Abstract
Congenital arrhythmia syndromes are rare genetic disorders that can cause a high risk of sudden cardiac death. Expert panels have affirmed 15 genes that are linked to congenital arrhythmias. These genes mostly encode cardiac ion channel proteins or associated regulatory proteins that generate the cardiac action potential. Common genetic variation modulates the risk of rare variants and partially explains the incomplete penetrance of these disorders. As genetic testing becomes more prevalent, a major challenge is that most detected variants are annotated as variants of uncertain significance (VUS). This review will highlight emerging methods that are refining our understanding of arrhythmia genetics, including phenotype risk scores, large cohorts, in vitro functional assays, structural models, and computational predictions.
Introduction
Congenital arrhythmia syndromes include the long QT syndrome (LQTS), Brugada syndrome (BrS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and short QT syndrome (SQTS). These rare syndromes (frequency of 1 in 2,000 to 1 in 10,000) are classically considered to be caused by rare, large-effect variants in arrhythmia genes. Atrial fibrillation (AFib) is the most common arrhythmia but is considered a common disease with a highly polygenic and environmental origin [1], and will largely not be discussed in this review. Arrhythmias can confer risk for sudden cardiac death (SCD) that partially can be prevented by drug therapy, avoidance of disease-specific triggers such as drugs or exercise, or implantation of cardioverter-defibrillators in high-risk individuals. Genetic testing is increasingly becoming a part of clinical care for arrhythmias. An expert statement from the American Heart Association [2] and a consensus statement from four international heart rhythm associations [3] both affirmed the utility of genetic testing in individuals with suspected congenital arrhythmia disorders or in close relatives of affected individuals, and outlined genetic testing considerations for specific conditions.
Studies over the last 30 years have identified the major genes underlying congenital arrhythmia syndromes: 1) genes encoding cardiac ion channels underlying the cardiac action potential, 2) genes affecting intracellular calcium handling, and 3) additional genes that regulate these processes (Figure 1). Dozens of gene-disease associations have been proposed, including over 20 genes for each of LQTS and BrS. Some purported gene-disease associations have only been supported by minimal evidence, but these genes were nevertheless added to expanding clinical genetic testing panels. In response, the Clinical Genomics Resource (ClinGen) systematically reassessed the validity of gene-disease associations, asserting that 15 genes have “definitive” or “strong” congenital arrhythmia associations (Table 1) [4-6].
Figure 1: Major genes associated with congenital arrhythmias.
A) Schematic of the mechanism of congenital arrhythmia genes. B) Major plasma membrane ion channels in the heart. C) Left: Diagram of a cardiomyocyte with major arrhythmia-associated proteins shown (not to scale). Right: Additional congenital arrhythmia-associated genes that are not plasma membrane-localized ion channels. B-C) Diseases affirmed by ClinGen at the definitive, strong, or moderate levels are in bold. Abbreviations: BrS—Brugada syndrome, LQT—long QT syndrome, DCM—dilated cardiomyopathy, SQT—short QT syndrome, JLNS—Jervell and Lange-Neilsen Syndrome, And.-Tawil— Andersen-Tawil syndrome, CPVT—catecholaminergic polymorphic ventricular tachycardia. RyR2 CRDS—RyR2 calcium release deficiency syndrome.
Table 1:
ClinGen reappraisal of genes associated with inherited arrhythmia syndromes
| ClinGen Class |
Long QT syndrome |
Brugada syndrome |
Catecholaminergic polymorphic VT |
Short QT syndrome |
Other |
|---|---|---|---|---|---|
| Definitive | KCNQ1, KCNH2, SCN5A, CALM1/2/3 | SCN5A | RYR2, CASQ2(AR), TRDN, TECRL | KCNH2 | CACNA1C (Timothy), KCNJ2 (Anderson-Tawil) |
| Strong | TRDN (AR) | — | — | KCNQ1, SLC4A3 * | KCNE1 (aLQTS), KCNE2 (aLQTS) |
| Moderate | CACNA1C | — | CASQ2(AD), CALM1/2/3 |
SLC4A3*, KCNJ2 |
— |
| Limited | CAV3, KCNE1, KCNJ2 | — | — | — | — |
| Disputed/ No evidence |
SNTA1, AKAP9, ANK2, KCNE2, KCNJ5, SCN4B | 20 genes | ANK2, KCNJ2, PKP2, SCN5A | CACNA1C, CACNA2D1, CACNB2, SCN5A, SLC22A5 ** | — |
The panel reviewing SLC4A3 was divided whether to classify it as having strong or moderate evidence.
This gene causes primary systemic carnitine deficiency which may mimic SQTS. Abbreviations: VT=ventricular tachycardia, AR=autosomal recessive, AD=autosomal dominant, aLQTS=acquired long QT syndrome. Timothy Syndrome and Anderson-Tawil Syndrome are characterized by long QT syndrome in conjunction with characteristic non-cardiac phenotypes. CALM1, CALM2, and CALM3 are 3 distinct genes that all encode an identical calmodulin protein.
