Abstract
Background
Many cases of monkeypox have been reported across Europe from early May 2022 onward. Initial publications suggest that nearly all of the affected persons to date have been men who have sex with men (MSM).
Methods
To characterize the German cases, an anonymous questionnaire was sent via the mailing lists of the German AIDS Society (Deutsche AIDS-Gesellschaft, DAIG) and the German Association of Outpatient Physicians for Infectious Diseases and HIV Medicine (Deutsche Arbeitsgemeinschaft ambulant tätiger Ärztinnen und Ärzte für Infektionskrankheiten und HIV-Medizin e.V., DAGNAE).
Results
301 PCR-verified cases had been registered as of 23 June 2022. All of the affected persons were MSM, including 141 (46.7%) with HIV infection and 135 (44.7%) with pre-exposure prophylaxis (PrEP). The great majority of skin lesions were in the anal and genital areas. The most common general symptoms were fever, headache, limb pain, and, often, painfully swollen lymph nodes. Most infections to date have taken a relatively mild course: 5.0% of the patients were hospitalized, and none died. A high frequency of sexually transmitted infections (STI) was noted: only 41.0% of the patients had not been given a diagnosis of an STI in the six months before their monkeypox infection.
Conclusion
Monkeypox seems to be establishing itself as a new type of STI among MSM. In view of the rising case numbers, there is a need for a rapid information and vaccination campaign in the population at risk. Heightened alertness among physicians is needed as well.
Monkeypox virus (MPXV) belongs to the genus orthopoxvirus. The natural hosts of the monkeypox virus are rodents; monkeys and humans are considered false hosts. The first case of monkeypox in humans was observed in 1970 in a nine-year-old boy in the Democratic Republic of Congo (1). Since then, monkeypox has mostly been identified in people from Central and West African countries. In recent years, sporadic outbreaks outside Africa was limited to a few cases, for example in the United Kingdom and Israel (2). This changed in 2022. Unusually high numbers of cases are now being reported from all over Europe. Initial publications suggest that, to date, almost exclusively men who have sex with men (MSM) are affected (3). In Germany, the first case from Munich was published on May 20 (4). By June 17, the Robert Koch Institute (RKI) was aware of 338 confirmed cases; a week later, this number had doubled to 676 (5).
Transmission of MPXV takes place from person to person through close physical contact with possibly not only symptomatic but also infected asymptomatic individuals. Smear and droplet infections are possible. The reasons for the current increase in case numbers are still unclear, and there are still unanswered questions about the transmission. Initial genome sequence data suggest a close relationship with the MPXV variant circulating in West Africa (6– 8). This one possibly causes milder disease and may have lower mortality rates than the variant first described in Central Africa (9, 10). However, in a cohort from Nigeria, from where relevant case numbers with the West African variant have been continually reported since 2017, case-mortality was 6%, and among the deaths were predominantly male and female patients with AIDS (11). Little is known so far about other risk factors for severe diseases courses; probably children and pregnant women in particular have an increased risk (12).
In view of the unexpected dynamics of the outbreak, the aim of the present article was to report on the affected patient population and clinical presentation.
