Abstract
We report a case of a man in his early 60s presenting with bilateral adrenal and pulmonary haemorrhages as an initial presentation of polycythaemia vera (PV). Symptomatology included severe compressive chest pain radiating to epigastrium, with unremarkable physical findings, parameters and ECG. Blood investigations showed an elevated haemoglobin (174 g/L, reference range (RR): 141-172g/L) and haematocrit (55.7%, RR: 40.4%–50.4%) levels.
Cross-sectional imaging excluded aortic dissection, but imaging repeated 48 hours after his admission for acute dyspnoea and worsening abdominal pain showed bilateral alveolar and adrenal haemorrhages. Cortisol level was 27 nmol/L (RR: 145–619 nmol/L). Investigations confirming PV included the presence of a Janus kinase 2 (JAK2V617F) gene mutation, hypercellularity with erythroid hyperplasia on bone marrow microscopy and a low serum erythropoietin (2.6 mIU/mL, RR: 4.3–29.0 mIU/mL). Aspirin, hydroxyurea, venesection and cortisol replacement were initiated to get good treatment outcome.
Keywords: Adrenal disorders, Haematology (incl blood transfusion), Respiratory medicine, Endocrine system
Background
Polycythaemia vera (PV) is a Philadelphia chromosome-negative myeloproliferative neoplasm. The course of PV is variable. Cardiovascular and thrombotic events are well-known complications, leading to increased mortality in patients with this condition.1 To date, there are no other case reports of patients with simultaneous bilateral adrenal and pulmonary haemorrhages as a complication of PV.
Case presentation
A man in his early 60s presented to the emergency department with sudden onset of severe chest pain radiating to his upper abdomen. The pain was constant and described as sharp, occasionally becoming compressive in nature, with no other associated symptoms. His medical history included a 9-year history of well-controlled hypertension on medication. His surgical history was unremarkable. His drug history included valsartan at a dose of 80 mg two times per day and reported no known drug allergies. The patient was an active smoker, with a 40 pack-year history, and used alcohol socially. He was independent in activities of daily living. There was no significant family history.
On clinical assessment, the patient was visibly distressed and in severe pain with a score of 9/10 despite paracetamol, needing escalation to intravenous morphine for pain relief. His blood pressure was 168 mm Hg systolic on 89 mm Hg diastolic, with a regular pulse of 91 beats per minute (bpm) and an oxygenation of 98% on room air. Full physical examination including chest and abdomen examinations were normal.
Forty-eight hours after hospital admission, the patient reported sudden-onset shortness of breath associated with severe generalised abdominal pain. The patient was tachypnoeic with a respiratory rate of 35 breaths/min, tachycardiac at 110 bpm, regular and hypoxic, with oxygen saturation of 83% on room air. His blood pressure was normal at 146/81 mm Hg with blood glucose test of 6.3 mmol/L. Positive findings on full physical examination revealed generalised abdominal tenderness with guarding. There was no rigidity and bowel sounds were normal. Despite maximum treatment, the patient remained hypoxic and required intensive care support.
Investigations
Initial blood investigations showed an elevated haemoglobin of 174 g/L (reference range (RR): 141-172g/L) with a haematocrit of 55.7% (RR: 40.4%–50.4%). Otherwise, blood tests including white cell count and platelets, renal profile, inflammatory markers, troponin, D-dimer, liver function tests and amylase were normal. Chest X-ray and baseline ECG were unremarkable. Given the severity of the chest pain, a CT of the arch of aorta was performed, which excluded aortic dissection. A CT of the abdomen was also performed, which showed non-specific asymmetrical thickening of the anterior gastric wall with no signs of mesenteric ischaemia or other acute abdominal causes.
The patient was admitted to a medical ward for further management. Repeat ECGs showed no interval changes; however, a repeat troponin showed a significant rise from 12 ng/L to 44 ng/L. Given this troponin rise, the case was discussed with cardiology who performed an echocardiogram, which was completely normal, with no signs of pericardial inflammation or pericardial effusion. By the following morning, the pain had settled completely without the need for further analgesia. A coronary angiogram was performed, which showed normal patent coronary arteries, excluding an ischaemic myocardial event.
Given the asymmetrical gastric thickening, an oesophagogastroduodenoscopy (OGD) was performed, which excluded any pathology.
During the acute episode of dyspnoea and abdominal pain, arterial blood gas showed compensated type 1 respiratory failure with a pH of 7.41, partial pressure of carbon dioxide (pCO2) of 31 mm Hg, partial pressure of oxygen (pO2) of 62 mm Hg, oxygen saturation of 84%, bicarbonate (HCO3)of 22 mmol/L, lactate of 0.9 mmol/L and glucose of 5.8 mmol/L (RR: pH 7.35–7.45; pCO2 35–45 mm Hg; pO2 80–100 mm Hg; HCO3 22–26 mmol/L). Repeated blood investigations were unremarkable.
