Abstract
Hydroxyanthracene derivatives are widely distributed in the plant kingdom, mainly in botanicals such as the Hypericum, Rheum, Rhamnus and Aloe genera. For centuries, plants containing hydroxyanthracene derivatives have been used as herbal remedies, mainly as laxatives. The root and underground stem (rhizome) are used to make medicine, primarily for digestive complaints including constipation, diarrhoea, heartburn, stomach pain, gastrointestinal bleeding, and preparation for certain gastrointestinal diagnostic procedures. The use of hydroxyanthracene-containing botanicals has raised the attention of European Food Safety Authority (EFSA) for the potential genotoxicity activity, that in 2018 concluded “[.] and that there is a safety concern for extracts containing hydroxyanthracene derivatives although uncertainty persists”. No genotoxic activity has been reported with other constituents such as rhein, physcion and chrysophanol. In the present study, Rhubarb ethanolic extract of ground rhubarb rhizome (hydroxyanthracene total content 1.39 %) was tested in the Ames Assay in Salmonella typhimurium and Escherichia coli, up to 5000 µg/plate and up to 5000 µg/mL in human lymphocytes Micronucleus Test (OECD 471 and 487 respectively) in vitro mutagenic and genotoxic effects. Under the experimental conditions used, the rhubarb rhizome extract showed no genotoxic activity.
Keywords: Herbal medicine, Herbal food supplements, Botanical extract, Genotoxicity, Ames test, in vitro micronucleus test, Hydroxyanthracenes
Graphical Abstract
Highlights
-
•
Negative results in Ames test with rhubarb treatment.
-
•
No genotoxicity in human lymphocytes after rhubarb treatment.
-
•
Experimental design for hazard assessment of herbal preparation.
1. Introduction
Botanicals are widely used as food supplements and medicinal products. Rheum palmatum (Chinese rhubarb) is one of these species and is used in traditional medicine to treat constipation, gastrointestinal haemorrhage, and ulcers [1], [2]. Its most remarkable feature is its union of a cathartic with astringent power. Given in small doses, rhubarb shows eupeptic properties. At higher doses, however, rhubarb acts as a laxative. Rhubarb also has cholagogue activities and is therefore used in the treatment of chronic liver diseases. In recent years, it has been shown to have antibacterial, antioxidant, and anti-inflammatory effects [3], [4].
Therapeutic extracts of rhubarb are commonly obtained from its rhizome; the most representative components of these extracts include hydroxyanthracene derivates such as emodin, aloe-emodin and rhein. The safety of hydroxyanthracenes was evaluated by the Panel of the European Food Safety Authority, Food Additives and Nutrient Sources added to Food (EFSA-ANS Panel) in 2018, which concluded that “hydroxyanthracene derivatives should be regarded as genotoxic and carcinogenic unless there are specific data to the contrary, [.] and that there is a safety concern for extracts containing hydroxyanthracene derivatives although uncertainty persists” [5]. No genotoxic activity has been reported for rhein, physcion and chrysophanol.
In 2021, preparations from the root or rhizome of Rheum palmatum L., Rheum officinale Baillon and their hybrids containing hydroxyanthracene derivatives were added to Part C of the Regulation (EC) No 1925/2006, and so are under Community scrutiny, that is subject to confirmation, together with extracts from the leaves and fruit of Cassia senna L., containing hydroxyanthracene derivatives, and extracts from the bark of Rhamnus frangula L., Rhamnus purshiana DC., which also contain hydroxyanthracene derivatives [6].
Whether rhubarb poses a risk of cancer, on the basis of the hydroxyanthracene derivatives, is controversial. Several studies have demonstrated beneficial effects of extracts obtained from this plant and its most representative components (aloe emodin, emodin, rhein, etc.), for example in inhibiting oxidative stress induced by hydrogen-peroxide in intestinal epithelial cells [7] as well as their anti-inflammatory and antibacterial properties [8], whereas other studies have raised concern on the potential genotoxic/carcinogenic effect of long-term administration [9]. The World Health Organization (WHO) has recommended limiting the consumption of products containing anthraquinone glycosides, identifying the average dose as 0.5–1.5 g of dried plant material or in decoction and a safe individual dose as 10–30 mg of hydroxyanthraquinones per day [10] mainly for their laxative properties, not be used for longer than 1–2 weeks, due to possible incidence of electrolyte imbalance. According to European Medicines Agency (EMA) monographs, the maximum dosage of hydroxyanthracene derivatives should not exceed 30 mg/day in medicinal products used as a laxative for adults, elderly and adolescents over 12 years [11].
