Abstract
Introduction
Regular intramuscular benzathine penicillin G injections have been the cornerstone of rheumatic heart disease (RHD) secondary prophylaxis since the 1950s. As the pharmacological correlate of protection remains unknown, it is difficult to recommend changes to this established regimen. Determining the minimum effective penicillin exposure required to prevent Streptococcus pyogenes infection will accelerate development of new long-acting penicillins for RHD prevention as well as inform opportunities to improve existing regimens. The CHIPS trial will address this knowledge gap by directly testing protection afforded by different steady state plasma concentrations of penicillin in an established model of experimental human S. pyogenes pharyngitis.
Methods and analysis
This is a double-blinded, placebo-controlled, randomised experimental human infection study. Sixty healthy adult volunteers aged 18–40 years will be recruited and randomised 1:1:1:1:1 to continuous intravenous penicillin infusions targeting five different steady state plasma concentrations of 0 (placebo), 3, 6, 12 and 20 ng/mL via a midline catheter. Each participant’s penicillin pharmacokinetic parameters will be established prior to the challenge, to ensure accurate dosing for the continuous infusion. Following the challenge with a well-characterised strain of S. pyogenes, participants will be observed for up to 6 days for the development of pharyngitis and treated with antibiotics prior to discharge. The primary objective is to determine the minimum effective steady-state plasma penicillin concentration required to prevent experimental pharyngitis. Secondary objectives will explore systemic and mucosal immunoinflammatory responses during pharyngitis, bacterial colonisation dynamics, environmental contamination and qualitative evaluation of the participant experience.
Ethics and dissemination
Ethical approval has been obtained (Bellberry Human Research Ethics Committee). Findings will be reported in peer-reviewed publications and presented at national/international stakeholder forums.
Trial registration number
ACTRN12621000751875.
Keywords: INFECTIOUS DISEASES, MICROBIOLOGY, CLINICAL PHARMACOLOGY
Strengths and limitations of this study
The Streptococcus pyogenes controlled human infection model provides a unique platform for a randomised, double-blinded, placebo-controlled study to evaluate the minimum concentration of penicillin required to prevent infection.
The S. pyogenes challenge strain was selected after extensive characterisation efforts, including a reproducible penicillin minimum inhibitory concentration (12 ng/mL).
Individualised pharmacokinetic modelling will be used to determine the intravenous penicillin infusion dose required for each participant to achieve very low steady-state target plasma concentrations.
The sample size (n=60) is adequately powered to detect the anticipated effect size and far smaller than what would be required in any field trial designed to address these questions.
Despite the use of a standardised pharyngitis case definition to ascertain the primary outcome, individual assessor variability in assessment of severity and diagnosis of pharyngitis cannot be fully accounted for, reflecting the practical complexity encountered in clinical practice.
Introduction
Recurrent infections with Streptococcus pyogenes (Group A beta-haemolytic Streptococcus; S. pyogenes) are associated with development of acute rheumatic fever (ARF) and rheumatic heart disease (RHD).1 RHD affects 40.5 million people globally and causes 306 000 deaths annually, mostly children and young adults living in low-income and middle-income countries.2 3 The efficacy of the intramuscular injections of 1.2 million units (MU; 900 mg) benzathine benzylpenicillin G (BPG) every 3–4 weeks for RHD secondary prophylaxis was first demonstrated in the 1950s and it remains the only proven and cost-effective protection against recurrent infection and progressive RHD.4–6 After deep intramuscular injection, BPG is slowly hydrolysed to benzylpenicillin G (penicillin) and absorbed into the plasma.7
While secondary prophylaxis has been shown to be moderately effective in adherent individuals, poor adherence to painful monthly intramuscular injections (recommended for a minimum of 5 years) limits coverage of secondary prophylaxis and its overall effectiveness.6 8–10 There is an urgent need to improve penicillin formulations for patients with ARF and RHD. Stakeholder consultations with consumers and RHD experts have consistently identified the ideal characteristics for secondary prophylaxis which include reducing dose frequency (ideally every 3–6 months), reducing pain of administration, alternative delivery strategies (including injectable implants or non-injection methods) and cold chain independence as key aspirations for an acceptable product.8 11 However, as the pharmacological correlate of protection remains unknown, it is difficult to recommend changes to the established regimen.
It has conventionally been assumed that critical pharmacological correlate for prevention of S. pyogenes infections is the time between intramuscular injections that plasma penicillin concentrations remain above 0.02 mg/L (20 ng/mL), a typical minimum inhibitory concentration (MIC) for S. pyogenes isolates.12 However, emerging evidence from a number of high-risk settings demonstrates that even the most adherent patients do not maintain these target concentrations for the majority of the interval between BPG injections.13 14 Given the apparent efficacy in adherent patients, it is possible that current regimens of BPG confer protection at lower, sustained inter-injection levels of plasma penicillin. Alternatively, transient peaks in serum concentration may be sufficient as an intermittent presumptive treatment.
