Graphical abstract
Keywords: Traceability, Apples, Isotope ratio mass spectrometry, Multi-isotopic analysis, Protected designations of origin
Highlights
-
•
Isotope ratio mass spectrometry (IRMS) was used to trace the orchard of provenance of apples.
-
•
The δ13C, δ15N, and δ34S values of apple parts were measured rather than the whole fruit.
-
•
The inner part of the seed is the most practical subfraction to be analyzed using IRMS.
-
•
Sulfur in seed is the best isotopic marker for tracing the regions of provenance of apples.
-
•
Linear discrimination analysis with the δ13C and δ34S of apple parts traced zones of production.
Abstract
Isotope ratio mass spectrometry is a well-known technique used to trace the origin of agri-food products from different countries. Here this method was tested to trace the exact orchard of provenance of Italian apples harvested at sites close to each other. We measured the δ13C, δ15N, and δ34S values of apple subfractions (peel, petiole, pulp, seed) from two orchards in Ferrara and one orchard in Trento. Sulfur represents the best marker for tracing the regions of provenance of samples because it is linked to the presence of sulfate (Ferrara1: +9.0 ‰; Ferrara 2: +7.3 ‰) and sulfide (Trento: −1.3 ‰) minerals in soils. However, the δ13C of apple subfractions combined with the δ34S of seed in a linear discrimination analysis better discriminated the three orchards. The isotopic fingerprint of apples is thus significantly affected by the relative terroir, and it can be used as “isotopic identity card” to certify “protected designations of origin”.
Introduction
Given the increasing awareness of consumers about the authenticity of the food they eat, the traceability of food products is gaining interest. Therefore, in recent decades, several analytical techniques have been developed to assess the authenticity and quality of foodstuffs: isotope ratio mass spectrometry, inductively mass spectrometry, near infrared spectroscopy, nuclear magnetic resonance spectrometry (Katerinopoulou, Kontogeorgos, Salmas, Patakas, & Ladavos, 2020 for a review). Although well-established, most of these techniques are laborious, time-consuming, and expensive (Eisenstecken et al., 2019, Jiang et al., 2020). Among them, the isotope traceability method is considered the most rapid and cost-effective technique to establish the origin of foodstuffs (Jiang et al., 2020) because it can determine several isotopes in a unique analysis process that uses a limited amount of sample without any pre-treatment processes. In nature, the stable isotope ratios of light bio-elements in living organisms (both plants and animals) such as carbon (C), nitrogen (N), sulphur (S), oxygen (O), and hydrogen (H), are strongly affected by climatic, pedological, geological, botanical, and agricultural factors, and any isotopic variations are ultimately incorporated into the organism tissues throughout eating, drinking, breathing, and exchange with the environment (Francois, Fabrice, & Didier, 2020). For example, in plant ecology, the isotopic compositions of vegetables and fruits reflect the photosynthetic pathways as well as the soil and environment in which the plant grows. The 13C/12C ratio of plants is mainly influenced by their photosynthetic pathways (O'Leary, 1988), but also by characteristic pedo-climatic settings of the area (temperature, humidity, soil pH; Brombin et al., 2020). The 15N/14N ratio is related to the type of fertilizer (organic or synthetic; Francois et al., 2020, Brombin et al., 2020). The 34S/32S ratio in plants primarily depends on the geological features of the soil, soil bacterial processes, and fertilizer uses (Cuchet et al., 2021, Pianezze et al., 2020).
Several studies have tested one or more isotopic parameters as tracers of the geographical provenance of various agri-foods, including olive oil, milk, honey, meat, vanilla, and seafoods (Camin et al., 2007; Camin et al., 2015, Schellenberg et al., 2010, Luo et al., 2015, Luo et al., 2016, Carrera and Gallardo, 2017, Dong et al., 2018, Pianezze et al., 2020, Won et al., 2021). However, in these studies, isotopic parameters were used to discriminate the origin of foodstuffs produced on different continents and/or countries with significantly distinct climatic and environmental conditions. Only a few studies have applied multi-stable isotopic analyses to trace the provenance of agri-foods products at smaller-scale, i.e., local scale. For example, Bianchini et al., 2021, Brombin et al., 2022 successfully applied multi-stable isotopic analysis to discriminate the provenance of manila clams collected in neighboring North Adriatic lagoons characterized by similar climatic and environmental conditions and Mimmo et al. (2015) used the stable isotope ratios of light elements to trace the specific region of apple fruit provenance in Italy. However, studies about the determination of the specific orchard where apple fruits grow are missing. Consequently, although the country or region of provenance of a fruit may be determined, the exact site of production remains unknown and even the application of orchard sustainable production processes and ethical working conditions cannot be ascertained.
Italy is the sixth largest producer of apples (Malus × domestica Borkh.) in the world and produces approximately 2.3 million tons of apples per year. Some Italian regions, such as Trentino, are famous for their “protected designations of origin” (PDO) or “protected geographical indication” (PGI) apple varieties, whereas other Italian regions, including Emilia-Romagna, aim to establish the PDO or PGI labels for their fruits. In this context, reliable techniques for the traceability of agri-foods at the regional scale must be established to ensure the correct recognition of products. Therefore, for the first time, we tested the reliability of the isotope ratio mass spectrometry (IRMS) technique to trace the origin of apples at the local scale and identify the exact orchard of the provenance of apples that were harvested at sites close to each other. We analyzed the isotopic signatures of C, N, and S of apples collected from two orchards of Ferrara, which are less than two kilometers apart, and one orchard from Trento (Northern Italy) to reveal the (i) different isotopic signatures between the two most important Italian regions for apple production and (ii) different isotopic signatures between orchards located in the same region and in close proximity. In this study, we also defined the ideal approach to trace the fruit through IRMS, evaluating the best isotopic parameter(s) to trace the origin of apples and comparing the isotopic signature of apple subfractions (i.e., peel, petiole, pulp, and inner part of the seed) to assess the variability of the fingerprints in the different apple parts.