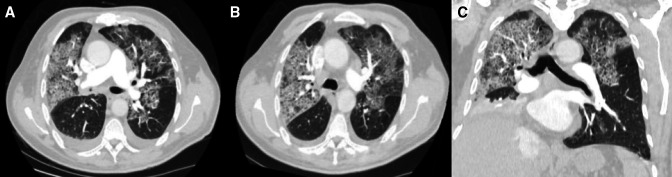
Given the clinical picture of acute type 1 respiratory failure and tachycardia with abdominal guarding, urgent CT pulmonary angiogram and CT scan of the abdomen were performed, which showed diffuse interlobular septal thickening and ground-glass changes in both lungs, mainly involving the upper lobes, in keeping with bilateral alveolar haemorrhages (figure 1A–C) and bilateral adrenal gland haemorrhages with a density of about 60 HU (figure 2A–C), respectively. His random cortisol level was 27 nmol/L (RR: 145–619 nmol/L). Serum sodium and potassium were normal at 140 mmol/L (RR: 135–145 mmol/L) and 4.1 mmol/L (RR: 3.5–5.1 mmol/L), respectively.
Figure 1.
Axial CT pulmonary angiogram (A, B) and coronal reformatted CT of the lungs (C) showing diffuse interlobular thickening and ground-glass changes in the bilateral lungs, giving a crazy paving appearance, more prominent in the bilateral anterior portions of the lungs. There were also small pleural effusions.
Figure 2.
CT scan of the abdomen and pelvis ((A, B) axial, (C) coronal) showing bilateral adrenal haemorrhage (marked with asterisk (*)) with right adrenal gland measuring 3.5 cm craniocaudally (CC)×2.1 cm transversely (TR)×3.3 cm anteroposteriorly (AP), and left adrenal gland measuring 3.7 cm AP×2.6 cm TR×3.7 CC with density of about 60 HU.
On review of the full blood count trends, it was noted that the patient had a normal blood count on the blood tests taken 16 months earlier; however, polycythaemia was present on the latest blood available 1 month prior to presentation, as presented in table 1. White cell and platelet counts were always within normal range.
Table 1.
Trend view of full blood cell counts over time
| Full blood count | Date: 4 years pre-hospital admission | Date: 1 year pre-hospital admission | Date: 2 months pre-hospital admission | Date: day of presentation | Date: 2 months post-discharge | Date: 4 months post-discharge | Reference range/unit |
| WCC (109/L) | 8.16 | 5.61 | 5.58 | 7.41 | 4.43 | 4.33 | 4.30–11.40×109/L |
| Hb (g/L) | 148 | 143 | 178 | 174 | 130 | 136 | 141-172g/L |
| HCT (%) | 42.1 | 40.9 | 60.4 | 55.7 | 40.1 | 42.5 | 40.4%–50.4% |
| RCC (×1012/L) | 4.70 | 4.70 | 9.24 | 8.94 | 5.11 | 4.9 | 4.60–5.90×1012/L |
| MCV (fL) | 89.6 | 90.5 | 65.2 | 62.3 | 79.4 | 86.7 | 79.0–93.0 fL |
| PLTS (×109/L) | 221 | 187 | 184 | 209 | 166 | 148 | 146–302×109/L |
Hb, haemoglobin; HCT, haematocrit; MCV, mean cell volume; PLTS, platelets; RCC, red cell count; WCC, white cell count.
JAK2 mutation screen (including exons 14 and 12) and serum erythropoietin (sEpo) levels were taken for the working diagnosis of PV. Results confirmed a mutation in JAK2V617F gene and a low sEpo at a level of 2.6 mIU/mL (RR: 4.3–29.0 mIU/mL). Bone marrow biopsy showed hypercellularity with erythroid hyperplasia. Putting all the findings together, a diagnosis of PV was confirmed. In addition, a full vasculitic screen and cyroglobulin levels were also taken to exclude other possible causes of pulmonary haemorrhages.
Differential diagnosis
This case describes a middle-aged man who presented with severe chest pain radiating to the abdomen.
Given the presenting signs and symptoms and the presence of possible hyperviscosity syndrome secondary to polycythaemia, the primary working diagnoses were that of an ischaemic myocardial event, which was excluded via a normal coronary angiogram; pulmonary embolism, which was ruled out by a normal D-dimer; and aortic dissection, which was excluded through a normal arch of aorta CT. In addition, a surgical abdominal aetiology, mainly ischaemic bowel or perforated viscus, was also a plausible differential, which was excluded via CT of the abdomen. OGD ruled out upper gastrointestinal pathology including malignancy, infection or inflammation.
