Abstract
Coronary CT angiography (CCTA) demonstrated high diagnostic accuracy for detecting coronary artery disease (CAD) and a key role in the management of patients with low-to-intermediate pretest likelihood of CAD. However, the clinical information provided by this noninvasive method is still regarded insufficient in patients with diffuse and complex CAD and for planning percutaneous coronary intervention (PCI) and surgical revascularization procedures. On the other hand, technology advancements have recently shown to improve CCTA diagnostic accuracy in patients with diffuse and calcific stenoses. Moreover, stress CT myocardial perfusion imaging (CT-MPI) and fractional flow reserve derived from CCTA (CT-FFR) have been introduced in clinical practice as new tools for evaluating the functional relevance of coronary stenoses, with the possibility to overcome the main CCTA drawback, i.e. anatomical assessment only. The potential value of CCTA to plan and guide interventional procedures lies in the wide range of information it can provide: a) detailed evaluation of plaque extension, volume and composition; b) prediction of procedural success of CTO PCI using scores derived from CCTA; c) identification of coronary lesions requiring additional techniques (e.g., atherectomy and lithotripsy) to improve stent implantation success by assessing calcium score and calcific plaque distribution; d) assessment of CCTA-derived Syntax Score and Syntax Score II, which allows to select the mode of revascularization (PCI or CABG) in patients with complex and multivessel CAD.
The aim of this Consensus Document is to review and discuss the available data supporting the role of CCTA, CT-FFR and stress CT-MPI in the preprocedural and possibly intraprocedural planning and guidance of myocardial revascularization interventions.
Rationale for the use of coronary CT angiography for planning coronary revascularization
Several prospective multicenter studies confirmed the high diagnostic accuracy of coronary CT angiography (CCTA) for detecting coronary artery disease (CAD) when compared to invasive coronary angiography (ICA).1,2,3,4,5,6,7,8,9,10,11 Although this imaging modality is now considered the preferable method in patients with low-to-intermediate pretest likelihood of CAD, functional imaging remains the first-line approach in patients with complex CAD.12,13,14,15 On the other hand, technology advancements, such as improved temporal and spatial resolution or capability of calcium removal by dual-energy CT, have shown to improve CCTA diagnostic accuracy in patients with diffuse and calcific stenoses.16 Recently, stress CT myocardial perfusion imaging (CT-MPI) and fractional flow reserve derived from CCTA (CT-FFR) have been introduced in clinical practice as new tools for evaluating the functional relevance of coronary stenoses, with the possibility to overcome the main CCTA drawback, i.e. anatomical assessment only.17 In addition, CCTA might be useful to overcome main ICA limitations, such as depiction of only a two-dimensional silhouette of the lumen, no assessment of atherosclerotic plaque burden, and vessel overlapping and foreshortening. Consequently, the number of patients referred to the catheterization laboratory after a non-invasive workup based on CCTA is expected to increase over the next decade. Although this diagnostic approach has not been implemented in everyday clinical practice, the possibility of evaluating with CCTA patients who are candidate for an invasive approach may lead to a better and more efficient organization of catheterization laboratory activity and an improvement of procedural planning. Indeed, allocation of resources and time can be tailored to case complexity. Moreover, in cases with complex lesions (e.g. bifurcations and CTO), dedicated personnel, longer time slots and specific resources can be made available before the procedure. Finally, after a CCTA-based diagnosis of a hemodynamically significant lesion, the catheterization laboratory can focus mainly on interventional activities avoiding to waste time in unnecessary diagnostic angiography.18 The role of CCTA to guide interventional procedures stems from the fact that this imaging tool allows a detailed evaluation of the extension, volume and composition of atherosclerotic plaques,18 unlike conventional ICA that only assesses coronary lumen narrowing. Although ICA limitations can be overcome by the systematic use of invasive intravascular imaging tools, they are used in less than 10% of PCI for several reasons, including that they are time consuming, increase procedural cost, have modest penetration in the real world and need experienced knowledge of image interpretation.19,20,21
Therefore, aim of this Consensus Document, which has been proposed by the Guidelines Committee and approved by the Board of Directors of the Society of Cardiovascular Computed Tomography (SCCT), is to expound the key technical aspects of CCTA and to review the available clinical data supporting the role of CCTA, CT-FFR and stress CT-MPI in the preprocedural and possibly intraprocedural planning and guidance of myocardial revascularization interventions.
Bullet points:
CCTA has emerged as a first-line noninvasive tool for the management of patients with chest pain and suspected CAD, at the same level of recommendation of stress echocardiography, cardiac magnetic resonance and nuclear imaging.
The integration of CCTA imaging in the work-up before coronary revascularization as a tool for procedural planning is supported by its accuracy for plaque and calcium characterization.
Methods
The CCT clinical indications were discussed and arranged according to the following two modalities: a brief paragraph dedicated to each indication, with the description of its clinical usefulness; and each indication was rated by the technical panel for appropriateness, using a score and a methodology previously described.22 In particular, the technical panel rated indications independently.
The technical panel scores each indication as follows:
Scores 7-9: ‘Appropriate’ test for a specific indication (the test is generally acceptable and is a reasonable approach for the indication).
Scores 4-6: Uncertain for a specific indication (the test may be generally acceptable and may be a reasonable approach for the indication). Uncertainty also implies that more research and/or patient information is needed to classify the indication definitively.
Scores 1-3: Inappropriate test for a specific indication (the test is not generally acceptable and is not a reasonable approach for the indication).
The final score is the arithmetic mean of the scores of each individual panelist.
Technical basis (minimal requirements)
The major technological advances of CT scanners currently available on the market and the scan techniques that are generally employed for CCTA are reported in the Supplementary data section.
Atherosclerosis analysis and implication for interventional procedures
Advanced atherosclerosis evaluation by CCTA is supported by several reports suggesting its accuracy vs. invasive imaging23 and its prognostic value.24,25 It is of importance for example that plaque calcification presence and extent evaluated with CCTA led to the use of specific procedural techniques.26 On the other hand, Nakazawa et al. reported that among 51 patients who underwent CCTA before PCI, transient no-reflow phenomenon (i.e. absence or slow coronary flow in the absence of significant coronary stenosis) which is associated with worse prognosis was more common in those with napkin ring sign and low attenuation plaque at CCTA.27 Similarly, Watabe et al. demonstrated that the prevalence of high-risk plaque features identified at CCTA (low-density plaque, positive remodeling and spotty calcification) was higher among patients with post-PCI cTnT elevation >3 times the upper limit of normal.28 High-risk plaque features have been linked to invasively determined myocardial ischemia as well. A sub-analysis from the NXT trial, enrolling 254 patients who underwent both CCTA and invasive fraction flow reserve (FFR) evaluation, demonstrated that elevated low-attenuation plaque volume quantified by CCTA was independently associated with myocardial ischemia (FFR < 0.8) regardless of the degree of lumen stenosis.29 Similarly, data from the CREDENCE trial suggested that complex coronary anatomy models might predict ischemia better that myocardial stress perfusion imaging.30 Finally, in the recent CCTA-FFR Registry31 high-risk morphologic features offered additive prognostic value to coronary physiology that allowed to optimize selection of treatment strategies by improving FFR-based risk predictions. Thus, although further research is needed to investigate the clinical impact of revascularization of high-risk plaque in the absence of significant stenosis and to assess whether CCTA could be used to identify these patients and guide decisions, these data support that advanced analysis of coronary atherosclerosis by CCTA may provide interventional cardiologists with relevant information to guide PCI. This analysis should be tailored on the specific characteristics of each patient and each particular PCI procedure. However, the presence of high-risk plaque features should be routinely included in CCTA reports, as suggested in the CADRADS reporting model.32 Moreover, recent data derived from the PARADIGM trial regarding the assessment of coronary plaque progression by CCTA underlined the possibility to identify plaques and patients at higher risk of progression. Among all different parameters, the percent atheroma volume (PAV), defined as [(plaque volume/vessel volume) x 100] was demonstrated as the best predictor of plaque progression from non-obstructive to more than 50% stenosis. However, volume changes of plaque subtypes have been recently adopted as an effective measure of plaque progression/regression after medical therapy.33 These data should be taken into consideration for appropriate follow-up planning when coronary revascularization is deferred (Table 1).
Table 1. Non-invasive atherosclerosis evaluation by CCTA.
| CCTA atherosclerosis evaluation | |
|---|---|
| Qualitative features | Quantitative analysis |
| – Positive remodeling – Low-attenuation plaque – Napking ring sign – Spotty calcification | – Low-attenuation plaque volume (mm3) – Non-calcified plaque volume (mm3) – Calcified plaque volume (mm3) |
| Clinical insight for the interventionalist | |
| – All adverse plaque features could be associated with higher rate of peri-procedural MI – Adverse plaque features could be associated with myocardial ischemia, independently from lumen stenosis degree at invasive angiography – Extensive presence of calcified plaque could be associated with the need of dedicated intra-procedural techniques (i.e., rotablator, intravascular lithotripsy) | |
Bullet points:
Non-invasive detection and characterization of coronary atherosclerosis of the entire coronary tree may be useful for PCI planning.
Plaque characterization should be part of every CCTA report.
Role of CT-FFR to guide coronary revascularization
CT-FFR is now an established method that allows non-invasive estimation of pressure drop during hyperemia from a standard CCTA with high accuracy and agreement with invasive FFR.34
There are growing clinical data on the accuracy and safety of CT-FFR in guiding ICA referral. The evidence supports ICA deferral in the setting of negative CT-FFR and prior studies demonstrated significantly higher PCI/ICA ratio when decisions to perform ICA are guided by CT-FFR as compared to CCTA alone35,36 (Figure 1).
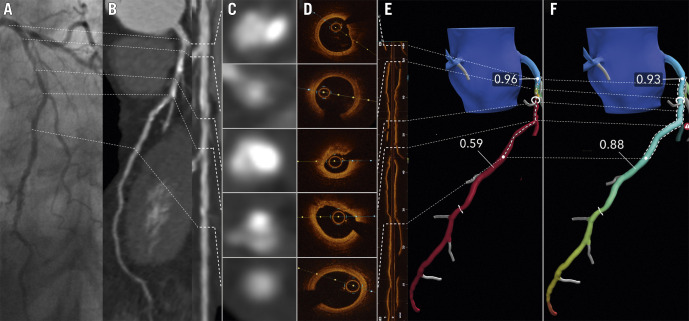
Figure 1. Image showing a co-registration of invasive coronary angiography (A), coronary CTA and straight MPR (panel B and C) with CTA cross sections (panel D), corresponding OCT cross sections and longitudinal OCT view (E).
Panel F shows the CT-FFR patient-specific model and panel G the result of the CT-FFR Planner. Invasive coronary angiography (A), coronary CT angiography (B) and straight MPR (C) showing a calcified fibro-atheroma with positive remodeling in the proximal mid LAD. The CT-FFR (panel F) reaches a value of 0.59 with a focal gradient at the level of the MLA (panel D and E mid). The OCT cross sections mirror the CTA images. The most distal CTA cross section exhibits a healthy landing zone for percutaneous stenting also visualized by OCT (E). Based on the CT-FFR model (F), an appropriate stent length and position is selected (dashed lines). In panel G the predicted functional result by the CT-FFR Planner mimicking PCI can be appreciated.
