Abstract
Background
Implantable cardioverter-defibrillator (ICD) implantation to prevent sudden cardiac death (SCD) in post-myocardial infarction (MI) patients varies by geography but remains low in many regions despite guideline recommendations.
Objectives
This study aimed to characterize the care pathway of post-MI patients and understand barriers to referral for further SCD risk stratification and management in patients meeting referral criteria.
Methods
This prospective, nonrandomized, multi-nation study included patients ≥18 years of age, with an acute MI ≤30 days and left ventricular ejection fraction <50% ≤14 days post-MI. The primary endpoint was defined as the physician’s decision to refer a patient for SCD stratification and management.
Results
In total, 1,491 post-MI patients were enrolled (60.2 ± 12.0 years of age, 82.4% male). During the study, 26.7% (n = 398) of patients met criteria for further SCD risk stratification; however, only 59.3% of those meeting criteria (n = 236; 95% CI: 54.4%-64.0%) were referred for a visit. Of patients referred for SCD risk stratification and management, 94.9% (n = 224) attended the visit of which 56.7% (n =127; 95% CI: 50.1%-63.0%) met ICD indication criteria. Of patients who met ICD indication criteria, 14.2% (n = 18) were implanted.
Conclusions
We found that ∼40% of patients meeting criteria were not referred for further SCD risk stratification and management and ∼85% of patients who met ICD indications did not receive a guideline-directed ICD. Physician and patient reasons for refusing referral to SCD risk stratification and management or ICD implant varied by geography suggesting that improvement will require both physician- and patient-focused approaches. (Improve Sudden Cardiac Arrest [SCA] Bridge Study; NCT03715790)
Key Words: cardiac resynchronization therapy–defibrillator, delivery of health care, implantable cardioverter-defibrillator, myocardial infarction
Abbreviations and Acronyms: CRT-D, cardiac resynchronization therapy-defibrillator; ICD, implantable cardioverter-defibrillator; ISC, India subcontinent; LVEF, left ventricular ejection fraction; MEACAT, Middle East, Africa, Central Asia, and Turkey; MI, myocardial infarction; OR, odds ratio; SEA, South East Asia; SCD, sudden cardiac death
Central Illustration
The burden of sudden cardiac death (SCD) varies by geography but remains a leading cause of death worldwide.1 Multiple clinical trials have provided evidence that implantable cardioverter-defibrillators (ICDs) can reduce mortality in patients at risk of SCD including post–myocardial infarction (MI) patients with reduced left ventricular ejection fraction (LVEF).2, 3, 4, 5, 6, 7, 8, 9 Evidence from these and other studies has led to current clinical practice guidelines recommending ICD use for prevention of SCD in post-MI patients with LVEF ≤40%; however, use remains low in many countries.10,11
Low ICD use despite guideline recommendations and mortality benefit suggests that barriers in the care pathway exist. Potential barriers include cost, especially in regions with few health care reimbursement options12,13; limited physician awareness of guidelines thereby limiting referrals to cardiovascular specialty services14,15; and limited patient understanding of ICD benefits.13 Although some efforts have been made to improve guideline-based ICD adoption, more efforts are needed to understand why ICD utilization remains low in certain geographies, especially in the post-MI setting.9
The Improve Sudden Cardiac Arrest (SCA) Bridge study assessed the real-world care pathway for post-MI patients and the associated barriers to appropriate referrals for SCD risk stratification and management in regions known to have low ICD therapy adoption rates. Understanding the current standard of practice for post-MI patients will enable the development of more effective care pathways to improve patient outcomes and mitigate SCD risk through adherence to medical guidelines.
Methods
Study design, planned enrollments, and eligibility
The Improve SCA Bridge study (NCT03715790) was a prospective, nonrandomized, multi-site, global, post-market study conducted in regions where ICD use in clinically indicated patients is low. A total of 6 protocol-defined regions were part of the study: 1) Mainland China; 2) Indian subcontinent (ISC), including India and Bangladesh; 3) South Korea; 4) Middle East, Africa, Central Asia, and Turkey (MEACAT), including Egypt, Pakistan, Saudi Arabia, South Africa, and Tunisia; 5) South East Asia (SEA), including Brunei, Indonesia, Malaysia, The Philippines, Singapore, and Thailand; and 6) Taiwan (a full list of participating centers and investigators can be found in Supplemental Table 1).
Upon study initiation, investigators and site clinical personnel (study coordinators, nurses, etc) were provided with informational study materials that addressed when patients should be referred for further SCD risk stratification and management according to guidelines with a strong emphasis on LVEF measurement. Investigators were encouraged to adhere to guidelines and recommend patients with criteria for further SCD risk stratification and management visits. Patient informational materials addressing the risk of SCD post-MI and potential mitigation strategies were also made available. All provided study and patient materials were based on the European Society of Cardiology guidelines.11
Patient inclusion criteria were: 1) age 18 years and older (and met age requirements per local law); 2) an acute MI ST-segment elevation myocardial infarction or non–ST-segment elevation myocardial infarction ≤30 days before enrollment; and 3) LVEF <50% measured ≤14 days post-MI. The protocol strongly recommended following the European Society of Cardiology guidelines for acute MI definition.11,16 More information regarding the inclusion criteria and a complete overview of the exclusion criteria are reported in Supplemental Table 2.