Materials and methods
Sampling
Forty-eight apple samples were collected during the commercial fruit harvest period in 2020 in Northern Italy in the provinces of Ferrara (Emilia-Romagna region) and Trento (Trentino region) (Fig. 1, 1a). Ferrara apples were harvested from two different orchards<2 km from each other. In the first orchard (44°50′38.03″N 11°43′24.21″E; “Ferrara 1” in this work) 16 samples of four apple varieties (Fuji, Gala, Granny Smith, and Modì) were collected, while in the second orchard (44°49'44.06“N 11°44'0.96”E; “Ferrara 2” in this work), 30 samples of two other apple varieties (Cripps Pink and Golden Delicious) were collected. The two orchards are located in the eastern part of the Po Plain (Fig. 1) at an altitude of approximately 4 m above sea level (a.s.l.). The soil of Ferrara 1 orchard was characterized by sand and silty sand deposited near the Po di Volano River, whereas the soil of Ferrara 2 orchard was characterized by clay deposited in the floodplain (Fig. 1b). The climate in this area is warm temperate, and fully humid, with hot summers. In Ferrara, the mean annual air temperature was 14.4 °C and the mean annual precipitation was 500 mm in 2020. The management of both orchards conformed with the regional integrated fruit production guidelines.
Fig. 1.
Location of the samples collected in Northern Italy (Fig. 1a) for this work and those studied in the literature by Mimmo et al. (2015). b) Simplified sedimentological map (modified from Stefani & Minarelli, 2018) of the study area reporting the location of the two Ferrara orchards.
For a comparison, we enlarged the sample suite to include 4 Fuji apples grown in an orchard located in the southern part of the province of Trento (45°59′8.50″N 11°7′1.91″E; Fig. 1), close to the Adige River, at an altitude of approximately 200 m a.s.l. In this area, the climate is warm temperate, and fully humid, with warm summers. The average annual temperature and average annual rainfall in Trento were 8.7 °C and 1221 mm in 2020, respectively. The orchard was managed using an organic management system.
Sample preparation
Before proceeding with the analyses, the fruits were washed and dried. The apple samples were partitioned into peel (with a thickness between 0.25 and 0.35 mm), petiole, pulp, and seed (without the seed coat). Each apple subfraction was dried at 40 °C, homogenized using an agate mill, and stored in Eppendorf tubes until analyses. For each apple samples, the peel, petiole, and pulp were analyzed for C isotopes. Only the seed was also analyzed for N and S isotopes because (i) it is the most practical subfraction for analyses (fast to dry and easy to mill) and (ii) its isotopic ratios are the easiest detectable by IRMS for the analyses, even with small amounts of material.
Stable isotope analyses
Isotopic analyses of the apple subfractions were carried out at the Department of Physics and Earth Science of the University of Ferrara (Italy). Analyses of C and N and their isotope ratios (13C/12C and 15N/14N) were performed using an elemental analyzer (EA) Vario MICRO Cube (Elementar) coupled with an Isoprime 100 IRMS system (Elementar). The analyses of S and corresponding isotope ratio 34S/32S were carried out using an EA vario PYRO Cube (Elementar) operating in combustion mode and coupled with an IRMS precisION (Elementar). C—N analyses were performed following the method described in detail by Bianchini et al. (2021), and S analyses were performed following the method described by Salani, Brombin, Natali, and Bianchini (2021).
Homogenous powdered samples (approximately 2 mg) were weighed in tin capsules, wrapped, and loaded in the EA autosampler of the Vario MICRO Cube and PYRO Cube for the C—N and S analyses, respectively. Samples were burnt in the furnaces of the EAs at 950 °C for the C—N or at 1150 °C for S. After complete combustion of the sample, the released gases passed into the IRMS to determine the isotopic ratios. The detections of distinct isotopic masses of the sample were bracketed between those of reference gases (N2, CO2, SO2; purity grade 5), which were calibrated using reference materials. Calibration of the instruments was performed using several in-house working standards: JLs-1 limestone (Kusaka & Nakano, 2014), Carrara Marble (Natali & Bianchini, 2015), and Jacupiranga carbonatite (Beccaluva, Bianchini, Natali, & Siena, 2017), which were previously calibrated against the international reference material Tibetan human hair powder USGS42 (Coplen & Qi, 2011).
The 13C/12C, 15N/14N, and 34S/32S isotopic ratios (R) were expressed with δ notation (in ‰ units):
where Rsam is the isotopic ratio of the sample and Rstd is the isotopic ratio of the international isotope standards Pee Dee Belemnite (PDB), air N2, and Canyon Diablo troilite (CDT) for C, N, and S, respectively. Analytical uncertainties (1σ) for the isotope analyses were of the order of ± 0.1 ‰ for δ13C and ± 0.3 ‰ for δ15N and δ34S, as indicated by repeated analyses of the samples and standards. Each sample was repeated three times and standards were measured every ten sample analyses.
Statistical analyses
Statistical analyses were performed using the R environment (R version 4.0.2; R Development Core Team, 2017). Analysis of variance (ANOVA) and Tukey’s HSD post-hoc test were applied to each isotopic variable to determine statistical differences within the apple subfractions and orchards. For all statistical tests, the cutoff value was set at p < 0.05, which indicated significant differences between the groups.
Correlation plots were used to investigate possible relationships among the isotopic parameters. Linear discriminant analysis (LDA) was used to perform multivariate analyses. LDA identifies linear combinations of measured variables that maximize the separation of groups. In this work, LDA was performed using stable isotope values of apple subfractions to trace the orchard where the apple samples were collected. Each linear discriminant function was validated with the leave-one-out method.
Results and discussion
The isotopic values of the investigated apple subfractions (peel, petiole, pulp, and seed) are reported in Table 1.
Table 1.
