Highlights
-
•
We firstly explored exogenous GABA could improve the quality of edamame.
-
•
Exogenous GABA improved edamame quality through enhancing endogenous GABA metabolism.
-
•
Exogenous GABA promoted the accumulation of amino acids and bioactive peptides.
-
•
Exogenous GABA enhanced flavonoids, sugars, and organic acid for edamame quality.
-
•
Exogenous GABA stimulated oxoglutarate to enter amino acid metabolism through GABA-T.
Abbreviations: GABA, γ-aminobutyric acid; GAD, glutamate decarboxylase; GABA-T, γ-aminobutyric acid transferase; Vc, vitamin C; GSH, glutathione; GR, glutathione reductase; γ-ECS, γ-glutamylcysteine synthetase; TCA cycle, tricarboxylic acid cycle; PAL, phenylalanine ammonia-lyase; CHS, chalcone synthase; CHI, chalcone isomerase; IFS, isoflavone synthase; UFGT, UDP-glycose flavonoid glycosyltransferase; ANS, anthocyanidin synthase; 4CL, 4-coumarate coenzyme A ligase; C4H, cinnamic acid 4-hydroxylase; DFR, dihydroflavanol 4-reductase; F3′H, flavanone 3′-hydroxylase; F3H, flavanone 3-hydroxylase; NR, nitrate reductase; GS, glutamine synthetase; GOGAT, glutamate synthase; GDH, glutamate dehydrogenase; GOT, glutamic-oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase
Keywords: Edamame, γ-Aminobutyric acid, Nutritional quality, Nitrogen metabolism, Flavonoid metabolism
Abstract
γ-aminobutyric acid (GABA) has been reported to improve stress resistance in plants. Nonetheless, little is known about the effects of GABA on the nutritional quality and regulatory mechanisms of edamame. Therefore, we analyzed the flavonoid and amino acid (AA) metabolism and the effects of GABA on the nutrient content of edamame seeds through physiological and metabolomic analyses. Exogenous GABA increased endogenous GABA metabolism and GABA transaminase activity and enhanced the oxoglutarate content, which entered into nitrogen metabolism and increased the activity and expression of nitrogen metabolism-related enzymes, to accumulate AAs and bioactive peptides. Meanwhile, exogenous GABA induced the metabolism of flavonoids, including total flavonoids, anthocyanins, 6′'-o-malonyglycitin, glycitin, ononin, cyanin, and ginkgetin, by increasing the activity and expression of flavonoid biosynthetic enzymes. This is the first study to reveal that GABA effectively improves the nutritional quality of edamame through the accumulation of AAs, bioactive peptides, isoflavones, anthocyanins, sugars, and organic acids.
Introduction
Edamame [Glycine max (L.) Merr.], also called “vegetable soybean,” “green edamame,” or “vegetable-type soybean,” is harvested when the pods and seeds are still green (R6 growth stage) (Fehr, 1971). Edamame is popularly consumed as a cooked or roasted snack and is also consumed as an addition to salads, soups, stews, stir-fried dishes, processed sweets, and desserts (Song et al., 2013).
With the improvement in living standards, nutrition has garnered increased scientific attention. Previous studies revealed that the content of nutrients, including free amino acids (4.581–10.180 mg g−1), sucrose (8.2 g 100 g−1), isoflavones (27.02 mg 100 g−1), and oil (212.6 g kg−1), in edamame seeds was higher than that in soybean grains (Song et al., 2013, Carneiro et al., 2021, Jiang et al., 2020).
Amino acids are the primary metabolites that play a vital role in growth, development, and health maintenance and serve as building blocks of proteins and polypeptides exhibiting a wide range of biological activities, including hypolipidemic, anti-diabetic, anti-hypertensive, and anti-cancerous properties (Daliri, Oh, & Lee, 2017). Additionally, exogenous reagents have been known to promote the accumulation of amino acids by affecting the expression and activity of enzymes involved in nitrogen metabolism (Wang et al., 2014, Wang et al., 2022, Zhang et al., 2020).
Flavonoids, including isoflavonoids, chalcones, flavones, flavonols, flavanones, and anthocyanins, are important phenolic secondary metabolites in plants synthesized through the phenylpropane metabolic pathway (Chen, Zhang, Zhang, Li, & Ma, 2019). They promote disease resistance in plants and exhibit anti-oxidant, anti-cancerous, and anti-aging properties in humans (Chen et al., 2019). However, they can also be modulated by multiple exogenous agents, as they are synthesized using a complex biochemical process involving different signaling pathways (Zhao et al., 2021, Jia et al., 2019).
Song et al. (2013) determined that edamame contained 23 amino acids, including all the essential amino acids. In addition, edamame is an excellent dietary source of natural flavonoids (Wang et al., 2018), whose content was higher in edamame than in other legumes (Kim et al., 2006). Edamame is also rich in organic acids, vitamins (C, E, and B1), minerals, phytochemicals, and other active compounds that have the potential to reduce the risk of several diseases such as cardiovascular disease, cancer, and osteoporosis (Jiang et al., 2020). Nevertheless, the mechanisms underlying the regulation of nutritional content in edamame have received limited attention. Therefore, we focused on the nutritional quality and regulatory mechanisms in edamame.
γ-aminobutyric acid (GABA) is a ubiquitous non-proteinogenic amino acid found in bacteria, fungi, plants, and animals and exhibits anti-hypertensive and anti-stress effects on human health (Ma, Wang, Chen, Gu, & Yang, 2018). As a functional food ingredient, GABA has been enriched in a myriad of foods, such as cereal-based foods, dairy products, beverages, meat, vegetables, and legumes, through lactic acid bacteria fermentation or manipulation of plant food material (Diana, Quílez, & Rafecas, 2014). In addition, GABA has been used as a food additive or supplement (Boonstra et al., 2015). While GABA constitutes a considerable portion of the free amino acid pool in plants, it is mainly synthesized and metabolized via the GABA shunt and catalyzed by glutamate (Glu) decarboxylase (GAD) and GABA transaminase (GABA-T) (Ma et al., 2018).