Follow-up visits occurred at 3, 6, and 12 months and were performed in-person or by phone by study investigators due to the COVID-19 pandemic. LVEF was assessed at the 3-month follow-up, as a study protocol requirement, and at the 6- and 12-month follow-up exams per standard practice. If at 3-, 6-, or 12-month follow-up the patient had an LVEF ≤40% or met one of the referral criteria associated with a higher risk of SCA (ie, sustained ventricular tachycardia, cardiac or unexplained syncope, clinically significant palpitations, new-onset bundle branch block, conduction abnormalities, or symptomatic bradycardia), the patient could be referred for SCD risk stratification and management based on the investigator’s discretion. If patients were not referred, the reason was collected, and referral status was assessed at subsequent visits. During subsequent study follow-up visits, the patients who were not referred previously could be referred at any time if they met referral criteria.
If the patient was referred and met the ICD/cardiac resynchronization therapy-defibrillator (CRT-D) implant indication (possible reasons for indication detailed in Supplemental Table 3 as defined by the American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines10), any brand or model of market released ICD/CRT-D could be implanted. Reasons for implant refusal were collected. At subsequent visits, patients were asked whether there was a change in their device status.
Study objectives and endpoints
The primary objective of the study was to characterize, at the regional level, the proportion of post-acute MI patients who were referred for SCD risk stratification and management. The primary endpoint was defined as the physician’s decision to refer a patient for SCD stratification and management.
Secondary objectives and related endpoints of the study, at the regional level, were to characterize: 1) the proportion of patients indicated for an ICD/CRT-D within 12 months post-MI; 2) the proportion indicated patients who received an ICD/CRT-D within 12 months post-MI; 3) the referral and implant refusal rationale of patients having met referral criteria, but not referred and/or patients having an ICD/CRT-D indication but who refused implant; and 4) the proportion of patients who experienced cardiovascular mortality.
Statistical considerations
Statistical analyses were performed within each region and for 3-, 6-, and 12-month follow-up visits. Specifically, we computed the proportion and Wilson score 95% CI of post-MI patients who were referred for SCD risk stratification and management and of patients who were indicated for an ICD/CRT-D. A logistic regression model was fitted across all regions to estimate the odds of being referred for SCD risk stratification and management. The odds were estimated depending on region, follow-up visit, referral occurrence during COVID-19, age, sex, having received a MI treatment (coronary artery bypass surgery or percutaneous coronary intervention), ST-segment elevation myocardial infarction diagnosis, time from index MI to hospital admission, LVEF at baseline, and referral physician specialty.
Reasons for having refused SCD risk stratification and management referral or ICD/CRT-D implant in indicated patients were summarized by means of proportions. Mortality experienced during the study period was summarized overall and as SCD or non-SCD for each region by means of proportions.
The time-to-event plot and cumulative incidence plots were computed using Kaplan-Meier estimates for the survival function and, when provided, the log-log transformation for its corresponding 95% CIs. Analyses were provided using SAS 9.4 (SAS Institute).
Ethics statements
The study was conducted in compliance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee at each participating site before enrollment.
Results
In total, 1,491 post-MI patients were enrolled. Of these, 1,170 had a 3-month visit, 916 had a 6-month visit, and 887 had a 12-month visit (shown regionally in Supplemental Figure 1). Further, 381 (25.5%) were exited from the study at the 3-month visit because of LVEF ≥50%, 88 (5.9%) died, 75 (5.0%) were lost to follow-up, 48 (3.2%) withdrew from the study, 9 (0.6%) were exited from the study due to sponsor request, and 2 (0.1%) were exited from the study due to physician decision (Figure 1).
Figure 1.
Study Cohort Flow Chart
Points at which patients were exited or continued through the study are shown along with a breakdown of when patients met referral criteria and were referred for sudden cardiac death (SCD) risk stratification and management as well as when patients met implantable cardioverter-defibrillator (ICD)/cardiac resynchronization therapy–defibrillator (CRT-D) implant criteria and were implanted with a device. “Lost to follow-up” means the patients were not able to be followed up any longer due to lost contact with the patient. “Missed visit” means that a patient continued in the study but missed a specific visit. “Missed patients were exited” means that a patient missed the last visit of the study and was therefore exited at that timepoint because there were no more visits to complete after that. ∗Single patient could meet referral criteria for SCD risk stratification and management at multiple visits. †Single patient could be referred for SCD risk stratification and management at multiple visits. ‡2 patients were recorded as having completed SCD risk stratification and management visits but not as having been referred. §1 patient was recorded as having met indication criteria at multiple visits. ¶1 patient had an implant recorded at study exit.
Baseline characteristics
Across all regions, the average age of the patients enrolled in the study was 60.2 ± 12.0 years, 82.4% were males, 35.6% had type 2 diabetes, and 49.8% had hypertension (Table 1).
Table 1.
