Abstract
The presence of five antibiotics (metronidazole, ciprofloxacin, amoxicillin, doxycycline, and chloramphenicol) and four analgesics (diclofenac, ibuprofen, paracetamol, and caffeine) were investigated in water and soil samples from the Sunyani municipality, Ghana. Liquid samples were collected from hospital effluents, sachet drinking water, municipal waterworks, river Tano, and dumpsite leachates, while soil samples were collected from dumpsites and municipal waterworks. All samples were prepared using solid-phase extraction (SPE) and analyzed via an HPLC- PDA method. All antibiotics analyzed, apart from metronidazole, were detected either in soil or water samples. Doxycycline and ciprofloxacin were present in almost all liquid samples. The investigated hospital effluents had antibiotic concentrations of up to 2.93 mg/L for doxycycline and 4.74 mg/L for ciprofloxacin. The highest concentration of any antibiotic found was 8.76 mg/L of amoxicillin in hospital effluents. The maximum concentration of analgesics in liquid samples analyzed was 3.20 mg/L (paracetamol) and 3.00 mg/kg (caffeine) in soil samples. Ecological risk assessment indicated that the pharmaceuticals pose a possible risk to some aquatic organisms. The findings from this study showed the presence of these pharmaceuticals at concentrations that could impact the ecosystem. Consistent monitoring of environmental levels and pursuing the development and implementation of a suitable remediation program is needed.
Keywords: Pharmaceutical residues, Risk assessment, Antibiotic resistance, Leachates, Hospital Effluents, Dumpsites
Graphical Abstract
Highlights
-
•
Pharmaceutical residues in the environment are a growing problem in Ghana, and globally.
-
•
Improper disposal practices a major contributor to the presence of pharmaceutical residues in the environment.
-
•
Antibiotic and analgesic residues are present in dumpsites, hospital effluents and municipal water work samples.
-
•
The levels of pharmaceuticals pose considerable risk to aquatic life.
-
•
Regular monitoring of pharmaceutical residues and implementation of remediation protocols are recommended.
1. Introduction
Public health care is achieved mainly by the use of pharmaceuticals [1]. Thousands of diverse pharmaceutically active compounds are used in huge quantities to prevent or treat human and animal diseases. Analgesics and antibiotics are among the most prescribed drugs worldwide [2]. The relevance of analgesics cannot be overlooked as far as pain relief is concerned. On the other hand, antibiotics are used to inhibit the growth of bacteria or kill them. These pharmaceuticals, especially analgesics, are in high demand on the market and can be bought over-the-counter without a prescription [3], [4]. The total annual market consumption of antibiotics worldwide is estimated to lie between 100,000 and 200,000 tons [5]. Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most frequently used drugs worldwide, consumed by more than 30 million people daily [6]. In the USA, an annual prescription of over 111 million NSAIDs are consumed, and this represents about 60% of the USA’s over the counter analgesic market [7].
Antibiotics and analgesics are indispensable in modern medicine. However, the extensive use of these pharmaceuticals has led to their presence and persistence in environmental matrices which can result in the development of drug resistance - a global concern. About 40–90% of pharmaceuticals administered are excreted in feces and urine as parent compounds (in the active form) into the environment [8]. Pharmaceuticals ultimately find their way into the environment through runoffs, leaks, and sewage systems, and these could contaminate soils, water bodies, and plants [8]. The use of huge amounts of pharmaceuticals in animal farming can result in agro-ecosystem contamination. This occurs when contaminated manure is applied on agricultural lands as fertilizer and crops are irrigated with wastewater. Another route through which pharmaceuticals enter the environment is improper disposal of unused pharmaceuticals. Pharmaceuticals also enter the environment by discharging industrial effluent, hospital effluent, sewage treatment plants, and septic tanks into streams and rivers [5], [8], [9].