Building on this evidence, attention has turned towards combining anatomy and physiology to guide not only decisions regarding ICA but also to help revascularization planning. This new paradigm is supported by the unique opportunity of having anatomy (lumen and plaque characteristics) and lesion specific physiology, a combined information that is not provided by other non-invasive imaging modalities. This concept has been tested in the SYNTAX II and III trials,37,38 where non-invasive anatomy and physiology with CCTA and CT-FFR resulted in similar revascularization guidance as that provided by invasive anatomy and physiology assessment with ICA and FFR. The invasive Pullback Pressure Gradient (PPG) allows to identify distinct CAD patterns (focal or diffuse).39 Comparably, virtual CT-FFR pullbacks provide a rapid and non-invasive identification of CAD patterns and may detect functional vs. anatomical mismatch.40,41 These data, along with growing clinical adoption increased the interest in a planned PCI approach and bolstered the Heart Team role for pre-procedural decision-making in a way similar to that used for structural heart disease.
How to interpret the CT-FFR analysis:
Pressure measurements should be performed approximately 2 cm distal to the coronary stenosis. A CT-FFR value < 0.8 distal to a stenosis is considered hemodynamic significant. However, as with invasive FFR, evidence suggests that CT-FFR values should be considered as a continuous variable with lower values associated with higher risk.42,43,44
The CT-FFR gradient across a lesion should be included in the clinical report and should be integrated in the decision-making regarding revascularization.45
Anatomical location: while further methods are being developed to provide a measure of the myocardium at risk, at present clinical reports of CT-FFR should comment on the location of the abnormal physiology, coronary dominance, and coronary branches distal to the physiological abnormality.
Recently, a novel tool that simulates PCI providing modeled CT-FFR values after stenosis opening by using CT-FFR (PCI Planner) has been tested in the prospective, multicenter P3 study (Precise Percutaneous coronary intervention Plan study)46 in 123 patients with chronic coronary syndromes undergoing PCI. As a main finding, the measured invasive post-PCI FFR was 0.88 ± 0.06 whereas the Planner CT-FFR predicted 0.86 ± 0.06 (mean difference 0.02 ± 0.07 FFR units). The high accuracy of the CT-FFR Planner was confirmed also in cases with focal and diffuse disease, and low and high calcium burden.47 Therefore, CT-FFR Planner can mimic PCI to predict the increase in coronary flow after coronary stenting. Finally, the CT-FFR Planner seems a promising tool in overcoming the limitations related to the assessment of serial lesions with invasive FFR (cross talk between lesions - difficult to predict physiological impact of PCI of one lesion) by allowing for a non-invasive detection of which lesion/s need to be treated to achieve an optimal result.48,49
Bullet points:
CT-FFR is a validated tool for non-invasive assessment of the hemodynamic impact of coronary stenosisfrom a standard CCTA with high accuracy and agreement with invasive FFR.
CT-FFR allows to combine anatomy and physiology (estimation of pressure drop across a lesion) to guide referral to ICA and to help revascularization planning.
The contribution of CT myocardial perfusion imaging in patients with untreated CAD
Compared to CCTA alone, the addition of CT-MPI improves the diagnostic accuracy for the functional assessment of CAD. The reported sensitivity and specificity of CT-MPI for detecting functionally significant CAD range between 82-92% and 73-86%,50 respectively, with invasive FFR as reference. Meta-analyses indicate that CT-MPI performance to identify FFR-positive CAD is equivalent to other non-invasive stress tests. However, no head-to-head comparisons have been reported to date.50 The diagnostic accuracy of CT-MPI is in the same range of CT-based FFR. Both functional applications of CT appear to have complementary diagnostic value, though CT-MPI may be preferred in patients with extensive coronary calcification, chronic occlusions and in those with previous revascularization.51
In the CRESCENT II trial, patients with suspected CAD were randomized to a tailored cardiac CT approach including dynamic CT-MPI or standard stress testing. The study showed a higher rate of severe CAD requiring revascularization in the CT group (88% vs. 50%) without increasing the overall invasive catheterization rates.52 The CATCH trial randomized patients after a recent hospital admission for acute chest pain to CCTA or CCTA combined with CT-MPI and found that the latter strategy decreased catheterization referral rates, without any effect on long-term outcome.53
In clinical practice, CT-MPI can follow a positive CCTA to assess the functional severity of coronary lesions, with the benefit of direct spatial correlation of stenotic branches and their dependent myocardium. Absolute quantification of myocardial blood flow may reduce the risk of missing balanced ischemia in the presence of diffuse CAD. The use of radiation and iodine-based contrast agent are drawbacks of CT-MPI. In particular, quantitative perfusion studies benefit from a regular rhythm and an adequately performed breath hold for up to 30 seconds. Therefore, the increased radiation exposure (particularly for the dynamic technique) and contrast medium are potential limitations of CT-MPI in comparison to other imaging modalities, such as stress-echocardiography or stress-cardiac MR. Advantages of CT-MPI are the short acquisition time, high spatial resolution and coverage of the entire left ventricle.
Bullet points:
Stress CT-MPI is a promising non-invasive tool to detect flow-limiting stenoses.
Functional assessment by CT-MPI may improve decision-making in patients with CAD on CCTA.
The contribution of CT-MPI in revascularized patients
PCI with stent implantation is the most common revascularization procedure performed worldwide for the treatment of CAD patients.54 CCTA is generally not recommended in patients with coronary stents due to artifacts resulting from metallic stent struts55,56 that may reduce the diagnostic accuracy, particularly in <3.0-mm diameter stents and in patients who underwent complex revascularization procedures (e.g. bifurcation lesions). Stress CT-MPI has recently emerged as a promising strategy to combine anatomical and functional evaluation in a single technique.57 Several data demonstrated that CT-MPI might improve CCTA diagnostic accuracy for detecting obstructive CAD57. However, only few and small studies investigated the potential value of cardiac CT that incorporates CT-MPI in stented patients, and showed that a combined CCTA + CT-MPI protocol improved the diagnostic accuracy as compared with CCTA alone.57 The recent ADVANTAGE study,58 performed with a 256-detector row scanner in 150 patients, showed an increased diagnostic rate (as a measure of image interpretability) of CT-MPI vs. CCTA (96% vs. 68% in a patient-based analysis) and a significantly higher diagnostic accuracy of CT-MPI vs. CCTA when both QCA and invasive FFR were used as gold standard (CT-MPI vs. CCTA diagnostic accuracy in comparison with invasive FFR of 75% vs. 30.5%, p=0.0002, respectively). These findings were associated with low radiation exposure (4.15 ± 1.5 mSv for CCTA + stress CT-MPI) and support the use of cardiac CT for the preprocedural planning of coronary revascularization also in stented patients (Figure 2). Indeed, the combined anatomical/functional information in patients with complex anatomy, such as those who previously underwent multivessel stenting, may allow focusing the interventional procedures on the target vessels identified non-invasively. However, it should be noted that CT-FFR is not yet available for stent evaluation.
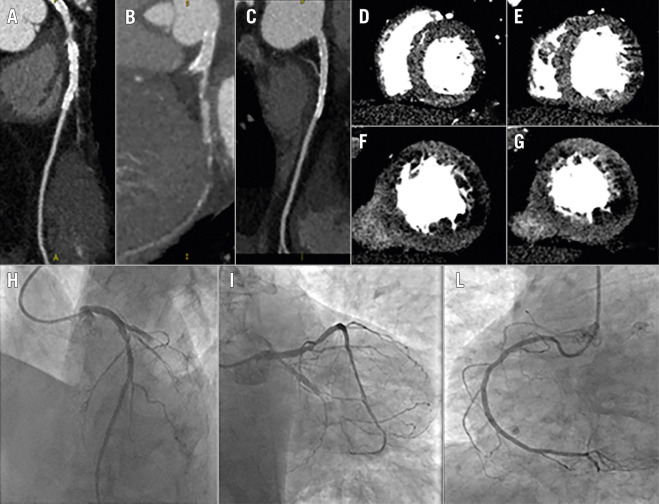
Figure 2. Stress CT-MPI for planning PCI in patients with known CAD.
A complete protocol of adenosine stress-rest CT-MPI was performed. In panels A-C coronary anatomy shows a critical LAD stenosis (panel A), occlusion of LCX (panel B) and patency of a previous stent in the RCA (panel C); the same scan demonstrates reversible perfusion deficit of the antero-septal wall and only partial reversible perfusion deficit of the posterolateral wall (panel D-G). Invasive coronary angiography confirms LCX occlusion and a critical stenosis of the LAD (panel H-L). The interventional cardiologist was able to plan the revascularization procedure and, more importantly, to decide with the Heart Team whether to treat LCX occlusion before bringing the patient to the cath lab.
Bullet points:
CCTA alone is still inaccurate for the assessment of some coronary stents (small diameter, overlapping, and bifurcations).
In patients with previous stent implantation the addition of CT-MPI to CCTA might increase the usefulness of CCTA alone as a non-invasive tool for selecting the patient to submit to invasive evaluation.
Chronic total occlusions
Chronic total occlusions (CTO) are defined as occluded coronary arteries with a Thrombolysis In Myocardial Infarction 0 flow with an estimated duration of at least 3 months.59 The prevalence of CTO in patients referred to CCTA has been shown to be lower (1.4%) than that reported in patients referred to ICA,60,61 similarly to the absolute rate of events in the population evaluated by CCTA.62 This is probably due to the different clinical profile of patients referred to each modality.