Baseline Patient Characteristics by Region
| Mainland China (n = 394) | ISC (n = 347) | South Korea (n = 237) | Taiwan (n = 120) | MEACAT (n = 197) | SEA (n = 196) | Overall (N = 1,491) | |
|---|---|---|---|---|---|---|---|
| Age, y | 63.0 ± 11.5 | 56.4 ± 11.2 | 64.5 ± 12.1 | 63.0 ± 11.1 | 57.8 ± 12.0 | 56.8 ± 11.3 | 60.2 ± 12.0 |
| Male | 302 (76.6) | 291 (83.9) | 195 (82.3) | 99 (82.5) | 167 (84.8) | 174 (88.8) | 1,228 (82.4) |
| STEMI | 257 (65.2) | 236 (68.0) | 147 (62.0) | 68 (56.7) | 132 (67.0) | 145 (74.0) | 985 (66.1) |
| LVEF | 41.3 ± 6.1 | 39.5 ± 5.7 | 40.2 ± 7.8 | 40.6 ± 8.1 | 38.3 ± 5.9 | 37.7 ± 7.7 | 39.8 ± 6.8 |
| Time from index MI to hospital admission, days | |||||||
| Mean ± SD | 2.1 ± 3.4 | 0.9 ± 1.7 | 0.5 ± 1.3 | 0.3 ± 0.6 | 0.4 ± 1.1 | 0.4 ± 0.7 | 1.0 ± 2.2 |
| Median | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Min to Max | -9 to 17 | -1 to 11 | 0 - 10 | 0 - 4 | 0 - 10 | 0 - 4 | -9 to 17 |
| Diabetes Type 1 Type 2 |
146 (37.1) 0 146 (37.1) |
153 (44.1) 19 (5.5) 134 (38.6) |
76 (32.1) 5 (2.1) 71 (30.0) |
50 (41.7) 1 (0.8) 49 (40.8) |
80 (40.6) 8 (4.1) 73 (37.1) |
59 (30.1) 1 (0.5) 58 (29.6) |
564 (37.8) 34 (2.3) 531 (35.6) |
| Hypertension | 207 (52.5) | 148 (42.7) | 131 (55.3) | 74 (61.7) | 87 (44.2) | 95 (48.5) | 742 (49.8) |
Values are mean ± SD or n (%).
ISC = India subcontinent; LVEF = left ventricular ejection fraction, MEACAT = Middle East, Africa, Central Asia, and Turkey; MI = myocardial infarction; SEA = South East Asia; STEMI = ST-segment elevated myocardial infarction.
Overall, 66.1% of enrolled patients with baseline data were patients with ST-segment elevation myocardial infarction, particularly in SEA, where ST-segment elevation myocardial infarction patients accounted for 74.0% of total enrollments (Table 1). Index MI was mostly treated with balloon angioplasty (detailed index MI information in Supplemental Table 4). Across all regions, most patients were admitted to hospital within 24 hours from index MI, except in Mainland China, where the average time from index MI to admission was 2.1 ± 3.4 days (Table 1).
SCD risk stratification and management
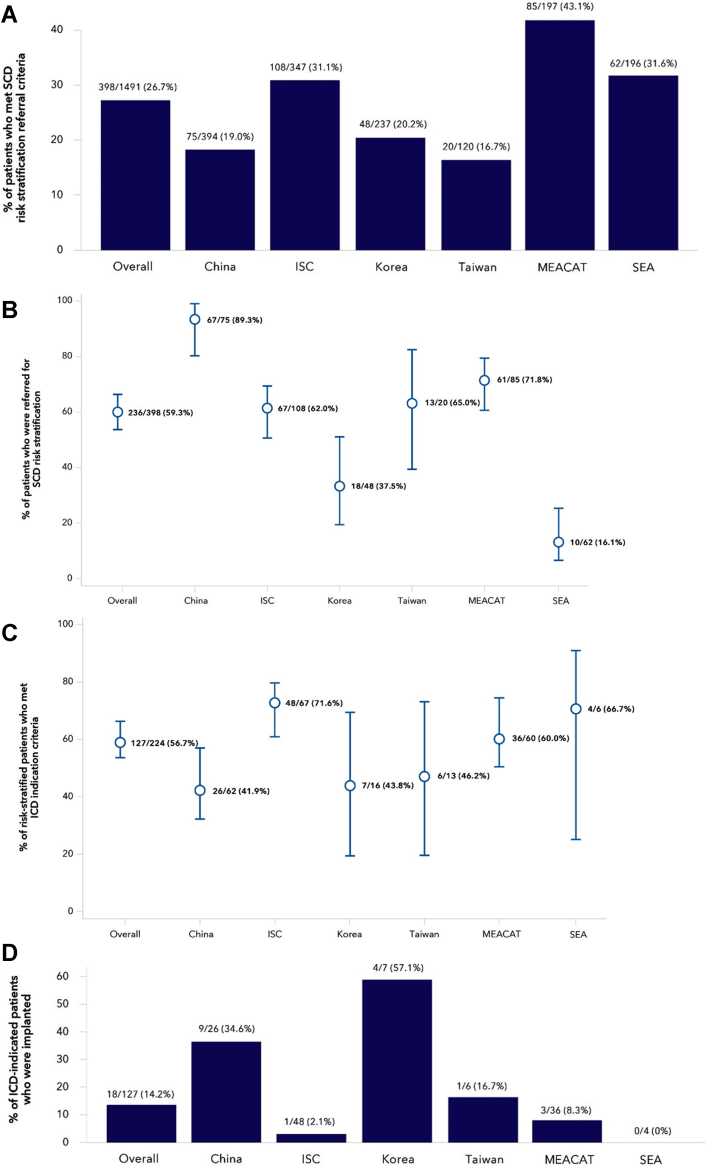
During the follow-up period, 26.7% of patients met referral criteria for SCD risk stratification and management at the 3-month visit and follow-up LVEF measure (incidence of patients meeting each criterion shown in Supplemental Figure 2). This proportion was higher in MEACAT (n = 85 of 197, 43.1%), ISC (n = 108 of 347, 31.1%), and SEA (n = 62 of 196, 31.6%) compared to Mainland China (n = 74 of 394, 19.0%), South Korea (n = 48 of 237, 20.2%), and Taiwan (n = 20 of 120, 16.7%) (Figure 2A).
