Abstract
Chronic granulomatous disease (CGD) is an inherited disorder of the NADPH oxidase in which phagocytes are defective in generating superoxide and downstream microbicidal reactive oxidants, leading to recurrent life-threatening bacterial and fungal infections. Xanthine oxidase (XO) is another enzyme known to produce superoxide in many tissues. Using the p47phox−/− mouse model of CGD, we evaluated the residual antibacterial activity of XO. Clearance of Burkholderia cepacia, a major pathogen in CGD, was reduced in p47phox−/− mice compared to that in wild-type mice and was further inhibited in p47phox−/− mice by pretreatment with the specific XO inhibitor allopurinol. Hepatic B. cepacia burden was similar in the two genotypes, but allopurinol significantly reduced net hepatic killing and killing efficiency only in p47phox−/− mice. Clearance and killing of intravenous Escherichia coli was intact in p47phox−/− mice and was unaffected by pretreatment with allopurinol. In CGD, XO may contribute to host defense against a subset of reactive oxidant-sensitive pathogens.
Chronic granulomatous disease (CGD) is an inherited disorder of the NADPH oxidase complex in which phagocytes are defective in generating superoxide anion and the downstream reactive oxidant products, hydrogen peroxide, hypohalous acid, and hydroxyl radical (2, 10, 30, 37, 38). As a result of the defect in this key host defense pathway, CGD patients suffer from recurrent life-threatening infections caused by catalase-positive bacteria and fungi (1, 27, 31, 33, 35).
NADPH oxidase is localized on the membranes of secondary granules in neutrophils and can be displayed on the phagocytic vacuoles and plasma membranes of myeloid cells (neutrophils, monocytes, and eosinophils). In response to phagocytosis and the chemoattractants interleukin 8, leukotriene B4, C5a, and platelet-activating factor, as well as nonphysiologic stimuli such as phorbol myristate acetate or the calcium ionophore A23817, a rapid and dramatic increase in oxygen consumption, termed the “respiratory burst,” whereby molecular oxygen is reduced to superoxide, occurs.
Xanthine oxidase (XO) is a ubiquitous enzyme involved in purine catabolism that, when activated from its constitutively expressed precursor, xanthine dehydrogenase, generates superoxide anion under certain circumstances. Moreover, in the presence of Fe3+, XO can generate a highly potent oxidant that behaves like the hydroxyl anion, via a Fenton reduction (13, 20). The role of XO in host defense has been supported by in vitro studies using isolated macrophages and Kupffer cells (28, 36) and by exacerbations of experimental infections in which animals received XO inhibitors (39, 40). However, the relative role of XO in superoxide generation has been difficult to determine because the NADPH oxidase system usually generates much more superoxide from phagocytes.
We hypothesized that in the absence of a functional NADPH oxidase, the role of XO in host defense against certain pathogens might be revealed. We have generated a knockout mouse model of CGD in which the p47phox subunit of the NADPH oxidase has been disrupted (15). Phagocytes from these mice are incapable of generating measurable reactive oxidants, and the mice are susceptible to a spectrum of pathogens similar to that to which CGD patients are susceptible (5, 15, 18). These mice are ideally suited to evaluating the relative contributions of the NADPH oxidase and XO superoxide generating systems.
p47phox−/− mice were generated as previously described (15). p47phox−/− and wild-type littermates (C57BL/6 × 129) were backcrossed five generations in the C57BL/6 lineage and maintained in specific pathogen-free conditions at the Taconic facility (Germantown, N.Y.). Experiments were conducted at the animal care facility at Johns Hopkins University School of Medicine (Baltimore, Md.). Mice were housed in a light-cycled, virus-isolated room with free access to chow and water. All experiments were conducted in accord with National Institutes of Health guidelines by a protocol preapproved by the Animal Care and Use Committee of Johns Hopkins University School of Medicine. Mice were age (10 to 20 weeks) and sex matched in each set of experiments.
p47phox−/− and wild-type mice were challenged with either Escherichia coli or Burkholderia cepacia, a virulent pathogen in patients with CGD that is highly sensitive to superoxide-derived reactive oxidants (24, 34). Hepatic phagocytic clearance and killing were then measured.