The opportunity to directly test the former hypothesis under the necessary controlled conditions has arisen with the advent of a new experimental human infection model of S. pyogenes pharyngitis in healthy adults.15 The CHIPS trial will address a key knowledge gap by directly testing protection against experimental human pharyngitis, in relation to different steady state plasma concentrations of penicillin, to inform strategies for pharmacological secondary prophylaxis of RHD, including development of new and more effective long-acting penicillin formulations or optimising dose and dosing intervals with currently available formulations.
Methods and analysis
Study design
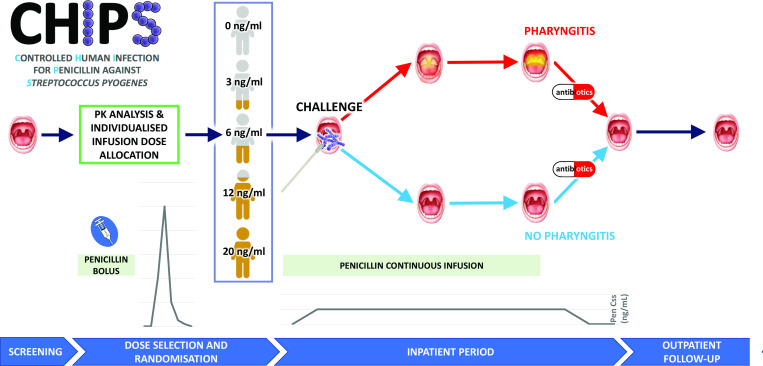
The CHIPS trial is a double-blinded, placebo-controlled, randomised human infection study to determine the minimum effective steady-state plasma penicillin concentration required to prevent pharyngitis following direct application of S. pyogenes to the oropharynx (figure 1). Based on the successful human challenge model developed in the CHIVAS-M75 study (a Controlled Human Infection model of S. pyogenes pharyngitis),15 healthy adult volunteers will be recruited through a private contract research organisation (CRO) and inoculated with the same S. pyogenes emm75 strain. A total of 60 participants will be recruited in four cohorts of 15 volunteers. Participants will be randomised 1:1:1:1:1 to receive continuous intravenous infusions of penicillin at five possible steady state plasma concentrations of 0 (placebo), 3, 6, 12 and 20 ng/mL. The study will be conducted within the CRO facility in Perth, Western Australia, with clinical support from a nearby tertiary hospital.
Figure 1.
Pictorial synopsis of the CHIPS study. Pen Css, penicillin steady state concentration; PK, pharmacokinetic.
Patient and public involvement
The need to improve RHD secondary prophylaxis is underpinned by extensive consumer engagement which has consistently identified pain and frequency associated with BPG injections as barriers to adherence.8 16 17 However, as this is a human infection study involving healthy volunteers, there is limited scope for health consumer input into the design and implementation of the study. While the methodology of our study involves S. pyogenes pharyngitis and its prevention using continuous penicillin infusion, the focus of our research and its intended beneficiaries are not sufferers of pharyngitis, but rather secondary prophylaxis for those living with ARF/RHD. Due to this indirect connection, involvement of the target patient population would be premature. For the healthy volunteers who participate in the study, they will be made aware of the results of this trial and informed of how to access the published findings.
Study objectives and outcomes
The primary objective is to determine the minimum plasma penicillin concentration associated with protection against experimental S. pyogenes pharyngitis following the challenge, assessed by the development of acute symptomatic pharyngitis (primary outcome) during the confinement period. This is assessed using the pharyngitis case definition from CHIVAS-M75 study, incorporating elements of clinical prediction rules based on Centor and McIsaac scores, change in tonsil size and real-time molecular point-of-care test for S. pyogenes (ID NOW Strep A2, Abbott).15 Secondary objectives are identification of plasma penicillin concentration required to prevent pharyngeal colonisation of S. pyogenes, and salivary penicillin concentration required to prevent S. pyogenes pharyngitis or colonisation. Exploratory objectives include characterisation of immune responses and inflammatory profiles comparing participants across penicillin dose bands and pharyngitis outcomes, examination of S. pyogenes potential for environmental contamination (with relevance to disease transmission) and exploration of motivations and the lived experiences of the volunteers who take part in human infection studies (listed in table 1 along with outcome/endpoint assessments).
Table 1.
