To the Editor:
The triple combination of CFTR modulators elexacaftor/tezacaftor/ivacaftor (ETI) improves the clinical status of patients with cystic fibrosis who are homozygous and heterozygous for F508 del and the fertility of women with cystic fibrosis (1, 2). An increase in the number of pregnancies is therefore expected among treated women, leading to the birth of infants heterozygous for CFTR mutations. Studies investigating the possible impact of this combination treatment on fetal development are therefore required (3). We investigated the effect of ETI on embryonic lung development during the pseudoglandular stage in cultured lung explants from heterozygous F508 del Cftr (Cftrtm1Eur/+) and wild-type (WT) mice (4). We used this experimental strategy to assess the potential impact on lung development in heterozygous fetuses from women with cystic fibrosis treated with ETI.
Some of the results of these studies have been previously reported in the form of a preprint (bioRxiv, 4 November 2021 https://www.biorxiv.org/content/10.1101/2021.11.01.466814v1).
Experiments were performed in accordance with European Directive 2010/63/UE, with approval from the ethics committee for animal experiments of Paris Descartes University (CEEA no. 34).
Lung explants were collected from E12.5 mouse embryos, as previously described (5), and cultured in Dulbecco's modified Eagle medium (DMEM) for 72 hours. They were analyzed at baseline (T0) and after 24 hours (T24), 48 hours (T48), and 72 hours (T72) of culture. The number and diameter of the terminal buds at the periphery of each sample were determined, as previously described (6) and illustrated in Figure E1 in the data supplement. All embryos of both sexes from a given litter were dissected and cultured. The results are expressed as the ratio of the number of dilations in the treated group to that reported in the DMEM/F12 littermate control group.
CFTR protein activity was modulated by supplementing the culture medium with ETI or with forskolin with and without Inh-172 for 72 hours at the following concentrations: elexacaftor (Selleckchem) (10 μM) + tezacaftor (Selleckchem) (10 μM) + ivacaftor (Selleckchem) (100 nM), or forskolin (Sigma Aldrich) (5 μM), to increase intracellular cAMP content and activate CFTR phosphorylation, with and without the CFTR-specific inhibitor Inh-172 (Sigma Aldrich) (5 μM). ETI and Inh-172 were diluted in DMSO (dimethyl sulfoxide), and forskolin was diluted in ethanol. We checked that DMSO and ethanol had no effect per se on lung branching in culture relative to the medium (Figure E2).
Real-time polymerase chain reaction analyses of the expression of Fgf10, Fgfr2IIIb, Shh, and Hhip were performed with RNA from E12.5 lung explants snap-frozen after 72 hours of culture (Table E1).
Cftr expression was detected in lung explants from E12.5 embryos (Figure E3). There was no significant difference between untreated lung explants from WT, F508 del heterozygous Cftrtm1Eur/+, and F508 del homozygous Cftrtm1Eur/tm1Eur embryos in terms of lung branching or terminal bud dilation, at any of the time points considered (Table E2 and Figure E3).