Genotoxicity experiments have recently been published on purified Aloe vera whole leaf dry juice assessed by mouse lymphoma tk assay and an in vivo comet assay in male F344 rats [12], and a mixture of aloin A and B in a commercial aloe gel beverage, which confirmed the absence of genotoxicity of these preparations [13].
Using a comet assay (OECD 489), we recently demonstrated the absence of genotoxicity in vivo in colon and kidney cells after treatments with Aloe ferox extract (rich in hydroxyanthracene derivatives) and pure aloe-emodin [14], [15]. In the present study we investigated the genotoxic potential of a Rheum palmatum extract in Salmonella typhimurium, TA1535, TA1537, TA98, TA100, and Escherichia coli (WP2 uvrA) strains (OECD 471) and in micronucleus assay in isolated human lymphocytes (OECD 487).
2. Materials and methods
2.1. Extract preparation
Rhubarb (dried rhizomes, from China) extract (solid soft extract obtained from a 60 % ABV ethanolic fluid extract of ground rhubarb rhizome) was kept at 4 °C and added immediately before use to dimethylsulphoxide (DMSO) to reach the concentration of 500 mg/mL. The characterization of the extract (provided by Davide Campari Milano N.V., Milan, Italy) is reported in Table 1.
Table 1.
Composition of rhubarb extract. The % represents w/w. The sum is 90.54%.
| Analyte | Assay (%, w/w) |
|---|---|
| Anthraquinones | |
| Aloin (A+B) | n.d. |
| Aloe-emodin | 0.37 |
| Rhein | 0.54 |
| Emodin | 0.31 |
| Chrysophanol | 0.10 |
| Physcion | 0.07 |
| Other analytes | |
| Water | 31.65 |
| Ethanol | 3.20 |
| Metals | 2.10 |
| Inorganic anions | 1.10 |
| Organic ions | 2.79 |
| Sugars (Glu+Fru+Suc) | 31.85 |
| Cellobiose | 4.00 |
| Amino acids (after acid hydrolysis) | 3.70 |
| Proteins (Kjeldahl) | 6.50 |
| Fatty acids | 0.48 |
| Fats | 1.10 |
| Fibres | 0.60 |
Mono- and disaccharides (fructose, galactose, glucose, fructose, lactose, maltose) were determined by using a gas chromatographic method after derivatization. Identification was confirmed by high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) analysis. Cellobiose and ethanol were determined using NMR. Anthraquinones were identified and quantified by ultra-HPLC, and chromones by NMR. Water content was determined by Karl Fisher titration, and total amino acids were determined by HPLC. Metals were assayed using inductively coupled plasma mass spectrometry, while inorganic and organic anions were assayed by ionic chromatography. Inorganic and organic anions were determined using ionic chromatography. Fibres and fats were determined using gravimetric methods. Fatty acids were determined using a gas chromatographic method and proteins were determined by the Kjeldahl method.
2.2. Bacterial strains
Four strains of Salmonella typhimurium (TA1535, TA1537, TA98, TA100) and one strain of Escherichia coli (WP2 uvrA) were used for this study.
TA1535 and TA100 are sensitive to base-pair mutagens, while TA1535 and TA98 are sensitive to frameshift mutagens. The histidine operon has an additional mutation that increases the sensitivity to some mutagenic compounds. The strains also have a rfa wall mutation that increases the permeability to certain classes of chemicals. All these strains are deficient in the DNA excision repair pathway. TA98 and TA100 are modified with a pKM101 plasmid which activates an error-prone DNA repair system.
WP2 uvrA is reverted from tryptophan dependence to tryptophan independence by base substitution mutagens and its tryptophan operon has a mutation which enhances its sensitivity to mutagenic compounds.
The cultures used in each experiment contained a high titre of viable bacteria (1–5 × 109 cells/mL).
2.3. Bacterial treatments
Oxoid Nutrient Broth No.2 (25 g) and Difco Bacto-agar (15 g) were prepared in 1 L of distilled water and used for non-selective growth of the tester strains. Minimal medium agar was prepared as 1.5 % Difco Bacto-agar in Vogel-Bonner Medium E, with 2 % of glucose. The overlay agar was prepared as 0.6 % Difco Bacto-agar and 0.5 % NaCl in distilled water. These solutions were autoclaved and poured into 9 cm Petri dishes and allowed to solidify and dry before use. Prior to use, 100 mL of the overlay agar were completed with 10 mL of sterile solution of 0.5 mM biotin and 0.5 mM histidine or tryptophan. The incubation was performed at 37 °C.