Study objectives and outcomes
| Objective(s) | Outcome(s)/endpoint(s) | |
| Primary | To determine the minimum plasma penicillin concentration required to prevent acute symptomatic Streptococcus pyogenes pharyngitis following a direct oropharyngeal challenge with S. pyogenes M75. | Development of S. pyogenes pharyngitis during confinement period, according to a predefined clinical and laboratory criteria. |
| Secondary | 1. To identify the target plasma penicillin concentration required to prevent S. pyogenes colonisation of the pharynx. | Development of S. pyogenes colonisation following the challenge, defined as S. pyogenes M75 isolation from throat swab in the absence of signs and symptoms of clinical pharyngitis after completing antibiotic treatment at the conclusion of confinement period. |
| 2. To identify the target salivary penicillin concentration required to prevent S. pyogenes pharyngitis or colonisation. | Assays to detect penicillin concentration in saliva from all participants. | |
| Exploratory | 1. To characterise plasma humoral and cellular immunological profiles of immune response to experimental challenge with S. pyogenes in healthy participants. | Laboratory assays to measure immunological and inflammatory responses to the challenge. |
| 2. To characterise plasma inflammatory (CRP and procalcitonin) and metabolomic profiles of S. pyogenes pharyngitis. | Measurement of inflammatory markers from blood samples. | |
| 3. To identify whether Cystatin C-based markers of renal function improve estimates of penicillin G renal clearance compared with creatinine-based measures. | Measurement of Cystatin-C from blood samples. | |
| 4. To explore microbiological and local factors associated with S. pyogenes adhesion to tonsillar mucosa. | Laboratory assays to measure mucosal response. | |
| 5. To explore S. pyogenes transcriptomic changes in response to penicillin exposure in S. pyogenes pharyngitis. | Transcriptomic analyses/genetic sequencing of S. pyogenes isolates. | |
| 6. To investigate potential environmental contamination of S. pyogenes via large respiratory droplets, airborne small respiratory droplets and surface contact. | Microbiological and culture analysis of participants’ contact surfaces and surroundings. | |
| 7. To explore motivations, attitudes and experiences of participating in clinical trials and human challenge studies. | Responses to questionnaires administered during study period by participants. |
Recruitment and eligibility
A database of healthy volunteers maintained by the CRO will be used for recruitment of study participants, along with multimedia advertisements (e.g., social media platforms of CRO and affiliated institutions) using materials approved by the ethics committee. Participants will be financially reimbursed of a value determined to be satisfactory by the ethics committee. Healthy adult males and non-pregnant, non-lactating females aged 18–40 years without pre-existing risk factors for severe S. pyogenes disease will be recruited. Strict eligibility criteria are in place to mitigate risks to potential participants (full eligibility criteria detailed in online supplemental material 1; copy of the participant information sheet and consent form provided in online supplemental material 2). In addition to usual ‘healthy adult’ inclusion and exclusion criteria, medical history and physical examination, prospective participants will undertake electrocardiography and transthoracic echocardiography to rule out undiagnosed subclinical cardiac pathology. They will also undergo screening throat swabs and a serum emm75 type-specific serology to exclude carriage and prior immunity to S. pyogenes emm75 strains, respectively.
bmjopen-2022-064022supp001.pdf (102.3KB, pdf)
bmjopen-2022-064022supp002.pdf (392.9KB, pdf)
Study interventions
Dose-finding pharmacokinetic study
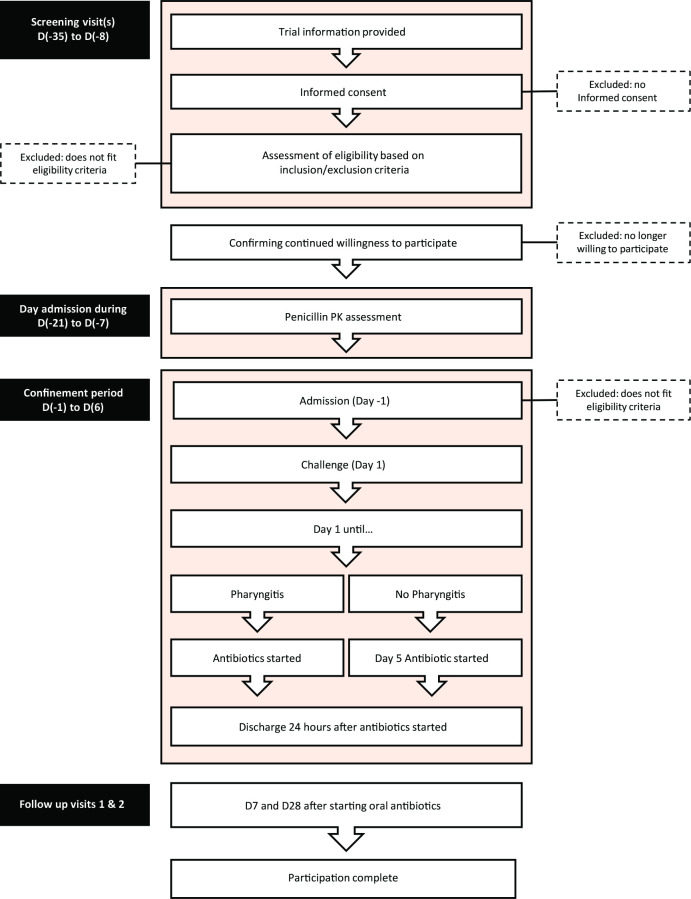
The overall journey of a participant from screening to completion of follow-up is illustrated in figure 2. At least several days prior to the inpatient challenge admission, each participant will have an individual pharmacokinetic dose-finding assessment. A 600 mg bolus dose of intravenous penicillin will be administered and serial venous blood samples will be collected for plasma penicillin concentration measurements at baseline, then 15, 30, 60, 120, 180, 240 and 360 min afterwards. Clearance and volume of distribution will be derived to enable calculation of individualised intravenous penicillin continuous infusion doses to attain the randomised target concentration for the S. pyogenes challenge admission.