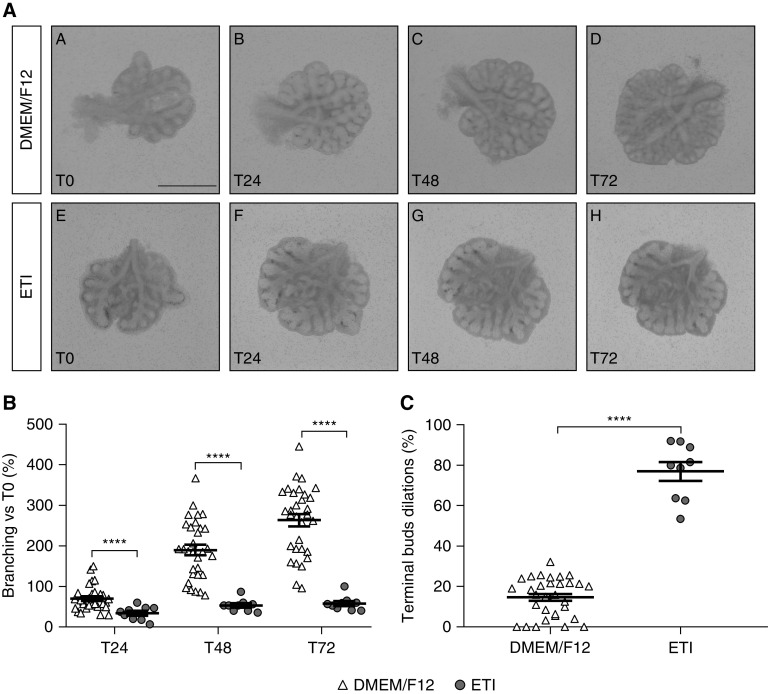
Exposure to ETI for 3 days significantly decreased branching in lung explants from Cftrtm1Eur/+ embryos, as demonstrated by comparison with Cftrtm1Eur/+ embryos incubated with medium alone. Mean lung branching in the ETI group was 51% ± 8% (not significant [NS]) lower at T24, 72% ± 3% (P < 0.0001) lower at T48, and 78% ± 2% (P < 0.0001) lower at T72 (Figure 1). ETI also significantly increased the percentage of terminal buds dilated in Cftrtm1Eur/+ lung explants by 5.3-fold (±0.3) (P < 0.001) after 72 hours, relative to Cftrtm1Eur/+ embryos incubated with medium (Figure 1).
Figure 1.
Effect of elexacaftor/tezacaftor/ivacaftor (ETI) on lung branching and bud dilation in lung explants from E12.5 Cftrtm1Eur/+ mouse embryos. (A) Lung explants from Cftrtm1Eur/+ embryos cultured for 3 days in Dulbecco's modified Eagle medium (DMEM)/F12 alone (A–D) (n = 31), or DMEM/F12 + ETI (E–H) (n = 9). Scale bar, 1 mm. (B) Number of terminal buds at T24, T48, and T72, expressed as the percentage increase in branching relative to baseline (T0). Data are expressed as the mean ± SEM and were analyzed by two-way ANOVA. ****P < 0.0001. (C) Number of terminal bud dilations at T72 relative to the mean number of terminal bud dilations in the medium-only control group. The number of terminal bud dilations is expressed as the mean ± SEM percentage of buds presenting an increase in diameter to over 2 SD from the mean value for the control group. Two group comparisons were performed in Mann-Whitney U tests. ****P < 0.0001.
Similar effects of ETI were observed in WT mouse lung explants. Incubation with ETI decreased lung branching by 39% ± 12% (NS) at T24, 75% ± 6% (P < 0.0001) at T48, and 79% ± 4% (P < 0.0001) at T72 in WT embryos, relative to incubation with medium alone. The percentage of terminal buds dilated was also significantly higher, by 5.7-fold (±0.3) (P = 0.004) after 72 hours of culture, in the ETI group than in WT embryos incubated with medium alone (Figure E5). Incubation with forskolin reproduced the abnormalities observed with ETI, with a significant decrease in lung branching at all 3 time points tested and a significant increase in the mean percentage of terminal buds displaying dilation for WT embryos incubated with forskolin relative to WT embryos incubated with medium alone (Figure E6).
The addition of Inh-172 to the culture medium, together with forskolin, rescued the lung branching phenotype and terminal bud formation (Figure E7). The concentrations of Fgf10, Fgfr2IIIb, Shh, and Hhip expression in lung explants from E12.5 Cftrtm1Eur/+ and WT embryos treated by ETI were significantly lower than those in the group of explants treated with medium alone. The same expression profile was observed in the presence of forskolin for Fgf10 and Hhip in lung explants of WT embryos, with a partial reversion in the presence of Inh-172. These findings contrast with the increase in Fgfr2IIIb and Shh expression observed after incubation with forskolin (Figure E8 and Table E3).