Figure 2.
Patient Referral, Device Indication, and Implant
(A) The percentage of patients that met referral criteria at any point during the study are shown overall and in each geographical region. The error bars represent the 95% CI. Overall 26.7%, Mainland China 19.0%, India subcontinent (ISC) 31.1%, South Korea 20.3%, Taiwan 16.7%, Middle East, Africa, Central Asia, Turkey (MEACAT) 43.1%, and South East Asia (SEA) 31.6%. (B) Of the 398 patients who met further SCD risk stratification and management criteria, the percent referred for SCD risk stratification and management is shown overall and by geography. (C) Of the 224 patients who had an SCD risk stratification and management visit, the percent who met an ICD/CRT-D indication is shown overall and by geography. Overall 56.7%, Mainland China 41.9%, ISC 71.6%, South Korea 43.8%, Taiwan 46.2%, MEACAT 60.0%, and SEA 66.7%. (D) Of the 127 patients who met ICD/CRT-D referral criteria, the percentage of ICD/CRT-D implants in each geographical region are shown: Overall 14.2%, Mainland China 34.6%, ISC 2.1%, South Korea 57.1%, Taiwan 16.7%, MEACAT 8.3%, and SEA 0.0%. Abbreviations as in Figure 1.
The proportion of patients referred for an SCD risk stratification and management visit overall was 59.3% (n = 236 of 398; 95% CI: 54.4%-64.0%) and was highest in Mainland China (n = 67 of 75, 89.3%; 95% CI: 80.3%-94.5%), high in ISC (n = 67 of 108, 62.0%; 95% CI: 52.6%-70.6%), Taiwan (n = 13 of 20, 65%; 95% CI: 43.3%-81.9%), and MEACAT (n = 61 of 85, 71.7%; 95% CI: 61.4%-80.2%), lower in South Korea (n = 18 of 48, 37.5%; 95% CI: 25.2%-51.6%), and lowest in SEA (n = 10 of 62, 16.1%; 95% CI: 9.0%-27.2%) (Figure 2B). After having adjusted for the confounding effect of all other factors shown in Table 2 as compared to Mainland China, patients were significantly less likely to be referred if they were from SEA (odds ratio [OR]: 0.01; 95% CI: 0.00-0.05), South Korea (OR: 0.29; 95% CI: 0.10-0.91), and ISC (OR: 0.36; 95% CI: 0.14-0.91). Finally, the chance of being referred was, respectively, 89% (P < 0.0001) and 92% (P = 0.002) lower if patients were referred by an interventional cardiologist or a non-interventional cardiologist as compared to an electrophysiologist (Table 2). Of the patients referred to an SCD risk stratification and management visit, between 89% and 100% had the visit in all regions, except in SEA, where this proportion was 60%.
Table 2.
Odds of Being Referred for a SCD Risk Stratification and Management Visit
| OR (95% CI) | P Value | |
|---|---|---|
| Region | ||
| Mainland China | Reference | |
| ISC | 0.36 (0.14-0.93) | 0.0351 |
| South Korea | 0.29 (0.10-0.91) | 0.0332 |
| Taiwan | 1.05 (0.32-3.44) | 0.9326 |
| SEA | 0.01 (0.00-0.05) | <0.0001 |
| MEACAT | 0.68 (0.25-1.87) | 0.4535 |
| Referral during COVID-19 | ||
| No | Reference | |
| Yes | 1.45 (0.80-2.63) | 0.2177 |
| Sex | ||
| Male | Reference | |
| Female | 1.86 (0.91-3.81) | 0.0906 |
| Age, 5 y increase | 0.96 (0.86-1.07) | 0.4237 |
| STEMI | ||
| No | Reference | |
| Yes | 1.29 (0.77-2.18) | 0.3327 |
| MI treatment (CABG or PCI) | ||
| No | Reference | |
| Yes | 0.77 (0.41-1.42) | 0.3968 |
| Time from index MI to hospital admission, 1-day increase | 1.13 (0.97-1.31) | 0.1204 |
| LVEF at baseline, 1% increase | 1.03 (0.99-1.06) | 0.1546 |
| Specialty of referral physician | ||
| Electrophysiologist | Reference | |
| Interventional cardiologist | 0.11 (0.06-0.20) | <0.0001 |
| Noninterventional cardiologist | 0.08 (0.01-0.39) | 0.0021 |
| Both electrophysiologist and interventional cardiologist | 0.37 (0.12-1.14) | 0.0838 |
| Other | 2.48 (0.56-10.99) | 0.2324 |
CABG = coronary artery bypass graft; LVEF = left ventricular ejection fraction; OR = odds ratio; PCI = percutaneous coronary intervention; SCD = sudden cardiac death; other abbreviations as in Table 1.
The top reasons for the lack of a referral to an SCD risk stratification and management visit are listed in Table 3 with a full list in Supplemental Table 5. The most common reasons were different for each region. In Mainland China, it was patients deciding a referral was not necessary (n = 11, 64.7%). In ISC, it was patients being asymptomatic (n = 39, 90.7%). In South Korea and SEA, it was physician preference to continue with medication (n = 25, 62.5% and n = 16, 22.5% respectively). In Taiwan, it was that the patients did not meet the national health insurance criteria (n = 3, 42.9%). The reasons and diversity of reasons varied by geography.
Table 3.