Figure 2.
Participants’ journey through the CHIPS study. PK, pharmacokinetic.
Randomisation procedure
First 45 participants will be randomised 1:1:1:1:1 to one of five different target steady state concentrations (0 (placebo), 3, 6, 12 and 20 ng/mL). To ensure there is at least one participant in each concentration for each group of 5 participants and three-per-concentration in each cohort of 15 participants, the volunteers will be block randomised in groups of five following an allocation sequence generated by the study statistician and stored on a secure server, accessible only to the unblinded pharmacy and analytical team members. All clinical staff and participants will remain blinded to the treatment allocation (concentration level) for the duration of the study.
Challenge procedures
Participants are considered enrolled from the time of commencing the penicillin infusion via a midline intravenous catheter on the day of admission (Day −1). On the day of the challenge (Day +1), a sterile Dacron swab is dipped in a 1 mL single-dose vial containing 1–3 × 105 colony-forming units (CFU) of S. pyogenes emm75 and applied directly to the participant’s oropharynx. The single-dose vials will be produced according to the principles of Good Manufacturing Practice.18 19 Each participant will be challenged once only, using a standardised procedure analogous to a diagnostic throat swab done ‘in reverse’, as previously described.18 Participants will be fasted for 90 min before and after the challenge.
Confinement and discharge
Following the challenge, participants will remain inpatients at the CRO facility until reaching the primary pharyngitis outcome or until 5 days after the challenge if they remain asymptomatic, whichever occurs first. The penicillin infusion will stop at that time and a separate oral antibiotic course will be initiated (azithromycin 500 mg one time per day for 5 days).20 All participants will be monitored as inpatients for at least 24 hours after their first dose of oral antibiotic prior to discharge. Subsequent safety follow-up visits will occur 7 and 28 days after the first dose of oral antibiotic. Participants will return unused antibiotic tablets which will allow monitoring of adherence to the remainder of the oral treatment.
Adding/removing treatment arms
After 45 participants (three cohorts), an interim analysis will be performed. Provided that the prespecified statistical thresholds are met, the investigators may adjust the target concentration arms (while retaining the placebo arm) for the last cohort of 15 participants (to concentrations up to 100 ng/mL) to increase the precision of the minimum effective concentration estimate.
Pharmaceutical handling of penicillin
For each participant, individual pharmacokinetic parameters will inform the penicillin dose required to prepare the intravenous infusion bags for all possible dose allocations. After randomisation, infusion bags will be prepared at an aseptic compounding facility according to the individualised calculated dose to attain the allocated steady state plasma penicillin concentration. The stability of benzylpenicillin in 0.9% w/v sodium chloride intravenous infusion bags and the optimum sodium citrate concentration has been formally evaluated for the CHIPS trial.21 These stability studies demonstrated excellent chemical preservation of buffered benzylpenicillin at room temperature, with <1% degradation after 24 hours for benzylpenicillin 25 µg/mL in sodium citrate 100 µg/mL in 0.9% w/v sodium chloride solution, whether exposed to or protected from artificial light. Continuous infusion bags will be routinely changed every 24 hours. A sample of remnant fluid from each bag will be collected, stored at −80°C and assayed to confirm the expected stability of penicillin over the 24-hour period.
Measurement of possible environmental contamination and transmission potential
At three post-challenge time points (+24, +36 and +48 hours), colistin–nalidixic acid (CNA) agar plates will be placed in the participant’s room for 4 hours to capture potential droplet or airborne transmission of S. pyogenes. Swabs will also be taken of the participant’s surroundings and personal devices (approximately 25 cm2). To detect droplet transmission potential, participants will read a short text at each time point with CNA plates placed at varying lengths (30 cm, 90 cm and 180 cm). All swabs and CNA plates collected will undergo transfer and processing for microbial culture for beta-haemolytic streptococci as per Clinical and Laboratory Standards Institute standards, including use of positive and negative controls. If no growth is detected after 24 hours, incubation will be extended for another 24 hours. Presence of S. pyogenes from beta-haemolytic colonies will be confirmed with agglutination kits using group specific antigens (Streptex, Thermo Scientific).
Study participants’ experience
Participants will complete surveys at three time points: admission, at diagnosis of pharyngitis or Day +3 (if asymptomatic) and immediately prior to discharge. These surveys will collect qualitative data using standardised questionnaires evaluating participant’s motivation for involvement in the study and how expectations or concerns held prior to admission compared with the experience. In addition, participants will also be asked to keep a diary and record elements of their challenge admission specific to their experience of participating in a human challenge study in a non-structured form. This qualitative data will be collected and collated into themes for analysis and reporting.