The somewhat lower risk of MACE associated with CTO detected by CCTA has important implications for management, as current guidelines consider revascularization strategies only for symptomatic relief and quality of life improvement in these patients.63 Indeed, no reduction in events has been documented thus far with PCI or surgical revascularization, while CTO interventions are associated with non-trivial periprocedural complications.64
With the aim of improving acute and long-term results of CTO interventions, several scores have been developed. Among them, the J-CTO score is the most widely used, as it is a predictor of successful vessel opening as well as a predictor of long-term events.65
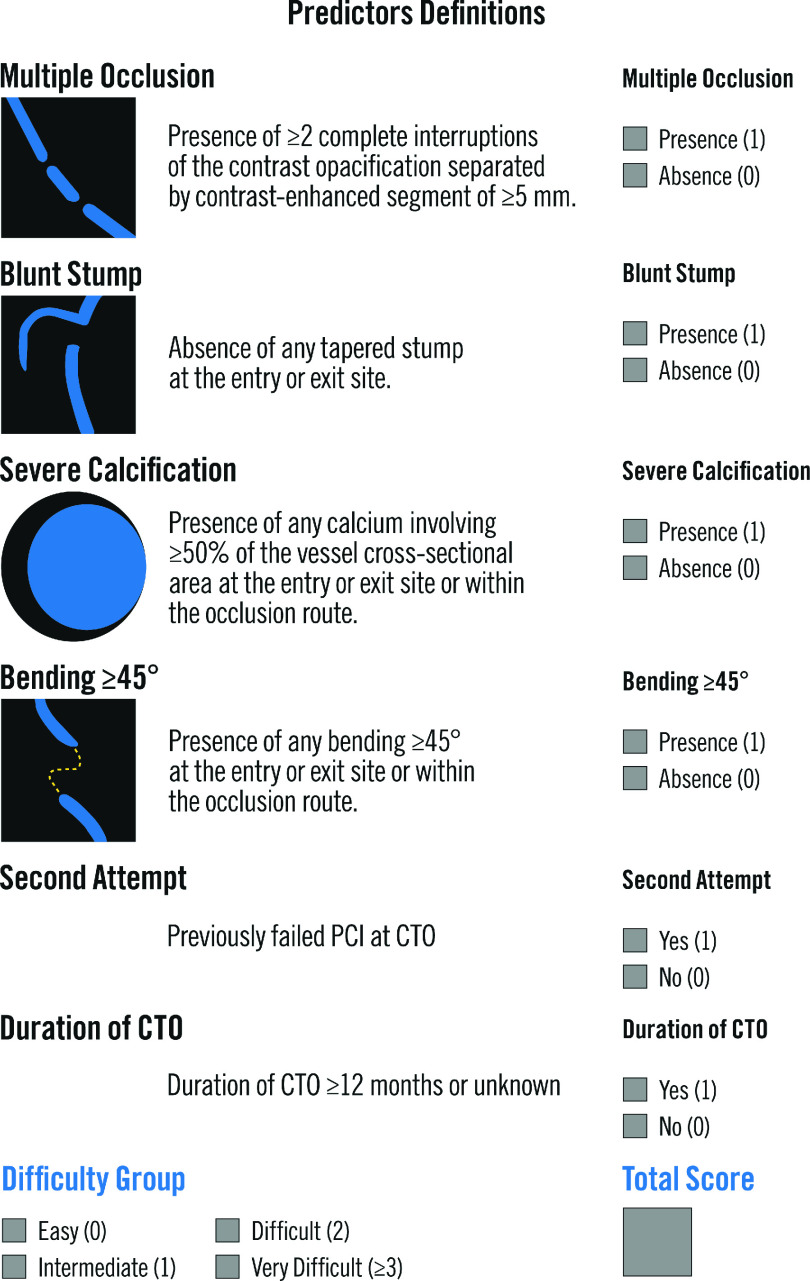
Early studies using CCTA identified several features associated with lower procedural success including occlusion length >15 mm, calcium >50% CSA (i.e., calcium occupies more than half of the cross-sectional area at the point of CTO maximum calcification), blunt stump, calcification at CTO entry point, calcification length >5.5 mm, percentage angulation, shrinkage of the vessel (identified as abrupt narrowing or severe tapering to less than 1 mm in cross-sectional diameter of the distal segment) and bending (defined as an angle of >45o in the trajectory of the vessel either of the occluded site or proximal vessel).66,67 Later, the CT-RECTOR multicenter registry was the first to derive a score from pre-procedural CCTA in 240 patients with CTO lesions. The score included six variables (Figure 3). The authors divided the CTO lesions as easy (score 0), intermediate (score 1), difficult (score 2) and very difficult (score≥3). The four groups were associated with successful lesions crossing within less than 30 min in 95%, 88%, 57% and 22% of the cases, respectively.68,69 Similarly, the rates of final procedural success were 95%, 91%, 66% and 40%, respectively. The authors concluded that the CCTA-derived score showed a higher discrimination than the J-CTO score.70
Figure 3. CT-Rector Score Calculator.
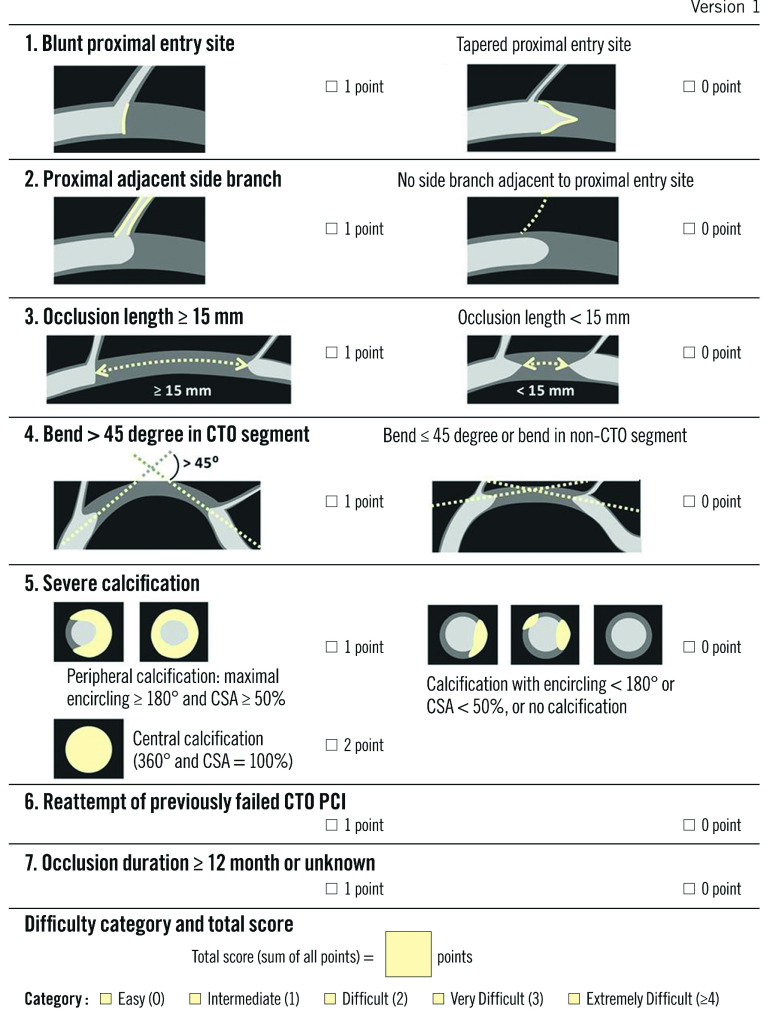
The Korean Multicenter CTO CT Registry (KCCT) included 684 CTO with pre-procedural CCTA. It incorporates seven clinical and CCTA-derived criteria to predict successful lesion crossing within less than 30 minutes as well as procedural success (Figure 4). Successful guidewire crossing occurred in 100% of the lesions with a score 0, in 84% of those with a score 1 or 2, in 51% of those with a score 3 and in 30% of those with a score 4 or above. Similarly, procedural success was 100% for a score 0 or 1, 94% for a score 2, 84% for a score 3 and 62% for a score 4 or higher. When compared to several other scores, the authors reported a higher discrimination for the KCCT.71
Figure 4. KCCT Score: definition and scoring system.
Finally, a third study compared the J-CTO score derived from ICA and CCTA. This study concluded that a score derived from CCTA had higher discrimination for procedural success as well as wire crossing within 30 minutes.72
Although no study to date have compared the three scores, it is unlikely that differences would be notable as several criteria are similar (blunt stump, severe calcification, bending >45o and second attempt after a previous failure to open the lesion).
Recently, the first randomized clinical trial comparing routine pre-procedural CTA to ICA in CTO patients demonstrated that coronary CTA resulted in a 10% absolute increase in the success rate with a reduction in periprocedural coronary perforations and periprocedural myocardial infarction.73
Although still in a feasibility phase, an additional application of CCTA prior to revascularization procedures is real-time fusion of CCTA and ICA images. This approach may be used to provide additional information on vessel tortuosity and calcification that can aid in procedural planning. However, data supporting this technology are still limited.74
In conclusion, the current literature indicates that CCTA is a useful tool to non-invasively diagnose and assess CTO. Additionally, CCTA-derived scores may be used to accurately predicting procedural success. Thus, CCTA may help in selecting patients who are unlikely to benefit from ICA and intervention and who are more adequately managed by optimal medical treatment or surgical intervention. When PCI is indicated, CCTA can also provide information to plan the procedure and select the adequate devices prior to PCI, and in some cases, to choose and guide the interventional technique (i.e., antegrade vs. retrograde approach).
Bullet points:
CCTA may help in identifying anatomical characteristics of CTO that are associated with increased complexity of CTO PCI.
Scores derived from the CT-RECTOR multicenter registry and Korean Multicenter CTO CT Registry (KCCT) allow predicting the procedural success of CTO PCI.
Usefulness of CCTA to optimize medical therapy in patients with evidence of coronary atherosclerosis
The potential implication of CCTA findings on medical therapy optimization are reported in the Supplementary material section.
How to integrate CCTA and CT-FFR in a non-invasive functional syntax score: CCTA for the decision-making in complex CAD
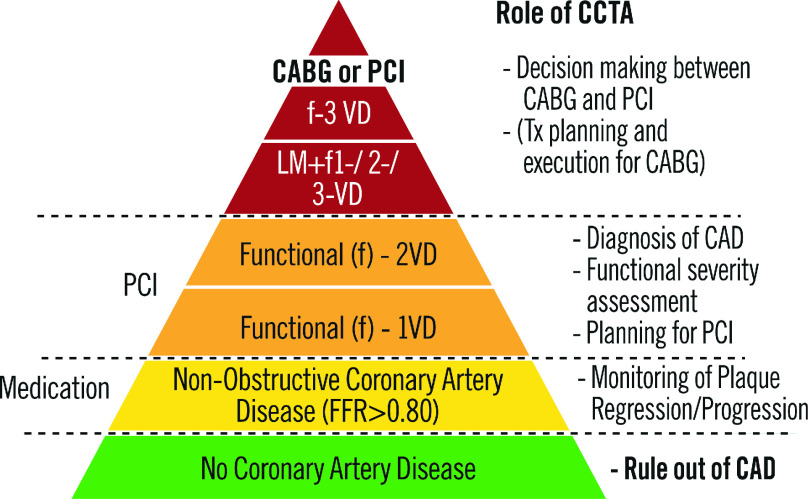
In addition to its primary use to rule-out CAD, CCTA is employed to diagnose and evaluate disease extension and severity (Figure 5). The anatomic Syntax score quantifies the extent and anatomic complexity of CAD based on the visual assessment of a significant stenosis (>50%) diagnosed by ICA.75 The functional SYNTAX score (FSS) adopting physiological parameter such as fractional flow ratio in anatomical SYNTAX score reclassifies a substantial number of patients with multi-vessel disease from high to lower risk of adverse events after PCI.76,77 Because of its significant prognostic value in patients with 3VD or LM disease, undergoing either PCI or CABG, the Syntax score is part of the guidelines and has been increasingly used during Heart Team discussion to decide the revascularization mode (CABG or PCI).
Figure 5. Role of CCTA in different degrees of CAD.
The SYNTAX score II combines anatomical Syntax Score and seven independent clinical predictors for 4-year all-cause mortality in patients undergoing either surgical or percutaneous revascularization.78,79,80 Recently, the prediction model was updated as Syntax Score 2020, which could predict 5-year MACE and 10-year all-cause mortality based on cross validation in the SYNTAX trial and on external validation in the FREEDOM, BEST and PRECOMBAT trials81 (Table 2).