Common Reasons for Refusal of Further SCD Risk Stratification and Management
| Mainland China | ISC | South Korea | Taiwan | MEACAT | SEA | Overall | |
|---|---|---|---|---|---|---|---|
| The patient was not referred by the physician | (n = 17) | (n = 43) | (n = 40) | (n = 7) | (n = 42) | (n = 71) | (N = 220)a |
| The patient was asymptomatic | 0 | 39 (90.7) | 1 (2.5) | 0 | 1 (2.4) | 1 (1.4) | 42 (19.1) |
| The patient was clinically stable | 0 | 2 (4.7) | 0 | 0 | 0 | 10 (14.1) | 12 (5.5) |
| Financial reasons | 0 | 1 (2.3) | 0 | 0 | 0 | 4 (5.6) | 5 (2.3) |
| The patient deemed referral not necessary | 11 (64.7) | 0 | 5 (12.5) | 2 (28.6) | 2 (4.8) | 1 (1.4) | 21 (9.5) |
| Physician decision to continue observation and postpone referral | 1 (5.9) | 0 | 3 (7.5) | 0 | 6 (14.3) | 11 (15.5) | 21 (9.5) |
| The physician preferred continuing with medication | 2 (11.8) | 1 (2.3) | 25 (62.5) | 2 (28.6) | 29 (69.0) | 16 (22.5) | 75 (34.1) |
| Improved symptoms | 0 | 0 | 0 | 0 | 0 | 5 (7.0) | 5 (2.3) |
| Other | 3 (17.6) | 0 | 6 (15.0) | 0 | 4 (9.5) | 10 (14.1) | 23 (10.5) |
| The patient refused referral | (n = 12) | (n = 0) | (n = 5) | (n = 4) | (n = 3) | (n = 2) | (N = 26)a |
| The patient was unwilling to change doctor | 9 (75.0) | 0 | 3 (60.0) | 3 (75.0) | 0 | 1 (50.0) | 16 (61.5) |
| Other | 3 (25.0) | 0 | 2 (40.0) | 1 (25.0) | 3 (100) | 1 (50.0) | 10 (38.5) |
Values are n (%).
Abbreviations as in Table 1.
Multiple reasons for refusal could be reported for a single patient and some patients did not have a reason for refusal listed.
Device indication and implant
Of patients who had an SCD risk stratification and management visit, 56.7% (n = 127 of 224; 95% CI: 50.1%-63.0%) met ICD/CRT-D indication criteria with most patients meeting criteria in ISC (n = 48 of 67, 71.6%; 95% CI: 59.9%-81.0%), MEACAT (n = 36 of 60, 60%; 95% CI: 47.3%-71.4%), and SEA (n = 4 of 6, 66.7%; 95% CI: 30.0%-90.3%). A smaller percentage of patients met criteria in Taiwan (n = 6 of 13, 46.2%; 95% CI: 23.2%-70.8%), South Korea (n = 7 of 16, 43.7%; 95% CI: 23.1%-66.8%), and Mainland China (n = 26 of 62, 41.9%; 95% CI: 30.5%-54.3%) (Figure 2C).
A total of 18 patients were implanted during the study period, with no implants occurring in SEA and 1 implant occurring in ISC and Taiwan (Figure 2D). Overall, within 1 year from enrollment, 14.2% of patients indicated for an ICD/CRT-D were implanted. In all regions, most of the implants occurred within 4 months of enrollment (Figure 3).
Figure 3.
Incidence of Device Implant Over Time
The incidence of device implant is shown over time (A) overall and (B) for each geographical region. Abbreviations as in Figure 2.
The most common reasons for device implant refusal despite indication are listed in Table 4 with a full list in Supplemental Table 6. Again, refusal reasons varied by geography. In Mainland China and ISC, the most common reason was that the patient was unable to pay for the device (n = 16, 39.0% and n = 41, 85.4%, respectively); in Taiwan it was that the patients did not want to incur risks associated with implantation (n = 6, 35.3%); in MEACAT it was tied between patients not wanting to incur risks associated with implantation (n = 22, 59.5%) and patients not believing in the benefit of ICD/CRT-D (n = 22, 59.5%); and in SEA it was that patients did not believe in the benefit of ICD/CRT-D (n = 3, 60%). There were no implant refusal reasons provided for patients in South Korea.
Table 4.
Reasons for ICD/CRT-D Implant Refusal in Indicated Patients
| Mainland China (n = 41) | ISC (n = 48) | South Korea (n = 0) | Taiwan (n = 17) | MEACAT (n = 37) | SEA (n = 5) | Overall (N = 148)a | |
|---|---|---|---|---|---|---|---|
| Patient does not want risk associated with implant | 12 (29.3) | 0 | 0 | 6 (35.3) | 22 (59.5) | 0 | 40 (27.0) |
| Patient does not believe in benefit of ICD/CRT-D | 8 (19.5) | 0 | 0 | 3 (17.6) | 22 (59.5) | 3 (60.0) | 36 (24.3) |
| Patient unable to pay for device | 16 (39.0) | 41 (85.4) | 0 | 5 (29.4) | 0 | 0 | 62 (41.9) |
| Patient unwilling to pay for device | 7 (17.1) | 0 | 0 | 1 (5.9) | 0 | 0 | 8 (5.4) |
| Other | 0 | 7 (14.6) | 0 | 2 (11.8) | 6 (16.2) | 2 (40.0) | 17 (11.5) |
Values are n (%).
CRT-D = cardiac resynchronization therapy–defibrillator; ICD = implantable cardioverter-defibrillator; other abbreviations as in Table 1.