Lately, pharmaceuticals have become micro-contaminants in soil and water as the rate of their introduction in the environment surpasses degradation [10]. Analgesics and antibiotics are among the most widely detected pollutants in the environment and are, hence, considered pseudo-persistent contaminants [11]. The negative impact of antibiotics on natural microbial communities could include the bactericidal and bacteriostatic actions of antibiotics. These actions cause the loss or destruction of some microbial groups involved in crucial ecosystem activities (direct effect) [12]. Exposure of oriental white-backed vultures to analgesics (diclofenac) has been shown to affect their kidney and thus resulting in kidney failure. This is reported to have led to a decline in vulture population and extinction in some parts of the world [13].
Pharmaceuticals have been detected in samples from dumpsites [14], hospital effluents [15], surface water [16], [17], and drinking water [18] all over the world. It has been noted that unwanted or expired medicines are most commonly thrown into trash cans or disposed of alongside household waste [19], [20], [21], [22]. A study conducted on landfill sites in Kumasi, Ghana, reported high concentrations of up to 18.25 ± 7.92 mg/L for metronidazole and 10.96 ± 6.93 mg/L for amoxicillin in leachate samples [14].
It is reported that about 25–68% of antibiotics are administered to hospitalized patients worldwide. That number is even higher in Ghana, where about 71% of hospitalized patients receive antibiotics as part of their treatment regimen [23]. Several studies have reported pharmaceutical residues in drinking water [18] due to runoffs from industries sewage, hospitals effluents, leachates from dumpsites, etc., which end up in water bodies. This makes rivers and drinking water samples possible hotspots as well. Pharmaceuticals have been detected in food, hospital effluents, dumpsite leachates, surface and drinking water in some parts of Ghana [14], [16], [24], [25], [26]. However, the residual concentrations, occurrence, and ecological effects of antibiotics and analgesics in the Sunyani Municipality are obscure. The purpose of this work was to investigate the occurrence of two classes of pharmaceuticals (antibiotics and analgesics) in hospital effluents, dumpsite soil and leachates, sachet water, and municipal waterworks samples and to investigate the risks associated with the presence of these pharmaceuticals in both water and soil samples.
2. Materials and methods
2.1. Reagents