Methods
Registered centers were contacted via the distribution lists of the German AIDS Society (DAIG) and the German Association of Outpatient Physicians for Infectious Diseases and HIV Medicine (DAGNAE) and invited to participate. The centers were encouraged to retrospectively document from May 19 to June 23, 2022, if possible, all cases from 2022 confirmed by polymerase chain reaction (PCR) from various swab samples. The anonymized, one-page questionnaire comprised questions on demographics (age, sex, ethnicity) and comorbidities, including possible HIV infection (stating current CD4 cell counts and HIV RNA copies/mL). Any previous viral hepatitis (hepatitis B and hepatitis C) was asked, as were sexually transmitted infections (STIs) in the previous six months. The date of diagnosis of monkeypox virus infection, probable country of infection, symptom onset, site and number of lesions were ascertained, as were the results of any throat swabs, previous smallpox vaccinations, and severity of monkeypox disease, including the number of lesions (categorized), as well as possible general symptoms such as fever, headache/aching limbs, night sweats, and lymph node swellings. Any specific treatments and any hospitalizations with their main reason were also asked. Virological PCR diagnostics were performed on a decentralized basis in the partner laboratories of participating centers and/or in the Consultant Laboratory for Poxviruses at the Robert Koch Institute. Statistical methods in the present article are limited to purely descriptive analyses. All patients gave their written informed consent for the clinical Figures. In cases of the same age, nationality and city of entry, a check was made for double entries. The study was submitted to the Ethics Committee of the Technical University of Munich for review prior to starting, in accordance with Section 67 (6) of the German Medicines Act (AMG), and approval was granted (file number 2022–338-S-NP).
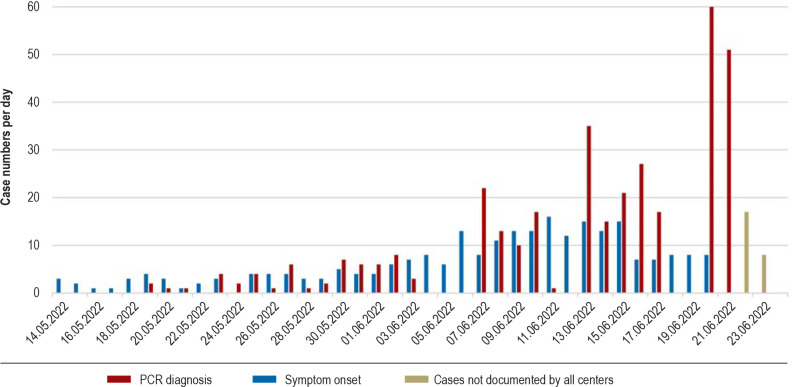
Figure 1.
Trend in case numbers per day
The exact date of symptom onset was not documented in all patients.
Results
As of June 23, 2022, a total of 301 PCR-confirmed cases had been reported and recorded from 32 centers (after excluding five double-reported cases). By far the most cases were reported from Berlin with 161 (53.3%), followed by Hamburg with 43 (14.2%) and Cologne with 29 (9.6%). Others came from Frankfurt (17), Munich (13), Düsseldorf (12), Ulm (5), Bonn (5), Stuttgart (4), Freiburg (4), Essen (3), Aachen (2), and Braunschweig, Magdeburg and Waake/Göttingen with one case each.
Figure 1 shows the number of cases confirmed by PCR per day. With a total of 227 (75.4%), the vast majority of patients had probably acquired the MPXV infection by autochthonous transmission in Germany. A total of 25 patients (8.3%) had acquired the infection in Spain, the majority in Gran Canaria. Many of these cases were primarily seen early in the outbreak; for example, among those cases diagnosed by May 30, more than one-third (11/31) cited Spain as the source of infection.
Affected groups, prevalent co-infections
The affected individuals were exclusively MSM, including 141 (46.7%) living with HIV, of whom the vast majority had normal immune status and adequate viral suppression on antiretroviral therapy (ART). Median CD4 cell counts were 691/µL (range 275–1603 cells/µL). Seven patients in total were viremic at their last test, with their HIV RNA above 50 copies/mL, including only three with HIV RNA above 200 copies/mL. In the case with the highest viral load, the HIV infection was diagnosed at the time of the presenting MPXV infection. The second largest affected group, with 135 (44.7%) cases, were MSM without HIV infection who were receiving pre-exposure prophylaxis (PrEP). The remaining 25 cases (8.3%) were MSM with no HIV infection and no PrEP. No heterosexual men, and no women or children were affected in our cohort. Characteristics and co-infections are listed in Table 1.