Isotopic compositions of carbon (C), nitrogen (N), and sulfur (S) among the apple subfractions (peel, petiole, pulp, and seed) collected in three orchards of the Northern Italy and the relative average values for each apple variety. Analytical uncertainties (1σ) are ± 0.1 ‰ for δ13C and ± 0.3 ‰ for δ15N and δ34S as indicated by repeated analyses of the samples and standards.
| Orchard | Variety | δ13C (‰) |
δ15N (‰) |
δ34S (‰) |
|||
|---|---|---|---|---|---|---|---|
| Peel | Petiole | Pulp | Seed | Seed | Seed | ||
| Ferrara 1 | Modi | −29.8 | −27.8 | −26.3 | −26.2 | +2.8 | +9.6 |
| −24.7 | −26.3 | −24.9 | −25.6 | +2.7 | +7.9 | ||
| −28.9 | −26.6 | −26.0 | −26.2 | +4.8 | +6.8 | ||
| Average | −27.8 | −26.9 | −25.7 | −26.0 | +3.4 | +8.1 | |
| Gala | −30.3 | −28.6 | −28.0 | −27.9 | +1.8 | +8.6 | |
| −28.7 | −27.2 | −26.5 | −25.6 | +1.7 | +9.2 | ||
| −30.9 | −29.1 | −28.3 | −27.5 | +1.6 | +8.9 | ||
| Average | −29.9 | −28.3 | −27.6 | −27.0 | +1.7 | +8.9 | |
| Fuji | −27.6 | −25.8 | −25.3 | −25.3 | +5.6 | +7.6 | |
| −28.9 | −27.6 | −26.7 | −27.0 | +2.2 | +9.1 | ||
| −30.5 | −28.1 | −26.9 | −27.6 | +3.5 | +10.4 | ||
| −29.3 | −27.9 | −25.6 | −24.6 | +2.1 | +11.2 | ||
| −30.7 | −28.4 | −26.9 | −26.9 | +1.2 | +8.6 | ||
| Average | −29.4 | −27.6 | −26.3 | −26.3 | +2.9 | +9.4 | |
| Granny | −30.4 | −28.5 | −26.8 | −26.9 | +1.5 | +10.1 | |
| −32.4 | −27.9 | −28.1 | −27.6 | +2.3 | +9.1 | ||
| −29.0 | −28.1 | −27.2 | −27.6 | +2.3 | +8.4 | ||
| −32.8 | −29.4 | −28.4 | −28.0 | +2.6 | +9.1 | ||
| −29.7 | −29.4 | −27.1 | −26.6 | +3.4 | +9.6 | ||
| Average | −30.9 | −28.7 | −27.5 | −27.4 | +2.4 | +9.3 | |
| Ferrara 2 | Pink | −32.0 | −29.3 | −27.8 | −27.0 | +2.2 | +7.5 |
| −30.1 | −27.5 | −27.2 | −25.7 | +2.8 | +10.1 | ||
| −30.4 | −29.7 | −26.9 | −26.9 | +2.3 | +9.0 | ||
| −31.3 | −29.2 | −28.5 | −26.8 | +2.6 | +7.9 | ||
| −30.6 | −28.1 | −27.4 | −26.4 | +1.9 | +7.3 | ||
| −30.6 | −28.9 | −27.3 | −26.2 | +2.4 | +8.4 | ||
| −33.2 | −29.1 | −28.3 | −27.7 | +2.0 | +7.8 | ||
| −31.6 | −29.2 | −28.4 | −27.4 | +1.5 | +6.8 | ||
| −31.6 | −29.1 | −28.8 | −26.9 | +3.2 | +6.4 | ||
| −32.8 | −29.6 | −29.4 | −28.1 | +2.3 | +7.1 | ||
| −32.3 | −29.9 | −31.6 | −27.5 | +2.4 | +7.8 | ||
| −31.7 | −29.1 | −28.1 | −27.1 | +3.7 | +6.8 | ||
| −30.5 | −27.6 | −27.3 | −25.5 | +2.3 | +8.0 | ||
| −32.1 | −30.0 | −29.4 | −28.6 | +2.2 | +6.6 | ||
| −32.2 | −29.8 | −28.8 | −27.7 | +1.9 | +7.0 | ||
| Average | −31.5 | −29.1 | −28.3 | −27.0 | +2.4 | +7.6 | |
| Golden | −28.1 | −27.4 | −25.9 | −25.6 | +2.6 | +8.4 | |
| −33.8 | −29.5 | −29.4 | −29.4 | +3.2 | +7.3 | ||
| −28.9 | −27.4 | −24.9 | −25.1 | +0.0 | +7.2 | ||
| −26.1 | −25.9 | −23.5 | −23.0 | +4.1 | +7.1 | ||
| −29.6 | −27.7 | −26.6 | −26.6 | +2.6 | +6.7 | ||
| −26.9 | −25.7 | −23.5 | −26.4 | +2.1 | +7.3 | ||
| −29.0 | −28.5 | −25.7 | −26.2 | +2.0 | +6.1 | ||
| −29.6 | −28.8 | −25.3 | −25.8 | +1.9 | +6.4 | ||
| −32.0 | −29.3 | −28.4 | −28.3 | +2.6 | +6.8 | ||
| −31.7 | −29.4 | −28.3 | −28.0 | +2.4 | +6.5 | ||
| −30.3 | −28.6 | −26.9 | −26.5 | +2.1 | +6.8 | ||
| −28.9 | −27.4 | −26.2 | −26.0 | +1.4 | +7.9 | ||
| −29.6 | −28.4 | −26.6 | −26.3 | +2.9 | +6.7 | ||
| −29.8 | −27.9 | −26.6 | −26.5 | +2.4 | +7.0 | ||
| −29.8 | −28.2 | −26.8 | −27.2 | +2.2 | +6.8 | ||
| Average | −29.6 | −28.0 | −26.3 | −26.5 | +2.3 | +7.0 | |
| Trento | Fuji | −30.0 | −28.9 | −28.0 | −28.1 | +1.9 | −0.2 |
| −30.3 | −27.8 | −28.7 | −27.8 | +1.9 | −1.7 | ||
| −33.4 | −29.7 | −29.1 | −29.4 | +1.8 | −2.1 | ||
| −30.4 | −28.7 | −27.9 | −27.6 | +2.3 | −1.2 | ||
| Average | −31.0 | −28.8 | −28.4 | −28.2 | +2.0 | −1.3 | |
In the following sub-sections, each isotopic parameter is evaluated as a potential key marker for the regional and local traceability of the northern Italian apples. Subsequently, any correlations among the isotopic parameters have been investigated to trace exactly the provenance of the fruits.