Exogenous GABA enhances resistance to hypoxia in melon and storage performance of postharvest citrus fruits by promoting the tricarboxylic acid (TCA) cycle and the accumulation of endogenous amino acids (Wang et al., 2014, Sheng et al., 2017). Moreover, as a key signaling molecule, GABA is involved in NaCl stress in barley (Ma et al., 2018) and soybean (Zhao et al., 2021), suggesting that exogenous GABA can upregulate the activity and expression of phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate coenzyme A ligase (4CL) to accumulate anthocyanins, phenolic compounds, and flavonoids. In addition, exogenous GABA can enhance the content of amino acids and soluble sugars in snap beans to ameliorate drought stress (Abd El-Gawad et al., 2021). However, there have been no reports on the direct effects of GABA on the quality of crops, especially edamame.
Therefore, we determined the effects of GABA on flavonoid and amino acid metabolism and the contents of sugars, vitamin C, organic acid, and other substances in edamame seeds using physiological and metabolomic methods for the first time to explore the how GABA regulates the nutritional quality of edamame.
2. Materials and methods
2.1. Plant growth conditions
Six treatments were established to screen for suitable concentrations of GABA (CAS NO.56–12-2, Sigma-Aldrich Co., ltd. CA). The leaves of edamame at the R1 stage were sprayed with 0, 2.5, 5, 10, 20, and 30 mmol/L (mM) of GABA. For each treatment, the leaves of the second or the third leaves from the top were harvested with three replicates after 6 days. Among them, the content of nutrients (including GABA, sucrose, and vitamin C), and the enzyme activity of glutamine synthetase (GS), glutamic-oxaloacetic transaminase (GOT), glutamic acid decarboxylase (GAD), and γ-aminobutyric acid transferase (GABA-T) related to nitrogen metabolism in edamame plants were determined (data not shown), and foliar application of 10 mM GABA exerted maximum benefits for phenotype. Subsequently, 10 mM GABA was used for experiments at the R5 stage to determine the effects of GABA on the nutrient content of edamame. The enzyme activity of leaves and substance content of seeds of plants treated after 6, 9, 12, 18, and 24 days were assayed. In addition, at samples at 12 h, 24 h and 3 d after treatments were used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis, and stored at −80 °C until analysis. Three biological replicates were used for all the test.
2.2. Determination of sugar content
All substances were extracted from fresh seeds (2.0 g). The content of sucrose, glucose, and fructose was assayed based on the methodologies of Du et al., 2020, Li et al., 2016. Sample was extracted with 80 % (v/v) ethanol (CAS NO. 64–17-5, Sigma-Aldrich Co., ltd. CA) at 80 °C for 30 min, followed by centrifugation at 10,000 × g for 10 min. The residue was extracted three times with 80 % ethanol. The three supernatants were combined and added with 80 % ethanol to a total volume of 5 mL, and then the content of sucrose, glucose, and fructose were determined using spectrophotometric method at the wavelength of A480, A625, and A291 nm, respectively.
2.3. Assay of the content of Vc and glutathione (GSH)
The content of Vc and GSH in edamame seeds were estimated by homogenizing fresh sample tissue at 5 % (w/v) metaphosphoric acid (CAS NO. 37267–86-0, Sigma-Aldrich Co., ltd. CA) and 5 % (w/v) sulpho-salicylic acid (CAS NO. 97–05-2, Sigma-Aldrich Co., ltd. CA), respectively (The content of GSH and Vc was estimated following the method of Jiao, Yang, Zhou, and Gu (2016).
2.4. Detection of the content of amino acids and related enzyme activity
The concentration of GABA and free amino acids was estimated following the method of Priya et al. (2019). The content of free amino acids was determined with ninhydrin (CAS NO. 485–47-2, Sigma-Aldrich Co., ltd. CA) reagent according to the method of Wang et al., 2014), respectively.
All samples for determination of enzymes activity were extracted from fresh leaves (0.3 g). The activity of nitrate reductase (NR), glutamine synthetase (GS), glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH) was determined according to the method of Wang et al. (2014). The activity of glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) was assayed according to Kaur’ s. (2015) method. The activity of glutamate decarboxylase (GAD) and GABA transaminase (GABA-T) was assayed according to Li et al. (2016).
2.5. Determination of flavonoids and related enzyme activity
The content of total flavonoid and total anthocyanins was determined using the method of Jia et al. (2019). The activity of phenylalanine ammonia lyase (PAL), chalcone isomerase (CHI), anthocyanidin synthase (ANS), dihydroflavanol 4-reductase (DRF), and UDP-glycose flavonoid glycosyltransferase (UFGT) was assayed following the methods described by Chen et al. (2019). The activity of cinnamate-4-hydroxylase (C4H) and 4-coumarateCoA ligase (4CL) was measured according to Ma et al. (2018). The activity of isoflavone synthase (IFS) and chalcone synthase (CHS) was determined by enzyme-linked immunoassay, using the IFS and CHS assay system kit (GE Healthcare), as described in the manufacturer’s instructions.
2.6. Sample preparation for LC–MS
The samples of edamame seeds treated with H2O (CK) and exogenous GABA for 18 days were grounded and mixed and then sent to BmK company for nontargeted LC-mass spectrometry (MS) detection. Each treatment was set up for 5 replicates. Data analysis was performed using BMK Cloud (www.biocloud.net).