Table 1. Demographic characteristics and presenting co-infections*.
| Case history details | Values |
| Age in years, median (range) | 39 (20–64) |
| Male sex, % (n) | 100 % (301/301) |
| Ethnicity Caucasian, % (n) | 85.9 % (231/269) |
| Nationality German, % (n) | 72.5 % (166/229) |
| Previous smallpox vaccination documented, % (n) | 12.5 % (28/224) |
| HIV infection | |
| documented HIV infection, % (n) | 46.8 % (141/301) |
| CD4 cells ≥500/μL, % (n) | 80.3 % (102/127) |
| CD4 cells 350–499/μL, % (n) | 16.5 % (21/127) |
| CD4 cells <350/μl, % (n) | 3.1 % (4/127) |
| HIV RNA <50 copies/ml, % (n) | 94.6 % (123/130) |
| HIV RNA ≥50–200 copies/mL, % (n) | 3.1 % (4/130) |
| HIV RNA ≥200 copies/mL, % (n) | 2.3 % (3/130) |
| Hepatitis B infection | |
| anti-HBc negative, % (n) | 88.3 % (234/265) |
| anti-HBc positive, HBsAG negative, % (n) | 10.9 % (29/265) |
| HBsAG positive, % (n) | 0.8 % (2/265) |
| Hepatitis C infection | |
| anti-HCV negative, % (n) | 85.7 % (87/273) |
| anti-HCV positive, HCV-PCR negative, % (n) | 11.0 % (30/273) |
| anti-HCV positive, HCV-PCR positive, % (n) | 0.4 % (1/273) |
* Note: Entries vary because not all information was available from all patients.
The median age of the patients was 39 years. Only a total of 26 (8.6%) patients were either under 25 (n = 6) or over 55 years of age (n = 20). Only three patients were over the age of 60. Altogether, 12.5% were documented as having been vaccinated against smallpox at least once. The majority of patients were of German nationality and Caucasian ethnicity. Relevant non-infectious and somatic comorbidities were present in very few patients (n = 11), namely psoriatic arthritis in three cases, and diabetes mellitus, bronchial asthma, arterial hypertension in two cases each. The proportion of patients who had suffered another STI within the previous six months was quite considerable (figure 2). More than half were diagnosed within the previous four weeks before MPXV infection or at the same time and most commonly with gonorrhea. Only 41.0% were not diagnosed with an STI within six months prior to MPXV infection.
Figure 2.

Percentage of documented sexually transmitted infections (STIs) in the past 6 months (acute = diagnosed within the past four weeks or concurrent with MPXV infection, subacute = diagnosed in the past six months but not acute).
Clinical presentation
Because a large proportion of infections were only diagnosed in the last few days of the study, the observation period was very short, with a median of three days (range 0–35 days). Only 130 patients had a follow-up of at least five days. The median time between symptom onset and MPXV diagnosis was four days (range 0–21 days). It is therefore to be expected that in subsequent days some of the cases will present themselves in an even more severe condition than before. Nevertheless, the disease characteristics will be briefly outlined below.
Most of the diseases so far appear to have been relatively mild. No deaths were reported. A total of 18 patients were admitted to hospital as inpatients, of whom at least three were clearly not due to the severity of the clinical picture or (imminent) complications but for quarantine reasons or for diagnostic purposes. A total of 15 hospitalizations (5.0% of all patients, 8.4% in patients with an observation period of at least five days) occurred because of the severity of the clinical picture or due to complications. These were predominantly massive lymph node and genital swellings, extensive involvement of the entire skin, bleeding, or due to refractory pain that could not be controlled on an outpatient basis, especially in cases of anal involvement. The average length of hospital stay was four days (range 3–6 days); however, seven of the 15 patients were still in hospital at the end of the observation period. At least one hospitalized patient received antiviral therapy with tecovirimat (off-label).
