino L, Vieira OV, et al. Neuropeptide Y inhibits interleukin-1β-induced phagocytosis by microglial cells. J Neuroinflammation . 2011;8:169. doi: 10.1186/1742-2094-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oztas B, Sahin D, Kir H, Eraldemir FC, Musul M, Kuskay S, et al. The effect of leptin, ghrelin, and neuropeptide-Y on serum Tnf-Α, Il-1β, Il-6, Fgf-2, galanin levels and oxidative stress in an experimental generalized convulsive seizure model. Neuropeptides . 2017;61:31–37. doi: 10.1016/j.npep.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 9. Rudders RA, Hensley GT. Bleomycin pulmonary toxicity. Chest . 1973;63:627–628. doi: 10.1378/chest.63.4.626. [DOI] [PubMed] [Google Scholar]
- 10. Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol . 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]