Abstract
Background
Street food has been a typical culinary feature of many countries. These foods, mainly, meats and fish, were often fried, and grilled with varied marinade and preparation. However, foods that contain a lot of protein after processing at high temperatures always have many risks, including cancer risks of which heterocyclic aromatic amines (HAAs) have been one of the typical compounds. However, there is a lack of data on HAAs in low- and medium-income countries to date.
Objective
The aim was to examine the concentration of HAAs including 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP); 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx); and 2-Amino-9H-pyrido[2,3-b]indole (AαC) in cooked meat and fish samples.
Methods
Three standards including PhIP, MeIQx, AαC, and three isotopically labeled internal standards PhIP-d3, MeIQx-d3, and AαC-15N3 were purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). Formic acid, HPLC-grade methanol, acetonitrile, water, sodium chloride, and magnesium sulfate were supplied by Sigma-Aldrich (St. Louis, MO, USA). We collected cooked meat and fish samples from street markets and restaurants in the area of Cau Giay district, Hanoi, Vietnam in 2020. The collected samples were prepared for LC-MS/MS analysis.
Results
Among 23 selected samples of cooked beef, fish, chicken, and pork, we have detected PhIP(ng/g) in 9 samples (the mean 2.68, standard deviation 2.41, median 2.40, minimum 0.33, and maximum 7.19); and AαC(ng/g) in 6 samples (the mean 0.74, standard deviation 0.75, median 0.45, minimum 0.12, and maximum 1.90); and MeIQx(ng/g) was not detected in all samples. Three grilled pork samples were positive with AαC at a concentration of 0.75–1.95 ng/g. Five fish samples have been detected to contain PhIP at the concentration of mean of 3.17; the standard deviation of 1.47, and the median of 3.90 ng/g. Two fried chickens have been detected to contain PhIP at the concentration of 0.41 and 7.19 ng/g.
Conclusions
We detected a considerable amount of PhIP concentration in the collected fried fish and other fried meat samples and AαC in grilled and fried pork, beef, and chicken samples. The findings warrant further measuring more compounds of the HAA group and extending the number of real samples, as well as types of samples for example cooked meats, fish, fried eggs, tofu, and other cooked food receipts by regions in Vietnam.
Keywords: Heterocyclic amines, Carcinogens, Cooking-methods, Diet
1. Background
Generally, heterocyclic aromatic amines (HAAs) are generated from the heating process of nitrogenous compounds due to the Maillard reaction [1], [2], [3]. The formation of HAAs is temperature-dependent, therefore, HAAs can be divided into two groups: thermic HAAs (forming at the temperature from 100 °C to 300 °C), and pyrolytic HAAs (forming above 300 °C). The HAA is often found in cooked animal-origin food [4], [5]. The level of HAAs in cooked food highly depends on the type of meat, time of cooking, and the temperature [6]. Many HAAs are reported as carcinogenic and induce tumors at multiple sites in rodents [7], [8]. Moreover, several studies have proved that frequent consumption of meats containing HAAs can result in elevated risks for colon, liver, stomach, and mammary cancers in animals [7], [8]. In 2002, 2-Amino-3-methylimidazo[4,5-f]quinoline (IQ) was the first time report listed in the Tenth Report on Carcinogens. In 2004, the other HAAs 2–Amino-3,4-dimethylimidazo[4,5–f]quinoline (MeIQ), 2–Amino-3,8-dimethylimidazo[4,5–f]quinoxaline (MeIQx), 2–Amino-1-methyl-6-phenylimidazo[4,5–b]pyridine (PhIP) were also the first time report listed in the Eleventh Report on Carcinogens. Based on sufficient evidence of carcinogenicity from studies in experimental animals, MeIQ, MeIQx, IQ, and PhIP are “reasonably anticipated to be human carcinogens” that have been stated by the National Toxicology Program, Department of Health and Human Services [9]. The MeIQ, MeIQx, and PhIP (Fig. 1) were classified as possibly carcinogenic to humans (IARC) [10].