Disease manifestations are listed in Table 2. Three cases remained without lesions and were diagnosed during STI screening. By far the majority of the monkeypox lesions manifested anally or genitally. In only 36 (12.0%) cases was there neither genital nor anal involvement. Other sites such as trunk or extremities were also affected in just under one half of cases. Only 16 patients (5%) had disease without any genital, anal, or oral manifestations. Only few patients had extensive findings with 50 or more lesions. These rates changed insignificantly when the analysis was restricted to cases with at least 5 or 10 days of illness (data not presented). The most common general symptoms were fever, headache, and aching limbs, and often painful swelling of the lymph nodes.
Table 2. Disease manifestations*.
| Case history details | Values |
| Site | |
| Genital, % (n) | 49.0 % (146/298) |
| of which exclusively genital, % (n) | 45.2 % (66/146) |
| Anal, % (n) | 50.8 % (152/299) |
| of which exclusively anal, % (n) | 35.5 % (54/152) |
| Oral, perioral, head, % (n) | 24.4 % (72/296) |
| of which exclusively oral/head, % (n) | 33.3 % (24/72) |
| Extremities and/or trunk, % (n) | 41.7 % (122/292) |
| of which exclusively, % (n) | 13.1 % (16/122) |
| Number of lesions | |
| none (only positive swabs), % (n) | 1.1 % (3/279) |
| 1–3, % (n) | 44.1 % (123/279) |
| 4–10, % (n) | 33.3 % (93/279) |
| 11–50, % (n) | 18.3 % (51/279) |
| >50, % (n) | 3.2 % (9/279) |
| General symptoms | |
| Fever, % (n) | 61.3 % (168/274) |
| Headache and aching limbs, % (n) | 46.7 % (126/270) |
| Night sweats, % (n) | 19.9 % (53/266) |
| Lymph node swelling, % (n) | 44.1 % (116/263) |
* in each case, only cases with available information listed
Discussion
In the present retrospective study, a total of 301 cases of PCR-confirmed MPXV infection from 32 participating centers were analyzed by June 23, corresponding to approximately 45% of the cases reported in 2022 to the RKI by that time. Several entries occurred just at the beginning of the outbreak from Gran Canaria, possibly on the occasion of the Maspalomas Gay Pride 2022, which had been taking place until May 15, 2022. Meanwhile, entries from other countries tend to be sporadic.
By far the most cases were reported from major cities, with 233 cases (77%) from Berlin, Hamburg and Cologne alone. The observed cases were found exclusively among MSM, more than 90% of whom either had HIV infection or were taking HIV PrEP. In over 90% of cases, the age range was between 25 and 55 years. It should be noted as a limitation that, to a considerable extent, the involved centers treat MSM with and without HIV infection and thus a bias is quite possible. It cannot therefore be ruled out that individuals outside the aforementioned affected group have already been infected and that they have either not yet been diagnosed or are being treated in centers that did not participate in this survey. A bias is therefore likely. However, it is also evident that awareness campaigns on risks and modes of transmission focus on major German cities and may initially remain target-group specific, such as via messages in dating apps, but without stigmatizing these people. The Joint United Nations Programme on HIV/AIDS (UNAIDS) has already stated that the fact that so far mainly MSM have been affected could create homophobic and racist stereotypes and reinforce stigmatization (13).
The majority of patients with HIV had a good immune status and were on viral suppression with antiretroviral therapy. Only three patients had viral loads above 200 copies/mL. In one case, HIV infection was diagnosed as a result of the MPXV infection, suggesting that diagnostic testing for HIV is likely to be worthwhile in HIV-negative persons with MPXV infection, as with other STIs.
The high number of other STIs diagnosed either in parallel or shortly before was particularly noteworthy. Almost two-thirds had been diagnosed, or documented, with at least one STI within the last six months, and almost one-third within the last month or even at the same time. Since there was no systematic screening for STI, it is quite possible that the documented cases are an underestimate of the actual rates. This high coincidence highlights the fact that in the current situation, when an STI is diagnosed, MPXV infection should definitely be considered as well (and vice versa).