Positive control treatments were used differently for the strains analysed. Solutions were prepared as follows: sodium azide (Moltox, Inc., batches 6378SA and 6383SA) and methylmethane sulphonate (MMS) (Aldrich, batch MKBX5165V) in sterile water, 9-aminoacridine (Sigma-Aldrich, batch BCBT2829), 2-nitrofluorene (Moltox, Inc., batch 2303NF), and 2-aminoanthracene (Moltox, Inc., batch 6466AA and Sigma, batch STBD3302V) in DMSO. As negative controls, untreated strains or vehicle (DMSO) were assessed.
Rhubarb extract was used at the concentrations of 313, 625, 1250, 2500, and 5000 µg/plate with all the tested strains (absence of toxicity was previously confirmed). Treatments were performed both in the presence and absence of S9 fraction (Moltox, Molecular Toxicology Inc). The mixture of S9 fraction and cofactors (S9 mix, Trinova Biochem GmbH), was prepared using: 1 mL of S9 tissue fraction, 400 µL of NADP (100 mM), 500 µL of G-6-P (100 mM), 1 mL of KCl (330 mM), 800 µL of MgCl2 (100 mM), 5 mL of phosphate buffer (pH 7.4, 200 mM), and 1.3 mL of distilled water.
2.4. The Ames test
For the selection of the doses to be tested, the toxicity has been assessed on the basis of a decline in the number of spontaneous revertants or a thinning of the background lawn.
The Ames test was performed in two different manners, with incorporation or pre-incubation methods. For the incorporation method, bacteria, Rhubarb extract, and S9 mix (or phosphate buffer) were added to molten overlay agar and vortexed. The solution was poured onto the surface of a minimal medium agar plate and allowed to solidify. Alternatively, for the pre-incubation method, bacteria, rhubarb extract, and S9 mix (or phosphate buffer) were added into an empty tube and vortexed. Tubes were kept at 37 °C for 30 min before addition of 2 mL of overlay agar and mixing again. The solutions were then poured onto the surface of a minimal medium agar plate and allowed to solidify. The plates were inverted and incubated for 72 h at 37 °C and then immediately scored by counting the number of revertant colonies for each plate.
2.5. Peripheral blood lymphocyte cultures
Lymphocytes were obtained from the whole blood of four donors (Biopredic International, France) healthy, young (under 35 years old), non-smoking individuals with no known recent exposure to radiation or drugs. The blood was collected into tubes treated with sodium heparin. Aliquots of blood (0.5 mL) were added to 4.5 mL of culture medium with phytohaemagglutinin (PHA) incubated at 37 °C for 48 h to isolate lymphocytes.
The culture medium for lymphocytes was RPMI 1640, supplemented with 10 % foetal calf serum, heat-inactivated, 200 mM of L-glutamine, and 1 % of antibiotic solution.
2.6. Lymphocyte treatments
Cells were treated with PHA for approximately 48 h after the cultures were initiated. Before treatment, cultures were centrifuged at 1000 rpm for 10 min. The culture medium with PHA was replaced by the treatment medium for 3 h (short treatment) or 31 h (continuous treatment).
Short treatments were performed for 3 h at 37 °C in the presence or absence of S9 metabolism. At the end, cells were centrifuged and washed twice with phosphate-buffered saline (PBS). Fresh medium with cytochalasin B (6 µg/mL) was added and cultures were incubated for 28 h at 37 °C before harvesting.
Continuous treatment lasted 31 h in the absence of S9 mix. Cytochalasin B (6 µg/mL) was added to the cultures which were incubated for 28 h before harvesting.
The tested concentrations were 195, 293, 439, 658, 988, 1480, 2220, 3330, and 5000 µg/mL for short and continuous treatments. Subsequently, a wider range of concentrations was analysed for continuous treatment (698, 873, 1010, 1210, 1450, 1740, 2080, 2500, and 3000 µg/mL), in the absence of S9 metabolism.
The mixture of S9 tissue fraction and cofactors (S9 mix) was prepared using: 1 mL of S9 tissue fraction, 400 µL of NADP (100 mM), 500 µL of G-6-P (100 mM), 200 µL of MgCl2 (100 mM), 5 mL of phosphate buffer (pH 7.4, 200 mM), and 2.9 mL of distilled water.
The positive control for the short treatments is Mitomycin-C in absence of S9 metabolism and Cyclophosphamide (CP) in presence of S9 metabolism, and for the continuous treatment is Colchicine (Col) without metabolic activation.
Osmolality and pH were measured and showed in Table S1 (Supplementary material).
2.7. The micronucleus test and slide evaluation
Cells were suspended in hypotonic solution and fresh methanol/acetic acid was used as a fixative. The fixative process was performed for several times after centrifugation and replacement of the fixative solution. A few drops of the cell suspension were placed on a glass slide. Three slides were prepared for each treatment. The slides were allowed to dry in air and kept at room temperature until they were stained with a solution of acridine orange (0.1 mg/mL in PBS).