We have previously described an in vivo assay that discriminantly quantitates hepatic phagocytic clearance and intracellular killing of bacterial targets double-labeled with 51Cr and 125I (16, 17). Briefly, an ampicillin-resistant XL1-BLUE strain of E. coli (American Type Culture Collection, Rockville, Md.) and a clinical isolate of B. cepacia from a CGD patient were grown on Mueller-Hinton agar, transferred to Trypticase soy broth (Beckton Dickinson, Cockeysville, Md.), and labeled with 0.1 mCi of 5-[125I]iodo-2′-deoxyuridine (125I-UdR; Amersham, Arlington Heights, Ill.). Cultures were incubated for 18 h at 37°C and then washed three times with normal saline. The 125I-labeled bacteria were pelleted and then incubated with 0.05 mCi of sodium chromate (Na251CrO4, CIS-11; Amersham). The washed suspension, stored for up to 4 h at 4°C, contained less than 2% free 51Cr and 125I-UdR. The bacterial concentration in suspension was determined by optical density (λ = 600 nm) following calibration with bacterial colony counts from serial 10-fold dilutions from a stock suspension. We have previously confirmed that these labels remain bound to the target bacteria (51Cr in the cytoplasm and 125I-UdR in the DNA) for at least 90 min, in vivo as well as in vitro (16). In previous studies in which mice and rats were inoculated intravenously with double-labeled E. coli, hepatic 51Cr and 125I-UdR content accurately reflected the hepatic uptake (phagocytosis) of the bacteria and the residual number of viable hepatic bacteria, respectively, as measured by quantitative culture (16).
The mice were administered the specific XO inhibitor allopurinol (50 mg/kg of body weight by gavage in 0.5 ml of dry milk suspended in normal saline) or vehicle only (an equal volume) at 36, 24, and 2 h prior to bacterial challenge. The mice were anesthetized with methoxyflurane, and double-labeled E. coli or B. cepacia (4 × 108 CFU) (n = 8 or 9 mice per group) was administered through the jugular vein. Previous studies in our laboratory have indicated that the optimal discrimination of hepatic radioisotope content is usually seen 90 min after bacterial challenge. Thus, at 90 min, mice were euthanized by cervical dislocation, their livers were removed, and hepatic levels of 51Cr and 125I were determined by 1 min of gamma counting using 50- to 175-kev and 0- to 20-kev windows, respectively. Total hepatic 51Cr content and 125I content were calculated and expressed as percentages of the total initial inoculum. To compare the degree of intrinsic hepatic bacterial killing under conditions of variable phagocytic uptake, the hepatic killing efficiency was calculated as follows: (hepatic 51Cr level − hepatic 125I level) × 100/hepatic 51Cr level. Hepatic killing efficiency therefore reflects the percentage of organisms trapped in the liver that are subsequently killed there. All experiments and data analysis were conducted in a blinded fashion with regard both to the genotype of mice and to allopurinol administration. Statistical significance was determined with the unpaired Student t test. A P value of 0.05 was considered significant.
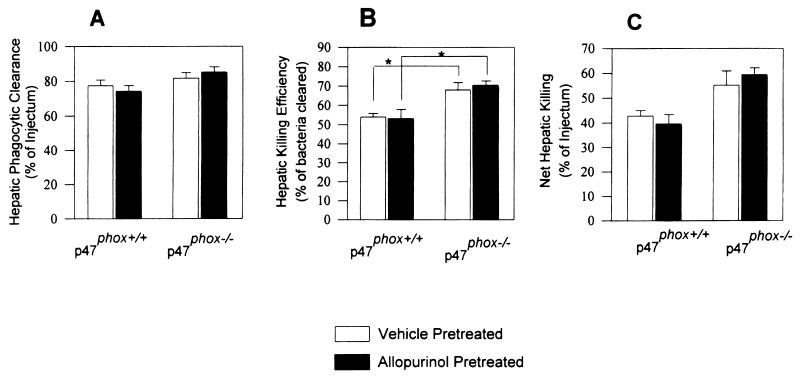
There was no significant difference in hepatic phagocytic clearance of E. coli between p47phox−/− and wild-type mice (Fig. 1). Net hepatic killing and efficiency of hepatic killing of E. coli were both modestly but significantly enhanced in p47phox−/− mice compared with those in the wild-type mice. Pretreatment with allopurinol did not significantly affect either phagocytic clearance or killing of E. coli in p47phox−/− or in wild-type mice.
FIG. 1.