Table 2. SYNTAX score: from anatomy to comorbidity and functional assessment.
| 1. Anatomic SYNTAX Score (2005): Anatomy (ICA or CTA), population strata outcome, PCI versus CABG – Predict MACCE (all-cause mortality, stroke, myocardial infarction, revascularization) prognosis from 1 to 5 years in the SYNTAX trial (PCI vs. CABG in three-vessel disease and left main) and mortality up to 10 years in the SYNTAXES study. |
| 2. SYNTAX Score II (2013): Anatomy (ICA) and comorbidity, PCI versus CABG – Predict 4-year all-cause mortality in the SYNTAX trial. – Used as an inclusion criteria for PCI population based on equipoise prediction of all- cause mortality after PCI versus surgery in three-vessel disease and left main (the SYNTAX II trial). |
| 3. Functional SYNTAX Score (2011, 2018): Anatomy (ICA or CTA) and functionality (iFR, FFR, CT-FFR), PCI population – Treatment decision making based on anatomy and functionality. |
| 4. Logistic clinical SYNTAX Score (2020): Anatomy (ICA) and comorbidity, individualized outcome for “all-comers” PCI – Predict all-cause mortality at 2 years in “all-comers” PCI trials. |
| 5. SYNTAX Score III: Anatomy (CTA), comorbidity, and functionality (CT-FFR), PCI and CABG – Treatment decision making between PCI and CABG in three-vessel disease and left main based solely on multi-slice CT scan with CT-FFR in the SYNTAX III REVOLU- TION trial. |
| 6. SYNTAX Score III: Anatomy (CTA), comorbidity, and functionality (CT-FFR), CABG population – Planning and execution of surgery in three-vessel disease and left main applying SYNTAX Score III derived solely on CTA scan with CT-FFR (the FASTTRACK CABG trial, First in men). |
| 7. SYNTAX Score 2020 (2020): Anatomy (ICA) and comorbidity, PCI versus CABG – Predict 5-year MACE and 10-year all-cause mortality based on cross validation in the SYNTAX trial and on external validation in the FREEDOM, BEST, and PRECOMBAT trials. |
| Evolution of the risk score algorithms derived from the historical SYNTAX I trial. CABG: coronary artery bypass grafting; CTA: computed tomography angiog- raphy; FFR: fractional flow reserve; ICA: invasive coronary angiography; iFR: instantaneous wave-free ratio; PCI: percutaneous coronary intervention |
Although initially developed as an angiographic score, the anatomic Syntax Score and its derivative scores are also derived from CCTA (CT-Syntax Score).82 CCTA overcomes the major limitations of ICA stemming from its 2D cine-fluoroscopic nature and lack of strong reproducibility due to subjective assessments of multiple observers. With its non-invasiveness and capability to assess coronary stenoses as well as their functional impact by CT-FFR, CCTA enables us to assess the severity of coronary lesions, the length of the lesion, the diffuse character of lumen reduction, the tortuosity of the target vessel, the single, double or triple involvement of a bifurcation and the degree of coronary calcification etc., in brief, all the individual components of the anatomic SYNTAX score.36
The potential role of CCTA in Heart Team discussion for decision-making between PCI and CABG was investigated in the Syntax III Revolution trial, which enrolled patients known for having 3VD with or without LM disease diagnosed by ICA. The agreement on treatment decision (surgery or PCI) of two heart teams was measured. The heart teams received, in order to make their decision, either a conventional ICA or CCTA of cases with 3VD with or without LM disease.37 The decision making of “CABG only”, “PCI only” or “equipoise CABG-PCI” was concordant between the two heart teams in 86% of the cases, with a Cohen's kappa of 0.82, qualifying the agreement as almost perfect according to the statistical Cohen's kappa categorization.37 This was a virtual trial since after signing off the decision-making, both Heart Teams were unblinded so that they had all the information available prior to the real clinical treatment, either in the interventional suite or in the operative room. The question remains whether cardiac surgeons would really be willing to perform surgery without prior ICA, using CCTA as the sole guidance of coronary grafting. In a survey among surgeons involved in the Syntax III trial, six surgeons reviewed 20 CCTA of 20 patients who underwent CABG performed previously by them during the course of the SYNTAX III trial.83 Each surgeon had to declare for himself whether the planning and the execution of surgery would be feasible. In 84% of the cases, they declared that patients would be eligible for surgery without prior conventional ICA. Subsequently, the Fast-Track CABG trial was designed and is currently ongoing to inves tigate whether the policy of surgical treatment without prior ICA and CCTA guidance only is feasible and safe (NCT04142021).84 So far, 45 patients have been enrolled in the FAST-TRACK CABG trial and in all cases, anatomical SYNTAX Score, SYNTAX II 2020 score and functional SYNTAX Scores were used to plan and guide the surgical bypass procedure.
Bullet points:
Syntax Score and its derivative have been introduced to assess on ICA the complexity and total burden of CAD, which is used to guide the selection of the revascularization mode (PCI or CABG), especially in patients with complex CAD (e.g. 3VD, LM disease).
With its 3-dimensional nature and physiological assessment (e.g. CT-FFR), CCTA allows to assess Syntax Score and Syntax Score II, which provide the Heart Team with a recommendation on the mode of revascularization (PCI or CABG) for patients with complex disease based on long-term mortality. The decision-making of two Heart Teams based on ICA or CCTA accounting for clinical characteristics and comorbidities was proven to have a high level of agreement in the Syntax III trial.
Pre-procedural surgical planning of coronary revascularization by CCTA
Although ICA is the preferred diagnostic method to guide the treatment decision between CABG and PCI, SYNTAX III Revolution trial has demonstrated the potential usefulness of CCTA in this clinical setting,37 whereas the addition of a functional assessment by CT-FFR further refines treatment decision and planning prior to revascularization.85
The identification of hemodynamic significance of a lesion, visualization of landing zone for the distal surgical anastomosis and distal runoff emerged as the most important factors supporting the feasibility of this approach. CCTA identifies non-diseased target segments for grafting. In addition, it provides insights into vessel course including an intramyocardial coronary path, presence of coronary anomalies and target vessel size. In case of CTO, assessment of the vessel distal to the occlusion proves usually feasible also providing information not always readily available with ICA, where only in case of well-developed collateral flow the course and size of the distal coronary bed is evaluable. Moreover, the amount of subtended myocardium and identification of potential myocardial scar by CCTA and optional CT-MPI can help omit futile grafting or optimize CABG strategies when limited grafting material is available.
CCTA 3D-volume rendering also facilitates the assessment of coronary anatomy. The course of and distance between the LAD and diagonal branches is relevant for planning minimal invasive sequential MIDCAB LIMA-LAD or sequential bypass grafting. Furthermore, CCTA can also assess the anatomy, caliber and length of the left and right internal mammary arteries by using the curved multiplanar reconstruction and the “lumen visualization” view. The presence of disease in the ascending aorta may prompt alternative surgical approaches such as no-touch aorta technique with the potential to minimize cerebral embolization during surgery. Maximal Intensity Projection (MIP) are particularly useful for the evaluation of aortic calcifications eventually changing cannulation or impacting the selection of a good location to sew the origin of the bypass graft.86 In patients referred to redo-CABG, CCTA informs about the distance between sternum and heart and warns about a potential pre-aortic graft path for sternotomy. Figure 6 summarizes the value of CCTA for guiding CABG.
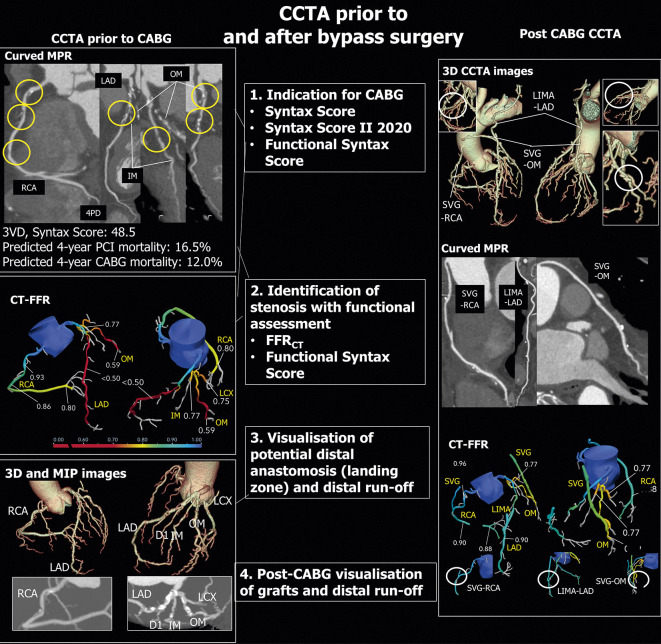
Figure 6. CCTA prior to and after Bypass surgery.
Left side: CCTA before CABG. Upper panel: Curved multiplanar reconstructions of major epicardial vessels showing the lesions scored in the SYNTAX score. Mid panel: CT-FFR analysis. Lower panel: 3D Volume rendering and MIP reconstructions. Right side: CCTA after CABG. Upper panel: 3D Volume rendering images showing the three grafts on LAD, OM and RCA. Mid panel: Curved multiplanar reconstructions of the three grafts. Lower panel: CT-FFR of native arteries and grafts. LAD = left anterior descending artery; MIP = maximum intensity projection; OM = obtuse marginal branch; RCA = right coronary artery; 3D = three dimensional.
The role of CCTA to assess graft patency is well recognized. Future research should confirm if CT-FFR, providing not only a distal CT-FFR value but also insights into the endotype of CAD (e.g., focal or diffuse disease) could predict long-term graft patency comparable to invasive FFR or angiography-derived functional assessment.87
Bullet points:
CCTA images may help cardiac surgeons in the planning of CABG procedure.
The ongoing multicenter FAST TRACK CABG study is aimed at assessing whether CCTA alone is able to support the cardiac surgeon in the procedural planning of CABG without information from ICA.
PCI guidance by integration of CCTA into the catheterization laboratory
Using CCTA, the location of the coronary ostia can be ascertained.88 Coronary ostia in their normal position can be easily cannulated with standard catheters and maneuvers. However, in cases of coronary anomalies or normal variants, knowledge of ostia position informs the operator on distinct maneuvers or special coronary catheter shapes required for selective cannulation.89 Moreover, the presence of an ostial or proximal lesion in the coronary artery prompts the operator to avoid deep cannulation that may concealed the lesion.