Under the 2015 American College of Medical Genetics-Association for Molecular Pathology (ACMG-AMP) classification scheme, variants are classified as benign, likely benign, variant of uncertain significance (VUS), likely pathogenic, or pathogenic [7]. A growing challenge is to decipher which rare variants in arrhythmia genes are linked to arrhythmias. Unfortunately, a majority of discovered variants in arrhythmia genes are annotated as variant of uncertain significance (VUS) [8]. This review will highlight emerging high-throughput methods that use patient datasets, in vitro functional assays, computational predictors, and protein hotspot and 3D structural analyses to help resolve the VUS Problem. This review will also discuss a major emerging theme of arrhythmia genetics—that so-called “Mendelian” arrhythmia syndromes are also influenced by common genetic variants (variants with a minor allele frequency of at least 5%). These common genetic variants can be aggregated and quantified in a Polygenic Risk Score (PRS), which may have utility in arrhythmia prediction [9].
Genes associated with congenital arrhythmias
Long QT syndrome (LQTS):
LQTS is characterized by a prolongation of the QT interval on the electrocardiogram, with risk of the torsades de pointes arrhythmia and SCD. Heterozygous dominant LQTS variants are termed Romano-Ward syndrome. Variants that lead to loss of function of the repolarizing potassium currents IKs (encoded by KCNQ1 and KCNE1), IKr (KCNH2), or IK1 (KCNJ2) can lead to LQTS [4,10]. In addition, gain of function variants in the SCN5A sodium channel or CACNA1C calcium channel genes cause excessive cation influx, leading to LQTS. Homozyous or compound heterozygous variants in KCNQ1 or KCNE1 can lead to Jervell and Lange-Neilsen Syndrome, characterized by marked QT prolongation and deafness [10,11].
Short QT syndrome (SQTS):
SQTS is a very rare syndrome characterized by a shortening of the QT (<340 ms) on the baseline ECG, with risk of subsequent arrhythmias. Gain of function variants in three potassium channel genes (KCNQ1, KCNH2, or KCNJ2) can cause SQTS, as well as loss of function variants in the anion exchanger SLC4A3 [6]. SLC22A5 loss of function causes primary systemic carnitine deficiency which may mimic SQTS [12].
Brugada syndrome (BrS):
BrS is characterized by ST-segment elevation in the right precordial leads on the electrocardiogram, with risk of SCD. Although over 20 genes have been linked with BrS, a ClinGen expert panel asserted that only dominant loss of function variants in the sodium channel gene SCN5A have definitive evidence, with the remaining genes disputed [5]. BrS appears to be more prevalent in individuals of Asian ancestry compared to other ancestries [13]. As is discussed below, BrS has a complex genetic basis with a large common genetic influence.
Catecholaminergic polymorphic ventricular tachycardia (CPVT):
CPVT is characterized by bidirectional or polymorphic ventricular tachycardia during exercise or adrenergic stimulation, which can lead to syncope or SCD. The most common cause of CPVT is dominant gain-of-function variants in the cardiac ryanodine receptor gene RYR2, which encodes the major channel involved in calcium-induced calcium release from the sarcoplasmic reticulum (SR) [14]. CPVT can also arise from dominant or recessive variants in the SR calcium-binding protein calsequestrin (encoded by CASQ2) [15] or recessive variants in the trans-2,3-enoyl-CoA reductase-like gene TECRL [16].
Other disorders:
Loss of function variants in RYR2 can cause the recently recognized RyR2 calcium release deficiency syndrome (CRDS), which (unlike CPVT) is not revealed by exercise testing, but also presents with ventricular arrhythmia and SCD [17]. A specific electrophysiological pacing protocol (a long-burst, long-pause, short-coupled stimulus) can induce ventricular arrhythmias in patients with CRDS [17]. Recessive null mutations in TRDN (triadin), a regulator of calcium release from the sarcomeric reticulum, can lead to triadin knockout syndrome (TKOS) [18]. In TKOS, young patients can present with T-wave inversions and QT prolongation or CPVT-like features, which can lead to recurrent ventricular arrhythmias even with treatment [18]. A high-penetrance form of LQTS presenting early in age can be caused by variants in the multi-functional calcium-binding protein calmodulin (encoded by 3 identical genes CALM1, CALM2, and CALM3) [19]. Variants in CALM1/2/3 can also lead to CPVT-like features. [19] A variety of additional congenital conduction disorders have been described, such as progressive cardiac conduction defect and sinus node dysfunction. In particular, SCN5A variants can give rise to a variety of conduction disorders including overlap syndromes with multiple different presentations [20].