Fig. 1.
: Chemical structures of PhIP, AαC, and MeIQx.
Thus, the regulation of HAAs levels in the human diet is necessary. Street food has been a typical culinary feature of many countries. These foods, mainly, meats and fish, were often fried, and grilled with varied marinade and preparation. However, foods that contain a lot of protein after processing at high temperatures always have many risks, including cancer risks of which heterocyclic aromatic amines (HAAs) have been one of the typical compounds. However, there is a lack of data on HAAs in low- and medium-income countries to date.
1.1. Objective
The aim was to examine the concentration of HAAs including 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP); 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx); and 2-Amino-9 H-pyrido[2,3-b]indole (AαC) in cooked meat and fish samples.
2. Methods
2.1. Materials
Three standards including PhIP, MeIQx, AαC, and three isotopically labeled internal standards PhIP-d3, MeIQx-d3, and AαC-15N3 were purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). Formic acid, HPLC-grade methanol, acetonitrile, water, sodium chloride, and magnesium sulfate were supplied by Sigma-Aldrich (St. Louis, MO, USA). For sample preparation, Oasis WCX cartridges (200 mg, 6 mL) were purchased from Waters Corporation (Dublin, Ireland).
2.2. Preparation of standard solution
Single stock solution (1000 μg/mL) of HAA standards and isotopically labeled internal standards were prepared by dissolving 1 mg of one standard in 1 mL of methanol. The mixture of 1 μg/mL HAA standards was prepared by mixing aliquots of each standard and diluting them with methanol. Similarly, a working solution of internal standards was prepared by mixing aliquots of all three stock solutions and diluting to 1 μg/mL of each internal standard. All stock and working solutions were stored in a refrigerator (4 °C) until use. The calibration curve from 0.2 to 5 ng/mL was established in the matrix by spiked with HAAs mixture.
2.3. Instrumentation
Sample and standard solutions were analyzed by Shimadzu UFLC XR (Columbia, MD, USA) coupled with an SCIEX triple quad 5500 (Framingham, MA, USA). The chromatographic separation of HAAs was conducted on a C18 Symmetry column (3.5 µm, 3150 mm). The injected sample was eluted by a linear gradient of mobile phase A (0.1% formic acid and 10 mM ammonium acetate in water) and mobile phase B (100% ACN) at a flow rate of 0.5 mL/min. In the beginning, mobile phase B was kept at 10% for 1 min, then increased to 100% in 2.5 min and kept for 3.5 min, after that, returned to 10% and kept for 3 min. The mass spectrometric analysis was carried out in the positive ion mode with the following parameters: Ion spray voltage at 5500 V, source heater temperature at 500 °C, curtain gas at 30 psi, ion source gas 1 at 40 psi, ion source gas 2 at 40 psi. The compound-dependent mass spectrometric parameters were presented in Table 1.
Table 1.
Compound-dependent mass spectrometric parameters for HAAs and their internal standards.
| Analytics | Precursor ions | Product ions | DP (V) | CE (V) | CXP (V) | Type of ion |
|---|---|---|---|---|---|---|
| AαC | 184.1 | 140.2 | 61 | 39 | 10 | Quantitation |
| 184.1 | 167.1 | 61 | 29 | 10 | Confirmation | |
| AαC-15N3 | 187.1 | 169.1 | 51 | 31 | 22 | Internal standard |
| MeIQx | 214.1 | 199.2 | 76 | 35 | 12 | Quantitation |
| 214.1 | 131.1 | 76 | 47 | 12 | Confirmation | |
| MeIQx-d3 | 217.1 | 199.2 | 51 | 33 | 10 | Internal standard |
| PhIP | 225.1 | 210.1 | 56 | 35 | 14 | Quantitation |
| 225.1 | 140.0 | 56 | 59 | 14 | Confirmation | |
| PhIP-d3 | 228.1 | 210.0 | 51 | 39 | 16 | Internal standard |
2.4. Samples
In this study, we collected cooked meat and fish samples from street markets and restaurants in the area of Cau Giay district, Hanoi, Vietnam in 2020. All the samples were coded and stored in a refrigerator (4 °C) until treatment (less than three hours).