The cytokinesis-block proliferation index (CBPI) was calculated as follows:
Five hundred cells per cell culture were analysed. CBPI was used to measure the cytotoxicity comparing the treatments (T) with the controls (C) using the following formula:
Micronuclei were identified if they met the following criteria: diameter less than 1/3 of the diameter of the nucleus, but greater than 1/16; no overlapping with the nucleus, non-refractile and with same staining intensity as the main nuclei. The highest doses were selected for scoring micronuclei since in absence of cytotoxicity. For the three selected doses, for the vehicle and the positive controls, 1000 binucleated cells per cell culture were scored to assess the frequency of micronucleated cells.
2.8. Statistical analysis
All the assays here enlisted were performed under good laboratory practice (GLP). Historical data are reported in Tables S2–3.
In the Ames test, the extract is considered mutagenic when two-fold (or more) increases in mean revertant number is observed at two consecutive dose levels or at the highest dose tested. There must be evidence of a dose-response relationship showing increasing numbers of mutant colonies with increasing dose levels [16].
For the micronuclei assay, a modified χ2 test was used to compare the number of cells with micronuclei in control and treated cultures. The Cochran-Armitage trend test (one-sided) was performed to aid determination of a concentration-response relationship.
Results are reported as the mean of three independent experiments ± standard deviation (SD).
3. Results
3.1. The Ames test
The results of the Ames test with the plate incorporation method are shown in Table 2, while the those related to the pre-incubation method are shown in Table 3. No toxicity was observed at any dose level, in the absence or presence of S9 metabolism, with any tester strain.
Table 2.
Plate incorporation method. Mean number of revertants of the different concentrations of rhubarb extract tested, the concentrations of rhubarb extract and positive controls (sodium azide, 2-aminoanthracene, 9-aminocridine and, 2-nitrofluorene and methylmethane sulphonate (MMS) are expressed in µg/plate. Values are expressed as mean ± SD, n = 3.
| Strain | TA1535 |
TA1537 |
TA98 |
TA100 |
WP2 uvrA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic activation | - S9 | + S9 | - S9 | + S9 | - S9 | + S9 | -S9 | + S9 | -S9 | + S9 |
| Untreated | 16.3 ± 2.8 | 16.3 ± 1.5 | 19.3 ± 1.3 | 17.3 ± 1.8 | 28.7 ± 2.3 | 38.7 ± 3.8 | 108.7 ± 0.9 | 117.7 ± 2.6 | 25.0 ± 1.5 | 33.3 ± 2.7 |
| Test item (μg/plate) | ||||||||||
| vehicle | 14.3 ± 0.9 | 18.7 ± 2.0 | 14.7 ± 0.7 | 17.0 ± 1.0 | 28.7 ± 2.4 | 31.3 ± 0.9 | 115.3 ± 1.8 | 119.7 ± 1.5 | 25.0 ± 1.0 | 28.3 ± 0.9 |
| 313 | 19.0 ± 1.0 | 19.0 ± 2.0 | 23.7 ± 1.5 | 17.7 ± 1.5 | 33.3 ± 1.9 | 38.7 ± 2.6 | 134.3 ± 3.8 | 121.3 ± 1.2 | 25.0 ± 2.3 | 28.0 ± 4.0 |
| 625 | 17.0 ± 1.5 | 18.7 ± 1.5 | 20.7 ± 1.5 | 18.0 ± 1.0 | 36.0 ± 3.1 | 37.0 ± 1.0 | 135.3 ± 8.6 | 124.0 ± 3.0 | 28.0 ± 1.7 | 30.0 ± 1.2 |
| 1250 | 20.3 ± 1.2 | 15.7 ± 0.7 | 17.7 ± 1.5 | 23.7 ± 1.3 | 35.3 ± 1.9 | 41.3 ± 1.8 | 130.3 ± 5.0 | 145.0 ± 11.8 | 29.0 ± 2.5 | 35.7 ± 1.7 |
| 2500 | 24.0 ± 0.6 | 24.0 ± 1.2 | 16.7 ± 0.3 | 36.3 ± 0.9 | 33.3 ± 1.3 | 39.0 ± 1.2 | 122.0 ± 5.9 | 138.7 ± 7.2 | 30.7 ± 3.3 | 38.7 ± 2.0 |
| 5000 | 23.0 ± 1.7 | 25.7 ± 0.9 | 25.3 ± 1.7 | 33.0 ± 1.2 | 42.0 ± 2.5 | 37.0 ± 2.9 | 119.3 ± 2.7 | 135.3 ± 1.5 | 34.0 ± 2.5 | 42.7 ± 1.5 |
| Sodium azide | ||||||||||
| 1 | 487.0 ± 15.7 | 587.7 ± 42.8 | ||||||||
| 2-Aminoanthracene | ||||||||||
| 1 | 134.7 ± 4.5 | 79.3 ± 3.7 | 520.7 ± 21.6 | 1124.7 ± 68.1 | ||||||
| 10 | 176.0 ± 1.5 | |||||||||
| 9-Aminoacridine | ||||||||||
| 50 | 130.3 ± 16.8 | |||||||||
| 2-Nitrofluorene | ||||||||||
| 2 | 159.0 ± 17.1 | |||||||||
| MMS | ||||||||||
| 500 | 147.3 ± 3.3 | |||||||||
Table 3.