No deaths were reported, and the clinical course was predominantly mild to moderate. It should be noted, however, that the observation period was very short and it is possible that the observed hospitalization rate may still increase somewhat, as may the number of observed lesions. However, the data did not change dramatically when analysis was limited to the (still quite small) number of patients with longer observation periods. On the other hand, it is also possible that patients with few symptoms may have escaped diagnosis and were therefore not enrolled in the study.
It should be noted that the patients were predominantly young and otherwise generally healthy. Whether the currently circulating West African variant is associated with low severity, as suggested by studies using the monkey model (9), remains to be seen. So far, little is known about risk factors associated with severe disease, but children and pregnant women in particular may be at increased risk of severe disease (12). So far, these populations have not been identified in the present cohort. Also, among the HIV-infected patients included in this cohort, there have been only very few individuals with severe immunodeficiency or viremia. Risk factors were not identified in the present study because of the small number of cases with severe courses of the disease.
In the present cohort, a total of 12.5% had evidence of smallpox vaccination. The date of smallpox vaccination was probably at least 40 years ago; mandatory vaccination in West Germany ended in 1976, and in East Germany in 1982. Laboratory studies and clinical observations suggest that previously administered smallpox vaccines provide some, but by no means complete, protection against monkeypox (14– 19). The present analysis also shows that smallpox vaccination does not give complete protection, although a certain degree of protection against severe infections cannot be excluded. The high proportion of young, unvaccinated patients was notable. Although this could also be due to absence of protective immunity from not having been vaccinated against smallpox, behavioral factors are equally possible.
For now, a prompt awareness and vaccination campaign targeted at at-risk populations is called for; the 240,000 vaccine doses (two doses per person) so far ordered by the German government may not be enough. The expected availability of the smallpox vaccine IMVANEX (modified form of the vaccinia virus Ankara, live) from Bavarian Nordic has not been studied in severely immunocompromised patients (20) and is not currently licensed for monkeypox vaccination in the European Union (although it is in the United States and Canada). Therefore, even in healthy individuals, available data are relatively limited, and the number of exposed individuals is still low. Response rates and seroconversion rates appear to be worse in people with HIV (21). Experiments using the monkey model would also suggest this (22). Therefore, a study to accompany the upcoming vaccination campaign would be desirable, which would also investigate seroconversion rates and evaluate possible breakthrough infections.
Apart from vaccination, a comprehensive awareness campaign is also needed, focused on the involved groups, as open as possible, close to the community, digital and on-site, low-threshold and non-judgmental. Physicians specializing in HIV and those who prescribe HIV PrEP are now called upon to do likewise. In particular, situations should be addressed where PrEP appears to be necessary and useful. Places where transmission is common (such as sex clubs and sex parties with large numbers of participants) should be avoided until the ongoing outbreak is under control.
According to RKI estimates, the risk for the general population in Germany is “currently low” (as of June 17, 2022). However, current outbreak kinetics suggest that monkeypox will establish itself as a new STI, and spread into populations outside MSM seems at least possible. It is likely that many patients will present outside of HIV-focused practices, especially urology, proctology, or even dermatology practices. Small lesions in sexually active MSM, especially genitoanal lesions, should also be examined for MPXV. In larger centers, infection is often already a visual spot diagnosis, but swabs are needed for confirmation and should also be tested for other STIs. A quick diagnosis is essential in order to prevent further infections – including infection of medical staff. In view of the considerable number of cases, it seems sensible to offer the medical staff of, among others, HIV-focused practices not only postexposure vaccinations, as recommended by the Standing Committee on Vaccination, but also indication-based vaccinations. Individual cases of nosocomial transmission have been reported (23– 25). The present study and especially the Figures should help to avoid this.
Acknowledgments
Translated from the original German by Dr. Grahame Larkin, MD
Acknowledgments
We would like to thank all our patients.