Hepatic phagocytic clearance (A), hepatic killing efficiency (B), and net hepatic killing (C) of E. coli. p47phox−/− (wild-type) and p47phox−/− (homozygous p47phox-deficient) mice were pretreated with allopurinol or vehicle only, followed by intravenous E. coli challenge. Net hepatic killing and hepatic killing efficiency in p47phox−/− were both modestly increased compared to those in wild-type mice (P < 0.05). Pretreatment with allopurinol had no significant effect on hepatic clearance or killing in either genotype. There were 8 or 9 mice per genotype per treatment group. Values are means ± standard deviations. Asterisks indicate P < 0.05.
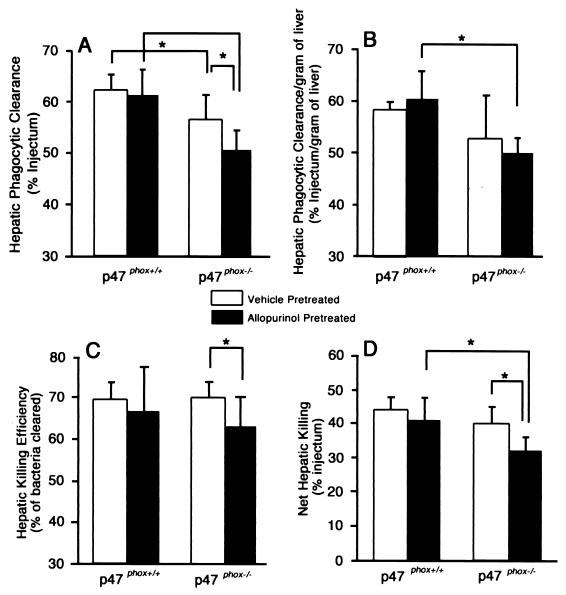
Following B. cepacia challenge, phagocytic clearance was significantly reduced in p47phox−/− mice compared to that in wild-type mice (P < 0.05), and pretreatment with allopurinol further increased the disparity between the two genotypes (Fig. 2). Net hepatic killing and hepatic killing efficiency were similar in p47phox−/− and wild-type mice pretreated with vehicle. In contrast, while allopurinol had no significant effect on either phagocytosis or killing of B. cepacia in wild-type mice, it significantly reduced phagocytic clearance, net hepatic killing, and hepatic killing efficiency in p47phox−/− mice. Phagocytosis and intracellular killing of B. cepacia were not affected by allopurinol pretreatment in commercial wild-type C57BL/6 mice (data not shown).
FIG. 2.
Hepatic phagocytic clearance (A), hepatic phagocytic clearance per gram of liver tissue (B), hepatic killing efficiency (C), and net hepatic killing (D) in wild-type and p47phox−/− mice challenged with B. cepacia. Hepatic phagocytic clearance of B. cepacia in p47phox−/− mice was decreased compared to that in wild-type mice, and this disparity was increased with XO inhibition by allopurinol. Hepatic killing efficiency and net hepatic killing were similar in untreated mice of the two genotypes. However, allopurinol pretreatment significantly reduced both hepatic killing efficiency and net hepatic killing in p47phox−/− mice but not in wild-type mice. There were 8 or 9 mice per genotype per treatment group. Values are means ± standard deviations. Asterisks indicate P < 0.05.
Based on the clinical experience with CGD, it is clear that NADPH oxidase constitutes a critical host defense pathway. We have previously posited that XO may also contribute to host defense in part by triggering a microvascular inflammatory response, leading to recruitment of neutrophils and trapping of circulating organisms (3, 4, 29). The proinflammatory mediators tumor necrosis factor alpha (TNF-α) C5a, and N-formyl peptide cause the rapid conversion (activation) of xanthine dehydrogenase to XO in cultured endothelial cells (9). Hepatic XO has been documented in hepatocytes, Kupffer cells, and the microvascular endothelium (25). In postischemic rat livers, rapid accumulation of neutrophils, centrolobular necrosis, and xanthine dehydrogenase-to-XO conversion all occurred most prominently in the pericentral sinusoids of the liver, a pattern that corresponds to the hepatic distribution of XO (19). Both neutrophil accumulation and hepatocellular injury were attenuated by XO inhibition by pretreatment with allopurinol (19). Taken together, these studies suggest that XO has a role in neutrophil recruitment and injury under various experimental conditions. In nature, XO may serve as a rapid, but perhaps redundant, trigger of reticuloendothelial function in response to bacteremia (3, 4).