Acetonitrile (HPLC grade) and methanol (Analytical grade) were obtained from VWR Chemicals (Accra, Ghana). Antibiotics (amoxicillin, chloramphenicol, metronidazole, doxycycline ciprofloxacin) and analgesics (paracetamol, caffeine, diclofenac, ibuprofen) standards were obtained as pure powders from Ernest Chemists (Accra, Ghana). Deionized water was used to prepare all solutions and calibration standards for HPLC analysis. Stock solutions of 1000 mg/L of each analyte were prepared. Working standard solutions were prepared from the stock standard solutions for both antibiotics and analgesics.
2.2. Study area
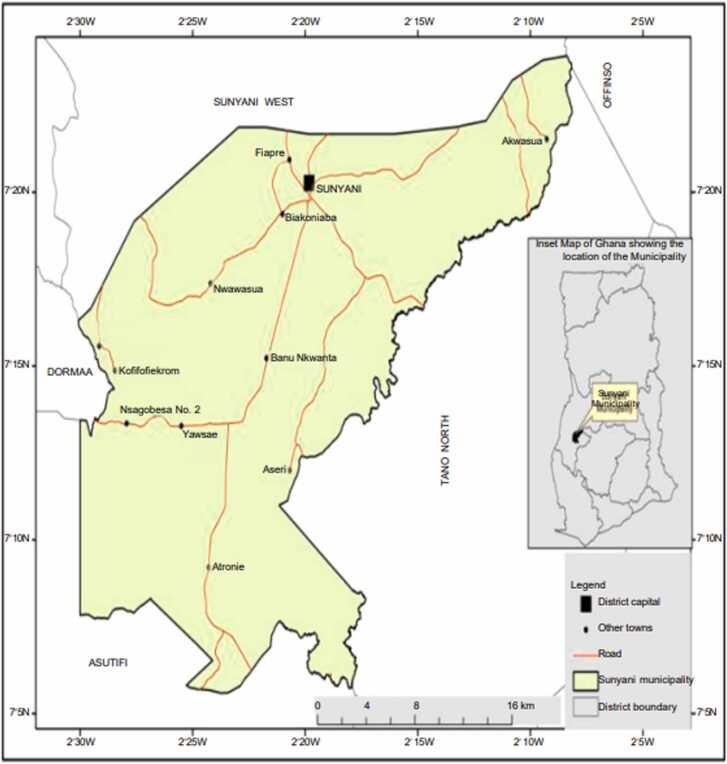
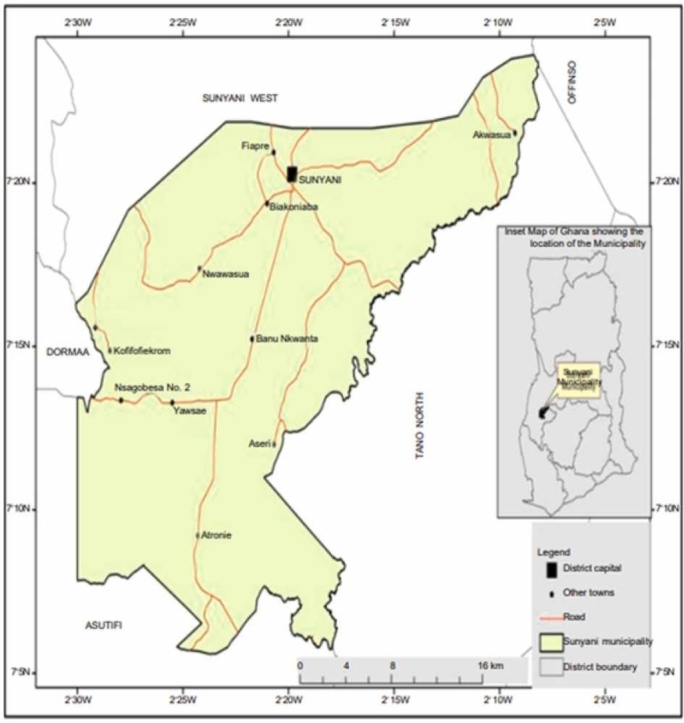
A map of the Sunyani municipality is shown in Fig. 1. Sunyani is known as the green city of Ghana and is the capital town of the Bono Region, Ghana. The Sunyani municipality is one of the Bono Region's 12 districts. Sunyani is inhabited by 152,567 people. A total of 125 retail pharmacies and 41 hospitals/health centers provides medical assistance in the region [27]. River Tano, the main source of water in the Sunyani Municipality, is located at Tanoso in the Bono Region. It is directed into a reservoir at the Sunyani Municipal waterworks station. At the station, it passes through different channels created (settled water chamber, final water chamber) for treatment and purification before it is distributed to various reception points.
Fig. 1.
Map of the Sunyani Municipality [27].
2.3. Sample collection
2.3.1. Soil samples
Soil and sediment samples were collected (0–20 cm depth) from dumpsites and the municipal waterworks station in the Sunyani Municipality using a stratified random sampling approach in September 2020.
2.3.2. Liquid samples
Pre-cleaned plastic bottles (1.5 L) were rinsed with each water sample to be collected before samples were fetched into the bottles. Dumpsite leachates, hospital effluents, water samples from the Sunyani Municipal waterworks station and River Tano were sampled for the study. Samples were collected about 10–20 cm deep within the banks of River Tano (latitude: 6.729490, longitude: −2.424003). Water samples were also taken from different reservoirs at the waterworks station. Different sachet drinking water sold in the Sunyani municipality was also sampled for analysis. In all, 60 water and 52 soil samples were collected. All the samples collected were labeled and packed into boxes and transported frozen in ice-chests maintained at 4 °C to the laboratory for analysis. In the laboratory, pH, total dissolved solids (TDS), and electrical conductivity (EC) were measured for all water and soil samples using procedures described elsewhere [28], [29].