2.5. Sample treatment
Our sample preparation was developed by simplifying and modifying the previous sample treatment of Robert J Turesky‘s group [11]. Each sample was homogenized and ground the whole including skin and meat, then weighed around 2.0 g into a conical tube. All the samples were added to an internal standard aliquot before proceeding analytical process: adding 10 mL of water, sonicating for 30 min, adding 1% formic acid in acetonitrile, mixing well for 1 min in vortex mixture, adding a mixture of 3.0 g magnesium sulfate and 1.0 g sodium chloride, mixing well for 1 min, centrifuging for 5 min, cleaning the upper layer by the Oasis MCX cartridges. Before loading the extract solution into an SPE cartridge, the column was activated by 3 mL methanol and 3 mL water. The column was rinsed with 3 mL water and 3 mL methanol; HAAs were eluted with 4.0 mL of 5% ammonia in methanol. The eluent, was then, dry under pure nitrogen gas and resuspended by 1.0 mL acetonitrile: 0.1% acid formic in water (v/v, 10:90). The final solution was filtrated through a 0.2 µm membrane and ready for LC-MS/MS analysis.
3. Results
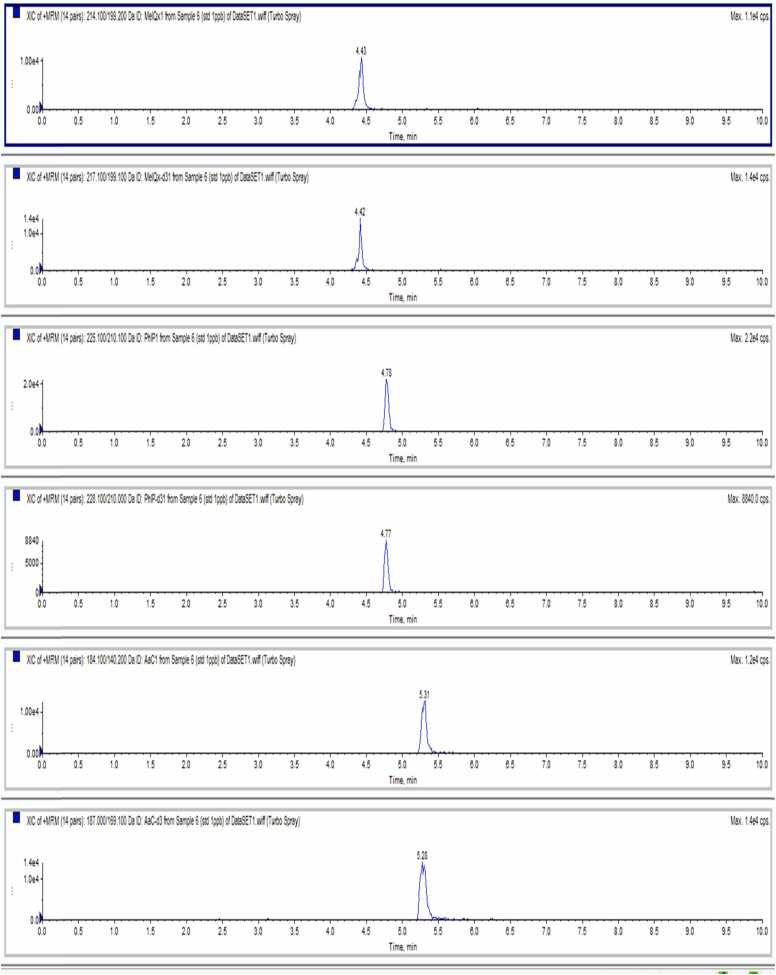
Three HAAs were separated and detected successfully. Extracted ion chromatograms of LC-MS/MS for three HAAs and their internal standard was shown in Fig. 2. The linearity was from 0.10 to 10.00 ng/mL, and the coefficient of determination was over 0.999. The limit of detection and the limit of quantitation were 0.03 and 0.10 ng/g, respectively.