In vitro studies using peritoneal macrophages and isolated Kupffer cells have indicated that the pharmacologic inhibition of either NADPH oxidase or XO causes a reduction in intracellular killing of Candida parapsilosis (28, 36). In vivo, XO inhibition caused increased mortality in mice challenged with E. coli, Klebsiella pneumoniae, or Plasmodium berghei (39). Umezawa et al. (40) reported that inhibition of either XO or the inducible isoform of nitric oxide synthase in mice challenged with Salmonella enterica serovar Typhimurium led to increased mortality and increased hepatic bacterial burden.
Host defense against B. cepacia is critically dependent on the NADPH oxidase. Speert et al. (34) reported that neutrophils from CGD patients had markedly defective in vitro bactericidal activity against B. cepacia, as did normal neutrophils mixed with the oxidant scavengers superoxide dismutase and catalase. B. cepacia is highly virulent in p47phox−/− mice, and stem cell-directed gene therapy, which restored NADPH oxidase function to a small proportion of myeloid cells, conferred protection against B. cepacia challenge (18).
In the current study, net hepatic killing efficiency and net hepatic killing were similar in p47phox−/− and wild-type mice. In CGD patients, B. cepacia infection is usually limited to the lungs and, in our experience, has never involved the liver. Moreover, intraperitoneal challenge with B. cepacia causes bacteremia and pneumonia without evidence of extrapulmonary organ involvement in p47phox−/− mice (unpublished observations). These findings suggest that local organ or tissue-specific factors are relevant in host defense in CGD and influence where sites of infection are likely to occur. We also found that hepatic phagocytic clearance, an indicator of phagocytosis, was modestly impaired in p47phox−/− mice. Impaired phagocytosis has not been observed in neutrophils from CGD patients based on in vitro bactericidal assays. We speculate that under more physiologic conditions, reactive oxidants may have a modest effect on Kupffer cell phagocytosis, perhaps through a cell-signaling function, leading to increased local expression of proinflammatory cytokines and chemokines, resulting in Kupffer cell activation and/or recruitment of circulating neutrophils (19).
We have therefore found that inhibition of XO can exacerbate the NADPH oxidase defect, presumably by suppression of an alternative pathway for superoxide generation. Another possible explanation is that allopurinol may reduce neutrophil accumulation within the liver (19). This finding raises the question of whether augmentation of XO function may be beneficial either as prophylaxis or as adjunctive therapy in CGD and whether therapy with allopurinol, as for gout, could be dangerous in CGD. These data also suggest the possibility that the XO system is redundant and supplementary to the NADPH oxidase system for the generation of oxidant-mediated phagocytic killing in normal hosts.
Prophylactic interferon-γ, a proinflammatory cytokine that activates macrophages, has been shown to reduce the rate of infections in CGD patients (14). Interferon-γ augments oxidative function in monocytes (22, 23) and enhances various oxidant-independent antimicrobial pathways, such as TNF-α production, tryptophan metabolism, granule protein synthesis, major histocompatibility complex class II expression, Fcγ receptor I expression and improved Fcγ-receptor-mediated phagocytosis (11, 32). Interferon-γ has not been shown to improve NADPH oxidase function or increase levels of its constituent proteins in patients with CGD (14, 21, 41). Interferons are known to augment xanthine dehydrogenase (the precursor of XO) gene expression in various cell cultures (6, 8, 26) and XO activity in vivo in several tissues in mice (7, 12). It is possible that part of the benefit of prophylactic interferon-γ in CGD may result from potentiation of the XO oxidant-generating system.
Acknowledgments
This work was supported by NIH grant no. DK31764 to Gregory B. Bulkley.