2.4. Sample preparation and extraction of antibiotics and analgesics
2.4.1. Soil
Soil/sediment samples were air-dried at room temperature. After drying, all samples from each site were mixed to form a composite. The samples were ground with mortar and pestle, sieved using a 200 µm mesh sieve into fine particles to ensure homogeneity, and then put into Ziplock bags.
2.4.2. Sonication and filtration
One hundred milliliters of acetonitrile was added to a 50 g soil sample, shaken vigorously, and allowed to stand overnight. The samples were sonicated in an ultrasonicator bath (VWR USC 600 T, 45 kHz, 120 W) for about 15 min the next day and centrifuged at 25 °C. The supernatant was decanted. Another 50 mL of acetonitrile was added to the sample and sonicated for 30 min. The supernatants were combined and successively filtered first with cotton wool and finally with Whatman 125 mm filter paper to obtain clear solutions prior to solid-phase extraction (SPE) [14].
2.4.3. Water and leachates
Solid impurities were removed from water and leachate samples by filtration, successively with cotton wool and Whatman 125 mm filter papers. Leachate samples that still had particles were centrifuged at 2000 rpm for 10 min at 25 °C to get the particles to settle, and the supernatants were then decanted. The filtered samples were then subjected to SPE [14].
2.5. Solid-phase extraction
2.5.1. Liquid samples
A hydrophilic-lipophilic balance cartridge (HLB 6 mL, 200 mg, 30 µm) was used. The SPE cartridges were conditioned with 5 mL methanol (MeOH) followed by 5 mL distilled water. Six hundred milliliter portions of liquid samples were loaded onto SPE cartridges at a flow rate of 1.5 mL/min. The cartridges were allowed to dry for a couple of minutes under vacuum. Analytes were eluted from the cartridges with 3 mL MeOH after washing with 3 mL water. Eluates were transferred into 2 mL autosampler HPLC vials for analysis [14].
2.5.2. Soil samples
For soil samples, the SPE cartridges were conditioned with 5 mL of methanol (MeOH) followed by 5 mL of distilled water. The combined filtrate from the extraction (100 mL) was loaded onto SPE cartridges at a flow rate of 1.5 mL/min and left to dry for a couple of minutes. The dried SPE cartridges were washed with 5 mL distilled water and then dried under vacuum for about 10 min. The sorbents were eluted with 3 mL methanol. Samples were then transferred to 2 mL autosampler HPLC vials for analysis [14].
2.6. HPLC analysis
Chromatographic separation was done on a Perkin Elmer Flexar HPLC coupled to a PDA detector. Antibiotics separation was attained on Agilent Zorbax 300SB C18 (250 ×4.6 mm, 5 µm) column. The mobile phase consisted of 0.05% TFA (A) and methanol (B). Separation of analgesics was achieved on a Phenomenex Luna C8 (150 ×4.6 mm, 5 µm) column. The mobile phase for analgesic separation consisted of 0.1% acetic acid (A) and methanol (B). Table 1, Table 2 give the detailed gradient program from the gradient elution performed at a flow rate of 1 mL/min with an injection volume of 20 µL for both antibiotics and analgesics.
Table 1.
HPLC flow program for antibiotics residue analysis.
| Step | Step Type | Step Time (min) | Flow (mL/min) | %A | %B |
|---|---|---|---|---|---|
| 0 | Equilibrate | 1.0 | 1.0 | 75 | 25 |
| 1 | Run | 4.0 | 1.0 | 75 | 25 |
| 2 | Run | 8.0 | 1.0 | 30 | 70 |
| 3 | Run | 5.0 | 1.0 | 75 | 25 |
Solvent A: 0.05% TFA, B: methanol
Table 2.