Governance
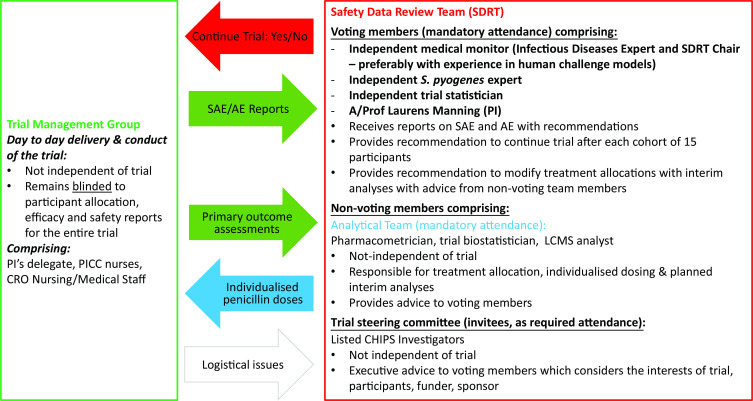
A unique governance structure, incorporating a Safety Data Review Team (SDRT), has been set up to meet the needs of this study as illustrated in figure 3. Day-to-day conduct of the study and reporting of its progress to the SDRT is done by the trial management group whose members will remain blinded to the randomised allocation until after completion of study. SDRT has voting members (chaired by an independent expert) who make decisions regarding continuation of trial after each cohort of 15 is completed, with non-voting members from the analytical team and trial steering committee providing an advisory role. An independent study monitor will be engaged who will ensure that the investigation is conducted according to the protocol and regulatory requirements. Strict data management plan will be adhered to protect participant confidentiality in compliance with Good Clinical Practice guidelines.
Figure 3.
Schematic illustration of governance structure and information flow. AE, adverse event; CRO, contract research organisation; LCMS, liquid crystallography mass spectrometry; PI, principal investigator; PICC, peripherally inserted central catheter; SAE, serious adverse event.
Safety
As in the CHIVAS-M7515 study, the following will be considered medically significant events in addition to the standard definitions—local and systemic complications of S. pyogenes infection, autoimmune sequelae of S. pyogenes infection (such as ARF, RHD and glomerulonephritis), recurrent pharyngitis in participants caused by the challenge strain and secondary cases of S. pyogenes infection with the challenge strain in non-participants.
Participant safety during confinement
Participants will be monitored closely during the confinement period in a purpose-built clinical trials facility with 24-hours clinical staffing and twice daily reviews. All adverse events will be recorded in real time and any serious adverse event will be reported to the SDRT within 24 hours of their occurrence. Starting a new cohort of participants will require approval by the SDRT following an interim review after each cohort’s confinement period is completed.
Long-term safety
The risk of long-term carriage of S. pyogenes is minimised with treatment using a non-beta-lactam antibiotic (azithromycin) prior to discharge. Additional reassurance comes from the CHIVAS-M75 study in which none of the 25 participants challenged with the emm75 strain had developed persistent carriage, systemic or autoimmune complications of S. pyogenes at completion of 6 months follow-up.15 The risk of secondary spread of infection from participants will be negligible as they will be confined in a clinical trial facility with stringent infection control measures and will have had 24 hours of oral antibiotic treatment by the time of discharge back to community (in keeping with public health recommendations for school exclusion).
Challenge strain
The S. pyogenes challenge strain (emm75, M75) was isolated from a patient with pharyngitis in 2011. It has been extensively characterised and selected for its suitability for human challenge. It is an infrequent emm-type in most published series but reliably causes pharyngitis. The particular challenge strain has favourable antibiotic susceptibility, and does not have a virulence profile typical of hypervirulent strains.19 The characterisation, selection, manufacture, storage and quality assurance approach for the emm75 challenge strain S. pyogenes has previously been described.15 18 In the CHIVAS-M75 study, at the starting dose level of 1–3 × 105 CFU in each single-dose vial, the pharyngitis attack rate was 85%.15
Sample size calculation
Based on simulations, a maximum of 60 participants are required (recruited in four cohorts of equal size; starting with five treatment arms) to detect a minimum effective dose (MED) between 0–20 ng/mL, with >80% power and Type 1 error <5%. Trial simulations were based on: (1) an anticipated 25% of placebo participants symptom-free at the end of study Day +5; (2) a monotonic normal dynamic linear model with weakly informative prior distributions; (3) equal allocation to all treatment arms; (4) interim analyses after each cohort has completed (ie, every 15 participants); (5) a high target of 90% symptom-free and a low target of 80% symptom-free in determining the MED; and (6) stopping rules for success if the posterior probability that the MED is greater than the low target is greater than 80% (ie, pr(MED>low target) >80%) and for futility if the posterior probability that the MED is greater than the upper target is less than 10% (ie, pr(MED>upper target) <10%). Trial operating characteristics were calculated for eight scenarios ranging from null efficacy to MED detected at the highest dose level.