2.7. RNA extraction and qRT-PCR assays
TRIzol® reagent (Invitrogen, Carlsbad, CA, United States) was used to extract total RNA. One microgram per RNA sample was used as the template for synthesis of the first-strand cDNA, using the ReverTra Ace™ qPCR RT Master Mix with gDNA Remover (TOYOBO Co., Osaka, Japan). Next, qRT-PCR was performed using SYBR® Select Master Mix qRT-PCR System (Takara) on an optical 96-well plate. Actin was used as an internal reference. All the primers used for gene expression analysis were shown in supplementary Table S1. The relative expression level was calculated with the formula 2−ΔΔCT (Livak & Schmittgen, 2001). Three independent biological replicates were analyzed.
2.8. Statistical analysis
At least three independent experiments were performed for each treatment. Data were expressed as mean ± standard error (SE) after a one-way analysis of variance (ANOVA) using the SPSS 17.0 program (SPSS Inc. Chicago, IL, United States). The data were statistically analyzed using the multiple range test of Duncan (P < 0.05). The PCA and OPLS-DA were performed using SIMCA v13.0.3 software (Umetrics, Umea, Sweden).
3. Results and discussion
3.1. Effects of exogenous GABA on nutritional content in edamame
Edamame contains nutrients such as carbohydrates, proteins, vitamins, minerals, and flavonoids (Jiang et al., 2020). Previous studies suggest that exogenous GABA can enhance the nutrient content in snap beans to improve drought stress resistance (Abd El-Gawad et al., 2021, Sheng et al., 2017) and might positively affect the storage quality of postharvest apple and citrus fruits (Li et al., 2021). In our study, we determined the contents of nine nutrients (GABA, free amino acids, glutathione (GSH), flavonoids, anthocyanins, vitamin C (Vc), glucose, sucrose, and fructose) in edamame seeds and revealed that exogenous GABA contributed to nutrient accumulation in edamame, especially endogenous GABA and glucose (Fig. 1).
Fig. 1.
The contents of GABA (A), free amino acids (B), GSH (C), flavonoid (D), anthocyanins (E), Vc (F), glucose (G), sucrose (H), and fructose (I)) in edamame fruit after 6, 9, 12, 18, and 24 d of H2O (control, CK) and GABA treatment. Data represent mean ± SE (n = 3). Asterisks (*) represent significant differences (Student’s t-test, P < 0.05) between control and GABA treatments.
Compared to the control, the content of endogenous GABA significantly increased by 22.4 %, 29.8 %, 59.6 %, and 18.2 % after 6, 9, 12 and 18 d of GABA foliar application, and glucose significantly increased by 38.6 %, 25.2 %, and 19.5 % after 9, 18, and 24 d of exogenous GABA treatment, respectively (Fig. 1A, 1G). In addition, the content of GSH increased by 21.7 % and 22.0 %, anthocyanins by 23.3 % and 88.0 %, and Vc by 22.0 % and 39.4 % after 18 and 24 d of exogenous GABA treatment, respectively (Fig. 1C, 1F). Moreover, compared to the control, the content of free amino acids markedly increased by 33.4 % and 92.4 % after 6 and 18 d of treatment, respectively (Fig. 1B). Exogenous GABA treatment remarkably enhanced total flavonoids by 38.0 % after 18 d and fructose content by 18.8 % after 12 d of treatment (Fig. 1D, 1I).
Overall, the contents of GABA, free amino acids, GSH, flavonoids, anthocyanins, Vc, and glucose significantly increased after 18 d of treatment, with the former five reaching maxima at 18 d after exogenous GABA treatment (Fig. 1A–G). Therefore, LC-MS analysis was performed using seeds after 18 d of treatment.
3.2. Non-targeted metabolomics analysis of edamame
To further determine the effects of exogenous GABA on the nutritional quality of edamame, we used LC–MS to identify the metabolites present in edamame seeds after 18 d of treatment with H2O (control, CK) and GABA. The total ion current (TIC) for the QC sample showed that the TIC metabolite detection curves exhibited a high degree of overlap (Supplementary Figures 1A, 1B), indicating that metabolite extraction and detection were reliable. This was important because unsupervised principal component analysis (PCA) of metabolites reflects the variability between and within sample groups. The scores of the sum of the first and second principal components of CK and GABA groups were greater than 75 % (Fig. 2A, 2B, Supplementary Figures 1C, 1D). Furthermore, OPLS-DA models indicated that R2Y was greater than 0.99 and Q2Y was greater than 0.85 in the positive and negative ion modes, which indicated good fitness and prediction of the experiment, respectively (Fig. 2C, 2D). In addition, the slope of the Q2Y fitting regression curve was greater than zero in permutation, illustrating the statistical validity of the OPLS-DA model (Fig. 2E, 2F).
Fig. 2.
Principal component analysis and PCA 2D score of CK and GABA-treated samples (A, B). The X-axis represents the first principal component, and Y-axis represents the second principal component. Point diagrams of OPLS-DA of the samples and model permutation verification diagram (C–F). A, C, and E are positive ion modes, whereas B, D, and F are negative ion modes. Note: Blue and red dots represent R2Y and Q2Y of the model after replacement, respectively. The two dotted lines indicate the regression curves of R2Y and Q2Y fitting in the permutation verification diagram. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Analysis of differentially accumulated metabolites (DAMs)
First, we analyzed the DAMs in the CK and GABA-treated edamame seeds in positive and negative ion modes. We identified 1,198 metabolites (Supplementary Table 2) and 284 DAMs with a fold change (FC) ≥ 1 or ≤ 0.5 and VIP ≥ 1. Compared to the CK, a total of 151 and 133 metabolites were significantly upregulated and downregulated in the GABA-treated group, respectively. These metabolites mainly included flavonoids, amino acids, unsaturated fatty acids, and bioactive peptides (Supplementary Fig. 2).