δ13C values of apple subfractions
The isotopic C fingerprint of the apple subfractions within the overall sample suite varied from −33.8 ‰ to −23.0 ‰ (Table 1). The 13C/12C ratio of plant-derived materials is mainly affected by the various photosynthetic pathways of the plant (i.e., the Calvin-C3, Hatch-Slack-C4, and Crassulacean Acid Metabolism-CAM pathway; O'Leary, 1988) used during the fixation of atmospheric CO2. In particular, C3 plants typically have more negative 13C/12C values (−33 ‰ to −24 ‰; O'Leary, 1988) than C4 (−16 ‰ to −10 ‰; O'Leary, 1988) and CAM (−20 ‰ to −10 ‰; O'Leary, 1988) plants, because of their stronger discrimination against 13C, the heaviest isotope. Therefore, the values of the apple subfractions in this study are in line with the botanical origin of the matrix: apple plants follow the C3 photosynthetic pathway, and their typical δ13C values range from −35 ‰ to −20 ‰ under natural conditions (Francois et al., 2020).
For C, we investigated the isotopic signature of each apple subfraction: therefore, we studied the potential differences in isotopic ratios among the organs irrespectively to the geographical origin of the samples. According to the one-way ANOVA test, a difference was observed among the apple subfractions (Fig. 2). In particular, the δ13C values of the peel and petiole were significantly different (p < 0.0001) from those of the pulp and seed, whose values were statistically very similar (p > 0.1). The δ13Cpeel ranged from −33.8 ‰ to −30.3 ‰ and showed the lowest values of δ13C in all samples (Table 1; Fig. 2), indicating that peels are always more depleted in 13C than the other apple parts. This correlation was also observed by Mimmo et al. (2015), who also analyzed apple subfractions in the Northern Italy. Such negative signature of the peel is probably related to the presence of phenolic compounds (Lee & Wrolstad, 1988), which are typically more abundant in the epidermal tissue of fruits (Francini & Sebastiani, 2013). The δ13Cpetiole ranges from −30.0 ‰ to −25.6 ‰ and generally present intermediate values between those of the peel (more negative) and the other two subfractions (pulp and seed, less negative). The δ13Cpulp and δ13Cseed vary from −31.6 ‰ to −23.5 ‰ and from −29.4 ‰ to −23.0 ‰, respectively (Table 1; Fig. 2), and almost overlap, with δ13Cpulp/δ13Cseed always close to 1.00, as also observed by Mimmo et al. (2015). This ubiquitous relationship suggests that the C isotopic ratios of the pulp and seed are comparable, and no fractionation processes occur during the growth and development of apples.
Fig. 2.
Box plots of the C isotopic values of apple subfractions. In each box plot, the black line and red cross represent the median and mean, respectively. Above the box plots the letters represent the results of Tukey’s HSD post-hoc test. Different letters denote significant differences among groups. The one-way ANOVA result for C isotopic signature is also reported (***p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
One-way ANOVA was used to verify whether the C isotopic ratios of the apple subfractions were affected by the geographical location of the orchards. The results are shown in the box plots in Fig. 3.
Fig. 3.
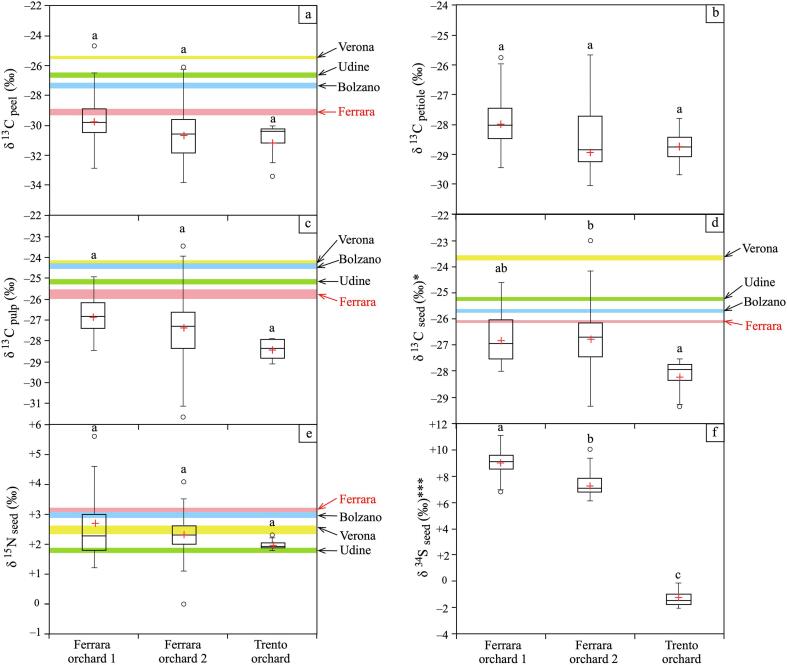
Box plots of the C, N, and S isotopic values of apple subfractions grouped according to the orchard of provenance. In each box plot the black line and red cross represent the median and mean, respectively. Above the box plots the letters represent the results of Tukey’s HSD post-hoc test. Different letters denote significant differences among groups. The one-way ANOVA results are also reported for each isotopic variable (*p < 0.01; ***p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The ANOVA test showed that δ13Cpeel, δ13Cpetiole, and δ13Cpulp were not significantly affected (p > 0.05) by the orchards where the apples grew. Instead, δ13Cseed seemed to be slightly more influenced by the provenance of apples (p ∼ 0.055), because the three orchards are characterized by C isotopic values that are slightly different and only partially overlapping (Ferrara 1: from −28.0 ‰ to −24.6 ‰; Ferrara 2: from −29.4 ‰ to −23.0 ‰; Trento: from −29.4 ‰ to −27.6 ‰; Table 1), which was also highlighted by the post-hoc test reported in Fig. 3d. Nonetheless, the δ13C signatures of the subfraction cannot clearly distinguish the orchard of the provenance of the apples, even if the orchards are in two different regions that are 200 km apart. This demonstrates that the C signature of fruits is mainly affected by the photosynthetic pathways of the plant, and not influenced by the climatic conditions of the area and/or the agricultural management used in the orchards. Other studies on traceability demonstrated that C cannot discriminate the country or continent of provenance of fruits or derived products (Gatzer et al., 2021, Rummel et al., 2010). Hence, for plants of the same species with the same metabolic process, the exact provenance is very difficult to discriminate. However, Mimmo et al. (2015) discriminated the geographical origin of apple samples collected from different Italian regions using the average values of δ13C of peel, pulp, and seed irrespectively to the fruit variety. Analogously, if we consider the mean values of apples in our study (red cross of the box plots of Fig. 3 a, c, d) instead of the ranges, then apples from Ferrara and Trento can be clearly differentiated. The average C signatures of the peel, petiole, and pulp of the apples of the two Ferrara orchards were very close to each other, but they did not overlap. The more negative δ13C values of the peel, petiole, and pulp of Ferrara 2 than Ferrara 1 were due to the presence of clay sediments in the soil of the second orchard, which tends to host more organic matter with a typical negative signature (Sarkar, Singh, Mandal, Churchman, & Bolan, 2018) than the sandy sediment of the soil of the first orchard.