Multiple reasons for refusal could be reported for a single patient and some patients did not have a reason for refusal listed.
Cardiovascular mortality
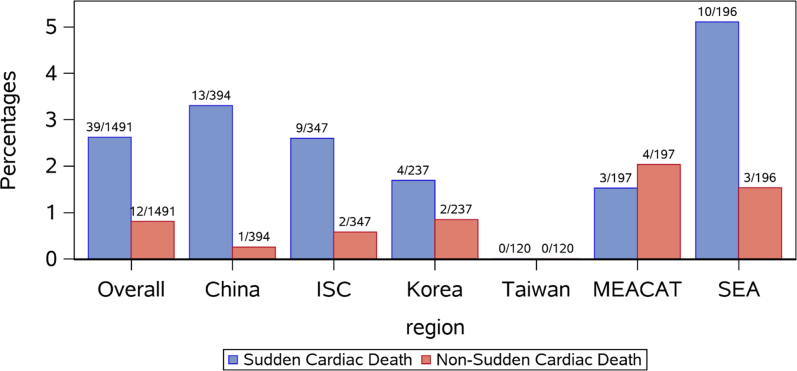
Cardiovascular mortality occurred for a total of 51 patients (3.4% of enrolled patients), of whom 39 (76.5%) experienced SCD and 12 (23.5%) experienced non-SCD. None of the patients enrolled in Taiwan experienced cardiovascular mortality, whereas the highest proportion of cardiovascular mortality was experienced in SEA, with 13 cardiovascular deaths (6.6% of the patients enrolled in this region), of which 10 (76.9%) were SCD and 3 (23.1%) were non-SCD. In the other 4 regions, most of the cardiovascular deaths were SCD, except in MEACAT which had a slightly higher proportion of non-SCD (Figure 4).
Figure 4.
Mortality in Post-MI Patients by Geography
The percentage of sudden cardiac death (blue) and non-sudden cardiac death (red) mortality are shown overall and by geography during the study period. Abbreviations as in Figure 2.
Discussion
The Improve SCA Bridge study was a prospective, observational, multi-national study that examined the current care pathway for post-MI patients in regions known to have low ICD use with several important findings. First, 26.7% of post-MI patients met the criteria associated with a higher risk of SCD and of those patients 59.3% were referred for an SCD risk stratification and management visit. Second, of risk-stratified patients, 56.7% were indicated for ICD/CRT-D; of these, 14.2% were implanted during the study with most of the implants occurring within 4 months from enrollment (Central Illustration). Finally, most cardiovascular patient deaths (n = 39 of 51, 76.5%) that occurred during the study were due to SCD.
Central Illustration.
Patient Movement Through the Post–Myocardial Infarction Care Pathway
Of the total patients who met referral criteria for further sudden cardiac death (SCD) risk stratification and management, the percent of patients that continued in the care path (blue), appropriately exited the care path (gray) or exited the care path while still at risk of SCD (red) are shown at each step of the care pathway. CRT-D = cardiac resynchronization therapy–defibrillator; ICD = implantable cardioverter-defibrillator.
The referral rate for patients who met further SCD risk stratification and management criteria varied depending on the region, suggesting that barriers to referral are strongest in SEA (16%) and South Korea (37.5%), less pronounced in ISC (62%), Taiwan (65%), and MEACAT (71.7%), and least pronounced in Mainland China (89%) (Figure 2). Patients were also much less likely to be referred if they were being treated by an interventional or non-interventional cardiologist (89% and 92%, respectively) as opposed to an electrophysiologist. LVEF at baseline was surprisingly not found to be a predictor of referral. However, patients were more likely to be referred at the 3-month visit than at the 6- or 12-month visit, likely because the clearest cases for referral, patients with a low LVEF 3-months post-MI, would have already been referred at the 3-month visit. Further, only 38.9% of patients with LVEF ≤25% were referred to an SCD risk stratification and management visit leaving 61.1% of high-risk patients without a referral (Supplemental Table 7).
Common barriers to patient referral included physician preference to continue with medication and the patient deeming the referral not necessary; however, the most prominent barrier varied by region. Physician preference to continue with medication was most pronounced in South Korea (62.5%) and MEACAT (69.0%), suggesting that this could be a particularly impactful target for effecting change. To our knowledge, this is the first study examining the rates of referral to SCD risk stratification and management and reasons for non-referral in post-MI patients from these regions.
A large gap remains in the number of patients receiving an ICD for the prevention of SCD in the studied regions vs those meeting guideline recommendations for ICD therapy. In this study, more than 40% of risk-stratified patients in Mainland China, South Korea, and Taiwan met ICD/CRT-D indication criteria, whereas between 60% and 70% of patients met indication criteria in ISC, MEACAT, and SEA. However, only 18 of 127 (14.2%) indicated patients were implanted across all regions. ICD use in indicated patients has been previously reported to vary across Asia from 1.5% in Indonesia to 17.9% in Mainland China to 52% in Japan.17 Similar to our study, a recent report from the ASIAN-HF (Asian Sudden Cardiac Death in Heart Failure) registry showed rates of ICD use to be ∼12% in Asia compared to ∼24% in Europe and 30% to 50% in the United States.7,17, 18, 19, 20, 21
This study found that common barriers to ICD/CRT-D implantation, despite guideline indication, included patients not wanting the risk associated with implant (Mainland China, Taiwan, and MEACAT), patients not believing in the benefit of ICT/CRT-D (Mainland China, Taiwan, MEACAT, and SEA), and patients being unable (Mainland China and ISC) or unwilling (Mainland China) to pay for the device. Previous reports also showed that barriers limiting ICD use included reimbursement for device therapy, the health care financing system, lack of health care provider awareness regarding ICD benefits, patient socioeconomic status, and patient lack of awareness of the therapy and its benefits.13,17 Together, this evidence suggests that barriers limiting ICD/CRT-D use are often related to cost and patient or physician awareness of ICD/CRT-D benefits.