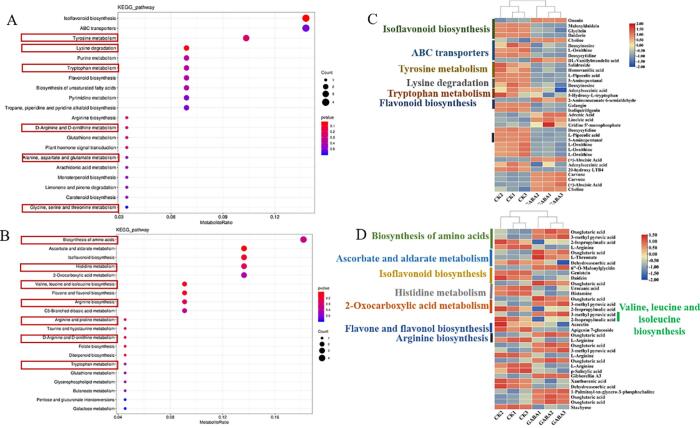
To identify the top 20 pathways involving DAMs in CK and GABA-treated edamame seeds, we conducted KEGG pathway enrichment analysis of the data from positive and negative ion modes (Fig. 3). The DAMs in edamame seeds were notably enriched in isoflavonoid biosynthesis (7 DAMs), particularly ononin and 6′'-o-malonylglycitin. Acacetin and galangin were enriched in flavonoid biosynthesis, and isoliquiritigenin and apigenin 7-glucoside were enriched in flavone and flavonol biosyntheses (Fig. 3C, 3D). As the enriched pathway is primarily involved in the biosynthesis of flavonoids, exogenous GABA significantly affected flavonoid biosynthesis in edamame.
Fig. 3.
Enrichment map of differential metabolite in the positive (A) and negative ion modes (B). Analysis of DAMs in each pathway in the positive (C) and negative ion modes (D) (n = 3).
In addition, DAMs were enriched in 13 pathways related to amino acid biosynthesis and metabolism (marked with red boxes in Fig. 3A, 3B). In these pathways, oxoglutaric acid, 3-methyl pyruvic acid, dl-vanillylmandelic acid, and 2-aminomuconate 6-semialdehyde were most significantly upregulated, suggesting that exogenous GABA significantly affected the amino acid biosynthesis and metabolism in edamame (Fig. 3C, 3D).
3.4. Effects of exogenous GABA on the metabolism of amino acids and their derivatives in edamame
GABA is synthesized from Glu by irreversible decarboxylation, which is catalyzed by glutamate decarboxylase (GAD) (Ma et al., 2018). Subsequently, GABA transaminase (GABA-T) and succinate semialdehyde dehydrogenase (SSADH) convert GABA into succinate, which then enters into the TCA cycle, namely the GABA shunt (Priya et al., 2019). This study showed that exogenous GABA directly enhanced GAD expression and activity, thereby increasing the content of endogenous GABA (by 28.2 % and 9.4 % after 6 d and 18 d of treatment), which was then transformed into succinic acid (SA) owing to the significant upregulation of GmGABA-T and GmSSADH (Fig. 4D, 4E). Thereafter, SA entered into the TCA cycle. Similar results were reported in the study on salt-stress resistance in wheat, which suggested that the increase in GABA shunt activity could overcome the inhibition of TCA cycle enzymes under salt stress (Li et al., 2021).
Fig. 4.
Bioactive peptides with significantly different contents (n = 5) (A). Amino acid content determined using LC-MS (B). Relative expression of GmGR and Gmγ-ECS (C). GAD, GABA-T, NR), GS, GOGAT, GDH, GPT, and GOT activity in edamame after 6 d and 18 d of treatment with H2O (CK) and exogenous GABA (D). Relative expression of GmGAD, GmGABA-T, GmSSADH, GmNR, GmNiR, GmGS, and GmGOGAT (E). Data in (B), (C), (D), and (E) represent mean ± SE (n = 3).. Asterisks (*) represent significant differences (Student’s t-test, P < 0.05) between control and GABA treatments.
In the TCA cycle, the contents of oxoglutaric acid and l-malic acid were significantly increased, especially oxoglutaric acid, whose content increased by 83.9 %. However, although oxoglutaric acid is a direct precursor of SA, the content of SA reduced significantly by 6.0 % (Supplementary Fig. 4), which might be attributed to the short half-life of GABA (Hijaz & Killiny, 2020). In addition, as the increase in TCA cycle metabolites promoted carbon fixation (Hijaz & Killiny, 2019), the contents of sucrose and glucose were significantly high probably because exogenous GABA promoted the TCA cycle (Fig. 1G, 1H). Similar findings were reported by Li et al. (2016). In summary, carbon skeletons of exogenous GABA entered the TCA cycle through the GABA shunt, further promoting carbon assimilation and accumulating organic acids, sucrose, and glucose to improve the nutritional content of edamame.
Oxoglutaric acid is also associated with nitrogen metabolism by glutamate dehydrogenase (GDH) and glutamate synthase (GOGAT) (Sorrequieta, Ferraro, Boggio, & Valle, 2010). We observed an increase in the expression of GmGOGAT and the activities of GDH (by 14.4 % after 6 d of treatment) and GOGAT (by 54.4 % and 14.3 % after 6 d and 18 d of treatment, respectively), which promoted oxoglutaric acid entering into the nitrogen metabolic pathway (Fig. 4D, 4E). Moreover, we observed a marked increase in GS expression (by 8.7 times after 12 h of treatment) and activity (34.3 % and 31.7 % after 6 d and 18 d of treatment), which probably improved the flux between Glu and glutamine (Gln) (Fig. 4D, 4E). Although the contents of Gln and Glu were not detected by LC-MS, the increase in GDH and GABA-T activities reflected the increase in Glu (Sorrequieta et al., 2010). Additionally, an enhanced GS/GOGAT cycle contributes to promoting the absorption of NH4+ and NO3− ions to improve nitrogen metabolism (Wang et al., 2014). In our study, exogenous GABA treatment upregulated nitrate reductase (GmNR) and nitrite reductase (GmNiR), which verified this fact (Fig. 4E). In addition, glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) convert Glu to other amino acids and proteins. In the present study, exogenous GABA significantly increased the activity of GOT (by 23.5 % after 6 d of treatment) (Fig. 4D), to accumulate amino acids.