Common genetic contributions to “Mendelian” arrhythmias
Classically, congenital arrhythmia syndromes were considered “Mendelian”, with rare large-effect variants conferring arrhythmia susceptibility. However, arrhythmia disorders were long-recognized to have incomplete penetrance—the phenomenon that only a fraction of individuals with a pathogenic variant demonstrate a phenotype. This led to the hypothesis that other genetic factors may be influencing arrhythmia risk, such as common genetic variation. The first genome-wide association study (GWAS) of a congenital arrhythmia syndrome, BrS, identified an unexpectedly large contribution of polygenic common loci near SCN5A-SCN10A and a transcription factor HEY2 [21]. The most recent BrS GWAS identified 21 significant associated common loci, including 8 independent loci near SCN5A and 6 loci near cardiac developmental transcription factors [22]. Genetic and functional studies identified MAPRE2, encoding a microtubule binding protein, as a regulator of NaV1.5 trafficking and BrS risk [22].
Common genetic variants discovered through a GWAS can be aggregated in a weighted average called a polygenic risk score (PRS) [9]. PRS’s typically follow a normal distribution, and individuals in the upper tail of the distributions of PRS might be especially predisposed to disease (Figure 2). A GWAS of AFib in over a half million individuals identified 97 significant loci, the largest of which is near the transcription factor PITX2 [23]. A PRS for AFib was deployed in the UK Biobank and explained 4.7% of the risk of AFib [24].
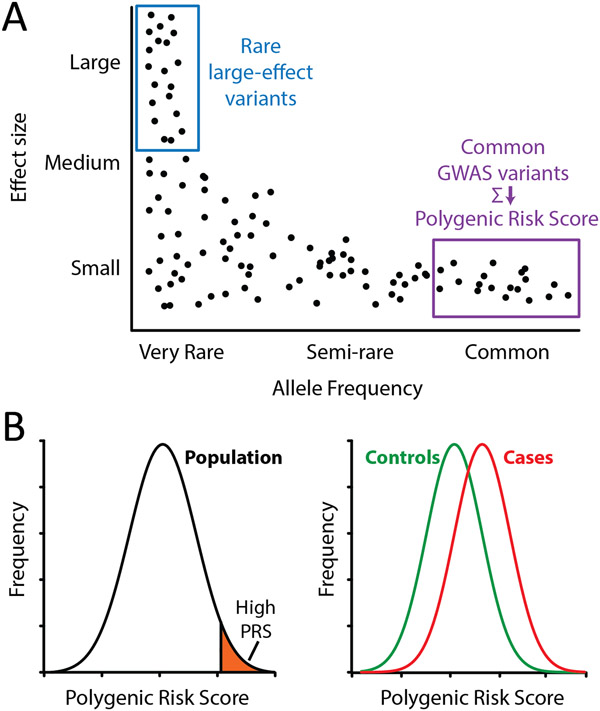
Figure 2: Common polygenic variants impact arrhythmia risk.
A) Schematic of the relationship between variant allele frequency and effect size (impact on arrhythmia risk). Rare large-effect variants and common variants identified through genome-wide association studies are highlighted. B) Polygenic risk scores (PRS) in the general population can identify individuals with high PRS who may be at an elevated risk for arrythmia disorders. Comparison of controls and cases may reveal altered distributions of PRS, indicating the influence of common genetic variants on disease risk.
In recent years, multiple studies have attempted to decipher the role of common genetic variation in influencing the QT interval and LQTS. A GWAS of baseline QT interval in 109,000 individuals identified over 35 common loci that explained ~10% of the variance in QT interval [25]. A 61 SNP PRS derived from the baseline QT GWAS explained 5-10% of QT variation in the general population [26]. The baseline QT PRS was found to be enriched in LQTS cases [27] and cases of drug-induced QT prolongation and arrhythmia [28]. In approximately 20% of cases of LQTS, no variant is found; these genotype-negative individuals were especially enriched for high PRS’s [27]. Another analysis of genotype-positive LQTS patients found that a QT PRS only explained 2% of the variance in QT interval, about 5 times lower than in the general population [29]. This result mirrors a result for BrS, where a PRS had the largest impact on BrS risk in genotype-negative cases [30]. Finally, a 1.1 million SNP PRS for QT interval (including non-genome-wide significant SNPs) was recently developed. Analyses deploying this PRS in individuals with a prolonged QT interval revealed a mixture of rare, pathogenic variants (classic LQTS) and polygenic common risk (high PRS) [31].
In summary, congenital arrhythmia syndromes have a polygenic common genetic component and PRS’s may help predict individual risk for arrhythmias, especially in genotype-negative individuals. One limitation of PRS’s is that they have lower predictive accuracy outside the ancestry group in which they were developed. Large diverse GWAS’s that include non-European ancestry individuals are needed to develop accurate arrhythmia PRS’s across ancestry groups.