The cost effectiveness of ICDs has been established previously in Western countries, and a recent analysis from Taiwan further supported ICD therapy cost-effectives in primary prevention patients regionally.22 Concerning the issue of patient awareness, a previous survey of 2,000 ICD nonrecipients in Asia found that a significant proportion of patients were unaware of the benefits (32.6%) and lacked information to decide (22.1%) on device therapy.17 Further, previous studies have suggested that physician awareness on the indications for ICD therapy is low.14,15 Our own data suggest that a greater opportunity may exist through targeting education regarding guideline recommendations for SCD prevention in post-MI patients to investigators in the interventional and non-interventional cardiologist specialty.
In this study, informational materials were made available to investigators and patients before site enrollment, suggesting that the real-life barriers to adoption of guideline-directed ICD therapy may be even more complicated and difficult to overcome than previously thought. Recently, Khan et al23 reported successfully using 3 strategies at the King’s College Hospital in London to improve evidence-based ICD programing. Briefly described, they first introduced institutional guidelines, circulated education materials, and encouraged compliance. Second, they kept printed summaries of the guidelines displayed prominently in applicable areas. Finally, they implemented monthly audit reports on guideline compliance which were circulated via email and displayed in applicable areas. These strategies could easily be applied at hospitals and clinics for improving guideline adherence for SCD prevention in post-MI patients. Another possible strategy, in areas where available, is using tags in electronic health records. In any case, more comprehensive, sustained quality improvement efforts may be necessary to increase guideline adherence in these regions to reduce SCD.
The results from this study suggest that focusing interventional efforts in the care pathways at different stages in different regions may be the most impactful approach. For instance, in Mainland China, focusing interventional efforts later in the post-MI patient care pathway in ICD/CRT-D–indicated patients may make the most sense. In South Korea, interventional strategies that target improving patient referral for SCD risk stratification and management may be more successful, whereas in Taiwan, ISC, MEACAT, and SEA, focusing interventional efforts at all stages may be required. Additional studies will be needed to determine the best interventional strategies for each region and what impact those strategies will have on reducing SCD risk and improving guideline-directed ICD/CRT-D therapy adoption. Overall, a significant portion of post-MI patients are still at risk of SCD and improvements to the care pathway can improve outcomes.
Study limitations
This study had several limitations that should be considered when applying the findings to other populations. This study was observational in nature and patients were enrolled prospectively based on prespecified enrollment criteria; however, patient characteristics vary between the populations being compared as no randomization or matching occurred. Analysis into how baseline characteristics impact different outcome measures (eg, ICD referral and implant rates), further analyses into mortality, and analyses on how reimbursement policies impact rates of referral and ICD implant are beyond the scope of the current analysis.
Additionally, a portion of this study occurred during the COVID-19 pandemic which may have impacted patient follow-up and the standard clinical care pathway. Our current analysis did not show a significant impact (P = 0.22) when comparing whether SCD risk stratification referral occurred before or during the COVID-19 pandemic.
Finally, reasons for ICD implant device refusal were not collected in South Korea and therefore could not be analyzed or discussed.
Conclusions
The results from this study show that many post-MI patients at risk of SCD are not getting referred for further SCD risk stratification or receiving guideline-directed ICDs. This suggests that opportunities exist for broad quality improvement efforts around the post-MI care pathway in the geographies included in this study. Physician and patient education on device risks and benefits may improve guideline adherence in indicated patients; however, optimal strategies to improve adherence will likely vary regionally.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Post-MI patients are at an increased risk for SCD. ICDs can reduce mortality in post-MI patients with reduced LVEF. However, ICD therapy adoption rates vary regionally and are particularly low in geographies underrepresented in clinical trials related to tachycardia and ICD therapy. More efforts are needed to understand why guideline-directed ICD use remains low in certain geographies, especially in post-MI patients at risk of SCD. The current study characterizes the care pathway of post-acute MI patients in 6 regions historically underrepresented in ICD trials. The observation of both referral rates to further SCD risk stratification and management and rates of ICD implant in indicated post-MI patients provides insight into where at-risk patients are most likely to fall out of the care pathway. Additionally, the collection of reasons for refusal to SCD risk stratification and management referral or ICD implant emphasizes why patients are not continuing through the care pathway. Taken together, this information can be used to develop targeted strategies to improve post-MI patient care through better guideline adherence for SCD prevention.
TRANSLATIONAL OUTLOOK: Many post-MI patients at risk of SCD do not receive guideline-directed ICDs in part due to low referral rates of SCD risk stratification and management in the geographies included in this study. The results from this study suggest that improvements in referral to SCD risk stratification and management and better adherence to guidelines are necessary. However, optimal strategies to realize these improvements will likely vary by region.