HPLC flow program for analgesics analysis.
| Step | Step type | Step time (min) | Flow (mL/min) | %A | %B |
|---|---|---|---|---|---|
| 0 | Equilibrate | 1.0 | 1.0 | 75.0 | 25.0 |
| 1 | Run | 4.0 | 1.0 | 75.0 | 25.0 |
| 2 | Run | 4.0 | 1.0 | 30.0 | 70.0 |
| 3 | Run | 8.0 | 1.0 | 30.0 | 70.0 |
| 5 | Run | 5.0 | 1.0 | 75.0 | 25.0 |
Solvent A: 0.1% acetic acid, B: methanol.
The total runtime for the analysis was 18 min for antibiotics and 22 min for analgesics, and all chromatographic work was carried out at ambient temperatures. Detection of metronidazole, ciprofloxacin, and doxycycline was at 320 nm and amoxicillin and chloramphenicol at 215 nm. Paracetamol and caffeine were monitored at 270 nm, diclofenac, and ibuprofen at 220 nm. Quantification was done using external calibration and peak area measurement.
2.7. Quality parameters
2.7.1. LOD and LOQ
Linearity was established for all test samples in concentrations ranging from 0.2 to 10 mg/. The limits of detection (LOD) and limits of quantification (LOQ) for each test sample were calculated with respect to signal-to-noise ratios.
2.7.2. Recoveries and quality assurance
A sample blank (water and soil matrices containing none of the analytes) was prepared and spiked with known concentrations of standard drugs. After the extraction and SPE clean-up procedures (as described earlier), the sample blank was analyzed by HPLC methods described earlier. The concentration of the drugs was determined from the chromatograms obtained. Standard solutions of the analytes were injected prior to analyses and after every 10 sample runs to ensure that the HPLC system was functioning properly. Blank samples were also injected after every 5 runs to monitor any sample interference. All sample injections were made in duplicates. Each batch of analyses was prepared to include a reagent blank to check background contamination [14]. Table 3 shows the recovery data of antibiotics and analgesics.
Table 3.
A summary of detection and quality assurance parameters obtained.
| Analyte | Retention time (mins) | Linear Range (mg/L) | LOD (mg/L) | LOQ (mg/L) | SPE Recoveries (%) |
||
|---|---|---|---|---|---|---|---|
| Metronidazolei | 4.746 | 0.2–10.0 | 0.0314 | 0.0952 | 85.51 | ||
| Ciprofloxacini | 12.096 | 0.2–10.0 | 0.0191 | 0.0579 | 89.12 | ||
| Amoxicillinii | 10.003 | 0.2–10.0 | 0.0358 | 0.1085 | 94.26 | ||
| Antibiotics | Chloramphenicolii | 12.372 | 0.2–10.0 | 0.0229 | 0.0694 | 91.16 | |
| Doxycyclinei | 13.688 | 0.2–10.0 | 0.0205 | 0.0621 | 91.04 | ||
| Diclofenaciv | 17.200 | 0.2–10.0 | 0.0394 | 0.1194 | 94.66 | ||
| Analgesics | Paracetamoliii | 3.000 | 0.2–10.0 | 0.0648 | 0.1964 | 97.02 | |
| Caffeineiii | 5.000 | 0.2–10.0 | 0.0049 | 0.0148 | 93.19 | ||
| Ibuprofeniv | 18.900 | 0.2–10.0 | 0.0710 | 0.2152 | 91.58 | ||
LOD – limit of detection; LOQ – limit of quantitation; SPE – solid phase extraction; all analytes with the same Roman numeral were detected and quantified with the same HPLC method
2.8. Ecological risk assessment
The risk that the presence of various pharmaceuticals in the environment poses was estimated using the Risk Quotient (RQ) as suggested by the European Medicines Evaluation Agency (EMEA, 2006). The ecological risk quotient (RQ) was calculated for each antibiotic and analgesic using the ratio between the maximal environmental concentrations (MEC) and predicted no-effect concentration (PNEC), as shown in equation 1.
| (1) |
RQ was calculated at different trophic levels of the ecosystem (algae, daphnid, fish, crustaceans). RQ < 0.1 indicates a minimal risk to aquatic organisms; 0.1 ≤ RQ ≤ 1.0 poses a medium risk and RQ ≥ 1.0, poses a possibly high risk and is likely to harm organisms in the environment [30].