Interestingly, the δ13C ranges of apple subfractions from Ferrara are the closest to the respective mean values of apples investigated by Mimmo et al. (2015), which were collected in the same province in 2012. However, the δ13C values of the two studies did not overlap, with the samples of this study showing systematically more negative values than those of Mimmo et al. (2015), which is probably related to the continuous decreasing trend of the δ13C value of the air due to the increase in anthropogenic emissions over time (Graven et al., 2017). According to the Scripps Institution of Oceanography Global CO2 Program (http://scrippsco2.ucsd.edu), the atmospheric δ13C value was −8.2 ‰ when Mimmo et al. (2015) sampled apples in 2012, and −8.6 ‰ during our sampling in 2020. Therefore, the C signature of plant-derived products is influenced by this initial difference, which should be considered in traceability studies that compare agri-food products from different locations and harvested at different times.
δ15N values of apple seed
The δ15N of the seeds ranged between −0.01 ‰ to +5.63 ‰ within the sample suite of this study (Table 1), this is indicative of the predominance of synthetic fertilizers in the three orchards. In fact, the principal factor that affects δ15N in cultured plants and derived products is the type of fertilizer applied to the soils. Synthetic fertilizers (e.g., urea, ammonium sulfate, and ammonium nitrate) are primarily produced using atmospheric nitrogen; therefore, they have δ15N values between −6 ‰ and +7 ‰ (Bateman, Kelly, & Woolfe, 2007), while organic fertilizers, such as raw or composted livestock manure, are characterized by δ15N values ranging from +3 ‰ to +37 ‰ (Choi et al., 2017). Therefore, N signatures can hardly be used as markers for traceability unless fertilizers with peculiar N compositions are employed in orchards (Mukome, Zhang, Silva, Six, & Parikh, 2013). In our study, the one-way ANOVA test showed that the δ15N of the seed was not statistically affected (p > 0.05) by the apple provenance orchard. Moreover, differentiating fruits from the Italian provinces where the apples were collected, such as in our work, is difficult because the ranges of δ15N in fruits from Ferrara and Trento overlap each other and with the mean δ15N values of the other provinces (Bolzano, Verona, and Udine) investigated by Mimmo et al. (2015) (Fig. 3e).
δ34S values of apple seed
Among studies of traceability of apple fruits, we analyzed for the first time the isotopic ratios of S in seeds. In general, the main sources of S for plants are bedrock, fertilizers, and natural atmospheric and anthropogenic sources (Sparks, Crowley, Rutherford, & Jaggernauth, 2019). As a result, δ34S in plant tissues is influenced by many factors, such as plant’ growth conditions, sulfides contents in bedrock, atmospheric sulfate deposition on the soil surface, synthetic fertilizers use, and seaspray (aerosolized seawater containing sulfates) deposition in areas close to the sea (Pianezze et al., 2020).
The δ34S values of our apple samples presented a wide range from −2.1 ‰ to +11.2 ‰, with the values of Trento (from −2.1 ‰ to −0.2 ‰; Table 1) being more negative than those of Ferrara (Ferrara 1: from +6.8 ‰ to +11.2 ‰; Ferrara 2: from +6.1 ‰ to +10.1 ‰; Table 1). According to the one-way ANOVA test, the δ34S of the seed was the parameter most significantly affected (p < 0.001) by the orchard of provenance. In Fig. 3f, the box plots and the post-hoc test show that the δ34Sseed is able to (i) discriminate apples from Ferrara and Trento and (ii) distinguish apples from the two orchards of Ferrara. The distinction between the apples of Ferrara and Trento is related to the different bedrocks that characterize the two provinces. The Trento area is famous for the presence of sulfide mineralization; therefore, sulfide minerals in this province, which usually have negative δ34S values, are predominant (Bianchini et al., 2019). On the other hand, the δ34S values of Ferrara reflect the presence of sulfate minerals in the soil, which are more enriched in 34S and are abundant in alluvial sediments deposited by the Po River in a large part of the province of Ferrara (Salani et al., 2021). Interestingly, according to the post-hoc test results (Fig. 3f), the S fingerprint could also discriminate the apples from the two Ferrara orchards. In fact, the δ34S values of Ferrara 1 tended to be more positive than those of Ferrara 2 (Table 1). This difference could be related to the sediment composition of the soils where the apple plants grew, which is related to the distance from Po di Volano, a branch of the Po River, the main Italian fluvial system (Marchina et al., 2016). The Po River collects sediments from the western and central Alps and north-western Apennines, where metamorphic and sedimentary rocks are present, including evaporites enriched in sulfate minerals (Manzi et al., 2007). The Ferrara 1 orchard is located in the inner meander bend of the Po di Volano, where most of the sediment load is deposited because the velocity of water flow is slower and the capacity to transport material is reduced, as indicated by the presence of coarse-medium sized sediments, such as sand in the areas next to the river (Fig. 1b). The Ferrara 2 orchard is located in the floodplain, far away from the river, where only fine sediments such as clay and silt are deposited (Fig. 1b). Commonly, clay minerals host impurities including sulfate and sulfide compounds (Zumaquero, Gilabert, Díaz-Canales, Gazulla, & Gómez-Tena, 2021). These minerals are characterized by reducing power; therefore, some sulfate compounds hosted in clay can be reduced into sulfides, which typically have negative signature (Hoving, Sander, Bruggeman, & Behrends, 2017). Consequently, soils with clay sediments, such as those of Ferrara 2, which have a mixture of sulfates and sulfides, have less positive S isotopic signature than soils mainly composed of sand hosting only sulfate. Such S signatures of the soils were also recorded in the respective fruit products. Therefore, δ34S appears to be a promising parameter for the traceability of the exact provenance of apple fruits, even when orchards are located in a narrow area, because the isotopic ratio of S is mainly related to the geological features of the soil, which can also change on a small scale. This feature was also demonstrated by the study of traceability of the northern Italian cheese by Pianezze et al. (2020), who distinguished the variety of cheese and the Italian region and the province of provenance using only the S isotopic signature.