We show that acute exposure to ETI at the pseudoglandular stage of lung development negatively affects lung branching and results in the formation of abnormal terminal dilations. These abnormalities are at least partly related to CFTR activation, as a similar, albeit milder, morphological pattern is observed in lungs from WT embryos cultured with forskolin, and this phenotype is partially reversed by the addition of the CFTR-specific inhibitor Inh-172 to the medium. In the developing human lung, CFTR expression is detected from Week 12 of gestation onwards (6) and is involved in lung fluid secretion and airway budding, as well as other less known cellular signaling pathways (7, 8, 9). Our data suggest that the pharmacological activation of WT CFTR may contribute to alterations in lung branching and the formation of epithelial cyst-like structures by increasing fluid secretion, as observed in the kidney epithelium (10). In the context of F508 del heterozygosity, this would combine excessive and continuous activation of the WT CFTR and the corrected F508 del CFTR.
These morphological changes also seem to involve other mechanisms, potentially off-target effects of the CFTR modulators, as these drugs decrease transcript concentrations for a number of genes involved in lung development and, more specifically, inhibit FGF10 signaling. In the human lung, defective FGF10 and FGFR2IIIb expression is associated with defective lung branching and cyst-like dilations of terminal buds (11). The SHH signaling pathway acts as a negative-feedback mechanism during lung branching morphogenesis by inhibiting the local FGF10 signal (11). In our dataset, the combined decrease in transcript concentrations for Fgf10 and its receptor, Fgfr2IIIb, after ETI exposure would ultimately lead to a total arrest of lung branching. By contrast, after forskolin treatment, the increase in Fgfr2IIIb expression would be expected to outcompete residual Shh expression, potentially accounting for the partial maintenance of lung branching in this condition.
The early mouse embryonic lung culture model is an extreme model of exposure to ETI. However, our results call for caution because ivacaftor, tezacaftor, and elexacaftor can cross the placenta (12, 13). Schneider and colleagues investigated the placental transfer and fetal concentrations of ivacaftor in the lungs and brain in E19 rats (14). Mean placental diffusion was 40% ± 20%, and the range of concentrations of ivacaftor in lungs revealed 10-fold variation, with a mean value of 12 ng/ml, because of high interindividual degrees of variability in pulmonary diffusion, which ranged from 50% to 410%. On the basis of this high degree of variability, the concentration of ivacaftor used here (100 nMol or 39 ng/ml) can be considered to lie in the same range. However, dose–response studies should be performed to obtain a better understanding of dose–response relationships for these drugs. No data are currently available for the fetal lung concentrations of tezacaftor and elexacaftor after passage across the placenta.
As reported in the U.S. Food and Drug Administration prescribing information for Trikafta (available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212273s004lbl.pdf), the oral administration during organogenesis of elexacaftor, tezacaftor, or ivacaftor to pregnant rats and rabbits, at doses leading to higher concentrations of maternal exposure than the maximum recommended dose for humans, resulted in no teratogenicity or adverse developmental effects. However, no animal studies are currently available concerning the effect of the simultaneous administration of these three drugs. Furthermore, few data are available concerning pregnancy outcomes in patients on Trikafta (2, 15, 16). The results available have revealed no alarming signals, with no obvious bronchial airway defects in the infants. The decision to treat patients with Trikafta during pregnancy is currently taken jointly by patients and physicians (15). Our results suggest ETI exposure during lung development may interfere with bronchial morphogenesis pathways. Even if it does not cause malformations, antenatal exposure may affect the future lung function trajectories of healthy heterozygous carrier infants with expression later in life, as shown by prematurity and maternal smoking, which are associated with abnormal lung growth and a risk of chronic obstructive pulmonary disease in adulthood (17). These findings argue for the close monitoring of such pregnancies and follow-up of the resulting children after birth.
Acknowledgments
Acknowledgment
The authors thank the Animal Core Facility of Institut Necker Enfants Malades.
Footnotes
Supported by Fonds de Recherche en Santé Respiratoire and la Fondation du Souffle (FR2017); the Cancer Research Society (grants. 22054 and 24217 [L.J.]); and Legs Poix 2017 – Chancellerie des Universités de Paris (A.H.).