Compared to the CK, d-aspartic acid, GABA, l-phenylalanine, d-proline, and pyroglutamic acid increased significantly by 15.9 %, 6.5 %, 17.3 %, 16.4 %, and 28.3 %, respectively, upon exogenous GABA treatment. Among these, d-aspartic acid is the most abundant amino acid in soybean. Although the contents of l-aspartic acid, l-arginine, l-asparagine, DL-o-tyrosine, d-tryptophan, and l-tyrosine decreased notably by 6.5 %, 16.5 %, 22.7 %, 15.8 %, 16.9 %, and 34.2 %, respectively, the contents of other amino acids did not exhibit significant difference upon treatment with exogenous GABA (Fig. 4C).
In addition, we identified 18 amino acids and 109 bioactive peptides (with 2–4 proteinogenic amino acids) involved in 13 pathways related to amino acid biosynthesis and metabolism (Fig. 4B) (Supplementary Fig. 2). The results revealed that exogenous GABA may serve as a signal molecule to increase the GABA shunt, improve nitrogen metabolism, and accumulate amino acid and bioactive peptides to enhance the nutritional quality of edamame. The 109 bioactive peptides identified using LC–MS might possess 2–4 proteinogenic amino acids (Supplementary Fig. 3) linked with each other by peptide bonds or released during enzyme proteolysis in soybean (Daliri et al., 2017). Among these, 27 bioactive peptides were significantly different, 20 of which were significantly upregulated and 7 were downregulated upon treatment with exogenous GABA (Fig. 4A). In addition, L-α-glutamyl-l-tyrosine, Pro-His, Ile-Val-Asn-Gly, Pro-Pro-Gln, and Lys-Pro-Asn exhibited a wide range of biological activities, such as hypolipidemic, anti-diabetic, anti-hypertensive, and anti-cancerous properties (Daliri et al., 2017), which were significantly enhanced upon treatment with exogenous GABA (Fig. 4A, Supplementary Fig. 5). According to Yin, Yang, Han, and Gu (2015), this might be because exogenous GABA promoted the dehydration and condensation of amino acids and increased the content of bioactive peptides. However, the specific mechanisms underlying this phenomenon need further studies.
It has been well documented that GSH, whose precursor is Glu, is a γ-tripeptide with an amide bond and sulfhydryl group and is mainly regulated by glutathione reductase (GR) and γ-glutamylcysteine synthetase (γ-ECS) (Jiao et al., 2016). We also showed that exogenous GABA treatment enhanced the content of GSH by upregulating GmGR and Gmγ-ECS (Fig. 1H, 4B).
Consequently, GABA acted as a signal molecule that enhanced endogenous GABA signaling, which stimulated the activity of key enzymes engaged in nitrogen metabolism and enhanced the contents of amino acids and active peptides to improve the nutritional quality of edamame.
3.5. Effects of exogenous GABA treatment on flavonoids metabolism in edamame
Flavonoids—important bioactive compounds in plants, such as soybean (Kim et al., 2006)—mainly include flavones, flavonols, flavanones, and isoflavonoids and have a variety of medicinal and healthcare benefits (Wu, Wang, Sciarappa, & Simon, 2004). To identify the flavonoids present in edamame seeds after treatment with exogenous GABA, the relevant compounds were rearranged according to their corresponding positions in the phenylpropanoid and flavonoid biosynthetic pathways established based on KEGG and previous studies (Ma, Ma, Gao, Wu, & Zhou, 2021) (Fig. 5A). In this study, exogenous GABA treatment significantly increased the content of l-phenylalanine (by 17.3 %) and promoted the accumulation of its metabolite cinnamaldehyde (Fig. 5A). Moreover, the contents of ginkgetin and cyanin, which were synthesized through anthocyanin and flavonoid biosynthetic pathways, respectively, increased significantly (by 27.22 % and 13.12 % respectively) compared to the CK (Fig. 5A).
Fig. 5.
Effects of GABA treatment on flavonoid biosynthesis in edamame seeds (A). PAL, C4H, 4CL, DFR, ANS, UFGT, CHI, CHS, and IFS activity in edamame seeds after 6 d and 18d of treatment with H2O (CK) and exogenous GABA (B). The relative expression levels of GmPAL1, GmC4H, Gm4CL, GmCHR, GmCHS8, GmCHI, GmIFS, GmF3′H, GmDFR, flavanone 3-hydroxylase (GmF3H), GmF3H, and GmANS in CK and exogenous GABA-treated seeds. Data represent mean ± SE (n = 3). Asterisks (*) represent significant differences (Student’s t-test, P < 0.05) between control and GABA treatments.
In addition, of the 12 major isoflavones in soybeans that have been classified into four major categories (Wu et al., 2004), we found 11 isoflavones, including malonylglycosides (6′'-o-malonyglycitin, malonyldaidzin, and 6′'-o-malonyldaidzin), β-glycosides (daidzin, glycitin, and genistin), aglycones (glycitein, daidzein, and genistein), acetylglycosides (6′'-o-acetyldaidzin), and ononin. Among these isoflavone groups, malonylglycosides were predominant in raw soybeans, followed by β-glycosides and acetylglycosides, whereas aglycones were rarely observed (Wang et al., 2018). The present study indicated that the contents of ononin, glycitin, and 6′'-o-malonyglycitinin increased significantly by 58.35 %, 30.06 %, and 23.90 %, respectively, upon GABA treatment, but the content of free isoflavones (glycitein, daidzein, and genistein) decreased (Fig. 5A). These results indicated that GABA treatment promoted the glycosylation and malonylation of isoflavones, which might play a crucial role in improving the nutritional quality of edamame. These findings are consistent with those of Zhao et al. (2021). In addition, the moisture content of soybean seeds decreased gradually with seed maturation (Gomes, Sinnecker, Tanaka, & Lanfer-Marquez, 2003), which might account for the reduced content of free isoflavones (Ziegler et al., 2016). However, the underlying mechanism remains to be elucidated.