REFERENCES
- 1.Abati A, Cajigas A, Holland S M, Solomon D. Chronic granulomatous disease of childhood: respiratory cytology. Diagn Cytopathol. 1996;15:98–102. doi: 10.1002/(SICI)1097-0339(199608)15:2<98::AID-DC3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Babior B M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 3.Bulkley G B. Physiology of reactive oxidant-mediated signal transduction: an overview. Biochem Soc Trans. 1997;25:804–812. doi: 10.1042/bst0250804. [DOI] [PubMed] [Google Scholar]
- 4.Bulkley G B. Reactive oxygen metabolites and reperfusion injury: aberrant triggering of reticuloendothelial function. Lancet. 1994;344:934–936. doi: 10.1016/s0140-6736(94)92276-4. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y C, Segal B H, Holland S M, Miller G F, Kwon-Chung K J. Virulence of catalase-deficient Aspergillus nidulans in p47phox−/− mice. Implications for fungal pathogenicity and host defense in chronic granulomatous disease. J Clin Investig. 1998;101:1843–1850. doi: 10.1172/JCI2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont G P, Huecksteadt T P, Marshall B C, Ryan U S, Michael J R, Hoidal J R. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Investig. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faggioni R, Gatti S, Demitri M T, Delgado R, Echtenacher B, Gnocchi P, Heremans H, Ghezzi P. Role of xanthine oxidase and reactive oxygen intermediates in LPS- and TNF-induced pulmonary edema. J Lab Clin Med. 1994;123:394–399. [PubMed] [Google Scholar]
- 8.Falciani F, Ghezzi P, Terao M, Cazzaniga G, Garattini E. Interferons induce xanthine dehydrogenase gene expression in L929 cells. Biochem J. 1992;285:1001–1008. doi: 10.1042/bj2851001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedl H P, Till G O, Ryan U S, Ward P A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989;3:2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- 10.Gallin J I, Buescher E S, Seligmann B E, Nath J, Gaither T, Katz P. NIH conference. Recent advances in chronic granulomatous disease. Ann Intern Med. 1983;99:657–674. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- 11.Gallin J I, Farber J M, Holland S M, Nutman T B. Interferon-gamma in the management of infectious diseases. Ann Intern Med. 1995;123:216–224. doi: 10.7326/0003-4819-123-3-199508010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ghezzi P, Bianchi M, Mantovani A, Spreafico F, Salmona M. Enhanced xanthine oxidase activity in mice treated with interferon and interferon inducers. Biochem Biophys Res Commun. 1984;119:144–149. doi: 10.1016/0006-291x(84)91630-9. [DOI] [PubMed] [Google Scholar]
- 13.Henderson W R, Klebanoff S J. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J Biol Chem. 1983;258:13522–13527. [PubMed] [Google Scholar]
- 14.The International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 15.Jackson S H, Gallin J I, Holland S M. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein A, Zhadkewich M, Margolick J, Winkelstein J, Bulkley G. Quantitative discrimination of hepatic reticuloendothelial clearance and phagocytic killing. J Leukoc Biol. 1994;55:248–252. doi: 10.1002/jlb.55.2.248. [DOI] [PubMed] [Google Scholar]
- 17.Klein A S, Zhadkevich M, Wang D, Margolick J B, Winkelstein J A, Bulkley G B. Discriminant quantitation of posttransplant hepatic reticuloendothelial function. The impact of ischemic preservation. Transplantation. 1996;61:1156–1161. doi: 10.1097/00007890-199604270-00006. [DOI] [PubMed] [Google Scholar]
- 18.Mardiney M, III, Jackson S H, Spratt S K, Li F, Holland S M, Malech H L. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood. 1997;89:2268–2275. [PubMed] [Google Scholar]
- 19.Mayumi T, Chan C K, Clemens M G, Bulkley G B. Zonal heterogeneity of hepatic injury following shock/resuscitation: relationship of xanthine oxidase activity to localization of neutrophil accumulation and central lobular necrosis. Shock. 1996;5:324–332. [PubMed] [Google Scholar]
- 20.Miles A M, Bohle D S, Glassbrenner P A, Hansert B, Wink D A, Grisham M B. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996;271:40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- 21.Muhlebach T J, Feickert H J, Welte K, Seger R A. Granulocyte-macrophage colony stimulating factor does not improve neutrophil oxidative metabolism in a patient with variant X-linked chronic granulomatous disease. Eur J Pediatr. 1991;150:575–578. doi: 10.1007/BF02072210. [DOI] [PubMed] [Google Scholar]