The VUS Problem and high-throughput functional analyses
A growing challenge in arrhythmia genetics is the “variant of uncertain significance (VUS) problem,” the issue that a large fraction of observed variants are VUS. This problem is especially acute for rare missense variants, which are plausible disease-causing variants [32] and are often classified as VUS. Over the past few years, the number of missense variants in arrhythmia-associated genes deposited in the ClinVar variant database has risen to over 5,000, and the percentage that are VUS or have conflicting classifications has risen as well (Figure 3A-B). In the past 10 years, 8.4% of arrhythmia variants have had a meaningful reclassification in ClinVar, and most reclassifications have been in the direction of diagnostic uncertainty, e.g. from pathogenic/likely pathogenic or benign/likely benign to VUS/conflicting [8]. Large genes pose an especially large problem. For example, 3% of individuals carry a rare variant in the very large (4,967 amino acid) gene RYR2, and most newly discovered RYR2 variants are annotated as VUS [14]. A likely reason that a large percentage of variants are VUS is that most variants are present at a very low frequency in the population and therefore have not have yet been the target of detailed patient or functional studies.
Figure 3: The variant of uncertain significance problem.
A,B) Prevalence of missense variants in arrhythmia genes in ClinVar over time. Yearly archives of the ClinVar dataset were downloaded and processed to include missense variants in the 15 ClinGen definitive or strong arrhythmia genes (Table 1). VUS=variant of uncertain significance, P/LP=pathogenic/likely pathogenic, B/LB=benign/likely benign. Panel B is identical to panel A but with a zoomed-in y-axis. C) High-throughput in vitro functional approaches can characterize variants at various levels of accuracy and scale. D) Schematic of computational and structural predictions of variant effect. E) Counts of P/LP, B/LB, or VUS/Conflicting variants by gene. The 15 ClinGen definitive or strong arrhythmia genes are shown. Clinvar data in E was obtained on February 2, 2022.
One approach to improved variant classification is to use improved patient phenotyping. Phenotype-enhanced variant classification, incorporating a clinical CPVT scorecard, resulted in lower rate of RYR2 VUS’s [33]. A similar approach incorporating type 1 LQT-specific clinical features also reduced the rate of KCNQ1 VUS’s [34].
Well-validated in vitro studies can be applied at a strong level in the ACMG classification scheme to aid variant classification [7], and several complementary in vitro functional approaches are being used (Figure 3C). Cardiac ion channel variants have classically been studied by low-throughput manual patch clamping. Automated planar patch clamping enables an increase in throughput, and has been recently deployed to study and reclassify dozens of variants in KCNQ1, KCNH2, and SCN5A [35-38]. These studies take place in non-cardiac cell lines, so an open question is how faithfully they reflect the function of channels in cardiomyocytes.
Human induced pluripotent stem cell-derived cardiomyocytes are a promising system to study arrhythmia variants and have been deployed in over 50 studies to date. iPSC-CMs can be studied from patient-derived lines with suspected arrhythmia conditions [39]. Alternately, variants can be introduced with CRISPR-Cas9 editing [40,41] or the endogenous gene can be knocked out and replaced with a variant gene [42,43]. iPSC-CMs are especially promising for the study of LQTS variants, because prolonged QT has a clear cellular correlate in iPSC-CMs—a prolonged action potential. iPSC-CMs also enable the study of non-coding variants, such as a cryptic deep intronic variant that affects KCNH2 splicing [44]. Challenges with iPSC-CMs remain, including challenges of throughput and cell variability. iPSC-CMs also have an immature phenotype with altered expression of several important arrhythmia-associated genes compared to CMs in the adult heart.
Deep mutational scanning is an emerging technology that can assay thousands of variants in a single multiplexed experiment. The process involves generating a library of variants, expressing that library in cells (1 variant per cell), and performing a selection using a cellular phenotype linked to the function of the protein of interest (e.g. fluorescence, cellular localization, or cell survival). High-throughput sequencing is used to identify functional and dysfunctional variants. Complete deep mutational scans of CALM1/2/3 [45] and KCNJ2 [46] have been published, as well as scans of partial regions of SCN5A and KCNH2 [47,48]. With deep mutational scans—as well as all the in vitro assays discussed above—a critical future question will be the throughput, cost, and accuracy of the assays in measuring variant function and disease risk (Figure 3C).
Computational and structural predictions
Computational predictors that incorporate multiple features, including three-dimensional protein structural data, may also help predict per-variant arrhythmia risk (Figure 3D). In the past several years, advances in cryo-electron microscopy has enabled the solving of structures of the channels encoded by RYR2 [49], KCNQ1 and calmodulin [50], KCNH2 [51], and SCN5A [52,53]. In addition, computational protein structure predictions have dramatically improved with the development of AlphaFold [54]. Improved structural knowledge enables the identification of hotspots or key functional residues that can be incorporated in variant effect predictors [14,55,56]. A recent study leveraged the homology of cardiac and non-cardiac voltage-gated sodium and calcium channels in a machine learning approach to separately predict loss or gain of function variants [57]. Other computational variant effect predictors have been developed for all genes [58,59] or specifically for arrhythmia-associated genes [60,61]. As computational and structural variant predictors continue to increase in accuracy, they can aid variant classification and improve understanding of underlying biological mechanisms.