Exogenous GABA increased the contents of phenylalanine (the synthetic substrate of flavonoids), total anthocyanins, total flavonoids (Fig. 1D, 1E), isoflavones (ononin, 6′'-o-malonyglycitin, and glycitin) (Fig. 5A) in edamame as GABA, being a signaling molecule, activated the phenylpropanoid pathway, leading to flavonoid enrichment and improving the nutritional quality of edamame. Ma et al., 2018, Zhao et al., 2021 also reported that GABA could activate the phenylpropanoid pathway and enhance isoflavones to resist NaCl stress.
In plants, the formation of flavonoids is mainly manifested by phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate coenzyme A ligase (4CL). These enzymes convert phenylalanine into p-coumaric acid (the general phenylpropanoid pathway) (Ma et al., 2021), which then enters into flavonoid metabolism, including flavonoid and anthocyanin biosynthetic pathways (Zhao et al., 2021, Chen et al., 2019) (Fig. 5B, 5C). In our study, the expression and activity of PAL, C4H, and 4CL, especially C4H, were affected by exogenous GABA treatment. GmC4H was significantly affected after 12 h, 24 h, and 3 d of treatment with exogenous GABA, and it activity was significantly increased by 2.8 and 2.8 times after 6 d and 18 d of the treatment, respectively. This indicated that exogenous GABA upregulated the expression and increased the activity of C4H and improved phenylpropanoid metabolism to promote the entry of its substrate into the flavonoid pathway. Singh, Kumar, Rani, Gulati, and Ahuja (2009) also demonstrated that PAL and C4H were the dominant regulators of flavonoid biosynthesis in tea.
In addition, chalcone reductase (CHR), chalcone synthase (CHS), and chalcone isomerase (CHI) are three key rate-limiting enzymes in the biosynthesis of flavonoids; their activity and expression determine flavonoid biosynthesis (Ma et al., 2021). In our study, exogenous GABA significantly enhanced the expression and activity of CHR, CHS, and CHI, especially CHI. The results revealed that GmCHI responded to exogenous GABA after 12 h, 24 h, and 3 d of treatment, and the activity of CHI was increased by 34.6 % and 17.0 % after 6 d and 18 d of treatment. This suggested that exogenous GABA contributed to flavonoid synthesis by increasing the expression and activity of CHI. Han, Vimolmangkang, Soria-Guerra, and Korban (2012) also believed that altered expression levels of CHI in apple were responsible for a lack of anthocyanins.
Furthermore, isoflavone synthase (IFS)—a key enzyme in isoflavone biosynthesis (Jiao et al., 2016)—was significantly upregulated after 24 h and 3 d of GABA treatment, and its activity notably increased by 23.2 % after 6 d of treatment with exogenous GABA. This may be the reason for the increase in the contents of glycitin, ononin, and 6′'-o-malonyglycitin. Subsequently, upon GABA treatment, flavanone 3′-hydroxylase (GmF3′H), which functions at the beginning of the anthocyanin biosynthetic pathway, was upregulated (Ma et al., 2021). Meanwhile, exogenous GABA enhanced anthocyanin content by significantly increasing the expression of dihydroflavanol 4-reductase (GmDFR) and anthocyanidin synthase (GmANS) and the activity of UDP-glycose flavonoid glycosyltransferase (UFGT) (2.4 and 3.5 times after 6 d and 18 d of treatment) and DFR (by 1.1 and 1.1 times after 6 d and 18 d of treatment). We also determined positive correlations between the expression levels of DFR, UFGT, and ANS and the content of anthocyanins, which have been consistently observed in many plants (Delgado et al., 2018, Chen et al., 2019, Han et al., 2012).
Overall, the results suggested that GABA served as a signaling molecule to enhance endogenous phenylalanine content and mediate phenylpropanoid metabolism, by enhancing the expression and activity of critical enzymes involved in the phenylpropanoid pathway and increasing isoflavone and anthocyanin levels to improve the nutritional quality of edamame.
Conclusions
To the best of our knowledge, this is the first study that revealed that the foliar application of GABA could effectively improve the nutritional quality of edamame through the accumulation of amino acids and bioactive peptides, isoflavones, anthocyanins, sugars, vitamin C, and organic acids. Exogenous GABA could increase endogenous GABA metabolism to increase the activity of GABA-T and further stimulate oxoglutaric acid, which is a TCA-cycle intermediate product that enters into amino acid metabolism, improves nitrogen metabolism, and helps in the accumulation of amino acids, bioactive peptides, and organic acids. Moreover, the application of exogenous GABA promoted flavonoid biosynthesis to enhance the content of phenylalanine and induced the metabolism of flavonoids, including anthocyanins, 6′'-o-malonyglycitin, glycitin, ononin, cyanin, and ginkgetin.
CRediT authorship contribution statement
Gaobo Yu: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. Fengqiong Chen: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Visualization. Yating Wang: Methodology, Investigation, Data curation, Formal analysis. Qiusen Chen: Methodology, Formal analysis. Hanlin Liu: Methodology, Formal analysis. Jin Tian: Methodology, Formal analysis. Mengxue Wang: Formal analysis. Chunyuan Ren: Formal analysis. Qiang Zhao: Formal analysis. Fengjun Yang: Formal analysis. Yunyan Sheng: Formal analysis. Jinpeng Wei: Formal analysis. Yuxian Zhang: Supervision, Project administration, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Heilongjiang Postdoctoral Scientific Research Startup Project (LBH-Q20052), the Fund Program for Overseas Returnees and the National Natural Science Foundation of China (31301769), the China Agriculture Research System of MOF and MARA (No. CARS-04-PS18), Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (TDJH202001), the Heilongjiang Province Science and Technology Project (No. 2021ZXJ05B02).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100511.