- 22.Nathan C F, Horowitz C R, de la Harpe J, Vadhan-Raj S, Sherwin S A, Oettgen H F, Krown S E. Administration of recombinant interferon gamma to cancer patients enhances monocyte secretion of hydrogen peroxide. Proc Natl Acad Sci USA. 1985;82:8686–8690. doi: 10.1073/pnas.82.24.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan C F, Kaplan G, Levis W R, Nusrat A, Witmer M D, Sherwin S A, Job C K, Horowitz C R, Steinman R M, Cohn Z A. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med. 1986;315:6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- 24.O'Neil K M, Herman J H, Modlin J F, Moxon E R, Winkelstein J A. Pseudomonas cepacia: an emerging pathogen in chronic granulomatous disease. J Pediatr. 1986;108:940–942. doi: 10.1016/s0022-3476(86)80934-9. [DOI] [PubMed] [Google Scholar]
- 25.Parks D A, Granger D N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- 26.Pfeffer K D, Huecksteadt T P, Hoidal J R. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J Immunol. 1994;153:1789–1797. [PubMed] [Google Scholar]
- 27.Pogrebniak H W, Gallin J I, Malech H L, Baker A R, Moskaluk C A, Travis W D, Pass H I. Surgical management of pulmonary infections in chronic granulomatous disease of childhood. Ann Thorac Surg. 1993;55:844–849. doi: 10.1016/0003-4975(93)90103-o. [DOI] [PubMed] [Google Scholar]
- 28.Potoka D A, Takao S, Owaki T, Bulkley G B, Klein A S. Endothelial cells potentiate oxidant-mediated Kupffer cell phagocytic killing. Free Radic Biol Med. 1998;24:1217–1227. doi: 10.1016/s0891-5849(97)00453-x. [DOI] [PubMed] [Google Scholar]
- 29.Qu X W, Rozenfeld R A, Huang W, Bulkley G B, Hsueh W. The role of xanthine oxidase in platelet activating factor induced intestinal injury in the rat. Gut. 1999;44:203–211. doi: 10.1136/gut.44.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos D. The genetic basis of chronic granulomatous disease. Immunol Rev. 1994;138:121–157. doi: 10.1111/j.1600-065x.1994.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 31.Ross J P, Holland S M, Gill V J, DeCarlo E S, Gallin J I. Severe Burkholderia (Pseudomonas) gladioli infection in chronic granulomatous disease: report of two successfully treated cases. Clin Infect Dis. 1995;21:1291–1293. doi: 10.1093/clinids/21.5.1291. [DOI] [PubMed] [Google Scholar]
- 32.Schiff D E, Rae J, Martin T R, Davis B H, Curnutte J T. Increased phagocyte Fc gammaRI expression and improved Fcγ-receptor-mediated phagocytosis after in vivo recombinant human interferon-γ treatment of normal human subjects. Blood. 1997;90:3187–3194. [PubMed] [Google Scholar]
- 33.Segal B H, DeCarlo E S, Kwon-Chung K J, Malech H L, Gallin J I, Holland S M. Aspergillus nidulans infection in chronic granulomatous disease. Medicine. 1998;77:345–354. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Speert D P, Bond M, Woodman R C, Curnutte J T. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J Infect Dis. 1994;170:1524–1531. doi: 10.1093/infdis/170.6.1524. [DOI] [PubMed] [Google Scholar]
- 35.Sponseller P D, Malech H L, McCarthy E F, Jr, Horowitz S F, Jaffe G, Gallin J I. Skeletal involvement in children who have chronic granulomatous disease. J Bone Jt Surg Am Vol. 1991;73:37–51. [PubMed] [Google Scholar]
- 36.Takao S, Smith E H, Wang D, Chan C K, Bulkley G B, Klein A S. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocytic killing. Am J Physiol. 1996;271:C1278–C1284. doi: 10.1152/ajpcell.1996.271.4.C1278. [DOI] [PubMed] [Google Scholar]
- 37.Tauber A I, Borregaard N, Simons E, Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine. 1983;62:286–309. [PubMed] [Google Scholar]
- 38.Thrasher A J, Keep N H, Wientjes F, Segal A W. Chronic granulomatous disease. Biochim Biophys Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 39.Tubaro E, Lotti B, Santiangeli C, Cavallo G. Xanthine oxidase increase in polymorphonuclear leucocytes and macrophages in mice in three pathological situations. Biochem Pharmacol. 1980;29:1945–1948. doi: 10.1016/0006-2952(80)90108-2. [DOI] [PubMed] [Google Scholar]
- 40.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodman R C, Erickson R W, Rae J, Jaffe H S, Curnutte J T. Prolonged recombinant interferon-gamma therapy in chronic granulomatous disease: evidence against enhanced neutrophil oxidase activity. Blood. 1992;79:1558–1562. [PubMed] [Google Scholar]