Multi-isotopic approach for tracing the Northern Italian apples
Combinations of two isotopes are commonly used in traceability studies in order to investigate positive or negative correlations among isotopic parameters (Pianezze et al., 2020) and ultimately to trace the origin of agri-food products because the use of more than one isotope increases the chance of separating groups of samples based on their provenance (Won et al., 2021). Therefore, in Fig. 4, we investigated in detail the correlations between the isotopic parameters of all the apple subfractions and whether they are useful for tracing the origin of apple fruits. As expected, although positive correlations were evident among the C signatures of all subfractions, they were not able to clearly trace either the orchard or the region of provenance of the samples because they were linked to the botanical origin of the plants rather than their geographical origin. The N signature of the seed did not correlate with any of the other isotopic ratios, indicating that the fertilization practices used did not affect the other isotopic parameters, including S, which can also be introduced through fertilization processes. A similar conclusion was reached by Pianezze et al. (2020), who investigated the relationship between N and S isotopes for the traceability of Italian cheese. The use of anthropic fertilizers containing N in all three orchards made it impossible to trace the origin of the apple fruits using the isotopic N signature as a discriminant parameter. The δ34S ratio of the seed does not correlate with any other isotopic ratio, indicating that S is affected by other factors, as also demonstrated by Pianezze et al. (2020). In fact, in this study, we demonstrated that S is mainly related to the composition of the sediments of the soils, particularly with the prevalence of sulfides or sulfate phases. This relation allows for the differentiation of the apple fruits of Trento from those of Ferrara because they cluster on the opposite sides of the plots with δ34S ratio in one axis. In addition, the overlap of the samples from the two Ferrara orchards was very limited, allowing tracing of the exact orchard of the provenance for most of the sample fruits. Therefore, the S signature of the seed shown in Fig. 3, Fig. 4 is the best parameter for tracing the northern Italian apples, although a small overlap between the two Ferrara orchards is observed in Fig. 4, thus reducing the accuracy of tracing results for any samples of unknown origin which fall into the overlapping area of the plot.
Fig. 4.
Correlation plots among the δ13C, δ15N, and δ34S of apple subfractions collected in three orchards in Northern Italy.
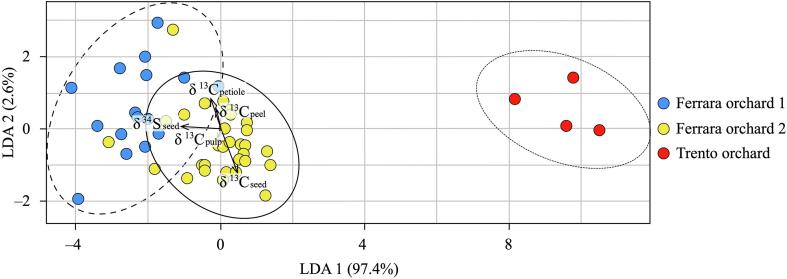
According to several studies on traceability the multi-element isotope approach involving different stable isotopic parameters (C, N, S, O, and H) is the “winning strategy” for tracing the geographical origin of different agri-food products, e.g., cheese (Camin et al., 2015, Pianezze et al., 2020), manila clams (Bianchini et al., 2021, Brombin et al., 2022, Won et al., 2021), and orange juice (Rummel et al., 2010). Recent studies have demonstrated that the combination of multiple isotopes is particularly effective for regional food discriminations among samples collected in different micro-areas with similar environments (Bianchini et al., 2021, Brombin et al., 2022). Multi-isotopic analysis combined with the linear discriminant analysis (LDA) is the most successful method in literature to trace the origin of foodstuff (Bianchini et al., 2021, Brombin et al., 2022, Won et al., 2021) because it combines the high sensitivity of the IRMS technique with the correlations of the environmental information of the origin of the products (Jiang et al., 2020). To perform the LDA with the data of this work, only the isotopic parameters whose average values were distinguishable among the three orchards were used (Table 1); therefore, the N isotopic parameter was excluded because the average δ15N values of the orchards overlap if we consider the analytical uncertainty of the analysis (i.e., ±0.3 ‰). Our LDA model was validated by cross-validation (CV) using the leave-one-out (LOO) procedure. In the resulting LDA (Fig. 5), the linear discriminant LD1 accounted for 97.4 % and LD2 accounted for 2.6 %. Sulfur is the principal isotopic parameter for distinguishing among the three orchards, as indicated in Fig. 3, Fig. 4, and the C signatures of the subfractions also allow for better separation also among the two Ferrara orchards, thus reducing the overlapping area and allowing for tracing of the exact orchard of provenance even when the fruit plants grow in areas that are near each other. In fact, the soils of the two orchards were characterized by different compositions (i.e., sandy soil vs clayey soils) which influenced the C signature of the plant-derived products of the two orchards. This means that even if the differences in C signatures between the soils of the two orchards are very small, they can contribute to the traceability of the fruits, indicating that the terroir plays a crucial role in the isotopic composition of plants and their products.
Fig. 5.
Linear discriminant analysis (LDA) of δ13C and δ34S of apple subfractions collected in three orchards in Northern Italy.