Data analysis plan
Study data will be collected using paper and electronic source documents and managed using a secure institution hosted electronic database (Research Electronic Data Capture, USA). For the primary endpoint, Bayesian analyses will be performed on the accumulating data after each cohort completes study Day +5 and the primary pharyngitis endpoint is determined for each participant. It is anticipated that up to 25% of the participants in the placebo arm may remain free from pharyngitis. A monotonic normal dynamic linear model will be used to assess the dose response and estimate the MED. After the completion of the second (n=30) and third (n=45) cohorts, we will evaluate stopping rules for success (pr(MED>low target) >80%) and for futility (pr(MED>upper target) <10%). All secondary outcomes will be summarised by treatment arm using appropriate statistics, including mean and SD for continuous variables with symmetrical distributions or median and IQR for asymmetric distributions. Categorical variables will be summarised using frequencies and percentages.
Ethics and dissemination
This protocol (Universal Trial Number U1111-1264-9535) has been reviewed and approved by the Bellberry Human Research Ethics Committee (Ref: 2021-03-295) and is registered on the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au). The sponsor is the Telethon Kids Institute and the study is indemnified under the existing institutional insurance policy. The results of the study will be of national and international significance. Our team has strong links to stakeholder groups and national and international profiles that will ensure dissemination of the results in peer-reviewed journals and presentation at relevant congresses.
Discussion
The Global Resolution on Rheumatic Fever and Rheumatic Heart Disease calls for new technological approaches to improving global RHD control, including the ‘development of a long-acting formulation of penicillin that might improve secondary prophylactic regimens’.22 The CHIPS trial aims to address a key knowledge gap toward achieving this goal, by challenging the dogma regarding what is the MED of penicillin for successful prevention of S. pyogenes infection and secondary RHD prophylaxis.
Experimental human infection, or challenge studies, have been core platforms for infectious diseases research since at least Edward Jenner’s 18th century smallpox vaccine studies.23 In recent decades, reinforced by rigorous ethical frameworks and independent safety reviews, modern human challenge trials have contributed to accelerating development of new drugs, vaccines and diagnostics.23 24 The CHIPS trial is the first to take advantage of the successful establishment of a human pharyngitis model in the CHIVAS-M75 study.15 Although human challenge trials have previously tested the efficacy of drugs, the use of randomised steady-state plasma concentration levels of penicillin aiming to demonstrate a prophylactic threshold is an innovative approach among modern human challenge trials.23–25 Even with a sample size of 60 participants, our study is adequately powered to detect the anticipated effect size.26–29 The minimum effective dose of penicillin established in the present study is expected to provide evidence that sustained low plasma penicillin concentrations (<20 ng/mL) do prevent S. pyogenes infection while shedding further light on the relationship between serum and tissue penicillin concentrations and the role they might play in host–pathogen interaction leading to pharyngitis. Pharmacokinetic correlates of protection identified will inform the target product profile for long-acting penicillin formulations and the development of implants or depot injections that provide a ‘smoother’ exposure profile to overcome the need for frequent painful injections associated with low adherence rates.6 8 30
Findings from the CHIPS trial may have wider applications beyond RHD secondary prophylaxis, for other indications for penicillin prophylaxis, such as recurrent lower extremity cellulitis. S. pyogenes is a leading cause of erysipelas and cellulitis, the latter commonly affecting older adults living in developed economies.31–34 In the USA alone, it is estimated to cost US$3.7 billion in healthcare expenditure from 14.5 million cases annually.35 In addition, improved long-acting formulations of penicillin could have advantages for treatment of certain conditions including syphilis and other treponemal syndromes.
Potential limitations of our approach include the uncertain generalisability of an MED related to a single strain. Nonetheless, notwithstanding recent concerns stemming from rare penicillin binding protein mutations, S. pyogenes remains universally susceptible to penicillin.36–38 The relationship between in vitro MIC of the emm75 challenge strain and the MED determined in the CHIPS trial will still be relevant to considering other strains with different MICs. Likewise, it is uncertain whether findings in healthy volunteers are generalisable to the relevant patient groups for RHD secondary prophylaxis. Certainly, future research will be needed to validate novel regimens of existing preparations or novel formulations which are underpinned by new knowledge obtained from this trial across a broad range of target patient populations. The CHIPS trial will also be a platform to build on insights into host–pathogen interactions already emerging from the CHIVAS-M75 trial, to exploring environmental determinants of transmission and adding to the literature exploring the experience of participants in human challenge trials.39 40
Supplementary Material
Footnotes
Twitter: @thel_hla
Contributors: Conceptualisation and design of the study: LM, JC, JO, ACS, JAM, KTB, SS, JM, TLS, JK, TKH. Data analysis plan: JAM, LM, TKH. Drafting of the protocol: TKH, LM, JO, SS, JAM, RB, SLE, KTB. Critical revisions of the manuscript: TKH, LM, JO, SS, KTB, JAM, JK, RB, SLE, TLS, JM, ACS, JC.