Pre-incubation method. Mean number of revertants of the different concentrations of rhubarb extract tested, the concentrations of rhubarb extract and positive controls (sodium azide, 2-aminoanthracene, 9-aminocridine and, 2-nitrofluorene and methylmethane sulphonate (MMS) are expressed in µg/plate. Values are expressed as mean ± SD, n = 3.
| Strain | TA1535 |
TA1537 |
TA98 |
TA100 |
WP2 uvrA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic activation | - S9 | + S9 | - S9 | + S9 | - S9 | + S9 | -S9 | + S9 | -S9 | + S9 |
| Untreated | 16.0 ± 1.7 | 20.0 ± 0.3 | 16.7 ± 1.5 | 20.3 ± 0.3 | 32.3 ± 3.0 | 40.3 ± 2.4 | 124.3 ± 2.4 | 132.0 ± 2.6 | 28.0 ± 1.2 | 34.0 ± 2.0 |
| Test item (μg/plate) | ||||||||||
| vehicle | 18.0 ± 0.6 | 15.0 ± 0.6 | 19.7 ± 0.3 | 19.3 ± 0.3 | 35.7 ± 0.9 | 41.0 ± 2.0 | 136.7 ± 1.9 | 129.3 ± 4.1 | 25.7 ± 1.8 | 36.7 ± 0.9 |
| 313 | 15.3 ± 1.5 | 20.7 ± 0.7 | 19.0 ± 2.5 | 22.7 ± 0.9 | 32.3 ± 0.9 | 39.0 ± 0.6 | 142.0 ± 5.0 | 125.7 ± 3.5 | 24.0 ± 0.0 | 34.0 ± 0.6 |
| 625 | 20.3 ± 1.3 | 18.3 ± 0.3 | 22.3 ± 1.2 | 22.3 ± 0.9 | 37.7 ± 0.9 | 33.3 ± 1.3 | 145.3 ± 4.7 | 125.7 ± 6.0 | 33.7 ± 0.9 | 29.7 ± 0.9 |
| 1250 | 16.7 ± 1.3 | 15.7 ± 1.2 | 19.3 ± 1.3 | 24.7 ± 2.2 | 35.0 ± 1.5 | 30.7 ± 2.6 | 129.3 ± 3.5 | 134.3 ± 3.8 | 30.7 ± 0.9 | 28.3 ± 2.6 |
| 2500 | 21.7 ± 1.3 | 18.0 ± 1.5 | 19.0 ± 1.7* | 26.7 ± 1.5 | 34.0 ± 0.6 | 34.0 ± 2.5 | 122.3 ± 2.0* | 131.3 ± 3.9 | 29.7 ± 0.3 | 32.0 ± 0.6 |
| 5000 | 18.7 ± 1.8 | 18.7 ± 0.9 | 17.3 ± 1.8** | 25.3 ± 1.5 | 39.0 ± 0.0 | 38.3 ± 1.5 | 107.7 ± 3.2** | 130.7 ± 4.5 | 34.3 ± 0.3 | 33.3 ± 0.3 |
| Sodium azide | ||||||||||
| 1 | 518.7 ± 34.3 | 657.3 ± 39.0 | ||||||||
| 2-Aminoanthracene | ||||||||||
| 1 | 103.3 ± 4.7 | 107.3 ± 1.5 | 612.7 ± 25.8 | 1238.7 ± 121.0 | ||||||
| 10 | 146.0 ± 6.7 | |||||||||
| 9-Aminoacridine | ||||||||||
| 50 | 110.3 ± 18.0 | |||||||||
| 2-Nitrofluorene | ||||||||||
| 2 | 197.7 ± 6.7 | |||||||||
| MMS | ||||||||||
| 500 | 183.0 ± 6.4 | |||||||||
Slight thinning of background lawn
Moderate thinning of background lawn
For the plate incorporation method, a marginally positive trend of increasing numbers of revertant colonies was noticed with the TA1535, TA1537 and WP2 uvrA tester strains in the presence of S9 metabolic activation and with TA1535, TA98 and WP2 uvrA tester strains in its absence. However, the number of mutant colonies did not reach twice the concurrent negative control value at any of the doses tested, with any tested strain, in the absence or presence of S9 metabolic activation.