Fig. 2.
Extracted ion chromatograms of LC-MS/MS for three HAAs and their internal standards.
The developed method was applied to estimate concentrations of three HAAs in 23 samples collected randomly from the local street markets. Among these 23 selected samples of cooked beef, fish, chicken, and pork, we have detected PhIP(ng/g) in 9 samples (mean 2.68, standard deviation 2.41, median 2.40, minimum 0.33, and maximum 7.19); and AαC(ng/g) in 6 samples (mean 0.74, standard deviation 0.75, median 0.45, minimum 0.12 and maximum 1.90); and MeIQx(ng/g) was not detected in all samples. The results showed that the extraction method is effective to extract HAAs from complicated matrices like cooked meats and fish. We have detected three grilled pork samples that were positive with AαC at a concentration of 0.75–1.95 ng/g and one roasted pork sample positive with PhIP at a concentration of 0.33 ng/g, over six collected ones. Among eight cooked chicken samples (including six fried, one grilled, and one boiled sample), two fried chickens have detected to contain PhIP at the concentration of 0.41 and 7.19 ng/g, and one grilled and one fried sample has AαC at the concentration of 0.12 and 0.16 ng/g. One grilled beef was positive with AαC at a concentration of 0.14 ng/g, and one fried beef was positive with PhIP at a concentration of 0.34 ng/g, over four collected ones. For five cooked-fish samples, the estimated PhIP concentration (ng/g) was the mean of 3.17; the standard deviation of 1.47, and the median of 3.90; (minimum 0.95, maximum 4.30), Table 2. Only one sample positive with both PhIP and AαC was fried chicken with skin, prepared by slicing it into mall pieces, covered with cornstarch, and fried twice with well-browned color.
Table 2.
Concentration of HAAs in collected samples.
| No. | Sample code | Sample | Describe and usual outside appearances | PhIP (ng/g) | AαC (ng/g) | MeIQx (ng/g) |
|---|---|---|---|---|---|---|
| 1 | 1209 | Boiled chicken | Lightly yellow | ND | ND | ND |
| 2 | 1210 | Fried beef | Lightly browned | ND | ND | ND |
| 3 | 1211 | Fried beef | Lightly browned | ND | ND | ND |
| 4 | 0501 | Grilled beef | Whole, lightly browned | ND | 0.14 ± 0.04 | ND |
| 5 | 0502 | Fried beef | Whole, well browned | 0.34 ± 0.10 | ND | ND |
| 6 | 1215 | Fried chicken | Sliced in small pieces and fried twice, lightly browned | 0.41 ± 0.12 | ND | ND |
| 7 | 1216 | Fried chicken | Sliced in small pieces and fried twice, lightly browned | ND | ND | ND |
| 8 | 1201 | Fried chicken thighs | Flour coated and seasoned, lightly browned | ND | ND | ND |
| 9 | 1205 | Fried chicken wings | None flour-coated, lightly browned | ND | ND | ND |
| 10 | 1212 | Fried chicken wings | Flour coated and seasoned, lightly browned | ND | ND | ND |
| 11 | 0503 | Grilled chicken | Sliced in small pieces, with skin, lightly browned | ND | 0.12 ± 0.04 | ND |
| 12 | 0504 | Fried chicken | Sliced in small pieces, with skin, covered with cornstarch, fried twice, well browned | 7.19 ± 2.16 | 0.16 ± 0.05 | ND |
| 13 | 1207# | Fried fish | Sliced in medium pieces and fried, blackened | 4.30 ± 1.30 | ND | ND |
| 14 | 1208# | Fried fish | Sliced in medium pieces and fried, blackened | 3.90 ± 1.17 | ND | ND |
| 15 | 1213# | Fried fish | Whole fish, well browned | 0.95 ± 0.29 | ND | ND |
| 16 | 1214# | Fried fish | Whole fish, well browned | 2.40 ± 0.72 | ND | ND |
| 17 | 1204# | Fried fish (sea fish) | Whole fish, blackened | 4.30 ± 1.30 | ND | ND |
| 18 | 1206 | Fried pork skewer | Sliced in small pieces and seasoned before frying, medium browned | ND | ND | ND |
| 19 | 1202 | Grilled pork skewer | Sliced in pieces and seasoned, medium browned | ND | 1.90 ± 0.57 | ND |
| 20 | 1203 | Grilled pork skewer | Sliced in pieces and seasoned, medium browned | ND | ND | ND |
| 21 | 0506 | Grilled pork skewer | Sliced in pieces and seasoned, medium browned | ND | 0.75 ± 0.23 | ND |