3. Results
The physicochemical parameters of the liquid and soil samples are presented in Table 4. Generally, water samples from the Sunyani municipal waterworks station were slightly acidic (pH range of 6.01–6.79). Sediment samples from the same station were slightly acidic to slightly basic (pH range of 5.92–7.63). Leachates and soil samples from dumpsites were slightly acidic (6.34–6.90) and slightly acidic to slightly basic (5.82 – 7.79), respectively. The pH of sachet water samples and hospital effluents ranged from 6.02 to 6.44 and 6.55–7.33, respectively. The total dissolved solids (TDS) values of all water and sediment samples from Sunyani municipal waterworks station ranged from 152.00 to 185.12 mg/L and 29.00 – 77.00 mg/L, respectively. TDS for leachates and soil samples from dumpsite were generally high, ranging from 230.00 to 1323.00 mg/L and 73.00 – 475.00 mg/L, respectively. Low TDS values ranging from 9.00 to 55.00 mg/L were recorded in sachet water samples and hospital effluents (161.00–1121.00 mg/L). The Electrical Conductivity (EC) of all water and sediment samples from Sunyani municipal waterworks station were in the range of 152.30 – 220.00 µS/cm and 31.60 – 86.00 µS/cm, respectively. The EC for leachates and soil samples from dumpsite were generally high (390.00 – 6557.25 µS/cm and 79.80 – 519.00 µS/cm, respectively). The lowest EC values were recorded in sachet water samples (9.25 – 57.75 µS/cm), with the EC for hospital effluents ranging from 187.15 to 1141.00 µS/cm.
Table 4.
Summary of the results of physicochemical parameters measured in liquid and soil samples from the Sunyani municipality.
| Sample type | pH |
EC (µS/cm) |
TDS (mg/L) |
||||
|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | ||
| Liquid | Sachet water | 6.02–6.44 | 6.14 ± 0.16 | 9.25–57.75 | 25.11 ± 18.51 | 9.00–55.00 | 24.50 ± 17.63 |
| Dumpsite leachates | 6.34–6.90 | 6.68 ± 0.23 | 390.00–6557.25 | 2022.35 ± 2557.77 | 230.00–1323.00 | 758.40 ± 473.61 | |
| Hospital effluents | 6.55–7.33 | 6.88 ± 0.40 | 187.15–1141.00 | 806.55 ± 536.99 | 161.00–1121.00 | 768.33 ± 528.24 | |
| Municipal waterworks | 6.01–6.79 | 6.48 ± 0.34 | 152.30–220.00 | 190.31 ± 28.53 | 152.00–219.00 | 188.25 ± 27.96 | |
| Soil | Dumpsites Soil |
5.82–7.79 | 7.03 ± 0.65 | 79.80–519.00 | 262.04 ± 163.50 | 73.00–475.00 | 238.33 ± 148.16 |
| Municipal water works sediment | 5.92–7.63 | 6.79 ± 0.86 | 31.60–86.00 | 62.10 ± 27.79 | 29.00–77.00 | 56.00 ± 24.56 | |
3.1. Occurrence of antibiotics and analgesics in water matrices
Of the 5 antibiotics analyzed, 4 were detected in at least one of the matrices. For analgesics, all target analytes were detected in either of the samples. The summary of the occurrence and concentration of antibiotics (liquid and solid samples), analgesics (liquid and solid samples), and the summary of the concentrations of both antibiotics and analgesics in liquid and solid samples are shown in Table 5, Table 6.
Table 5.