Contributor Information
Gaobo Yu, Email: yugaobo81@163.com.
Yuxian Zhang, Email: zyx_lxy@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abd El-Gawad H.G., Mukherjee S., Farag R., Abd Elbar O.H., Hikal M., Abou El-Yazied A.…Ibrahim M. Exogenous γ-Aminobutyric Acid (GABA)-Induced Signaling Events and Field Performance Associated with Mitigation of Drought Stress in Phaseolus Vulgaris L. Plant Signaling & Behavior. 2021;16(2):1853384. doi: 10.1080/15592324.2020.1853384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra E., De Kleijn R., Colzato L.S., Alkemade A., Forstmann B.U., Nieuwenhuis S. Neurotransmitters As Food Supplements: The Effects of GABA on Brain and Behavior. Frontiers in Psychology. 2015;6:1520. doi: 10.3389/Fpsyg.2015.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zhang M., Zhang G., Li P., Ma F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. International Journal of Molecular Sciences. 2019;20(23):6060. doi: 10.3390/ijms20236060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliri E.B., Oh D.H., Lee B.H. Bioactive Peptides. Foods (Basel, Switzerland) 2017;6(5):32. doi: 10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado L.D., Zúñiga P.E., Figueroa N.E., Pastene E., Escobar-Sepúlveda H.F., Figueroa P.M., Garrido-Bigotes A., Figueroa C.R. Application of a JA-Ile Biosynthesis Inhibitor to Methyl Jasmonate-Treated Strawberry Fruit Induces Upregulation of Specific MBW Complex-Related Genes and Accumulation of Proanthocyanidins. Molecules (Basel, Switzerland) 2018;23(6):1433. doi: 10.3390/molecules23061433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M., Quílez J., Rafecas M. Gamma-Aminobutyric Acid as A Bioactive Compound in Foods: A review. Journal of Functional Foods. 2014;10:407–420. doi: 10.1016/j.jff.2014.07.004. [DOI] [Google Scholar]
- Du Y., Zhao Q., Chen L., Yao X., Zhang W., Zhang B., Xie F. Effect of Drought Stress on Sugar Metabolism in Leaves and Roots of Soybean Seedlings. Plant physiology and biochemistry. 2020;146:1–12. doi: 10.1016/j.plaphy.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Fehr, W. R. (1971). Stage Of Development Descriptions for Soybeans, Glycine Max (L.) Merrill1. Merrill Crop, 11.
- Gomes M.S., Sinnecker P., Tanaka R.T., Lanfer-Marquez U.M. Effect of Harvesting and Drying Conditions on Chlorophyll Levels of Soybean (Glycine Max L. Merr) Journal of Agricultural and Food Chemistry. 2003;51(6):1634–1639. doi: 10.1021/jf011227w. [DOI] [PubMed] [Google Scholar]
- Han Y., Vimolmangkang S., Soria-Guerra R.E., Korban S.S. Introduction of Apple ANR Genes into Tobacco Inhibits Expression of Both CHI and DFR Genes in Flowers, Leading to Loss of Anthocyanin. Journal of Experimental Botany. 2012;63(7):2437–2447. doi: 10.1093/jxb/err415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz F., Killiny N. Exogenous GABA Is Quickly Metabolized to Succinic Acid and Fed into The Plant TCA Cycle. Plant Signaling & Behavior. 2019;14(3):e1573096. doi: 10.1080/15592324.2019.1573096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz F., Killiny N. The Use of Deuterium-Labeled Gamma-Aminobutyric (D6-GABA) to Study Uptake, Translocation, and Metabolism of Exogenous GABA in Plants. Plant Methods. 2020;16:24. doi: 10.1186/s13007-020-00574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Ma Y., Zou P., Cheng G., Zhou J., Cai S. Effects Of Different Oligochitosans on Isoflavone Metabolites, Antioxidant Activity, and Isoflavone Biosynthetic Genes in Soybean (Glycine Max) Seeds During Germination. Journal of Agricultural and Food Chemistry. 2019;67(16):4652–4661. doi: 10.1021/acs.jafc.8b07300. [DOI] [PubMed] [Google Scholar]
- Jiang G.L., Katuuramu D.N., Xu Y., Ren S., Rutto L.K. Analysis and Comparison of Seed Protein, Oil, and Sugars in Edamame Dried Using Two Oven-Drying Methods and Mature Soybeans. Journal of The Science of Food and Agriculture. 2020;100(10):3987–3994. doi: 10.1002/jsfa.10443. [DOI] [PubMed] [Google Scholar]
- Jiao C., Yang R., Zhou Y., Gu Z. Nitric Oxide Mediates Isoflavone Accumulation and The Antioxidant System Enhancement in Soybean Sprouts. Food Chemistry. 2016;204:373–380. doi: 10.1016/j.foodchem.2016.02.147. [DOI] [PubMed] [Google Scholar]
- Kaur B., Asthir B., Bains N.S. Apical Stem Culturing to Enhance Cell Sap Assimilates Towards Grain Sucrose and Glutamine Metabolism in Wheat. Cereal Research Communications. 2015;43:403–414. doi: 10.1556/crc.2014.0051. [DOI] [Google Scholar]
- Kim S.L., Berhow M.A., Kim J.T., Chi H.Y., Lee S.J., Chung I.M. Evaluation of Soyasaponin, Isoflavone, Protein, Lipid, and Free Sugar Accumulation in Developing Soybean Seeds. Journal of Agricultural and Food Chemistry. 2006;54(26):10003–10010. doi: 10.1021/jf062275p. [DOI] [PubMed] [Google Scholar]
- Li C., Zhu J., Sun L., Cheng Y., Hou J., Fan Y., Ge Y. Exogenous γ-Aminobutyric Acid Maintains Fruit Quality of Apples Through Regulation of Ethylene Anabolism and Polyamine Metabolism. Plant Physiology and Biochemistr. 2021;169:92–101. doi: 10.1016/j.plaphy.2021.11.008. [DOI] [PubMed] [Google Scholar]