| 22 | 0507 | Grilled pork skewer | Sliced in pieces and seasoned, medium browned | ND | 1.37 ± 0.41 | ND |
| 23 | 0505 | Roasted pork | Whole, well browned | 0.33 ± 0.10 | ND | ND |
ND: Not detected; # for five cooked-fish samples, the estimated PhIP concentration (ng/g), mean 3.17; standard deviation 1.47, median 3.90; (minimum 0.95, maximum 4.30)
4. Discussions
We detected a considerable amount of PhIP and AαC concentration in cooked beef, fish, chicken, and pork collected from street markets and restaurants located in the Northern population of Hanoi city, Vietnam. We have also successfully developed the method of LC-MS/MS analysis to examine local samples. The recovery of our method is over 50% for all compounds. We have developed a simple and effective sample treatment for complex matrices. The concentration of HAAs was measured by liquid chromatography coupled tandem mass spectrometer. The method was applied to estimate HAAs concentration in real samples collected from the local market in Hanoi, Vietnam.
Research and study on HAAs concentration in real samples are timely in Vietnam, a country that had a population number of 97,338,583 people in 2020. In this year, the estimated new cases and death from cancer were 182,563 and 122,690, respectively [12]. The HAA named MeIQ, MeIQx, and PhIP have been evaluated as possibly carcinogenic to humans by the International Agency for Research on Cancer [10]. About 35% of cancer sites might be caused by an unhealthy diet [13] and what is the underlying etiology of this association? The HAA will play an important role in the development of cancer.
PhIP is the most abundant HAAs in the human diet and our present finding is consistent with this conclusion by IARC [10], [14], [15]. The highest PhIP levels were found in very well done oven-broiled bacon meat as high as 30.3 ng/g (Samples were cooked to very well done by investigators) [16] which is about 10 times higher than our findings of 3.17 ± 1.47 ng/g. The reason for this difference is that our real sample was collected from street markets and restaurants located in the Northern population of Hanoi city, Vietnam.
These are the preliminary results of our project in Vietnam. From the analysis results of real cooked meat and fish samples, we can see that the fried fish has a high risk of containing HAA compounds, while the others also can be contaminated by HAAs. The research has just detected three common HAAs in a restricted number of real samples. We are going to broaden the research to measure more compounds of the HAA group and extend the number of real samples, as well as types of samples for example cooked meats, fish, fried eggs, tofu, and other cooked food receipts by regions in Vietnam. Furthermore, after getting certain data on HAAs concentration in cooked food, we plan to estimate HAA concentration in fresh food cooked by specific recipes. In this article, we have presented our results of the HAA analysis in some types of cooked meat. Our project will extend further in the number of types of food, the number of samples, and estimate the HAAs may contain in cooked food according to the number of fresh materials.
4.1. Limitations
This study continues a previous project that utilized the same standard materials. Therefore, the standard materials were opened 6 months before we started these measurements. We are a bit worried about the quality of the standard solution. Despite some limitations, the findings are important to observe a considerable amount of PhIP concentration in the collected fried fish and other fried meat samples and AαC in grilled and fried pork, beef, and chicken samples. The findings warrant further measuring more compounds of the HAA group and extending the number of real samples, as well as types of samples for example cooked meats, fish, fried eggs, tofu, and other cooked food receipts by regions in Vietnam.