Based on these results, the pre-incubation method does not show either toxicity, or relevant increases in revertant numbers with any tester strain, at any dose level, in the absence or presence of S9 metabolism.
3.2. Cytokinesis-block proliferation index
The CBPI was calculated for each of the treatment series and the results are presented in Fig. 1(A-D). As shown in Fig. 1A-B slight cytotoxicity was observed at the highest dose level tested after short exposure in the absence and presence of S9 metabolism (approximately 20 %). No remarkable cytotoxicity was observed over the remaining dose range. As shown in Fig. 1C, continuous treatment induced severe cytotoxicity at the highest dose levels of 5000 and 3330 µg/mL, when few mononucleated cells were recovered. Marked cytotoxicity was observed also at the dose level of 2220 µg/mL (69 %), while mild to no cytotoxicity was seen over the remaining dose range.
Fig. 1.
Evaluation of the cytokinesis-block proliferation index (CBPI) in human lymphocytes. (A) CBPI obtained after short treatment (3 h) with the rhubarb extract in the absence of the S9 fraction. (B) Results of the short treatment (3 h) in the presence of the S9 fraction. (C) Continuous treatment (31 h) in the absence of the S9 fraction (D) Continuous treatment (31 h) in the absence of the S9 fraction with a narrow range of concentration of rhubarb extract used. The concentrations of rhubarb extract, colchicine and cyclophosphamide are expressed in µg/mL. Values are expressed as mean ± SD, n = 3, n.d. = not detectable.
The continuous treatment performed with a narrow interval of concentrations showed marked cytotoxicity at the highest dose level tested (3330 µg/mL, 65%), while no cytotoxicity was present over the remaining dose range (Fig. 1D).
3.3. Micronuclei analysis
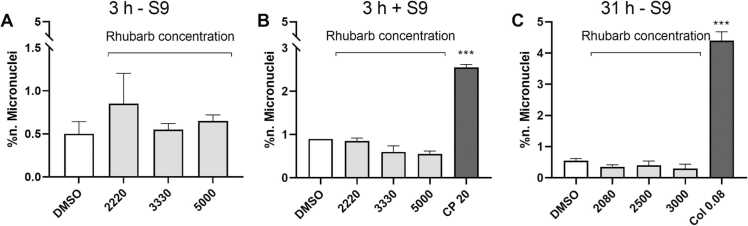
Statistically significant increases in the incidence of micronucleated cells were observed following treatments with the positive controls cyclophosphamide and colchicine, indicating the correct functioning of the assay. Following treatment with the test item, no statistically significant increase in the incidence of micronucleated cells over the control value was observed at any dose level, in any treatment series and no concentration-related increase was seen, as shown in Fig. 2A-C. Thus, no statistically significant increase, nor dose relationship was seen for these treatments. Rhubarb extract does not induce micronuclei in human lymphocytes after in vitro treatment.
Fig. 2.
Evaluation of the number of micronuclei in human lymphocytes. (A) Short treatment without the S9 fraction, (B) short treatment with the S9 fraction, (C) continuous treatment without the S9 fraction. The concentrations of rhubarb extract, colchicine and cyclophosphamide are expressed in µg/mL. Values are expressed as mean ± SD, n = 3.
4. Discussion
Botanicals, originating from herbs, roots, flowers, fruits, leaves or seeds are widely present on the global market in the form of or as a part of food supplements. Examples are Aloe species, Rheum palmatum L., Rheum officinale Baillon and their hybrids, extracts from the leaves and fruit of Cassia senna L., extracts from the bark of Rhamnus frangula L., Rhamnus purshiana DC., etc. Normally these products are labelled as natural foods and they are claimed to have various possible health benefits. They can be purchased in pharmacies, supermarkets, specialised stores and on the Internet. Although most of these products have been in use for a very long time, for some of them safety and quality concerns cannot be ruled out.
The assessment of the safety of plant preparations is problematic because of the large number and chemical diversity of their components. These components, separated from the botanical complex and tested individually at doses enormously far from their actual concentration present in the complex matrix of the plant preparation under examination, may be able to produce adverse effects unlikely to be attributable to the molecule investigated, of which toxicokinetics, toxicodynamics and the mechanism of action are strictly influenced by the dose and the matrix in which it is present.
Data regarding a pure substance may overestimate/underestimate effects of a single component in the botanical matrix and suitable evidence should be provided to demonstrate the likely occurrence of the matrix effect and its magnitude.