Given the recurrent episodes of unexplained significant chest pain radiating to the abdomen, positive clinical findings of abdominal tenderness and guarding, and subsequent tachycardia and hypoxia, further chest and abdominal imaging were warranted. These excluded pulmonary embolism but surprisingly showed bilateral diffuse alveolar haemorrhage. A random cortisol level was low in keeping with adrenal insufficiency; however, there was no haemodynamic compromise or electrolyte disturbances.
An immunology screen done to further investigate the pulmonary and adrenal haemorrhages was negative. Haematological screen confirmed a positive JAK2 gene mutation, low sEpo level and hypercellularity with erythroid hyperplasia on bone marrow biopsy. These confirmed the final diagnosis of bilateral pulmonary and adrenal haemorrhages as a complication of primary PV.
Treatment
Based on the findings of bilateral adrenal haemorrhage on CT and a low random cortisol level, a stat dose of 200 mg of intravenous hydrocortisone was given followed by regular hydrocortisone—50 mg every 6 hours intravenously to cover for possibility of glucocorticoid insufficiency. Despite maximum oxygen flow via non-rebreather mask at 15 L/min, the patient remained severely hypoxic with a maximum oxygenation of 90% and was thus transferred to the intensive therapy unit (ITU) for respiratory support, needing high-flow oxygen. During his stay, the patient’s blood pressure, glucose levels and blood electrolytes remained within normal ranges.
A haematologist was consulted and hydroxyurea was initiated at a dose of 500 mg daily upon confirmation of JAK2 mutation. The patient was transferred to a medical ward after a total of 4 days in the ITU, needing no respiratory or cardiovascular support. Hydrocortisone was switched to an oral regimen, at a dose of 20 mg–10 mg–10 mg daily, together with aspirin 75 mg daily. In terms of aiming for haematocrit decline to <45%, a phlebotomy was performed during admission to modify haematocrit level at 47%.
Outcome and follow-up
The patient was discharged after a total of 15 days in hospital on hydrocortisone 10 mg–5 mg–5 mg daily, hydroxyurea 1 g daily and aspirin 75 mg daily. Sick day rules were explained. He returned to his previous performance status within 4 weeks of discharge, needing no oxygen support and fully independent in his activities of daily living. A repeat chest X-ray, performed 8 weeks after his initial presentation, showed radiological resolution of the previously documented bilateral ground-glass changes and pleural effusions (figure 3A, B).
Figure 3.
(A) Scout view of CT of the lung showing bilateral ground-glass changes with pleural effusion compared with (B) repeat chest X-ray after 8 weeks showing normal lungs with complete resolution of ground-glass changes and pleural effusions.
At present, the patient is being followed up regularly by both the haematology and endocrinology teams on an outpatient basis. Haemoglobin and haematocrit levels remained well controlled, with no need for further venesections. His latest haemoglobin level is 143g/L with a haematocrit of 41%. A high adrenocorticotropic hormone level of 813 pg/mL (normal range 10–48 pg/mL), taken 3 months after discharge, confirmed adrenal insufficiency secondary to bilateral adrenal haemorrhage. Electrolytes remained within normal range and blood pressure was well controlled with the same dose of valsartan, without postural drops; thus, mineralocorticoid replacement was not required.
Discussion
PV is a Philadelphia chromosome-negative myeloproliferative neoplasm,1 which is characterised by clonal proliferation of haematopoietic stem cells resulting in excessive production of erythrocytes, leucocytes and platelets. The incidence is approximately 2.3 per 100 000, with a slightly higher incidence among men (1.2:1) and typically diagnosed between 60 and 65 years of age.2
A mutation in exon 12 or 14 of JAK2, commonly JAK2V617F allele, is present in >95% of patients with PV. The JAK2V617F mutation represents a gain of function mutation, caused by valine to phenylalanine substitution, occurring in the pseudokinase domain (JH2) of the JAK2 gene. This results in constitutive activation of signal transducer and activator of transcription (STAT) pathway. The JAK-STAT pathway is needed for normal haematopoiesis and development of immune function.3–5
Diagnosis of PV is made using the updated 2016 WHO diagnostic criteria, as shown in table 2. One of the major changes in the 2016 WHO criteria, compared with the previous 2008 WHO criteria, is the lowering of the diagnostic haemoglobin threshold values (> 165g/L(men) and > 160g/L(women) vs haemoglobin >185g/L (men) and >165g/L (women) in 2008 WHO criteria).6 The main rationale is to ensure that patients with ‘masked PV’, meaning that patients with PV-consistent bone marrow, JAK2 mutations and PV-like features, but low haemoglobin levels (≤185g/L in men, ≤165g/L in women), are not underdiagnosed. This is important because such patients have similar incidence of thrombosis when compared with those with overt PV (high haemoglobin levels); and moreover, patients with masked PV have a higher rate of progression to myelofibrosis (MF) and acute myeloid leukaemia (AML).7 Our patient fulfilled all the 2016 WHO criteria.