Thanks to its 3-dimensional nature, CCTA can be potentially useful to overcome some limitations of conventional ICA such as vessel foreshortening, which affects the evaluation of lesion length that is subsequently needed to select stent length. Although clear evidence supporting this approach is lacking, foreshortening might be minimized choosing upfront the optimal angulation based on CCTA. Optimal selection of angiographic projection is of upmost importance in two lesion subsets, namely bifurcation and ostial lesions.90 In coronary bifurcations, correct visualization of the side branch ostium is crucial to define PCI strategy and the need of additional interventions at the level of the side branch. Likewise, in ostial lesions, the precise demarcation of the ostium is essential for correct stent positioning and deployment.90 Although fixed projections for right and left coronary ostia evaluation and visualization of LAD/Diagonal or LCX/obtuse marginal bifurcations have been proposed, CCTA imaging allows determining a patient-specific angulation for a given coronary segment.91 CCTA may also help predicting the risk of side branch occlusion during bifurcation PCI91. Pre-PCI assessment is based on the evaluation of plaque extension, composition and characteristics. Lesion length is assessed as the distance between healthy (i.e., plaque free) landing zones proximal and distal to the coronary stenosis.92 Of note, lesion length derived from CCTA is unaffected by foreshortening. Thus, this approach has the potential to improve the selection of device length. The presence of a severely calcified plaque with circumferential and lengthy calcifications have been shown to be associated with stent under-expansion and sub-optimal result, which have been consistently identified as predictors of adverse events after PCI12. Therefore, in vessels with high calcium burden detected by CCTA, use of additional techniques like rotational or orbital atherectomy and intracoronary lithotripsy may improve stent expansion. In the other spectrum of the atherosclerotic disease, namely non-calcified plaque with positive remodeling and low-attenuation components, PCI has been associated with periprocedural myocardial infarction and no-reflow phenomenon. Thus, plaque characterization by CCTA may help in stratifying the risk of periprocedural complications.91,92 The potential future of CCTA is to join ICA in daily practice. Real-time automated co-registration of CCTA and live fluoroscopic images has been shown to be feasible and has the potential to improve PCI results.93 Indeed, further studies are required to establish the clinical benefit of this approach (Figure 7). Figure 8 summarizes the CCTA-derived parameters potentially useful for the preprocedural planning and intraprocedural guidance of PCI.
Figure 7. Prototype of coronary CT angiography catheterization laboratory viewer for online PCI guidance.
Software prototype allowing for visualization of the coronary anatomy and plaque components extracted from coronary CT angiography (CCTA) inside the catheterization laboratory. The central panel shows a three-dimensional (3D) reconstruction of a left coronary artery (red) with colored-coded visualization of the plaque components (white for calcium and green for non-calcified plaque). The inferior panel shows a straight MPR reconstruction with three markers (green, blue and red lines) related to 3D reconstruction. This tool allows for the measurement of lesion length for stent length selection. The top right panel shows a CCTA cross-sectional view of the left anterior descending artery displaying the lumen and vessel contours (yellow and orange lines). The dimension of the lumen and vessel, both mean diameter and area, are shown in the right mid-panel, and in the bottom right corner, the diameter function line in the y-axis and length in the x-axis. Software prototype QAngio Cath Lab from Medis Medical Imaging. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Figure 8. Main features of Cardiac CT-guided PCI.
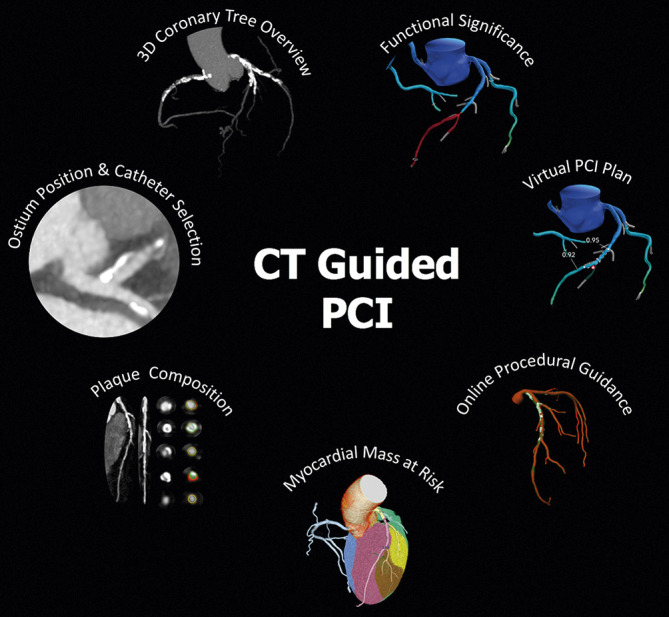
CCTA-derived parameters for PCI planning and guidance. The figure shows the main features of a CT-guided PCI approach, including assessment of coronary ostium position for guiding catheter selection, 3-vessel overview of coronary tree for evaluation of calcium extent and distribution, plaque composition and quantification analysis, functional significance of stenosis and virtual PCI planning by CT-FFR, estimation of myocardial mass at risk and online procedural guidance.
Bullet points:
Before PCI, CCTA can be utilized to overcome several limitations of conventional ICA, including vessel foreshortening and difficulties in selecting optimal projections. In this regard, CCTA is of upmost importance in two lesion subsets, namely bifurcation and ostial lesions.
Plaque characterization by CCTA may help in stratifying the risk of periprocedural complications.
Evidence gaps that need focused research
The SYNTAX III Revolution study opens new perspectives on CCTA use as a tool to provide interventionalists and cardiac surgeons with a non-invasive roadmap for planning myocardial revascularization strategies and raises the question if a cardiac surgeon might be confident in using only the non-invasive coronary information provided by CCTA to plan CABG. The intriguing hypothesis was tested first in a theoretical feasibility survey study83 and is now evaluated in a “first-in-man”, proof-of-concept feasibility and safety trial.84 The further step might be a large, international, prospective and randomized trial on the revascularization outcome by comparing patients who underwent a novel non-invasive vs. a traditional invasive roadmap. In addition, the interactive planner, which is a new application of CT-FFR, might improve treatment selection while tailoring procedural strategy based on assessment of functional outcome after virtual treatment.45 However, prospective, multicenter studies on this tool are needed in order to demonstrate its diagnostic accuracy in predicting the functional impact of the interventional treatment. Of note, a part from few clinical cases94 showing its potential usefulness, the CT-FFR calculation is still not validated and available for routine use in stented vessels. Finally, further evidence are needed to demonstrate the utility of CCTA as an alternative to intracoronary imaging, in particular for guiding stent sizing, stent length and lesion preparation with dedicated lesion modification strategies (i.e., rotational atherectomy, intravascular lithotripsy).
Bullet points:
Studies are underway to assess the feasibility of CABG planning based on CCTA only and the effectiveness of interactive PCI planner.
Randomized studies are needed to evaluate the outcome of coronary revascularization based on CCTA-directed procedures (PCI and CABG).
Critical appraisal
The final score on the Appropriate Use Criteria for the Pre-procedural Planning of Coronary Revascularization by CCTA is reported in Table 3. With the introduction of CCTA into clinical practice over 15 years ago, non-invasive evaluation of CAD has become an important anatomical alternative to traditional functional imaging modalities. Technological advances have enabled to progressively improve image quality, reduce radiation dose and limit artifacts. A major advantage of CCTA, not provided by functional imaging, is the additional characterization of plaque features, which hold important prognostic and therapeutic consequences even in the absence ischemia-inducing lesions. As a consequence, CCTA should now be considered the initial test of choice to analyze the presence of CAD in the majority of patients. The major drawback of mere anatomical imaging without information on the functional significance of coronary lesions have been overcome by the development of CT-FFR and, to a lesser extent, CT-MPI. Multiple studies have demonstrated the additional diagnostic value of CT-FFR as well as its beneficial impact to limit referral for (futile) ICA. Besides enabling a more judicious referral to the catheterization laboratory, more recently CT-FFR-based software tools have attempted to predict the effects of stent implantation on actual FFR with the aim of optimizing PCI result without the need of invasive interrogation. Although not ready for clinical use at this time, these developments are quite promising and may facilitated CT-guided PCI in the near future. Thus far, CCTA has been primarily regarded as a useful tool in patients without known CAD but this paradigm is quickly shifting to patients with known CAD showing features that are more complex. For example, pre-procedural CT acquisition is now commonly accepted for the routine planning of CTO PCI in patients with complex anatomy. Recent studies have also shown the feasibility to plan Heart Team decisions in patients with multivessel disease based on CCTA in conjunction with CT-FFR alone, paving the way for a future in which ICA may increasingly lose its dominant role for decision-making regarding revascularization procedures. Although many hurdles still need to be overcome, the available evidence indicates that CCTA may emerge in the field of interventional cardiology no longer as a mere diagnostic tool. However, further prospective multicenter studies will be needed to confirm that this noninvasive imaging modality may play a pivotal clinical role for planning and guiding revascularization procedures.95
Table 3. Pre-procedural planning of coronary revascularization by CCTA: appropriate use criteria.
| Indication | Appropriate use score |
|---|---|
| Rationale for the use of coronary CT angiography for planning coronary revascularization | |
| CCTA has emerged as a first-line noninvasive tool for the management of patients with chest pain and suspected CAD, at the same level of recommendation of stress echocardiography, cardiac magnetic resonance and nuclear imaging. | A (9) |
| The integration of CCTA imaging in the work-up before coronary revascularization as a tool for procedural planning is supported by its accuracy for plaque and calcium characterization. | A (7) |
| Atherosclerosis analysis and implication for interventional procedures | |
| Non-invasive detection and characterization of coronary atherosclerosis of the entire coronary tree may be useful for PCI planning. | A (7) |
| Plaque characterization should be part of every CCTA report. | A (8) |
| Role of CT-FFR to guide coronary revascularization | |
| CT-FFR is a validated tool for non-invasive assessment of the hemodynamic impact of coronary stenosisfrom a standard CCTA with high accuracy and agreement with invasive FFR. | A (8) |
| CT-FFR allows to combine anatomy and physiology (estimation of pressure drop across a lesion) to guide referral to ICA and to help revascularization planning. | A (7) |
| The contribution of CT myocardial perfusion imaging in patients with untreated CAD | |
| Stress CT-MPI is a promising non-invasive tool to detect flow-limiting stenoses. | U (6) |
| Functional assessment by CT-MPI may improve decision-making in patients with CAD on CCTA. | U (6) |
| The contribution of CT-MPI in revascularized patients | |
| CCTA alone is still inaccurate for the assessment of some coronary stents (small diameter, overlapping, and bifurcations). | A (8) |
| In patients with previous stent implantation the addition of CT-MPI to CCTA might increase the usefulness of CCTA alone as a non-invasive tool for selecting the patient to submit to invasive evaluation. | A (7) |
| Chronic total occlusions | |
| CCTA may help in identifying anatomical characteristics of CTO that are associated with increased complexity of CTO PCI. | A (8) |
| Scores derived from the CT-RECTOR multicenter registry and Korean Multicenter CTO CT Registry (KCCT) allow predicting the procedural success of CTO PCI. | A (8) |
| How to integrate CCTA and CT-FFR in a non-invasive functional Syntax score: CCTA for the decision-making in complex CAD | |
| Syntax Score and its derivative have been introduced to assess on ICA the complexity and total burden of CAD, which is used to guide the selection of the revascularization mode (PCI or CABG), especially in patients with complex CAD (e.g. 3VD, LM disease). | A (8) |
| With its 3-dimensional nature and physiological assessment (e.g. CT-FFR), CCTA allows to assess Syntax Score and Syntax Score II, which provide the Heart Team with a recommendation on the mode of revascularization (PCI or CABG) for patients with complex disease based on long-term mortality. The decisionmaking of two Heart Teams based on ICA or CCTA accounting for clinical characteristics and comorbidities was proven to have a high level of agreement in the Syntax III trial. | A (7) |
| Pre-procedural surgical planning of coronary revascularization by CCTA | |
| CCTA images may help cardiac surgeons in the planning of CABG procedure. | U (6) |
| The ongoing multicenter FAST TRACK CABG study is aimed at assessing whether CCTA alone is able to support the cardiac surgeon in the procedural planning of CABG without information from ICA. | A (7) |
| PCI guidance by integration of CCTA into the catheterization laboratory | |
| Before PCI, CCTA can be utilized to overcome several limitations of conventional ICA, including vessel foreshortening and difficulties in selecting optimal projections. In this regard, CCTA is of upmost importance in two lesion subsets, namely bifurcation and ostial lesions. | A(7) |
| Plaque characterization by CCTA may help in stratifying the risk of periprocedural complications. | A (7) |
| CABG: coronary artery bypass grafting; CCTA: coronary computed tomography angiography; FFR: fractional flow reserve; ICA: invasive coronary angiography; MPI: myocardial perfusion imaging; PCI: percutaneous coronary intervention | |
Declaration of competing interest
Carlos Collet reports receiving Consultant/Honoraria: Abbott Vascular, HeartFlow, Siemens and Grants/research support: Abbott Vascular, HeartFlow, Siemens. Patrick Serruys reports consultancy fees from Abbott, Biosensors, Medtronic, Micell, Qualimed, Sinomedical Sciences, St. Jude Medical, Stentys, Svelte Medical Systems, Philips/Volcano, Xeltis, StentIt and HeartFlow. Koen Nieman reports unrestricted institutional research support from Siemens Healthineers, HeartFlow Inc, Bayer. Marcio Bittencourt reports Consultant/Honoraria: Bayer, Research funding: Sanofi and Speaker's bureau: NovoNordisk, EMS, Novartis, GE Healthcare. Jonathon Leipsic reports Consultant/Honoraria: Circle CVI, HeartFlow, MVRX; Grants/Research Support: Abbott, Boston Scientific, GE Healthcare, Edwards, Medtronic; Speaker's Bureau: Philips; Stock and stock options: HeartFlow, Circle CVI. Giulio Stefanini reports research grant (to the Institution) from Boston Scientific, and speaker fees Abbott Vascular and Boston Scientific.