Furthermore, we would like to thank Uta Annweiler (Waake), Christina Appelhans (Frankfurt), Daniel Beer (Aachen), Max Bender (Berlin), Richard Betten (Köln), Oliver Blaukat (Braunschweig), S. Falkenau (Düsseldorf), Stefan Fenske (Hamburg), Annette Haas (Frankfurt), Heiko Hanel (Frankfurt), Walter Heise (Berlin), Judith Herbst (Berlin), Cornelius Hörner (Cologne), Julia Isselstein (Cologne), Bastian Kalb (Cologne), Pavel Khaykin (Frankfurt), Heribert Knechten (Aachen), Tim Kümmerle (Cologne), Marcel Lee (Munich), Renate Lingen (Waake), Romina Michalski (Berlin), Malte Monin (Bonn), Kim Selina Müller (Berlin), Matthias Müller (Freiburg), H. M. Orth (Düsseldorf), André Puls (Waake), Marc Da Silva Ribeiro (Berlin), Jürgen Rockstroh (Bonn), Stefan Reiner (Cologne), Andreas Roder (Köln), Monja Rößler (Frankfurt), Clemens Roll (Stuttgart), Jörg-Andres Rump (Freiburg), Sven Schellberg (Berlin), Carl-Knud Schewe (Hamburg), Luca Schifignano (Berlin), Stefan Schlabe (Bonn), Wolfgang Schmidt (Berlin), Stephan Schneeweiß (Cologne), Timo Schultheiß (Berlin), Richard Sinzig (Cologne), Kerstin Stein (Magdeburg), Luca Stein (Berlin), Jan Thoden (Freiburg), Heidi Topic (Cologne), Christian Träder (Berlin), Gunnar Urban (Berlin), Kathrin van Bremen (Bonn), Esther Voigt (Cologne), Christoph Weber (Berlin)
Footnotes
*Additional authors Jörn Teichmann, Christoph Wyen, Sebastian Noe, Peter Kreckel, Siegfried Köppe, Anja-Sophie Krauss, Christoph Schuler, Markus Bickel, Johannes Lenz, Stefan Scholten, Gerd Klausen, Harm-Henning Lindhof, Björn Jensen, Tobias Glaunsinger, Ramona Pauli, Georg Härter, Billy Radke, Stefan Unger, Simone Marquardt, Anja Masuhr, Stefan Esser, Tim Oliver Flettner, Guido Schäfer, Jochen Schneider, Christoph D. Spinner
Infectious Disease Medical Center Hamburg: Dr. med. Guido Schäfer
Practice of Jessen² + Colleagues Heiko Jessen and Arne Jessen, Berlin: Jörn Teichmann
Practice at Ebertplatz, Cologne: PD Dr. med. Christoph Wyen
Department for Internal Medicine I, University Hospital Cologne: PD Dr. med. Christoph Wyen
Medical Care Center Munich at Goetheplatz, Munich: PD Dr. med. Sebastian Noe
Group Practice for Internal Medicine m-50.de, Berlin: Peter Kreckel, Siegfried Köppe
Doctors’ Forum for Infectious Diseases, Seestrasse, Berlin: Dr. med. Anja-Sophie Krauss
Turmstrasse Practice, Berlin: Dr. med. Christoph Schuler, Johannes Lenz
Infectious Diseases Center, Frankfurt: PD Dr. med. Markus Bickel
Dr. Scholten & Schneeweiss Partnership, Cologne: Dr. med. Stefan Scholten
Subspecialized Practice for Infectious Disease at the Oranienburg Gate, Berlin: Dr. med. Gerd Klausen
Department of Dermatology, University Hospital Düsseldorf: Dr. med. Harm-Henning Lindhof
Department for Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf: Dr. med. Björn Jensen
Prenzlauer Berg Practice, Berlin: Dr. med. Tobias Glaunsinger
Medical Care Center at the Isartor, Munich: Dr. med. Ramona Pauli
Medical Care Centre Medicover Ulm: Dr. med. Georg Härter
Checkpoint Berlin: Billy Radke
Institute of Interdisciplinary Medicine (ifi), Medical Care Center ifi-Institute, Block L at the Asklepios Hospital St. Georg, Hamburg: Stefan Unger
Frietsch Roll Marquardt Group Practice, Stuttgart (West): Dr. med. Simone Marquardt
Auguste Victoria Clinic, Berlin: Dr. Anja Masuhr
University Hospital Essen, Clinic and Outpatient Clinic for Dermatology, Venerology, and Allergology, HPSTD Outpatient Department, Essen: PD Dr. med. Stefan Esser
Pempelfort Family Practice, Düsseldorf: Tim Oliver Flettner
Rechts der Isar University Hospital of the Technical University of Munich, Clinic and Outpatient Department for Internal Medicine II, Munich PD Dr. med. Jochen Schneider, PD Dr. med. Christoph D. Spinner
Conflict of interest statement
Christoph Boesecke has received lectures fees and/or consulting fees from AbbVie, Gilead, Janssen, MSD, ViiV.