Conclusions
In the past few years, expert panel reappraisals, high-throughput functional studies, and computational and structural predictors have helped decipher which genes and variants are linked to congenital arrhythmia syndromes. Increasingly large patient datasets are being used for genetic discovery and to clarify the contribution of common genetic variation. An improved understanding of genotype-phenotype relationships in congenital arrhythmias ultimately has promise to help identify at-risk patients and prevent morbidity and mortality associated with these disorders.
Acknowledgements
Dr. Glazer is supported by National Institutes of Health grant R00HG010904.
Footnotes
Declaration of interest: none.
References
* (special)
** (outstanding)
- 1.Roselli C, Rienstra M, Ellinor PT: Genetics of Atrial Fibrillation in 2020: GWAS, Genome Sequencing, Polygenic Risk, and Beyond. Circ Res 2020, 127:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musunuru K, Hershberger RE, Day SM, Klinedinst NJ, Landstrom AP, Parikh VN, Prakash S, Semsarian C, Sturm AC, Council AHA, et al. : Genetic Testing for Inherited Cardiovascular Diseases: A Scientific Statement From the American Heart Association. Circulation-Genomic and Precision Medicine 2020, 13. [DOI] [PubMed] [Google Scholar]
- 3.Wilde AAM, Semsarian C, Marquez MF, Shamloo AS, Ackerman MJ, Ashley EA, Sternick EB, Barajas-Martinez H, Behr ER, Bezzina CR, et al. : European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace 2022. [DOI] [PubMed] [Google Scholar]
- 4. Adler A, Novelli V, Amin AS, Abiusi E, Care M, Nannenberg EA, Feilotter H, Amenta S, Mazza D, Bikker H, et al. : An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation 2020, 141:418–428. [*] This ClinGen expert panel reappraisal of LQTS genes identified 8 genes with moderate, strong, or definitive evidence of causality in LQTS.
- 5.Hosseini SM, Kim R, Udupa S, Costain G, Jobling R, Liston E, Jamal SM, Szybowska M, Morel CF, Bowdin S, et al. : Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation 2018, 138:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh R, Adler A, Amin AS, Abiusi E, Care M, Bikker H, Amenta S, Feilotter H, Nannenberg EA, Mazzarotto F, et al. : Evaluation of gene validity for CPVT and short QT syndrome in sudden arrhythmic death. Eur Heart J 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. : Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015, 17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosamilia MB, Lu IM, Landstrom AP: Pathogenicity Assignment of Variants in Genes Associated With Cardiac Channelopathies Evolve Toward Diagnostic Uncertainty. Circ Genom Precis Med 2022:101161CIRCGEN121003491. [DOI] [PubMed] [Google Scholar]
- 9.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, et al. : Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nature Genetics 2018, 50:1219-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JD, Asaki SY, Mazzanti A, Bos JM, Tuleta I, Muir AR, Crotti L, Krahn AD, Kutyifa V, Shoemaker MB, et al. : An International Multicenter Evaluation of Type 5 Long QT Syndrome: A Low Penetrant Primary Arrhythmic Condition. Circulation 2020, 141:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, Shkolnikova M, Berul CI, Bitner-Glindzicz M, Toivonen L, et al. : The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation 2006, 113:783–790. [DOI] [PubMed] [Google Scholar]
- 12.Roussel J, Labarthe F, Thireau J, Ferro F, Farah C, Roy J, Horiuchi M, Tardieu M, Lefort B, Francois Benoist J, et al. : Carnitine deficiency induces a short QT syndrome. Heart Rhythm 2016, 13:165–174. [DOI] [PubMed] [Google Scholar]
- 13.Vutthikraivit W, Rattanawong P, Putthapiban P, Sukhumthammarat W, Vathesatogkit P, Ngarmukos T, Thakkinstian A: Worldwide Prevalence of Brugada Syndrome: A Systematic Review and Meta-Analysis. Acta Cardiol Sin 2018, 34:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapplinger JD, Pundi KN, Larson NB, Callis TE, Tester DJ, Bikker H, Wilde AAM, Ackerman MJ: Yield of the RYR2 Genetic Test in Suspected Catecholaminergic Polymorphic Ventricular Tachycardia and Implications for Test Interpretation. Circ Genom Precis Med 2018, 11:e001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng K, Titus EW, Lieve KV, Roston TM, Mazzanti A, Deiter FH, Denjoy I, Ingles J, Till J, Robyns T, et al. : An International Multicenter Evaluation of Inheritance Patterns, Arrhythmic Risks, and Underlying Mechanisms of CASQ2-Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation 2020, 142:932–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster G, Aburawi EH, Chaix MA, Chandler S, Foo R, Islam A, Kammeraad JAE, Rioux JD, Al-Gazali L, Sayeed MZ, et al. : Life-threatening arrhythmias with autosomal recessive TECRL variants. Europace 2021, 23:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B, Yao J, Ni M, Wei J, Zhong X, Guo W, Zhang L, Wang R, Belke D, Chen YX, et al. : Cardiac ryanodine receptor calcium release deficiency syndrome. Sci Transl Med 2021, 13. [DOI] [PubMed] [Google Scholar]