Brian Ghoshhajra reports Grants and research support: Siemens Healthineers. The other authors report no conflict of interest.
Supplementary data
Author relationships with industry - pre-procedural planning of coronary revascularization by cardiac-CT.
Reviewer relationships with industry - pre-procedural planning of coronary revascularization by cardiac-CT.
Contributor Information
Daniele Andreini, Centro Cardiologico Monzino, IRCCS, Milan, Italy; Department of Biomedical and Clinical Sciences “Luigi Sacco”, University of Milan, Milan, Italy.
Carlos Collet, Cardiovascular Center Aalst, OLVZ Aalst, Belgium.
Jonathon Leipsic, St Paul's Hospital & University of British Columbia, Vancouver, British Columbia Vancouver, Canada.
Koen Nieman, Stanford University School of Medicine, Departments of Medicine and Radiology, USA.
Marcio Bittencurt, Division of Internal Medicine, University Hospital, University of São Paulo, São Paulo, Brazil; DASA, São Paulo, Brazil; Division of Cardiology and the Heart and Vascular Institute, University of Pittsburgh Medical Center.
Johan De Mey, Department of Radiology, Vrije Universiteit Brussel (VUB), Universitair Ziekenhuis Brussel (UZ Brussel), Brussel, Belgium.
Nico Buls, Department of Radiology, Vrije Universiteit Brussel (VUB), Universitair Ziekenhuis Brussel (UZ Brussel), Brussel, Belgium.
Yoshinobu Onuma, Clinical Science Institute, National University of Ireland, Galway, Ireland.
Saima Mushtaq, Centro Cardiologico Monzino, IRCCS, Milan, Italy.
Edoardo Conte, Centro Cardiologico Monzino, IRCCS, Milan, Italy; Department of Biomedical Sciences for Health, University of Milan, Milan, Italy.
Antonio L. Bartorelli, Centro Cardiologico Monzino, IRCCS, Milan, Italy; Department of Biomedical and Clinical Sciences “Luigi Sacco”, University of Milan, Milan, Italy.
Giulio Stefanini, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele-Milan, Italy; Humanitas Research Hospital IRCCS, Rozzano-Milan, Italy.
Jeroen Sonck, Cardiovascular Center Aalst, OLVZ Aalst, Belgium; Department of Advanced Biomedical Sciences, University of Naples, Federico II, Naples, Italy.
Paul Knaapen, Vrije Universiteit Medical Center, Amsterdam, Netherlands.
Brian Ghoshhajra, Massachusetts General Hospital, Harvard University, Boston, MA, USA.
Patrick W. Serruys, Clinical Science Institute, National University of Ireland, Galway, Ireland.
References
- Stein PD, Yaekoub AY, Matta F, Sostman HD. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med. 2008;121(8):715–25. doi: 10.1016/j.amjmed.2008.02.039. [DOI] [PubMed] [Google Scholar]
- Schroeder S, Achenbach S, Bengel F, et al. Working group nuclear cardiology and cardiac CT; European Society of cardiology; European Council of nuclear cardiology. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a writing group deployed by the working group nuclear cardiology and cardiac CT of the European Society of cardiology and the European Council of nuclear cardiology. Eur Heart J. 2008;29(4):531–56. doi: 10.1093/eurheartj/ehm544. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- Marano R, De Cobelli F, Floriani I, et al, NIMISCAD Study Group. Italian multicenter, prospective study to evaluate the negative predictive value of 16- and 64-slice MDCT imaging in patients scheduled for coronary angiography (NIMISCAD-Non Invasive Multicenter Italian Study for Coronary Artery Disease). Eur Radiol. 2009;19(5):1114–23. doi: 10.1007/s00330-008-1239-8. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Kalia N, Cole J, Nakanishi R, Nezarat N, Thomas JL. Diagnostic accuracy of visipaque enhanced coronary computed tomographic angiography: a prospective multicenter trial. Coron Artery Dis. 2017;28(1):52–6. doi: 10.1097/MCA.0000000000000440. [DOI] [PubMed] [Google Scholar]
- Budoff M, Li D, Kazerooni E, Thomas G, Mieres J, Shaw L. Diagnostic accuracy of noninvasive 64-row computed tomographic coronary angiography (CCTA) compared with myocardial perfusion imaging (MPI): the PICTURE study, a prospective multicenter trial. Acad Radiol. 2017;24(1):22–9. doi: 10.1016/j.acra.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A, Miller J, Rochitte C, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (coronary artery evaluation using 64-row multidetector computed tomography angiography) international multicenter study. J Am Coll Cardiol. 2012;59(4):379–87. doi: 10.1016/j.jacc.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neglia D, Rovai D, Caselli C, et al, EVINCI Study Investigators. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;8(3):e002179. doi: 10.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- Pontone G, Andreini D, Quaglia C, Ballerini G, Nobili E, Pepi M. Accuracy of multidetector spiral computed tomography in detecting significant coronary stenosis in patient populations with differing pre-test probabilities of disease. Clin Radiol. 2007;62:978–85. doi: 10.1016/j.crad.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Vavere AL, Arbab-Zadeh A, Rochitte CE, et al. Coronary artery stenoses: accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification-a subanalysis of the CORE-64 trial. Radiology. 2011;261(1):100–08. doi: 10.1148/radiol.11110537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force Members. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013. American College of Cardiology Foundation Appropriate Use Criteria Task Force. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Andreini D, Martuscelli E, Guaricci AI, et al. Clinical recommendations on cardiac-CT in 2015: a position paper of the working group on cardiac-CT and nuclear cardiology of the Italian Society of cardiology. Working group on cardiac-CT and nuclear cardiology of the Italian Society of cardiology. J Cardiovasc Med. 2016;17(2):73–84. doi: 10.2459/JCM.0000000000000318. [DOI] [PubMed] [Google Scholar]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- Andreini D, Pontone G, Mushtaq S, et al. Diagnostic accuracy of rapid kilovolt peakswitching dual-energy CT coronary angiography in patients with a high calcium score. JACC Cardiovasc Imaging. 2015;8(6):746–48. doi: 10.1016/j.jcmg.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Conte E, Sonck J, Mushtaq S, et al. CT-FFR and CT perfusion: a review on the evaluation of functional impact of coronary artery stenosis by cardiac CT. Int J Cardiol. 2020;300:289–96. doi: 10.1016/j.ijcard.2019.08.018. [DOI] [PubMed] [Google Scholar]
- Collet C, Sonck J, Leipsic J, et al. Implementing coronary computed tomography angiography in the catheterization laboratory. JACC Cardiovasc Imaging. 2021;14(9):1846–55. doi: 10.1016/j.jcmg.2020.07.048. [DOI] [PubMed] [Google Scholar]
- Mentias A, Sarrazin MV, Saad M, Panaich S, Kapadia S, Horwitz PA, Girotra S. Long-Term Outcomes of Coronary Stenting With and Without Use of Intravascular Ultrasound. JACC Cardiovasc Interv. 2020;13:1880–90. doi: 10.1016/j.jcin.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha, Pinto DS, Osborn EA. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e013678. doi: 10.1161/JAHA.119.013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la, Escaned J, Hill J, Prati F, Colombo A, di Mario, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G ESC Scientific Document Group. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of cardiology Foundation appropriate Use criteria Task Force, the Society of Cardiovascular computed tomography, the American College of Radiology, the American heart association, the American Society of echocardiography, the American Society of nuclear cardiology, the North American Society for Cardiovascular imaging, the Society for Cardiovascular angiography and interventions, and the Society for Cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Conte E, Mushtaq S, Pontone G, et al. Plaque quantification by coronary computed tomography angiography using intravascular ultrasound as a reference standard: a comparison between standard and last generation computed tomography scanners. Eur Heart J Cardiovasc Imaging. 2020;21(2):191–201. doi: 10.1093/ehjci/jez089. [DOI] [PubMed] [Google Scholar]