Table 2.
2016 WHO diagnostic criteria for polycythaemia vera (PV)
| PV diagnosis requires meeting either all three major criteria or the first two major criteria and one minor criterion | |
| Major criteria | Hb >165g/L (men)/>160g/L (women) or HCT >49% (men)/>48% (women) or increased red cell mass more than 25% above mean normal predicted value |
| BM biopsy showing hypercellularity for age with trilineage growth including prominent erythroid, granulocytic and megakaryocytic proliferation with pleomorphic mature megakaryocytes (difference in size) | |
| Presence of JAK2 or JAK2 exon 12 mutation | |
| Minor criteria | Subnormal serum erythropoietin level |
| Criterion number 2 (BM biopsy) may not be required in cases with sustained absolute erythrocytosis: Hb levels 185g/L in men (HCT 55.5%) or 165g/L in women (HCT 49.5%) if major criterion 3 and the minor criterion are present. However, initial myelofibrosis (present in up to 20% of patients) can only be detected by performing a BM biopsy; this finding may predict a more rapid progression to overt myelofibrosis | |
BM, bone marrow; Hb, haemoglobin; HCT, haematocrit.
Clinical presentation of PV varies from an incidental finding on a routine blood test in an asymptomatic patient to vague symptoms of hyperviscosity, such as headaches, tinnitus, dizziness, pruritus (especially after a hot bath), to life-threatening PV-associated complications. Thrombotic complications, involving both the arterial (ischaemic strokes, myocardial infarctions, transient ischaemic attacks and peripheral arterial thrombosis) and venous systems (deep vein thrombosis and pulmonary embolism), are well-known complications of PV.8 9 Such complications are high in patients with advanced age, a history of thrombosis, high haematocrit, leucocytosis and high JAK2V617F allele burden (the genetic ratio of the JAK2V617F mutation to JAK2 wild-type in granulocytes).5 Other known complications include neoplasms, especially haematological transformations to MF and AML (up to 10% and 15% of patients every 10 years, respectively).8
Although haemorrhagic complications are well-known complications of PV, to date, there are no other case reports of patients who presented with simultaneous bilateral adrenal haemorrhage and pulmonary haemorrhage as direct consequences of PV. There are three case reports of bilateral adrenal haemorrhage in patients with already known PV.10–12 To our knowledge, there are no case reports of acute pulmonary haemorrhage as a complication of PV. There have been reports of pulmonary extra-medullary haematopoiesis (EMH)—the presence of haematopoietic precursor cells in the lungs.13 14 Unlike the typical chronic, progressive symptomatic presentation seen in pulmonary EMH, our patient showed acute respiratory symptoms together with sudden radiological lung changes.
Common causes of adrenal and pulmonary haemorrhages are listed in boxes 1 and 2, respectively. The adrenal glands receive rich arterial blood supply from the three main suprarenal arteries; however, venous return is only through a single adrenal vein draining directly into the left renal vein and the inferior vena cava. This ‘vascular dam’ is thought to be a predisposing factor to adrenal haemorrhage.15 This can be extremely painful due to increased intracapsular pressure, resulting in capsulitis. On the other hand, the pathophysiology underlying pulmonary haemorrhage is either direct erosion into nearby blood vessels (such as in mycobacterial infections and carcinomas) or vascular rupture secondary to ongoing inflammation or coughing, such as in bronchiectasis.16
Box 1. Causes of adrenal haemorrhage.
Idiopathic.
Trauma.
Infections (HIV, tuberculosis).
Anticoagulation.
Iatrogenic (post-biopsy).
Acute stress (sepsis, surgery, shock).
Neoplastic disease.
Meningococcal septicaemia/Waterhouse-Friderichsen syndrome.
Autoimmune disease (antiphospholipid antibody syndrome, systemic lupus erythematosus).
Examples are shown in parentheses.
Box 2. Causes of pulmonary haemorrhage.
Infections (tuberculosis, mycetoma, pneumonia, lung abscess).
Bronchiectasis.
Trauma.