For mixtures that contain individual components for which there are concerns regarding potential genotoxicity, as suggested for hydroxyanthracene derivatives (EFSA-ANS Panel, 2018) [5], studies in Hsd:ICR (CD-1) male mice showed a lack of genotoxic activity in the comet assay in vivo in single cell preparations of kidney and colon following oral gavage of doses of 250, 500, 1000 and 2000 mg/kg bw/day high-titre aloe-emodin [15]. In addition, in three in vivo studies (micronucleus assay in bone marrow cells of NMRI mice; chromosome aberration assay in bone marrow cells of Wistar rats; mouse spot test [DBA/2 J × NMRI]) no indication of a genotoxic activity of aloe-emodin was found. Furthermore, information about a possible reaction of aloe-emodin with DNA was derived from an in vivo UDS assay. Hepatocytes of aloe-emodin-treated male Wistar rats did not show DNA damage via repair synthesis. Aloe-emodin interacts with DNA under certain in vitro conditions. However, the in vivo negative results did not indicate an aloe-emodin genotoxic potential. Therefore, it may be assumed that a genotoxic risk for man might be unlikely [17].
Aloe-emodin is present in all botanical preparations under Community scrutiny and in particular quantified (0.37 %) in the extract of the rhubarb extract that is the object of the present study and whose composition has been almost completely characterized (90.56 %). Dismissing the genotoxicity of aloe emodin per se because, in vivo, this is transformed very quickly into rhein and is thus recognized not to cause any toxicity concerns, we investigated the in vitro mutagenic and genotoxic effects of an extract of rhubarb (whole mixture) obtained in accordance with European Pharmacopoeia of Rhamnus purshiana in both bacteria and human lymphocytes through the OECD protocols of the Ames assay and micronucleus tests in the absence and presence of S9 metabolism. Since the results of the two in vitro tests were clearly negative, the mixture could be considered as of no concern with respect to genotoxicity and no further testing is recommended for its hazard characterization in according to EFSA document on genotoxicity assessment of chemical mixtures [18].
In addition to the evident lack of genotoxic effects, a lyophilized powder of Rhubarb Extract (each gram of the powder extract was equivalent to 3.7037 g of crude rhubarb extraction by Cryodesiccation conducted under Good Manufacturing Practices) was dissolved with distilled water and orally administered to male and female Sprague-Dawley (SD) rats and according to the results of acute oral toxicity test (LD50 > 7.5 g/kg bw), three groups were dosed at 0.65, 1.62, 4.05 g/kg bw/day for 90 days.
No adverse related changes were observed in the 0.65 g/kg bw group. Based on the results, the no observed adverse effect level (NOAEL) of rhubarb extract in rats was established at 0.65 g/kg bw/day equivalent to an exposure of 45.5 g a day for an adult weighing 70 kilos [19].
Funding
This work was supported by the Italian Society of Toxicology (SITOX).
CRediT authorship contribution statement
Gloria Melzi: data management, investigation, formal analysis. Corrado Galli: study concept, methodology, writing, including preparation of the original draft. Paola Ciliutti: resources, data management, investigation, formal analysis. Cristina Marabottini: resources, data management, investigation, formal analysis. Marina Marinovich: supervision, writing, reviewing and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Eric De Combarieu and Ernesto Marco Martinelli (Indena SpA, Italy), for their fundamental support in the characterization of Rheum palmatum (Rhubarb) extract, the material tested in this study. We also thank Rachel Stenner and Robert Burns, of Language Consulting Congressi (Milan, Italy), for their language editing of this article.
Partly supported by TWINALT-EU project 952404.
Handling Editor: Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.07.017.
Appendix A. Supplementary material
Supplementary material.
.
Data availability
Data will be made available on request.
References
- 1.Zhong-zhi Q., Yang D., Yan-ze L., Yong P. Pharmacopoeia of the People’s Republic of China: A Milestone in Development of China’s Healthcare. Chinese Herbal Medicines. 2010;2(2):157–159. [Google Scholar]
- 2.European Pharmacopoeia, European Directorate for the Quality of Medicines, 4th edition, Strasbourg, 2001. European Directorate for the quality of Medicines & Health care. Council of Europe.