- 18.Clemens DJ, Tester DJ, Giudicessi JR, Bos JM, Rohatgi RK, Abrams DJ, Balaji S, Crotti L, Faure J, Napolitano C, et al. : International Triadin Knockout Syndrome Registry. Circ Genom Precis Med 2019, 12:e002419. [DOI] [PubMed] [Google Scholar]
- 19.Crotti L, Spazzolini C, Tester DJ, Ghidoni A, Baruteau AE, Beckmann BM, Behr ER, Bennett JS, Bezzina CR, Bhuiyan ZA, et al. : Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur Heart J 2019, 40:2964–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porretta AP, Probst V, Bhuiyan ZA, Davoine E, Deliniere A, Pascale P, Schlaepfer J, Superti-Furga A, Pruvot E: SCN5A overlap syndromes: An open-minded approach. Heart Rhythm 2022. [DOI] [PubMed] [Google Scholar]
- 21.Bezzina CR, Barc J, Mizusawa Y, Remme CA, Gourraud JB, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi F, et al. : Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet 2013, 45:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barc J, Tadros R, Glinge C, Chiang DY, Jouni M, Simonet F, Jurgens SJ, Baudic M, Nicastro M, Potet F, et al. : Genome-wide association analyses identify new Brugada syndrome risk loci and highlight a new mechanism of sodium channel regulation in disease susceptibility. Nat Genet 2022, 54:232–239. [**] This study carried out a genome-wide association study of BrS in 2,820 BrS cases and 10,001 controls and detected 21 independent association signals impacting BrS risk. This included 8 independent loci near SCN5A and 6 near cardiac transcription factor genes.
- 23.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, et al. : Multi-ethnic genome-wide association study for atrial fibrillation. Nature Genetics 2018, 50:1225-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, Jurgens SJ, Weng LC, Pirruccello JP, Roselli C, Chaffin M, Lee CJ, Hall AW, Khera AV, Lunetta KL, et al. : Monogenic and Polygenic Contributions to Atrial Fibrillation Risk: Results From a National Biobank. Circ Res 2020, 126:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arking DE, Pulit SL, Crotti L, Van der Harst P, Munroe PB, Koopmann TT, Sotoodehnia N, Rossin EJ, Morley M, Wang X, et al. : Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nature Genetics 2014, 46:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg MA, Lubitz SA, Lin H, Kosova G, Castro VM, Huang P, Ellinor PT, Perlis RH, Newton-Cheh C: Validation of Polygenic Scores for QT Interval in Clinical Populations. Circ Cardiovasc Genet 2017, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahrouchi N, Tadros R, Crotti L, Mizusawa Y, Postema PG, Beekman L, Walsh R, Hasegawa K, Barc J, Ernsting M, et al. : Transethnic Genome-Wide Association Study Provides Insights in the Genetic Architecture and Heritability of Long QT Syndrome. Circulation 2020, 142:324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss DG, Vicente J, Johannesen L, Blinova K, Mason JW, Weeke P, Behr ER, Roden DM, Woosley R, Kosova G, et al. : Common Genetic Variant Risk Score Is Associated With Drug-Induced QT Prolongation and Torsade de Pointes Risk A Pilot Study. Circulation 2017, 135:1300-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turkowski KL, Dotzler SM, Tester DJ, Giudicessi JR, Bos JM, Speziale AD, Vollenweider JM, Ackerman MJ: Corrected QT Interval-Polygenic Risk Score and Its Contribution to Type 1, Type 2, and Type 3 Long-QT Syndrome in Probands and Genotype-Positive Family Members. Circulation-Genomic and Precision Medicine 2020, 13. [DOI] [PubMed] [Google Scholar]
- 30.Wijeyeratne YD, Tanck MW, Mizusawa Y, Batchvarov V, Barc J, Crotti L, Bos JM, Tester DJ, Muir A, Veltmann C, et al. : SCN5A Mutation Type and a Genetic Risk Score Associate Variably With Brugada Syndrome Phenotype in SCN5A Families. Circulation-Genomic and Precision Medicine 2020, 13:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nauffal V, Morrill VN, Jurgens SJ, Choi SH, Hall AW, Weng LC, Halford JL, Austin-Tse C, Haggerty CM, Harris SL, et al. : Monogenic and Polygenic Contributions to QTc Prolongation in the Population. Circulation 2022. [*] This study performed a genome-wide association study of the QTc interval in the UK Biobank, from which a 1.1 million SNP PRS was developed. Individuals in a second cohort with a QTc above 480 had a mix of rare large-effect variants and a high PRS, implicating a mix of rare and common genetics.