Congenital lung malformations (bronchogenic cyst).
Vascular disorders (haemangiomas, arteriovenous malformation).
Coagulopathy.
Neoplastic disease.
Iatrogenic.
Autoimmune disease (antiphospholipid antibody syndrome, systemic lupus erythematosus).
Examples are shown in parentheses.
In our case, the patient was managed with aspirin, hydroxyurea and venesection. It is recommended that high-risk patients (age >60 years and with previous thrombotic events) are managed with aspirin, and the use of cytoreduction agents, such as hydroxyurea, interferon-alpha and busulfan.5 8 A more novel drug, ruxolitinib, a selective JAK1 and JAK2 inhibitor, has shown both good symptomatic response and haematocrit control in patients with PV.5 On the other hand, low-risk patients (age <60 years and with no previous thrombotic events) can be managed with low-dose aspirin and venesection, aiming haematocrit levels of <45%, as first line.
Learning points.
The course of polycythaemia vera (PV) is variable. Cardiovascular and thrombotic events are well-known complications, leading to increased mortality in patients with PV.
Advanced age, history of thrombosis, high haematocrit, leucocytosis and high JAK2V617F allele burden have all been associated with increased risk of thrombotic events.
Patients are typically managed with low-dose aspirin and phlebotomy, aiming at haematocrit level of <45%, with or without cytoreduction drugs.
Both adrenal and pulmonary haemorrhages are acute, life-threatening conditions needing prompt recognition and management.
Long-term follow-up is mandatory for patients with PV, especially in view of risk of malignant haematological transformation.
Footnotes
Contributors: KC wrote and designed the manuscript. MB and JB helped in editing the final draft, all under the supervision of EF.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health organization criteria and point-of-care diagnostic algorithms. Leukemia 2008;22:14–22. 10.1038/sj.leu.2404955 [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Polycythemia vera: a comprehensive review and clinical recommendations. Mayo Clin Proc 2003;78:174–94. 10.4065/78.2.174 [DOI] [PubMed] [Google Scholar]
- 3.Passamonti F, Maffioli M, Caramazza D, et al. Myeloproliferative neoplasms: from JAK2 mutations discovery to JAK2 inhibitor therapies. Oncotarget 2011;2:485–90. 10.18632/oncotarget.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia 2008;22:1299–307. 10.1038/leu.2008.113 [DOI] [PubMed] [Google Scholar]
- 5.Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev 2015;29:215–21. 10.1016/j.blre.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbui T, Thiele J, Vannucchi AM, et al. Rationale for revision and proposed changes of the who diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis. Blood Cancer J 2015;5:e337. 10.1038/bcj.2015.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbui T, Thiele J, Gisslinger H, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol 2014;89:52–4. 10.1002/ajh.23585 [DOI] [PubMed] [Google Scholar]
- 8.Raedler LA. Diagnosis and management of polycythemia vera: proceedings from a multidisciplinary roundtable. Am Health Drug Benefits 2014;7:S36–47. [PMC free article] [PubMed] [Google Scholar]
- 9.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32. 10.1200/JCO.2005.07.062 [DOI] [PubMed] [Google Scholar]
- 10.Bhandari S, Agito K, Krug EI. Bilateral adrenal hemorrhage in polycythemia vera. J Community Hosp Intern Med Perspect 2016;6:32416. 10.3402/jchimp.v6.32416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelfand ML. Bilateral adrenal hemorrhage in polycythemia vera. N Y State J Med 1992;92:3623. [PubMed] [Google Scholar]
- 12.Gönen MS, Ipekçi SH, Göveç N, et al. Polycythaemia vera presented with bilateral adrenal haemorrhage and adrenal insufficiency: a case report. Acta Clin Belg 2011;66:132–3. 10.2143/ACB.66.2.2062532 [DOI] [PubMed] [Google Scholar]
- 13.Trow TK, Argento AC, Rubinowitz AN, et al. A 71-year-old woman with myelofibrosis, hypoxemia, and pulmonary hypertension. Chest 2010;138:1506–10. 10.1378/chest.10-0973 [DOI] [PubMed] [Google Scholar]
- 14.Singh I, Mikita G, Green D, et al. Pulmonary extra-medullary hematopoiesis and pulmonary hypertension from underlying polycythemia vera: a case series. Pulm Circ 2017;7:261–7. 10.1177/2045893217702064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehmood KT, Sharman T, Mehmood KT, et al. Adrenal Hemorrhage. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555911/ [Google Scholar]
- 16.Shee B, Anjum F, Rockoff BI. Pulmonary Hemorrhage. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK538278/ [PubMed] [Google Scholar]