- 3.Wang J., Zhao H., Kong W., Jin C., Zhao Y., Qu Y., Xiao X. Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine. 2010;17:684–689. doi: 10.1016/j.phymed.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Öztürk M., Aydoǧmuş-Öztürk F., Duru M.E., Topçu G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): an edible medicinal plant. Food Chem. 2007;103:623–630. doi: 10.1016/j.foodchem.2006.09.005. [DOI] [Google Scholar]
- 5.EFSA ANS Panel, Younes M., Aggett P., Aguilar F., Crebelli R., Filipi M., Frutos M.J., Galtier P., Gott D., Gundert-Remy U., Kuhnle G.G., Lambré C., Leblanc J., Lillegaard I.T., Moldeus P., Mortensen A., Oskarsson A., Stankovic I., Waalkens-Berendsen I., Woutersen R.A., Andrade R.J., Fortes C., Mosesso P., Restani P., Pizzo F., Smeraldi C., Papaioannou A., Wright M. Safety of hydroxyanthracene derivatives for use in food. EFSA J. 2018;16:1–97. doi: 10.2903/j.efsa.2018.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Commission, Commission Regulation (EU) 2021/468 of 18 March 2021 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards botanical species containing hydroxyanthracene derivatives, 2021.
- 7.Zhuang S., Yu R., Zhong J., Liu P., Liu Z. Rhein from rheum rhabarbarum inhibits hydrogen-peroxide-induced oxidative stress in intestinal epithelial cells partly through PI3K/Akt-mediated Nrf2/HO-1 pathways. J. Agric. Food Chem. 2019;67:2519–2529. doi: 10.1021/acs.jafc.9b00037. [DOI] [PubMed] [Google Scholar]
- 8.Chinsembu K.C. Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Trop. 2016;154:6–18. doi: 10.1016/j.actatropica.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Xiang H., Zuo J., Guo F., Dong D. What we already know about rhubarb: a comprehensive review. Chin. Med. 2020;15:1–22. doi: 10.1186/s13020-020-00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . World Health Organisation; Geneva: 1999. WHO Monographs on Selected Medicinal Plants; pp. 39–40. [Google Scholar]
- 11.Committee on Herbal Medicinal Products (HMPC), Assessment report on Rheum palmatum L. and Rheum officinale Baillon, radix, 31 (2019). www.ema.europa.eu/contact.
- 12.Hu J., Lloyd M., Hobbs C., Cox P., Burke K., Pearce G., Streicker M.A., Gao Q., Frankos V. Absence of genotoxicity of purified Aloe vera whole leaf dry juice as assessed by an in vitro mouse lymphoma tk assay and an in vivo comet assay in male F344 rats. Toxicol. Rep. 2021;8:511–519. doi: 10.1016/j.toxrep.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes A.W., Clemens R.A., Pressman P. The absence of genotoxicity of a mixture of aloin A and B and a commercial aloe gel beverage. Toxicol. Mech. Methods. 2022;0:1–10. doi: 10.1080/15376516.2021.2023828. [DOI] [PubMed] [Google Scholar]
- 14.Galli C.L., Cinelli S., Ciliutti P., Melzi G., Marinovich M. Lack of in vivo genotoxic effect of dried whole Aloe ferox juice. Toxicol. Rep. 2021;8:1471–1474. doi: 10.1016/j.toxrep.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli C.L., Cinelli S., Ciliutti P., Melzi G., Marinovich M. Aloe-emodin, a hydroxyanthracene derivative, is not genotoxic in an in vivo comet test. Regul. Toxicol. Pharmacol. 2021;124 doi: 10.1016/j.yrtph.2021.104967. [DOI] [PubMed] [Google Scholar]
- 16.Chu K.C., Patel K.M., Lin A.H., Tarone R.E., Linhart M.S., Dunkel V.C. Evaluating statistical analyses and reproducibility of microbial mutagenicity assays. Mutat. Res. 1981;85 doi: 10.1016/0165-1161(81)90027-3. [DOI] [PubMed] [Google Scholar]
- 17.Heidemann A., Völkner W., Mengs U. Genotoxicity of aloeemodin in vitro and in vivo. Mutat. Res. 1996;367:123–133. doi: 10.1016/0165-1218(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 18.EFSA Scientific Committee, More S., Bampidis V., Benford D., Boesten J., Bragard C., Halldorsson T., Hernandez-Jerez A., Hougaard-Bennekou S., Koutsoumanis K., Naegeli H., Nielsen S.S., Schrenk D., Silano V., Turck D., Younes M., Aquilina G., Crebelli R., Gürtler R., Hirsch-Ernst K.I., Mosesso P., Nielsen E., Solecki R., Carfı M., Martino C., Maurici D., Morte J.P., Schlatter J. Genotoxicity assessment of chemical mixtures. EFSA J. 2019;17:1–11. doi: 10.2903/j.efsa.2019.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., Liu J., Zheng Y., Qu J., Yang W., Tang X., Bai H., Fan B. Subchronic oral toxicity study of rhubarb extract in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2021;123 doi: 10.1016/j.yrtph.2021.104921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.