Jochen Schneider has received fees and/or funding from Gilead Sciences, Janssen-Cilag, CORAT Therapeutics GmbH, Dr. Falk Pharma GmbH, AbbVie.
Christoph Spinner has received fees and/or funding from B. Braun Melsungen, BioNtech, Gilead Sciences, Janssen-Cilag, Eli Lilly, Formycon, Pfizer, Roche, MSD, Apeiron, Cepheid, GSK, Molecular partners, SOBI, AbbVie, Synairgen und ViiV Healthcare.
Stefan Scholten has received fees von Abbvie, Gilead, Janssen, GSK, MSD, ViiV Healthcare, Theratechnologies.
The other authors declare that no conflict of interest exists.
References
- 1.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states. MMWR Morb Mortal Wkly Rep. 2022;71:764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noe S, Zange S, Seilmaier M, et al. Clinical and virological features of first human monkeypox cases in Germany. Infection. 2022:1–6. doi: 10.1007/s15010-022-01874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert-Koch-Institut. Internationaler Affenpocken-Ausbruch: Fallzahlen und Einschätzung der Situation in Deutschland. Stand: 17.6.2022. https://www.rki.de/DE/Content/InfAZ/A/Affenpocken/Ausbruch-2022-Situation-Deutschland.html (last accessed on 20 July 2022) [Google Scholar]
- 6.Giorgi FM, Pozzobon D, Di Meglio A, Mercatelli D. Genomic analysis of the recent monkeypox outbreak. bioRxiv. 2022 doi: 10.1016/j.vaccine.2023.12.086. 06.01.494368. [DOI] [PubMed] [Google Scholar]
- 7.Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. bioRxiv. 2022 doi: 10.1126/science.add4153. 06.10.495526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022 doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Li G, Liszewski MK, Atkinson JP, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS. UNAIDS warns that stigmatizing language on monkeypox jeopardises public health. https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2022/may/20220522_PR_MONKEYPOX (last accessed on 17 June 2022) [Google Scholar]
- 14.Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 15.Karem KL, Reynolds M, Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol. 2007;14:1318–1327. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilchuk I, Gilchuk P, Sapparapu G, et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016,;167:684–694. doi: 10.1016/j.cell.2016.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine PE, Zezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 18.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen E, Abubakar I, Ihekweazu C, Heymann D, Ntoumi F, Blumberg L. Monkeypox—enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022,;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imvanex, Fachinformation. https://wwwema.europa.eu/en/documents/product-information/imvanex-epar-product-information_de.pdf (last accessed on 20 July 2022) [Google Scholar]
- 22.Edghill-Smith Y, Bray M, Whitehouse CA, et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 23.Fleischauer AT, Kile JC, Davidson M, et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40:689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adler H, Gould S, Hine P, et al. NHS England high consequence infectious diseases (airborne) network Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022,;22:1473–3099. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]