- 32.Whiffin N, Minikel E, Walsh R, O'Donnell-Luria AH, Karczewski K, Ing AY, Barton PJR, Funke B, Cook SA, MacArthur D, et al. : Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med 2017, 19:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giudicessi JR, Lieve KVV, Rohatgi RK, Koca F, Tester DJ, van der Werf C, Martijn Bos J, Wilde AAM, Ackerman MJ: Assessment and Validation of a Phenotype-Enhanced Variant Classification Framework to Promote or Demote RYR2 Missense Variants of Uncertain Significance. Circ Genom Precis Med 2019, 12:e002510. [DOI] [PubMed] [Google Scholar]
- 34.Bains S, Dotzler SM, Krijger C, Giudicessi JR, Ye D, Bikker H, Rohatgi RK, Tester DJ, Bos JM, Wilde AAM, et al. : A phenotype-enhanced variant classification framework to decrease the burden of missense variants of uncertain significance in type 1 long QT syndrome. Heart Rhythm 2022, 19:435–442. [DOI] [PubMed] [Google Scholar]
- 35.Glazer AM, Davogustto G, Shaffer CM, Vanoye CG, Desai RR, Farber-Eger EH, Dikilitas O, Shang N, Pacheco JA, Yang T, et al. : Arrhythmia Variant Associations and Reclassifications in the eMERGE-III Sequencing Study. Circulation 2022, 145:877–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glazer AM, Wada Y, Li B, Muhammad A, Kalash OR, O'Neill MJ, Shields T, Hall L, Short L, Blair MA, et al. : High-Throughput Reclassification of SCN5A Variants. Am J Hum Genet 2020, 107:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng CA, Perry MD, Liang W, Smith NJ, Foo B, Shrier A, Lukacs GL, Hill AP, Vandenberg JI: High-throughput phenotyping of heteromeric human ether-a-go-go-related gene potassium channel variants can discriminate pathogenic from rare benign variants. Heart Rhythm 2020, 17:492–500. [*] This study performed automated planar patch clamping of 30 variants in KCNH2. The assay successfully discriminated 17/17 pathogenic/likely pathogenic/benign control variants and identified novel dysfunctional LQT2-associated variants.
- 38.Vanoye CG, Desai RR, Fabre KL, Gallagher SL, Potet F, DeKeyser JM, Macaya D, Meiler J, Sanders CR, George AL Jr.: High-Throughput Functional Evaluation of KCNQ1 Decrypts Variants of Unknown Significance. Circ Genom Precis Med 2018, 11:e002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg P, Oikonomopoulos A, Chen H, Li Y, Lam CK, Sallam K, Perez M, Lux RL, Sanguinetti MC, Wu JC: Genome Editing of Induced Pluripotent Stem Cells to Decipher Cardiac Channelopathy Variant. J Am Coll Cardiol 2018, 72:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandao KO, van den Brink L, Miller DC, Grandela C, van Meer BJ, Mol MPH, de Korte T, Tertoolen LGJ, Mummery CL, Sala L, et al. : Isogenic Sets of hiPSC-CMs Harboring Distinct KCNH2 Mutations Differ Functionally and in Susceptibility to Drug-Induced Arrhythmias. Stem Cell Reports 2020, 15:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavali NV, Kryshtal DO, Parikh SS, Wang L, Glazer AM, Blackwell DJ, Kroncke BM, Shoemaker MB, Knollmann BC: Patient-independent human induced pluripotent stem cell model: A new tool for rapid determination of genetic variant pathogenicity in long QT syndrome. Heart Rhythm 2019, 16:1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dotzler SM, Kim CSJ, Gendron WAC, Zhou W, Ye D, Bos JM, Tester DJ, Barry MA, Ackerman MJ: Suppression-Replacement KCNQ1 Gene Therapy for Type 1 Long QT Syndrome. Circulation 2021, 143:1411–1425. [DOI] [PubMed] [Google Scholar]
- 43.Pierre M, Djemai M, Poulin H, Chahine M: Na(V)1.5 knockout in iPSCs: a novel approach to study Na(V)1.5 variants in a human cardiomyocyte environment. Scientific Reports 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobert KE, Tester DJ, Zhou W, Haglund-Turnquist CM, Giudicessi JR, Ackerman MJ: Genome sequencing in a genetically elusive multigenerational long QT syndrome pedigree identifies a novel LQT2-causative deeply intronic KCNH2 variant. Heart Rhythm 2022. [DOI] [PubMed] [Google Scholar]
- 45.Weile J, Sun S, Cote AG, Knapp J, Verby M, Mellor JC, Wu Y, Pons C, Wong C, van Lieshout N, et al. : A framework for exhaustively mapping functional missense variants. Mol Syst Biol 2017, 13:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coyote-Maestas WN D; He Y; Schmidt D: Determinants of trafficking, conduction, and disease within a K+ channel revealed through multiparametric deep mutational scanning. Elife 2022. [*] This study performed a deep mutational scan of Kir2.1 (KCNJ2). Study of 7,429 missense mutations revealed variants affecting cell surface trafficking and/or channel function.