Author Contributions: Conception and design: I.S.-G. and A.H. Experiments: M.L., L.A., E.D., M.-L.F.-M., K.L.-T., and N.H. Analysis and interpretation: M.L., L.A., E.D., L.J., I.S.-G., and A.H. Drafting the manuscript and revision for important intellectual content: M.L., S.C., A.H., A.E., C.D., L.J., I.S.-G., and A.H.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med . 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Connor KE, Goodwin DL, NeSmith A, Garcia B, Mingora C, Ladores SL, et al. Elexacafator/tezacaftor/ivacaftor resolves subfertility in females with CF: a two center case series. J Cyst Fibros . 2021;20:399–401. doi: 10.1016/j.jcf.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res . 2019;5:00082–2019. doi: 10.1183/23120541.00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Moral P-M, Warburton D.Ward A. Tosh D. Mouse cell culture. Totowa, NJ: Humana Press; 2010. Explant culture of mouse embryonic whole lung, isolated epithelium, or mesenchyme under chemically defined conditions as a system to evaluate the molecular mechanism of branching morphogenesis and cellular differentiation; pp. 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carraro G, del Moral P-M, Warburton D. Mouse embryonic lung culture, a system to evaluate the molecular mechanisms of branching. J Vis Exp . 2010;30:2035. doi: 10.3791/2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boucherat O, Nadeau V, Bérubé-Simard F-A, Charron J, Jeannotte L. Crucial requirement of ERK/MAPK signaling in respiratory tract development. Development . 2015;142:3801. doi: 10.1242/dev.131821. [DOI] [PubMed] [Google Scholar]
- 7. Marcorelles P, Montier T, Gillet D, Lagarde N, Ferec C. Evolution of CFTR protein distribution in lung tissue from normal and CF human fetuses. Pediatr Pulmonol . 2007;42:1032–1040. doi: 10.1002/ppul.20690. [DOI] [PubMed] [Google Scholar]
- 8. McCray PB, Jr, Bettencourt JD, Bastacky J, Denning GM, Welsh MJ. Expression of CFTR and a cAMP-stimulated chloride secretory current in cultured human fetal alveolar epithelial cells. Am J Respir Cell Mol Biol . 1993;9:578–585. doi: 10.1165/ajrcmb/9.6.578. [DOI] [PubMed] [Google Scholar]
- 9. Meyerholz DK, Stoltz DA, Gansemer ND, Ernst SE, Cook DP, Strub MD, et al. Lack of cystic fibrosis transmembrane conductance regulator disrupts fetal airway development in pigs. Lab Invest . 2018;98:825–838. doi: 10.1038/s41374-018-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Yang W, Mendes F, Amaral MD, Sheppard DN. Impact of the cystic fibrosis mutation F508del-CFTR on renal cyst formation and growth. Am J Physiol Renal Physiol . 2012;303:F1176–F1186. doi: 10.1152/ajprenal.00130.2012. [DOI] [PubMed] [Google Scholar]
- 11. Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development . 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 12. Trimble A, McKinzie C, Terrell M, Stringer E, Esther CR., Jr Measured fetal and neonatal exposure to lumacaftor and ivacaftor during pregnancy and while breastfeeding. J Cyst Fibros . 2018;17:779–782. doi: 10.1016/j.jcf.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins B, Fortner C, Cotey A, et al. Drug exposure to infants born to mothers taking elexacaftor, tezacaftor, and ivacaftor. J Cyst Fibros . 2022;21:725–727. doi: 10.1016/j.jcf.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu F, Habgood M, Schneider-Futschik EK. The Balance between the safety of mother, fetus, and newborn undergoing cystic fibrosis transmembrane conductance regulator treatments during pregnancy. ACS Pharmacol Transl Sci . 2020;3:835–843. doi: 10.1021/acsptsci.0c00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jain R, Taylor-Cousar JL. Fertility, pregnancy and lactation considerations for women with CF in the CFTR modulator era. J Pers Med . 2021;11:418. doi: 10.3390/jpm11050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kendle AM, Roekner JT, Santillana EC, Kis LE, Cain MA. Cystic fibrosis transmembrane conductance regulator modulators during pregnancy: a case series. Cureus . 2021;13:e17427. doi: 10.7759/cureus.17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med . 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]