- Li Z., Yu J., Peng Y., Huang B. Metabolic Pathways Regulated By γ-Aminobutyric Acid (GABA) Contributing to Heat Tolerance in Creeping Bentgrass (Agrostis Stolonifera) Scientific Reports. 2016;6:30338. doi: 10.1038/srep30338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Y., Ma X., Gao X., Wu W., Zhou B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. International Journal of Molecular Sciences. 2021;22(20):11116. doi: 10.3390/ijms222011116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang P., Chen Z., Gu Z., Yang R. GABA Enhances Physio-Biochemical Metabolism and Antioxidant Capacity of Germinated Hulless Barley Under Nacl Stress. Journal of Plant Physiology. 2018;231:192–201. doi: 10.1016/j.jplph.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Priya M., Sharma L., Kaur R., Bindumadhava H., Nair R.M., Siddique K., Nayyar H. GABA (γ-Aminobutyric Acid), as A Thermo-Protectant, to Improve the Reproductive Function of Heat-Stressed Mungbean Plants. Scientific Reports. 2019;9(1):7788. doi: 10.1038/s41598-019-44163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renata C. V. Carneiro, Yun Yin, Susan E. Duncan, and Sean F. O’Keefe (2021). Edamame Flavor Characteristics Driving Consumer Acceptability in the United States: A Review. ACS Food Science & Technology 1 (10), 1748-1756. https://doi.org/ 10.1021/acsfoodscitech.1c00261.
- Sheng L., Shen D., Luo Y., Sun X., Wang J., Luo T.…Cheng Y. Exogenous γ-Aminobutyric Acid Treatment Affects Citrate and Amino Acid Accumulation to Improve Fruit Quality and Storage Performance of Postharvest Citrus Fruit. Food Chemistry. 2017;216:138–145. doi: 10.1016/j.foodchem.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Singh K., Kumar S., Rani A., Gulati A., Ahuja P.S. Phenylalanine Ammonia-Lyase (PAL) and Cinnamate 4-Hydroxylase (C4H) and Catechins (Flavan-3-Ols) Accumulation in Tea. Functional & Integrative Genomics. 2009;9(1):125–134. doi: 10.1007/s10142-008-0092-9. [DOI] [PubMed] [Google Scholar]
- Song J.F., Liu C.Q., Li D.J., Gu Z.X. Evaluation Of Sugar, Free Amino Acid, and Organic Acid Compositions of Different Varieties of Vegetable Soybean (Glycine Max [L.] Merr) Industrial Crops and Products. 2013;50:743–749. doi: 10.1016/j.indcrop.2013.08.064. [DOI] [Google Scholar]
- Sorrequieta A., Ferraro G., Boggio S.B., Valle E.M. Free Amino Acid Production During Tomato Fruit Ripening: A Focus on L-Glutamate. Amino acids. 2010;38(5):1523–1532. doi: 10.1007/s00726-009-0373-1. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Li J.R., Xia Q.P., Wu X.L., Gao H.B. Influence of Exogenous Gamma-Aminobutyric Acid (GABA) on GABA Metabolism and Amino Acid Contents in Roots of Melon Seedling Under Hypoxia Stress. Ying Yong Sheng Tai Xue Bao. 2014;25(7):2011–2018. [PubMed] [Google Scholar]
- Wang H., Ren C., Cao L., Zhao Q., Jin X., Wang M.…Zhang Y. Exogenous Melatonin Modulates Physiological Response to Nitrogen and Improves Yield in Nitrogen-Deficient Soybean (Glycine Max L. Merr.). Frontiers. Plant Science. 2022;13 doi: 10.3389/fpls.2022.865758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu Y., Li X., Xu Q., Feng Y., Yang S. Isoflavones from Green Vegetable Soya Beans and Their Antimicrobial and Antioxidant Activities. Journal of the Science of Food and Agriculture. 2018;98(5):2043–2047. doi: 10.1002/jsfa.8663. [DOI] [PubMed] [Google Scholar]
- Wu Q., Wang M., Sciarappa W.J., Simon J.E. LC/UV/ESI-MS Analysis of Isoflavones in Edamame and Tofu Soybeans. Journal of Agricultural and Food Chemistry. 2004;52(10):2763–2769. doi: 10.1021/jf035053p. [DOI] [PubMed] [Google Scholar]
- Yin Y., Yang R., Han Y., Gu Z. Comparative Proteomic and Physiological Analyses Reveal the Protective Effect of Exogenous Calcium on The Germinating Soybean Response to Salt Stress. Journal of Proteomics. 2015;113:110–126. doi: 10.1016/j.jprot.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li S., Cai Q., Wang Z., Cao J., Yu T., Xie T. Exogenous Diethyl Aminoethyl Hexanoate Ameliorates Low Temperature Stress by Improving Nitrogen Metabolism in Maize Seedlings. PloS One. 2020;15(4):e0232294. doi: 10.1371/journal.pone.0232294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xie C., Wang P., Gu Z., Yang R. GABA Regulates Phenolics Accumulation in Soybean Sprouts Under Nacl Stress. Antioxidants (Basel, Switzerland) 2021;10(6):990. doi: 10.3390/antiox10060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler V., Vanier N.L., Ferreira C.D., Paraginski R.T., Monks J.L., Elias M.C. Changes in The Bioactive Compounds Content of Soybean as a Function of Grain Moisture Content and Temperature During Long-Term Storage. Journal of Food Science. 2016;81(3):H762–H768. doi: 10.1111/1750-3841.13222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.