Funding
There was no funding for the present study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. R.J. Turesky, Dr. N. T. M. Tu and Ms. D. T. Linh for their initial development of the method of LC-MS/MS analysis and continuing advice in performing laboratory works to complete this pilot project.
Authors/role participation
Le Thi Hong Hao, Dang Thu Hien, Cao-Son Tran, Nguyen Thi Minh Hoa, Nguyen Thi Hong Ngoc: performing laboratory works to analyze heterocyclic aromatic amines in cooked food samples; Cao-Son Tran, Ngoan Tran Le: Prepare manuscript and revise. Le Thi Hong Hao, Binh Thanh Nguyen, Ngoan Tran Le: Designed the study and ran the project.
Handling Editor
References
- 1.Gibis M. Heterocyclic aromatic amines in cooked meat products: causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016;15:269–302. doi: 10.1111/1541-4337.12186. [DOI] [PubMed] [Google Scholar]
- 2.Hodge J.E. Dehydrated foods, the chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953;1:928–943. [Google Scholar]
- 3.Maillard L.C. Action of amino acids on sugars. Formation of melanoidins in a methodical way. Compt. Rend. 1912;154:66. [Google Scholar]
- 4.ur Rahman Ubaid, Sahar Amna, Khan Muhammad Issa, Nadeem Mudasar. Production of heterocyclic aromatic amines in meat: Chemistry, health risks, and inhibition. A review. LWT - Food Sci. Technol. 2014;59:229–233. [Google Scholar]
- 5.Turesky R.J. Formation, and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol. Lett. 2007;168:219–227. doi: 10.1016/j.toxlet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Skog K., Steineck G., Augustsson K., Jagerstad M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis. 1995;16:861–867. doi: 10.1093/carcin/16.4.861. [DOI] [PubMed] [Google Scholar]
- 7.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21:387–395. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 8.Sugimura T. Overview of carcinogenic heterocyclic amines. Mutat. Res. 1997;376:211–219. doi: 10.1016/s0027-5107(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 9.NTP, Report on Carcinogens, Thirteenth Edition: Heterocyclic Amines (Selected), 〈http://ntp.niehs.nih.gov/pubhealth/roc/roc13/index.html〉, U.S. Department of Health and Human Services: National Toxicology Program 2014.
- 10.IARC, Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins, in WHO-IARC (Ed.), IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, WHO-IARC, Lyon France, 1993, pp. 165–229.
- 11.Ni W., McNaughton L., LeMaster D.M., Sinha R., Turesky R.J. Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray ionization/tandem mass spectrometry. J. Agric. Food Chem. 2008;56:68–78. doi: 10.1021/jf072461a. [DOI] [PubMed] [Google Scholar]
- 12.IARC, WHO, Vietnam - Globocan 2020; 〈https://gco.iarc.fr/today/data/factsheets/populations/704-viet-nam-fact-sheets.pdf〉, International Agency for Research on Cancer, Lyon France, 2020.
- 13.Doll R., Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 14.Le N.T., Michels F.A., Song M., Zhang X., Bernstein A.M., Giovannucci E.L., Fuchs C.S., Ogino S., Chan A.T., Sinha R., Willett W.C., Wu K. A Prospective Analysis of Meat Mutagens and Colorectal Cancer in the Nurses’ Health Study and Health Professional Follow-up Study. Environ. Health Perspect. 2016 doi: 10.1289/EHP238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha R., Rothman N., Brown E.D., Salmon C.P., Knize M.G., Swanson C.A., Rossi S.C., Mark S.D., Levander O.A., Felton J.S. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo- [4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res. 1995;55:4516–4519. [PubMed] [Google Scholar]
- 16.Sinha R., Knize M.G., Salmon C.P., Brown E.D., Rhodes D., Felton J.S., Levander O.A., Rothman N. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem. Toxicol. 1998;36:289–297. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]