Conclusion
In recent decades consumer consciousness has increased, with people preferring regional sustainable products to support local farmers and the national/regional economy. Consequently, farmers are interested in exhibiting certification of agri-food product provenance. In this context, the development of rapid and cost-effective analytical approaches is encouraged for the correct labeling of commodities. We tested the reliability of C, N, and S isotopic fingerprints to trace the exact orchard of provenance of apples collected in three orchards in Northern Italy. According to our results, the δ34S of apple seeds has been demonstrated to be the best isotopic parameter for tracing the province of provenance of apples (i.e., Ferrara or Trento) because it is linked to the geological nature of bedrock. Sulfur can also discriminate the apples of the two Ferrara orchards, which are located very close to each other, with a discrete grade of confidence. However, the C fingerprints of apple peel, pulp, petiole, and seed together with the S fingerprint of the seed in a linear discriminant analysis (LDA) could better discriminate the apples of the two orchards, demonstrating that the differences between the combined isotopic ratios of C and S of the apple subfractions are relevant, and the isotopic fingerprints of plant-derived products, such as fruits, are affected by the relative terroir. This work demonstrates that multi-isotopic analysis is a valid method for the local traceability of agri-food, even when the harvested areas are only a few kilometers apart. In line with this, to improve the traceability of fruits, an “isotopic identity card” should be established for individual orchards to distinguish products from various orchards and ultimately to create an exhaustive archive for determining the provenance of food by matching the compositions of unknown samples with genuine control samples, which will also in order to reduce food fraud.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank four anonymous reviewers for their comments, as well as the guest and chief editors for the thoughtful editorial handling.
Data availability
Data will be made available on request.
References
- Bateman A.S., Kelly S.D., Woolfe M. Nitrogen isotope composition of organically and conventionally grown crops. Journal of Agricultural and Food Chemistry. 2007;55:2664–2670. doi: 10.1021/jf0627726. [DOI] [PubMed] [Google Scholar]
- Beccaluva L., Bianchini G., Natali C., Siena F. The alkaline-carbonatite complex of Jacupiranga (Brazil): Magma genesis and mode of emplacement. Gondwana Research. 2017;44:157–177. doi: 10.1016/j.gr.2016.11.010. [DOI] [Google Scholar]
- Bianchini G., Brombin V., Carlino P., Mistri E., Natali C., Salani G.M. Traceability and authentication of manila clams from North-Western Adriatic lagoons using C and N stable isotope analysis. Molecules. 2021;26 doi: 10.3390/molecules26071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini G., Natali C., Ferretti P., Casagrande L., Conedera M., Marchina C. Trace-element distribution on sulfide mineralization in Trento province, NE Italy. Minerals. 2019;9 doi: 10.3390/min9120736. [DOI] [Google Scholar]
- Brombin V., Mistri E., De Feudis M., Forti C., Salani G.M., Natali C.…Bianchini G. Soil carbon investigation in three pedoclimatic and agronomic settings of Northern Italy. Sustainability. 2020;12 doi: 10.3390/su122410539. [DOI] [Google Scholar]
- Brombin V., Natali C., Frijia G., Schmitt K., Casalini M., Bianchini G. Isotope geochemistry for seafood traceability and authentication: the northern Adriatic manila clams case study. Foods. 2022;11:3054. doi: 10.3390/foods11193054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camin F., Bertoldi D., Santato A., Bontempo L., Perini M., Ziller l.…Larcher R. Validation of methods for H, C, N and S stable isotopes and elemental analysis of cheese: Results of an international collaborative study. Rapid Communications in Mass Spectrometry. 2015;29:415–423. doi: 10.1002/rcm.7117. [DOI] [PubMed] [Google Scholar]
- Carrera M., Gallardo J.M. Determination of the geographical origin of all commercial Hake species by Stable Isotope Ratio (SIR) analysis. Journal of Agricultural and Food Chemistry. 2017;65:1070–1077. doi: 10.1021/acs.jafc.6b04972. [DOI] [PubMed] [Google Scholar]
- Choi W.-J., Kwak J.-H., Lim S.-S., Park H.-J., Chang S.X., Lee S.-M.…Kim H.Y. Synthetic fertilizer and livestock manure differently affect d15N in the agricultural landscape: A review. Agriculture, Ecosystems and Environment. 2017;237:1–15. doi: 10.1016/j.agee.2016.12.020. [DOI] [Google Scholar]
- Coplen T.B., Qi H. USGS42 and USGS43: Human-hair stable hydrogen and oxygen isotopic reference materials and analytical methods for forensic science and implications for published measurement results. Forensic Science International. 2011;214:135–141. doi: 10.1016/j.forsciint.2011.07.035. [DOI] [PubMed] [Google Scholar]
- Cuchet A., Anchisi A., Telouk P., Yao Y., Sciets F., Fourel F.…Casabianca H. Multi-element (13C, 2H and 34S) bulk and compound-specific stable isotope analysis for authentication of Allium species essential oils. Food Control. 2021;126 doi: 10.1016/j.foodcont.2021.108086. [DOI] [Google Scholar]
- Dong H., Xiao K., Xian Y., Wu Y. Authenticity determination of honeys with non-extractable proteins by means of elemental analyzer (EA) and liquid chromatography (LC) coupled to isotope ratio mass spectroscopy (IRMS) Food Chemistry. 2018;240:717–724. doi: 10.1016/j.foodchem.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Eisenstecken D., Stürz B., Robatscher P., Lozano L., Zanella A. The potential of near infrared spectroscopy (NIRS) to trace apple origin: Study on different cultivars and orchard elevations. Postharvest Biology and Technology. 2019;147:123–131. doi: 10.1016/j.postharvbio.2018.08.019. [DOI] [Google Scholar]
- Francini A., Sebastiani L. Phenolic compounds in apple (Malus x domestica Borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants. 2013;2:181–193. doi: 10.3390/antiox2030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois G., Fabrice V., Didier M. Traceability of fruits and vegetables. Phytochemistry. 2020;173 doi: 10.1016/j.phytochem.2020.112291. [DOI] [PubMed] [Google Scholar]