- Andreini D, Magnoni M, Conte E, et al. Coronary plaque features on CTA can identify patients at increased risk of cardiovascular events. JACC Cardiovasc Imaging. 2020;13:1704–17. doi: 10.1016/j.jcmg.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Hong YJ, Mintz GS, Kim SW, et al. Impact of plaque composition on cardiac troponin elevation after percutaneous coronary intervention: an ultrasound analysis. J Am Coll Cardiol Img. 2009;2:458–68. doi: 10.1016/j.jcmg.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Sekimoto T, Akutsu Y, Hamazaki Y, et al. Regional calcified plaque score evaluated by multidetector computed tomography for predicting the addition of rotational atherectomy during percutaneous coronary intervention. J Cardiovasc Comput Tomogr. 2016;10(3):221–28. doi: 10.1016/j.jcct.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Tanabe K, Onuma Y, et al. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am Heart J. 2008;155(6):1150–57. doi: 10.1016/j.ahj.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Watabe H, Sato A, Akiyama D, et al. Impact of coronary plaque composition on cardiac troponin elevation after percutaneous coronary intervention in stable angina pectoris: a computed tomography analysis. J Am Coll Cardiol. 2012;59(21):1881–88. doi: 10.1016/j.jacc.2012.01.051. [DOI] [PubMed] [Google Scholar]
- Gaur S, Øvrehus KA, Dey D, et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia causing lesions. Eur Heart J. 2016;37:1220–27. doi: 10.1093/eurheartj/ehv690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuijfzand WJ, van Rosendael AR, Lin FY, et al, CREDENCE Investigators. Stress myocardial perfusion imaging vs coronary computed tomographic angiography for diagnosis of invasive vessel-specific coronary physiology: predictive modeling results from the computed tomographic evaluation of atherosclerotic determinants of myocardial ischemia (CREDENCE) trial. JAMA Cardiol. 2020;5(12):1338–48. doi: 10.1001/jamacardio.2020.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Koo BK, Hwang D, Zhang J, Hoshino M, Lee JM, Murai T, Park J, Shin ES, Doh JH, Nam CW, Wang J, Chen S, Tanaka N, Matsuo H, Akasaka T, Chang HJ, Kakuta T, Narula J. High-Risk Morphological and Physiological Coronary Disease Attributes as Outcome Markers After Medical Treatment and Revascularization. JACC Cardiovasc Imaging. 2021;14:1977–89. doi: 10.1016/j.jcmg.2021.04.004. [DOI] [PubMed] [Google Scholar]
- Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) coronary artery disease -reporting and data system. An expert consensus document of the Society of Cardiovascular computed tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular imaging (NASCI). Endorsed by the American College of cardiology. J Cardiovasc Comput Tomogr. 2016;10(4):269–81. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925–32. doi: 10.1093/eurheartj/ehaa652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen, Leipsic JA, Knuuti J, Underwood SR, van de, van Rossum, Taylor CA, Knaapen P. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J Am Coll Cardiol. 2019;73:161–73. doi: 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Patel MR, Nørgaard BL, Fairbairn TA, et al. 1-Year impact on medical practice and clinical outcomes of CT-FFR: the ADVANCE registry. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):97–105. doi: 10.1016/j.jcmg.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Fairbairn TA, Nieman K, Akasaka T, et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE registry. Eur Heart J. 2018;39(41):3701–11. doi: 10.1093/eurheartj/ehy530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Miyazaki Y, Ryan N, Asano T, Tenekecioglu E, Sonck J, Andreini D, Sabate M, Brugaletta S, Stables RH, Bartorelli A, de Winter, Katagiri Y, Chichareon P, De Maria, Suwannasom P, Cavalcante R, Jonker H, Morel MA, Cosyns B, Kappetein AP, Taggart DT, Farooq V, Escaned J, Banning A, Onuma Y, Serruys PW. Fractional Flow Reserve Derived From Computed Tomographic Angiography in Patients With Multivessel CAD. J Am Coll Cardiol. 2018;71:2756–69. doi: 10.1016/j.jacc.2018.02.053. [DOI] [PubMed] [Google Scholar]
- Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S, La Meir, Miyazaki Y, De Mey, Gaemperli O, Ouda A, Maureira JP, Mandry D, Camenzind E, Macron L, Doenst T, Teichgräber U, Sigusch H, Asano T, Katagiri Y, Morel MA, Lindeboom W, Pontone G, Lüscher TF, Bartorelli AL, Serruys PW. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018;39:3689–98. doi: 10.1093/eurheartj/ehy581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, Argacha JF, Schoors D, Colaiori I, Di Gioia, Kodeboina M, Suzuki H, Van ’t, Bartunek J, Barbato E, Cosyns B, De Bruyne. Measurement of Hyperemic Pullback Pressure Gradients to Characterize Patterns of Coronary Atherosclerosis. J Am Coll Cardiol. 2019;74:1772–84. doi: 10.1016/j.jacc.2019.07.072. [DOI] [PubMed] [Google Scholar]
- Mizukami T, Tanaka K, Sonck J, et al. Evaluation of epicardial coronary resistance using computed tomography angiography: a proof concept. J Cardiovasc Comput Tomogr. 2020;14(2):177–84. doi: 10.1016/j.jcct.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Lodi Rizzini, Nagumo S, Gallo D, et al. Mismatch between morphological and functional assessment of the length of coronary artery disease. Int J Cardiol. 2021;334:1–9. doi: 10.1016/j.ijcard.2021.04.046. [DOI] [PubMed] [Google Scholar]
- Norgaard BL, Fairbairn TA, Safian RD, et al. CCTA-Derived fractional flow reserve testing in patients with stable coronary disease- recommendations on interpretation and reporting. Radiol Cardiothorac Imaging. 2019;1(5):e190050. doi: 10.1148/ryct.2019190050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh SH, Mooney J, Ohana M, et al. Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J Cardiovasc Comput Tomogr. 2017;11(6):462–67. doi: 10.1016/j.jcct.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Takagi H, Ishikawa Y, Orii M, et al. Optimized interpretation of fractional flow reserve derived from computed tomography: comparison of three interpretation methods. J Cardiovasc Comput Tomogr. 2019;13(2):134–41. doi: 10.1016/j.jcct.2018.10.027. [DOI] [PubMed] [Google Scholar]
- Ihdayhid AR, Norgaard BL, Gaur S, et al. Prognostic value and risk continuum of noninvasive fractional flow reserve derived from coronary CT angiography. Radiology. 2019;292(2):343–51. doi: 10.1148/radiol.2019182264. [DOI] [PubMed] [Google Scholar]
- Nagumo S, Collet C, Norgaard BL, et al. Rationale and design of the precise percutaneous coronary intervention plan (P3) study: prospective evaluation of a virtual computed tomography-based percutaneous intervention planner. Clin Cardiol. 2021;44(4):446–54. doi: 10.1002/clc.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonck Jeroen. Clinical validation of a virtual coronary interventions planner. https://www.pcronline.com/Courses/EuroPCR/Programme/Late-Breaking-Trials.
- Modi BN, van de Hoef TP , Piek JJ, Perera D. Physiological assessment of left main coronary artery disease. EuroIntervention. 2017;13(7):820–27. doi: 10.4244/EIJ-D-17-00135. [DOI] [PubMed] [Google Scholar]
- Takx RA, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8(1):e002666. doi: 10.1161/CIRCIMAGING.114.002666. [DOI] [PubMed] [Google Scholar]
- Coenen A, Rossi A, Lubbers MM, et al. Integrating CT myocardial perfusion and CTFFR in the work-up of coronary artery disease. JACC Cardiovasc Imaging. 2017;10:760–70. doi: 10.1016/j.jcmg.2016.09.028. [DOI] [PubMed] [Google Scholar]
- Pontone G, Baggiano A, Andreini D, et al. Dynamic stress computed tomography perfusion with a whole-heart coverage scanner in addition to coronary computed tomography angiography and fractional flow reserve computed tomography derived. JACC Cardiovasc Imaging. 2019;12(12):2460–71. doi: 10.1016/j.jcmg.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Lubbers M, Coenen A, Kofflard M, et al. Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: the multicenter, randomized CRESCENT-II trial. JACC Cardiovasc Imaging. 2018;11:1625–36. doi: 10.1016/j.jcmg.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Sørgaard MH, Linde JJ, Kühl JT, et al. Value of myocardial perfusion assessment with coronary computed tomography angiography in patients with recent acute-onset chest pain. JACC Cardiovasc Imaging. 2018;11(11):1611–21. doi: 10.1016/j.jcmg.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Moschovitis A, Cook S, Meier B. Percutaneous coronary interventions in Europe in 2006. EuroIntervention. 2010;6:189–94. doi: 10.4244/EIJV7I6A31. [DOI] [PubMed] [Google Scholar]
- Schuetz GM, Walther S, Schlattmann P. Meta-analysis: noninvasive angiography by computed tomography for the evaluation of coronary stents. http://www.cardiacctjournal.com/issues.