Funding: This study is funded by the National Health and Medical Research Council of Australia (NHMRC) Clinical Trials and Cohort Studies—grant number APP1187743.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2016;2:15084. 10.1038/nrdp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med 2007;357:439–41. 10.1056/NEJMp078039 [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denny FW, Wannamaker LW, Hahn EO. Comparative effects of penicillin, aureomycin and terramycin on streptococcal tonsillitis and pharyngitis. Pediatrics 1953;11:7–14. 10.1542/peds.11.1.7 [DOI] [PubMed] [Google Scholar]
- 5.Stollerman GH, Rusoff JH, Hirschfeld I. Prophylaxis against group A streptococci in rheumatic fever; the use of single monthly injections of benzathine penicillin G. N Engl J Med 1955;252:787–92. 10.1056/NEJM195505122521901 [DOI] [PubMed] [Google Scholar]
- 6.Wyber R, Carapetis J, Evolution CJ. Evolution, evidence and effect of secondary prophylaxis against rheumatic fever. Journal of the Practice of Cardiovascular Sciences 2015;1:9–14. 10.4103/2395-5414.157554 [DOI] [Google Scholar]
- 7.Ginsburg CM, McCracken GH, Zweighaft TC. Serum penicillin concentrations after intramuscular administration of benzathine penicillin G in children. Pediatrics 1982;69:452–4. 10.1542/peds.69.4.452 [DOI] [PubMed] [Google Scholar]
- 8.Wyber R, Boyd BJ, Colquhoun S, et al. Preliminary consultation on preferred product characteristics of benzathine penicillin G for secondary prophylaxis of rheumatic fever. Drug Deliv Transl Res 2016;6:572–8. 10.1007/s13346-016-0313-z [DOI] [PubMed] [Google Scholar]
- 9.Wood HF, Feinstein A, Taranta A. Rheumatic fever in children and adolescents: a long-term epidemiologic study of subsequent prophylaxis, streptococcal infections, and clinical sequelae: III. Comparative effectiveness of three prophylaxis regimens in preventing streptococcal infections and rheumatic recurrences. Ann Intern Med 1964;60:31–46. 10.7326/0003-4819-60-2-31 [DOI] [PubMed] [Google Scholar]
- 10.RHDAustralia (ARF/RHD writing group) . The 2020 Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease (3.2 edition, March 2022, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Sika-Paotonu D, Laing B, Cockburn A. The BPG reformulation preferences of children and young people – an interim analysis. In: Xavier Louisiana school of pharmacy health disparities conference. New Orleans, 2019. [Google Scholar]
- 12.Craig WA. Re-evaluating current antibiotic therapy. Respir Med 2001;95:S12–19. 10.1016/S0954-6111(01)90023-X [DOI] [PubMed] [Google Scholar]
- 13.Hand RM, Salman S, Newall N, et al. A population pharmacokinetic study of benzathine benzylpenicillin G administration in children and adolescents with rheumatic heart disease: new insights for improved secondary prophylaxis strategies. J Antimicrob Chemother 2019;74:1984–91. 10.1093/jac/dkz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ketema EB, Gishen NZ, Hailu A, et al. High risk of early sub-therapeutic penicillin concentrations after intramuscular benzathine penicillin G injections in Ethiopian children and adults with rheumatic heart disease. PLoS Negl Trop Dis 2021;15:e0009399. 10.1371/journal.pntd.0009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osowicki J, Azzopardi KI, Fabri L, et al. A controlled human infection model of Streptococcus pyogenes pharyngitis (CHIVAS-M75): an observational, dose-finding study. Lancet Microbe 2021;2:e291–9. 10.1016/S2666-5247(20)30240-8 [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain-Salaun J, Mills J, Kevat PM, et al. Sharing success - understanding barriers and enablers to secondary prophylaxis delivery for rheumatic fever and rheumatic heart disease. BMC Cardiovasc Disord 2016;16:166. 10.1186/s12872-016-0344-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huck DM, Nalubwama H, Longenecker CT, et al. A qualitative examination of secondary prophylaxis in rheumatic heart disease: factors influencing adherence to secondary prophylaxis in Uganda. Glob Heart 2015;10:63–9. 10.1016/j.gheart.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Osowicki J, Azzopardi KI, Baker C, et al. Controlled human infection for vaccination against Streptococcus pyogenes (CHIVAS): establishing a group A Streptococcus pharyngitis human infection study. Vaccine 2019;37:3485–94. 10.1016/j.vaccine.2019.03.059 [DOI] [PubMed] [Google Scholar]
- 19.Osowicki J, Azzopardi KI, McIntyre L, et al. A Controlled Human Infection Model of Group A Streptococcus Pharyngitis: Which Strain and Why? mSphere 2019;4. doi: 10.1128/mSphere.00647-18. [Epub ahead of print: 13 Feb 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therapeutic Guidelines Ltd . Therapeutic Guidelines - Antibiotic (eTG December 2020, 2021. [Google Scholar]