- Gatzer X., Chun K.P., Boner M., Hermanowski R., Mäder R., Breuer L.…Orlowski N. Assessment of multiple stable isotopes for tracking regional and organic authenticity of plant products. Isotopes in Environmental and Health Studies. 2021;57:281–300. doi: 10.1080/10256016.2021.1905635. [DOI] [PubMed] [Google Scholar]
- Graven H., Allison C.E., Etheridge D.M., Hammer S., Keeling R.F., Levin I.…White J.W.C. Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geoscientific Model Development. 2017;10:4405–4417. doi: 10.5194/gmd-10-4405-2017. [DOI] [Google Scholar]
- Hoving A.L., Sander M., Bruggeman C., Behrends T. Redox properties of clay-rich sediments as assessed by mediated electrochemical analysis: Separating pyrite, siderite and structural Fe in clay minerals. Chemical Geology. 2017;457:149–161. doi: 10.1016/j.chemgeo.2017.03.022. [DOI] [Google Scholar]
- Jiang D., Lin D., Guo Y., Ma J., Li X., Han L.…Qian Y. Potential use of stable isotope and multi-element analyses for regional geographical traceability of bone raw materials for gelatin production. Food Analytical Methods. 2020;13:762–769. doi: 10.1007/s12161-019-01687-1. [DOI] [Google Scholar]
- Katerinopoulou K., Kontogeorgos A., Salmas C.E., Patakas A., Ladavos A. Geographical origin authentication of agri-food products: A review. Foods. 2020;9 doi: 10.3390/foods9040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka S., Nakano T. Carbon and oxygen isotope ratios and their temperature dependence in carbonate and tooth enamel using GasBench II preparation device. Rapid Communications in Mass Spectrometry. 2014;28:563–567. doi: 10.1002/rcm.6799. [DOI] [PubMed] [Google Scholar]
- Luo D., Dong H., Luo H., Xian Y., Guo X., Wu Y. Multi-Element (C, N, H, O) Stable isotope ratio analysis for determining the geographical origin of pure milk from different regions. Food Analytical Methods. 2016;9:437–442. [Google Scholar]
- Luo D., Dong H., Luo H., Xian Y., Wan J., Guo X., Wu Y. The application of stable isotope ratio analysis to determine the geographical origin of wheat. Food Chemistry. 2015;174:197–201. doi: 10.1016/j.foodchem.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Manzi V., Roveri M., Gennari R., Bertini A., Biffi U., Giunta S.…Taviani M. The deep-water counterpart of the messinian lower evaporites in the apennine foredeep: The fanantello section (northern Apennines, Italy) Palaeogeography, Palaeoclimatology, Palaeoecology. 2007;251:470–499. doi: 10.1016/j.palaeo.2007.04.012. [DOI] [Google Scholar]
- Marchina C., Bianchini G., Knoeller K., Natali C., Pennisi M., Colombani N. Natural and anthropogenic variations in the Po river waters (northern Italy): Insights from a multi-isotope approach. Isotopes in Environmental and Health Studies. 2016;52:649–672. doi: 10.1080/10256016.2016.1152965. [DOI] [PubMed] [Google Scholar]
- Mimmo T., Camin F., Bontempo L., Capici C., Tagliavini M., Cesco S., Scampicchio M. Traceability of different apple varieties by multivariate analysis of isotope ratio mass spectrometry data. Rapid Communications in Mass Spectrometry. 2015;29:1984–1990. doi: 10.1002/rcm.7306. [DOI] [PubMed] [Google Scholar]
- Mukome F.N.D., Zhang X., Silva L.C.R., Six J., Parikh S.J. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. Journal of Agricultural and Food Chemistry. 2013;61:2196–2204. doi: 10.1021/jf3049142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali C., Bianchini G. Thermally based isotopic speciation of carbon in complex matrices: A tool for environmental investigation. Environmental Science and Pollution Research. 2015;22:12162–12173. doi: 10.1007/s11356-015-4503-x. [DOI] [PubMed] [Google Scholar]
- O'Leary M.H. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. doi: 10.2307/1310735. [DOI] [Google Scholar]
- Pianezze S., Bontempo L., Perini M., Tonon A., Ziller L., Franceschi P., Camin F. δ34S for tracing the origin of cheese and detecting its authenticity. Journal of Mass Spectrometry. 2020;55 doi: 10.1002/jms.4451. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: a language and environment for statistical computing. Available online. https://www.R–project.org/ (Accessed 22 June 2020).
- Rummel S., Hoelzl S., Horn P., Rossmann A., Schlicht C. The combination of stable isotope abundance ratios of H, C, N and S with 87Sr/86Sr for geographical origin assignment of orange juices. Food Chemistry. 2010;118:890–900. doi: 10.1016/j.foodchem.2008.05.115. [DOI] [Google Scholar]
- Salani G.M., Brombin V., Natali C., Bianchini G. Carbon, nitrogen, and sulphur isotope analysis of the Padanian Plain sediments: Backgrounds and provenance indication of the alluvial components. Applied Geochemistry. 2021;135 doi: 10.1016/j.apgeochem.2021.105130. [DOI] [Google Scholar]
- Sarkar B., Singh M., Mandal S., Churchman G.J., Bolan N.S. In: The future of soil carbon its conservation and formation. Garcia C., Nannipieri P., Hernandez T., editors. Academic Press; Elsevier: 2018. Chapter 3 - Clay minerals-organic matter interactions in relation to carbon stabilization in soils; pp. 71–86. [Google Scholar]
- Schellenberg A., Chmielus S., Schlicht C., Camin F., Perini M., Bontempo L.…Horacek M. Multielement stable isotope ratios (H, C, N, S) of honey from different European regions. Food Chemistry. 2010;121:770–777. doi: 10.1016/j.foodchem.2009.12.082. [DOI] [Google Scholar]
- Sparks J.M., Crowley B.E., Rutherford M.G., Jaggernauth D. Coastal proximity, orientation, and precipitation amount drive spatial variability in δ34S values on the Caribbean Island of Trinidad. Applied Geochemistry. 2019;108 doi: 10.1016/j.apgeochem.2019.104372. [DOI] [Google Scholar]
- Stefani, M., & Minarelli L. (2018). QC1.2.3 - Carta geologica del Comune di Ferrara, 1:36.000 - RUE 2018.
- Won E.-J., Kim S.H., Go Y.-S., Kumar K.S., Kim M.-S., Yoon S.-H.…Shin K.-H. A multi-elements isotope approach to assess the geographic provenance of manila clams (Ruditapes philippinarum) via recombining appropriate elements. Foods. 2021;10 doi: 10.3390/foods10030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumaquero E., Gilabert J., Díaz-Canales E., Gazulla M.F., Gómez-Tena M. Study on sulfide oxidation in a clay matrix by the hyphenated method. Minerals. 2021;11 doi: 10.3390/min11101121. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.