- Park DW, Kim YH, Yun SC, et al. Complexity of atherosclerotic coronary artery disease and long-term outcomes in patients with unprotected left main disease treated with drug-eluting stents or coronary artery bypass grafting. J Am Coll Cardiol. 2011;57(21):2152–59. doi: 10.1016/j.jacc.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Mushtaq S, Conte E, Pontone G. State-of-the-art-myocardial perfusion stress testing: Static CT perfusion. J Cardiovasc Comput Tomogr. 2019 Sep 12; doi: 10.1016/j.jcct.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Andreini D, Mushtaq S, Pontone G, et al. Additional diagnostic value of CT perfusion over coronary CT angiography in patients with suspected in-stent restenosis or coronary artery disease progression the ADVANTAGE prospective study. JACC Img. 2020;13(3):732–42. doi: 10.1016/j.jcmg.2019.05.031. [DOI] [PubMed] [Google Scholar]
- Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh, Alaswad K, Araya M, Avran A, Azzalini L, Babunashvili AM, Bayani B, Bhindi R, Boudou N, Boukhris M, Božinović NŽ, Bryniarski L, Bufe A, Buller CE, Burke MN, Büttner HJ, Cardoso P, Carlino M, Christiansen EH, Colombo A, Croce K, Damas de, De Martini, Dens J, Di Mario, Dou K, Egred M, ElGuindy AM, Escaned J, Furkalo S, Gagnor A, Galassi AR, Garbo R, Ge J, Goel PK, Goktekin O, Grancini L, Grantham JA, Hanratty C, Harb S, Harding SA, Henriques JPS, Hill JM, Jaffer FA, Jang Y, Jussila R, Kalnins A, Kalyanasundaram A, Kandzari DE, Kao HL, Karmpaliotis D, Kassem HH, Knaapen P, Kornowski R, Krestyaninov O, Kumar AVG, Laanmets P, Lamelas P, Lee SW, Lefevre T, Li Y, Lim ST, Lo S, Lombardi W, McEntegart M, Munawar M, Navarro Lecaro, Ngo HM, Nicholson W, Olivecrona GK, Padilla L, Postu M, Quadros A, Quesada FH, Prakasa Rao, Reifart N, Saghatelyan M, Santiago R, Sianos G, Smith E, C Spratt, Stone GW, Strange JW, Tammam K, Ungi I, Vo M, Vu VH, Walsh S, Werner GS, Wollmuth JR, Wu EB, Wyman RM, Xu B, Yamane M, Ybarra LF, Yeh RW, Zhang Q, Rinfret S. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation. 2019;140:420–33. doi: 10.1161/CIRCULATIONAHA.119.039797. [DOI] [PubMed] [Google Scholar]
- Tsai TT, Stanislawski MA, Shunk KA, et al. Contemporary incidence, management, and long-term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: insights from the VA CART program. JACC Cardiovasc Interv. 2017;10:866–75. doi: 10.1016/j.jcin.2017.02.044. [DOI] [PubMed] [Google Scholar]
- Opolski MP, Hartaigh BO, Berman DS, et al. Current trends in patients with chronic total occlusions undergoing coronary CT angiography. Heart. 2015;101:1212–18. doi: 10.1136/heartjnl-2014-306616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello SD, Boukhris M, Giubilato S, Marzà F, Garbo R, Contegiacomo G, Marzocchi A, Niccoli G, Gagnor A, Varbella F, Desideri A, Rubartelli P, Cioppa A, Baralis G, Galassi AR. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. 2015;36:3189–98. doi: 10.1093/eurheartj/ehv450. [DOI] [PubMed] [Google Scholar]
- Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, Rumoroso JR, Erglis A, Christiansen EH, Escaned J, di Mario, Hovasse T, Teruel L, Bufe A, Lauer B, Bogaerts K, Goicolea J, Spratt JC, Gershlick AH, Galassi AR, Louvard Y EUROCTO trial investigators. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484–93. doi: 10.1093/eurheartj/ehy220. [DOI] [PubMed] [Google Scholar]
- Elias J, van Dongen, Ramunddal T, et al. Long-term impact of chronic total occlusion recanalisation in patients with ST-elevation myocardial infarction. Heart. 2018;104:1432–38. doi: 10.1136/heartjnl-2017-312698. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Wyman RM, Alaswad K, et al. Clinical utility of the Japan-chronic total occlusion score in coronary chronic total occlusion interventions. Circ Cardiovasc Interv. 2015;8:e002171. doi: 10.1161/CIRCINTERVENTIONS.114.002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JR, Kim YJ, Ahn CM, et al. Quantification of regional calcium burden in chronic total occlusion by 64-slice multi-detector computed tomography and procedural outcomes of percutaneous coronary intervention. Int J Cardiol. 2010;145:9–14. doi: 10.1016/j.ijcard.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Ehara M, Terashima M, Kawai M, et al. Impact of multislice computed tomography to estimate difficulty in wire crossing in percutaneous coronary intervention for chronic total occlusion. J Invasive Cardiol. 2009;21:575–82. [PubMed] [Google Scholar]
- García-García HM, van Mieghem, Gonzalo N, et al. Computed tomography in total coronary occlusions (CTTO registry): radiation exposure and predictors ofsuccessful percutaneous intervention. EuroIntervention. 2009;4:607–16. doi: 10.4244/eijv4i5a102. [DOI] [PubMed] [Google Scholar]
- Luo C, Huang M, Li J, et al. Predictors of interventional success of antegrade PCI for CTO. J Am Coll Cardiol Cardiovasc Imaging. 2015;8:804–13. doi: 10.1016/j.jcmg.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Opolski MP, Achenbach S, Schuhbäck A, Rolf A, Möllmann H, Nef H, Rixe J, Renker M, Witkowski A, Kepka C, Walther C, Schlundt C, Debski A, Jakubczyk M, Hamm CW. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion: insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc Interv. 2015;8:257–67. doi: 10.1016/j.jcin.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Yu CW, Lee HJ, Suh J, Lee NH, Park SM, Park TK, Yang JH, Song YB, Hahn JY, Choi SH, Gwon HC, Lee SH, Choe YH, Kim SM, Choi JH. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion: Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Imaging. 2017;10:e005800. doi: 10.1161/CIRCIMAGING.116.005800. [DOI] [PubMed] [Google Scholar]
- Fujino A, Otsuji S, Hasegawa K, et al. Accuracy of J-CTO score derived from computed tomography versus angiography to predict successful percutaneous coronary intervention. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):209–17. doi: 10.1016/j.jcmg.2017.01.028. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Kim BK, Cho I, Kim HY, Rha SW, Lee SH, Park SM, Kim YH, Chang HJ, Ahn CM, Kim JS, Ko YG, Choi D, Hong MK, Jang Y CT-CTO Investigators. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention: A Randomized Trial. JACC Cardiovasc Imaging. 2021;14:1993–2004. doi: 10.1016/j.jcmg.2021.04.013. [DOI] [PubMed] [Google Scholar]
- Ghoshhajra BB, Takx RAP, Stone LL, et al. Real-time fusion of coronary CT angiography with x-ray fluoroscopy during chronic total occlusion PCI. Eur Radiol. 2017;27:2464–73. doi: 10.1007/s00330-016-4599-5. [DOI] [PubMed] [Google Scholar]
- Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63(2):285–99. doi: 10.1161/01.cir.63.2.285. [DOI] [PubMed] [Google Scholar]
- Nam CW, Mangiacapra F, Entjes R, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011;58(12):1211–8. doi: 10.1016/j.jacc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Asano T, Katagiri Y, Chang CC, Kogame N, Chichareon P, Takahashi K, Modolo R, Tenekecioglu E, Collet C, Jonker H, Appleby C, Zaman A, van Mieghem, Uren N, Zueco J, Piek JJ, Reiber JHC, Farooq V, Escaned J, Banning AP, Serruys PW, Onuma Y. Angiography-Derived Fractional Flow Reserve in the SYNTAX II Trial: Feasibility, Diagnostic Performance of Quantitative Flow Ratio, and Clinical Prognostic Value of Functional SYNTAX Score Derived From Quantitative Flow Ratio in Patients With 3-Vessel Disease. JACC Cardiovasc Interv. 2019;12:259–70. doi: 10.1016/j.jcin.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Escaned J, Collet C, Ryan N, De Maria, Walsh S, Sabate M, Davies J, Lesiak M, Moreno R, Cruz-Gonzalez I, Hoole SP, Ej West, Piek JJ, Zaman A, Fath-Ordoubadi F, Stables RH, Appleby C, van Mieghem, van Geuns, Uren N, Zueco J, Buszman P, Iñiguez A, Goicolea J, Hildick-Smith D, Ochala A, Dudek D, Hanratty C, Cavalcante R, Kappetein AP, Taggart DP, van Es, Morel MA, de Vries, Onuma Y, Farooq V, Serruys PW, Banning AP. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J. 2017;38:3124–34. doi: 10.1093/eurheartj/ehx512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYNTAX score working-group. SYNTAX score calculator. http://www.syntaxscore.com.
- Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, Colombo A, Holmes DR, Jr, Mack M, Feldman T, Morice MC, Ståhle E, Onuma Y, Morel MA, Garcia-Garcia HM, van Es GA, Dawkins KD, Mohr FW, Serruys PW. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–50. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- Thuijs DJFM, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ, Holmes DR, Curzen N, Davierwala P, Noack T, Milojevic M, Dawkins KD, da Costa, Jüni P, Head SJ SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. 2019;394:1325–34. doi: 10.1016/S0140-6736(19)31997-X. [DOI] [PubMed] [Google Scholar]
- Papadopoulou SL, Girasis C, Dharampal A, et al. CT-SYNTAX score: a feasibility and reproducibility Study. JACC Cardiovasc Imaging. 2013;6(3):413–15. doi: 10.1016/j.jcmg.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Sonck J, Miyazaki Y, Collet C, et al. Feasibility of planning coronary artery bypass grafting based only on coronary computed tomography angiography and CTderived fractional flow reserve: a pilot survey of the surgeons involved in the randomized SYNTAX III revolution trial. Interact CardiovascThorac Surg. 2019;29:209–16. doi: 10.1093/icvts/ivz046. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Pompilio G, Andreini D, et al. Safety and feasibility evaluation of planning and execution of surgical revascularisation solely based on coronary CTA and CT-FFR in patients with complex coronary artery disease: study protocol of the FASTTRACK CABG study. BMJ Open. 2020;10(12):e038152. doi: 10.1136/bmjopen-2020-038152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini D, Modolo R, Katagiri Y, et al. Impact of fractional flow reserve derived from coronary computed tomography angiography on heart team treatment decision making in patients with multivessel coronary artery disease: insights from the SYNTAX III REVOLUTION trial. Circ Cardiovasc Interv. 2019;12(12):e007607. doi: 10.1161/CIRCINTERVENTIONS.118.007607. [DOI] [PubMed] [Google Scholar]
- Nykonenko A, Feuchtner G, Nykonenko O. Feasibility of coronary CT angiography for guidance of CABG. J Cardiovasc Comput Tomogr. 2020 Sep 18; doi: 10.1016/j.jcct.2020.09.005. [DOI] [PubMed] [Google Scholar]
- Gigante C, Mizukami T, Sonck J, et al. Graft patency and progression of coronary artery disease after CABG assessed by angiography-derived fractional flow reserve. Int J Cardiol. 2020;316:19–25. doi: 10.1016/j.ijcard.2020.04.083. [DOI] [PubMed] [Google Scholar]
- Pursnani A, Jacobs JE, Saremi F, et al. Coronary CTA assessment of coronary anomalies. J Cardiovasc Comput Tomogr. 2012;6(1):48–59. doi: 10.1016/j.jcct.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Kočka V, Thériault-Lauzier P, Xiong TY, Ben-Shoshan J, Petr R, Laboš M, Buithieu J, Mousavi N, Pilgrim T, Praz F, Overtchouk P, Beaudry JP, Spaziano M, Pelletier JP, Martucci G, Dandona S, Rinfret S, Windecker S, Leipsic J, Piazza N. Optimal Fluoroscopic Projections of Coronary Ostia and Bifurcations Defined by Computed Tomography Coronary Angiography. JACC Cardiovasc Interv. 2020;13:2560–70. doi: 10.1016/j.jcin.2020.06.042. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee JM, Song YB. Prediction of side branch occlusions in percutaneous coronary interventions by coronary computed tomography: the CT bifurcation score as a novel tool for predicting intraprocedural side branch occlusion. EuroIntervention. 2019;15(9):e788–95. doi: 10.4244/EIJ-D-18-00113. [DOI] [PubMed] [Google Scholar]
- Opolski MP, Achenbach S. CT angiography for revascularization of CTO: crossing the borders of diagnosis and treatment. JACC Cardiovasc Imaging. 2015;8(7):846–58. doi: 10.1016/j.jcmg.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Uetani T, Amano T, Kunimura A, et al. The association between plaque characterization by CT angiography and post-procedural myocardial infarction in patients with elective stent implantation. JACC Cardiovasc Imaging. 2010;3(1):19–28. doi: 10.1016/j.jcmg.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Wink O, Hecht HS, Ruijters D. Coronary computed tomographic angiography in the cardiac catheterization laboratory: current applications and future developments. Cardiol Clin. 2009;27(3):513–29. doi: 10.1016/j.ccl.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Andreini D, Mushtaq S, Pontone G, Rogers C, Pepi M, Bartorelli AL. Severe in-stent restenosis missed by coronary CT angiography and accurately detected with CT-FFR. Int J Cardiovasc Imaging. 2017;33(1):119–20. doi: 10.1007/s10554-016-0971-4. [DOI] [PubMed] [Google Scholar]
- Andreini D, Onuma Y, Bartorelli AL, Serruys PW. The time has come to use coronary CT angiography in patients with multivessel coronary artery disease. Eur Heart J. 2019 Feb 26; doi: 10.1093/eurheartj/ehz070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author relationships with industry - pre-procedural planning of coronary revascularization by cardiac-CT.
Reviewer relationships with industry - pre-procedural planning of coronary revascularization by cardiac-CT.