- 47.Glazer AM, Kroncke BM, Matreyek KA, Yang T, Wada Y, Shields T, Salem JE, Fowler DM, Roden DM: Deep Mutational Scan of an SCN5A Voltage Sensor. Circ Genom Precis Med 2020, 13:e002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozek KA, Glazer AM, Ng CA, Blackwell D, Egly CL, Vanags LR, Blair M, Mitchell D, Matreyek KA, Fowler DM, et al. : High-throughput discovery of trafficking-deficient variants in the cardiac potassium channel KV11.1. Heart Rhythm 2020, 17:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N: Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 2016, 354. [DOI] [PubMed] [Google Scholar]
- 50.Sun J, MacKinnon R: Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 2017, 169:1042-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, MacKinnon R: Cryo-EM Structure of the Open Human Ether-a-go-go-Related K(+) Channel hERG. Cell 2017, 169:422–430 e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang D, Shi H, Tonggu L, Gamal El-Din TM, Lenaeus MJ, Zhao Y, Yoshioka C, Zheng N, Catterall WA: Structure of the Cardiac Sodium Channel. Cell 2020, 180:122–134 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Jin X, Wu T, Huang G, Wu K, Lei J, Pan X, Yan N: Structural Basis for Pore Blockade of the Human Cardiac Sodium Channel Nav 1.5 by the Antiarrhythmic Drug Quinidine*. Angew Chem Int Ed Engl 2021, 60:11474–11480. [DOI] [PubMed] [Google Scholar]
- 54.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, et al. : Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz PJ, Moreno C, Kotta MC, Pedrazzini M, Crotti L, Dagradi F, Castelletti S, Haugaa KH, Denjoy I, Shkolnikova MA, et al. : Mutation location and IKs regulation in the arrhythmic risk of long QT syndrome type 1: the importance of the KCNQ1 S6 region. Eur Heart J 2021, 42:4743–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walsh R, Lahrouchi N, Tadros R, Kyndt F, Glinge C, Postema PG, Amin AS, Nannenberg EA, Ware JS, Whiffin N, et al. : Enhancing rare variant interpretation in inherited arrhythmias through quantitative analysis of consortium disease cohorts and population controls. Genetics in Medicine 2021, 23:47–58. [**] This study examined mutational patterns in over 1800 LQTS and 3300 BrS patients. They discovered disease-associated hotspot regions in KCNQ1, KCNH2, and SCN5A that could be integrated into revised ACMG-AMP classification criteria.
- 57. Heyne HO, Baez-Nieto D, Iqbal S, Palmer DS, Brunklaus A, May P, Johannesen KM, Lauxmann S, Lemke JR, Moller RS, et al. : Predicting functional effects of missense variants in voltage-gated sodium and calcium channels. Science Translational Medicine 2020, 12. [*] Loss and gain of function variants in voltage-gated sodium and calcium channels are associated with multiple arrhythmia and non-arrhythmia diseases. This study trained a deep learning model across the SCNxA/CACNA1x family to predict loss and gain of function variants.
- 58.Frazer J, Notin P, Dias M, Gomez A, Min JK, Brock K, Gal Y, Marks DS: Disease variant prediction with deep generative models of evolutionary data. Nature 2021, 599:91–95. [DOI] [PubMed] [Google Scholar]
- 59.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M: CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 2019, 47:D886–D894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Draelos RL, Ezekian JE, Zhuang F, Moya-Mendez ME, Zhang Z, Rosamilia MB, Manivannan PKR, Henao R, Landstrom AP: GENESIS: Gene-Specific Machine Learning Models for Variants of Uncertain Significance Found in Catecholaminergic Polymorphic Ventricular Tachycardia and Long QT Syndrome-Associated Genes. Circ Arrhythm Electrophysiol 2022, 15:e010326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozek K, Wada Y, Sala L, Denjoy I, Egly C, O'Neill MJ, Aiba T, Shimizu W, Makita N, Ishikawa T, et al. : Estimating the Posttest Probability of Long QT Syndrome Diagnosis for Rare KCNH2 Variants. Circ Genom Precis Med 2021, 14:e003289. [DOI] [PMC free article] [PubMed] [Google Scholar]