Funding Support and Author Disclosures
This study was funded by Medtronic Inc. Dr Zhang has received speaker fees/consulting fees from Boston Scientific, Medtronic, Abbott, and Biotronik; and has received steering committee fees from Medtronic. Dr Chen has received honorariums from Medtronic, Biotronik, Abbott, and Boston Scientific. Dr Liew has received speaker fees and honorarium from Medtronic and Boston Scientific. Dr Haggui has received honorariums from Medtronic, Abbott, and Boston Scientific. Dr Ong has received speaker/consultant fees from Boston Scientific, Medtronic, Abbott Vascular, Biotronic, OrbusNeich, Alvimedica, B Braun, Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr Rungpradubvong has received honoraria from Medtronic, Abbott, Boston Scientific, Biotronik, Johnson & Johnson, Pfizer, Daiichi Sankyo, Boehringer Ingelheim, and Bayer. Dr Wang has received honorariums from Medtronic, Abbott, and Biotronik. JinKyung Jeon, Grace Wong, Dr Lemme, Brian Van Dorn, and Dr Lexcen are employees of Medtronic Inc. Dr Huang has received speaker fees/consulting fees from Boston Scientific, Bayer, Boehringer-Ingelheim, and Abbott. All other authors have reported that there are no relationships relevant to the contents of this paper to disclose.
Acknowledgements
The authors thank Amy Molan, PhD, and Cody Lensing, PhD, for medical writing assistance; Bart Gerritse, PhD, and Valentine Obidigbo, MS, for help with statistical analysis; and Michelle Cercioglu for critical review.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For additional methods, figures, and tables, please see the online version of this paper.
Appendix
References
- 1.Pan H., Hibino M., Kobeissi E., Aune D. Blood pressure, hypertension and the risk of sudden cardiac death: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2020;35:443–454. doi: 10.1007/s10654-019-00593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss A.J., Hall W.J., Cannom D.S., et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 3.Moss A.J., Zareba W., Hall W.J., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Bardy G.H., Lee K.L., Mark D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Buxton A.E., Lee K.L., Fisher J.D., et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 6.Kadish A., Dyer A., Daubert J.P., et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 7.Rohde L.E., Chatterjee N.A., Vaduganathan M., et al. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter-defibrillator use and heart failure cause: a PARADIGM-HF analysis. J Am Coll Cardiol HF. 2020;8:844–855. doi: 10.1016/j.jchf.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Schrage B., Uijl A., Benson L., et al. Association between use of primary-prevention implantable cardioverter-defibrillators and mortality in patients with heart failure: a prospective propensity score-matched analysis from the Swedish Heart Failure Registry. Circulation. 2019;140:1530–1539. doi: 10.1161/CIRCULATIONAHA.119.043012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S., Ching C.K., Huang D., et al. Utilization of implantable cardioverter-defibrillators for the prevention of sudden cardiac death in emerging countries: Improve SCA clinical trial. Heart Rhythm. 2020;17:468–475. doi: 10.1016/j.hrthm.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:1677–1749. doi: 10.1016/j.jacc.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Priori S.G., Blomström-Lundqvist C., Mazzanti A., et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S. Sudden cardiac death in China: current status and future perspectives. Europace. 2015;17(suppl 2):ii14–18. doi: 10.1093/europace/euv143. [DOI] [PubMed] [Google Scholar]
- 13.Singh B., Zhang S., Ching C.K., et al. Improving the utilization of implantable cardioverter defibrillators for sudden cardiac arrest prevention (Improve SCA) in developing countries: clinical characteristics and reasons for implantation refusal. Pacing Clin Electrophysiol. 2018;41:1619–1626. doi: 10.1111/pace.13526. [DOI] [PubMed] [Google Scholar]
- 14.Hubinette C., Lund L.H., Gadler F., Stahlberg M. Awareness of indications for device therapy among a broad range of physicians: a survey study. Europace. 2014;16:1580–1586. doi: 10.1093/europace/eut416. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos J.M., Smith L.M., Varosy P.D., Dehlendorf C., Marcus G.M. Referring physicians' discordance with the primary prevention implantable cardioverter-defibrillator guidelines: a national survey. Heart Rhythm. 2012;9:874–881. doi: 10.1016/j.hrthm.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 17.Chia Y.M.F., Teng T.K., Tan E.S.J., et al. Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes. 2017;10(11) doi: 10.1161/CIRCOUTCOMES.116.003651. [DOI] [PubMed] [Google Scholar]
- 18.John Camm A., Nisam S. European utilization of the implantable defibrillator: has 10 years changed the “enigma”. Europace. 2010;12:1063–1069. doi: 10.1093/europace/euq282. [DOI] [PubMed] [Google Scholar]
- 19.Hoang A., Shen C., Zheng J., et al. Utilization rates of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death: a 2012 calculation for a midwestern health referral region. Heart Rhythm. 2014;11:849–855. doi: 10.1016/j.hrthm.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra M.R., Yancy C.W., Albert N.M., et al. Evidence of clinical practice heterogeneity in the use of implantable cardioverter-defibrillators in heart failure and post-myocardial infarction left ventricular dysfunction: findings from IMPROVE HF. Heart Rhythm. 2009;6:1727–1734. doi: 10.1016/j.hrthm.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez A.F., Fonarow G.C., Liang L., et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook R., Higuera L., Wherry K., et al. Implantable cardioverter defibrillator therapy is cost effective for primary prevention patients in Taiwan: an analysis from the Improve SCA trial. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan P., Gopinath B., Jahagirdar N., et al. Improving adoption of evidence-based implantable cardioverter defibrillator programming — a single centre experience. Heart Rhythm. 2022;19(6):1011–1012. doi: 10.1016/j.hrthm.2022.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.