- 21.Batty KT, Page-Sharp M, Salman S, et al. Stability of benzylpenicillin for continuous intravenous infusions: an isotonic formulation for therapeutic use and a low-dose formulation for clinical trial. J Infect Chemother 2022;28:1225–30. 10.1016/j.jiac.2022.04.016 [DOI] [PubMed] [Google Scholar]
- 22.Vekemans J, Gouvea-Reis F, Kim JH, et al. The path to group A Streptococcus vaccines: World Health organization research and development technology roadmap and preferred product characteristics. Clin Infect Dis 2019;69:877–83. 10.1093/cid/ciy1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roestenberg M, Hoogerwerf M-A, Ferreira DM, et al. Experimental infection of human volunteers. Lancet Infect Dis 2018;18:e312–22. 10.1016/S1473-3099(18)30177-4 [DOI] [PubMed] [Google Scholar]
- 24.Sekhar A, Kang G. Human challenge trials in vaccine development. Semin Immunol 2020;50:101429. 10.1016/j.smim.2020.101429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalil JA, Halperin SA, Langley JM. Human challenge studies: a review of adequacy of reporting methods and results. Future Microbiol 2012;7:481–95. 10.2217/fmb.12.15 [DOI] [PubMed] [Google Scholar]
- 26.D'Alessandri R, Plotkin G, Kluge RM. Protective studies with group A streptococcal M protein vaccine. III. Challenge of volunteers after systemic or intranasal immunization with Type 3 or Type 12 group A Streptococcus. J Infect Dis 1978;138:712–8. [DOI] [PubMed] [Google Scholar]
- 27.Fox EN, Waldman RH, Wittner MK, et al. Protective study with a group A streptococcal M protein vaccine. infectivity challenge of human volunteers. J Clin Invest 1973;52:1885–92. 10.1172/JCI107372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polly SM, Waldman RH, High P. Protective studies with a group A streptococcal M protein vaccine. II. Challange of volenteers after local immunization in the upper respiratory tract. J Infect Dis 1975;131:217–24. [DOI] [PubMed] [Google Scholar]
- 29.Waldman RH, Lee JD, Polly SM, et al. Group A streptococcal M protein vaccine: protection following immunization via the respiratory tract. Dev Biol Stand 1975;28:429–34. [PubMed] [Google Scholar]
- 30.Montagnat OD, Webster GR, Bulitta JB, et al. Lessons learned in the development of sustained release penicillin drug delivery systems for the prophylactic treatment of rheumatic heart disease (RhD). Drug Deliv Transl Res 2018;8:729–39. 10.1007/s13346-018-0482-z [DOI] [PubMed] [Google Scholar]
- 31.Bernard P, Bedane C, Mounier M, et al. Streptococcal cause of erysipelas and cellulitis in adults. A microbiologic study using a direct immunofluorescence technique. Arch Dermatol 1989;125:779–82. [PubMed] [Google Scholar]
- 32.Leppard BJ, Seal DV, Colman G, et al. The value of bacteriology and serology in the diagnosis of cellulitis and erysipelas. Br J Dermatol 1985;112:559–67. 10.1111/j.1365-2133.1985.tb15264.x [DOI] [PubMed] [Google Scholar]
- 33.Cannon J, Dyer J, Carapetis J, et al. Epidemiology and risk factors for recurrent severe lower limb cellulitis: a longitudinal cohort study. Clin Microbiol Infect 2018;24:1084–8. 10.1016/j.cmi.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 34.Cannon J, Rajakaruna G, Dyer J, et al. Severe lower limb cellulitis: defining the epidemiology and risk factors for primary episodes in a population-based case-control study. Clin Microbiol Infect 2018;24:1089–94. 10.1016/j.cmi.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 35.Raff AB, Kroshinsky D. Cellulitis: a review. JAMA 2016;316:325–37. 10.1001/jama.2016.8825 [DOI] [PubMed] [Google Scholar]
- 36.Horn DL, Zabriskie JB, Austrian R, et al. Why have group A streptococci remained susceptible to penicillin? report on a symposium. Clin Infect Dis 1998;26:1341–5. 10.1086/516375 [DOI] [PubMed] [Google Scholar]
- 37.Macris MH, Hartman N, Murray B, et al. Studies of the continuing susceptibility of group A streptococcal strains to penicillin during eight decades. Pediatr Infect Dis J 1998;17:377–81. 10.1097/00006454-199805000-00006 [DOI] [PubMed] [Google Scholar]
- 38.Vannice KS, Ricaldi J, Nanduri S, et al. Streptococcus pyogenes PBP2x mutation confers reduced susceptibility to β-lactam antibiotics. Clin Infect Dis 2020;71:201–4. 10.1093/cid/ciz1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson J, Imran S, Frost HR, et al. Immune signature of acute pharyngitis in a Streptococcus pyogenes human challenge trial. Nat Commun 2022;13. 10.1038/s41467-022-28335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation 2009;119:1442–52. 10.1161/CIRCULATIONAHA.107.742775 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064022supp001.pdf (102.3KB, pdf)
bmjopen-2022-064022supp002.pdf (392.9KB, pdf)