Summary of the occurrence of antibiotics in liquid and solid samples.
| Sample Type | Pharmaceutical | Range (mg/L) | Mean ± Sd (mg/L) | Maximal Environmental Concentration (MEC, mg/L) | Frequency of Detection % | |
|---|---|---|---|---|---|---|
| Liquid | Hospital Effluents | Doxycycline | 1.33–2.93 | 2.13 ± 1.32 | 2.93 | 66.70 |
| Ciprofloxacin | 0.59–4.74 | 2.67 ± 0.29 | 4.74 | 66.70 | ||
| Amoxicillin | 3.58–8.76 | 8.76 ± 0.00 | 8.76 | 33.30 | ||
| Chloramphenicol | 1.00–1.05 | 1.02 ± 0.39 | 1.05 | 66.70 | ||
| Dumpsite Leachates | Doxycycline | 1.78–2.22 | 2.00 ± 0.31 | 2.22 | 40.00 | |
| Ciprofloxacin | 0.25–1.91 | 0.25 ± 0.00 | 0.25 | 20.00 | ||
| Amoxicillin | ND | NA | NA | 0.00 | ||
| Chloramphenicol | 2.29–4.66 | 4.66 ± 0.00 | 4.66 | 20.00 | ||
| Solid | Municipal Water Works | Amoxicillin | 0.01–3.58 | 0.01 ± 0.00 | 0.01 | 20.00 |
ND: Not Detected; NA – Not Applicable; Metronidazole was not detected in any of the liquid samples; Doxycycline, chloramphenicol, ciprofloxacin, and metronidazole were not detected in dumpsite soil samples.
Table 6.
Summary of the occurrence of analgesics in liquid and solid samples.
| Type | Pharmaceutical | Range (mg/L) | Mean ± SD (mg/L) | Maximal Environmental Concentration (MEC, mg/L) | Frequency of Detection (%) | |
|---|---|---|---|---|---|---|
| Liquid | Drinking Water | Caffeine | 0.01–0.20 | 0.20 ± 0.00 | 0.20 | 17.00 |
| Diclofenac | 0.10–0.50 | 0.20 ± 0.20 | 0.50 | 67.00 | ||
| Ibuprofen | 0.30–0.50 | 0.40 ± 0.10 | 0.50 | 33.00 | ||
| Paracetamol | 0.07–3.20 | 3.20 ± 0.00 | 3.20 | 17.00 | ||
| Hospital Effluents | Caffeine | ND | NA | NA | 0.00 | |
| Diclofenac | 0.10–0.20 | 0.15 ± 0.07 | 0.20 | 67.00 | ||
| Ibuprofen | 0.10–0.30 | 0.20 ± 0.10 | 0.30 | 100.00 | ||
| Paracetamol | 0.07–1.11 | 1.10 ± 0.00 | 1.10 | 17.00 | ||
| Leachates | Caffeine | 0.01–0.20 | 0.20 ± 0.00 | 0.20 | 20.00 | |
| Diclofenac | 0.20–1.02 | 0.60 ± 0.40 | 1.20 | 80.00 | ||
| Ibuprofen | 0.10–0.20 | 0.20 ± 0.06 | 0.20 | 60.00 | ||
| Paracetamol | ND | NA | NA | 0.00 | ||
| Municipal Water Works | Caffeine | 0.01–0.20 | 0.20 ± 0.00 | 0.20 | 25.00 | |
| Diclofenac | 0.20–0.40 | 0.30 ± 0.10 | 0.40 | 100.00 | ||
| Ibuprofen | 0.10–1.70 | 0.80 ± 0.80 | 1.70 | 75.00 | ||
| Paracetamol | 0.07–1.10 | 1.10 ± 0.00 | 1.10 | 25.00 | ||
| Solid | Dumpsite | Caffeine | 0.60 – 2.70 | 1.95 ± 1.10 | 2.70 | 33.00 |
| Diclofenac | 0.30 – 0.90 | 0.50 ± 0.30 | 0.90 | 44.00 | ||
| Ibuprofen | 0.30 – 1.50 | 0.60 ± 1.50 | 1.50 | 55.00 | ||
| Paracetamol | 0.07 – 2.10 | 2.10 ± 0.00 | 2.10 | 11.00 | ||
| Municipal Water Works | Caffeine | 0.01 – 3.00 | 3.00 ± 0.00 | 3.00 | 50.00 | |
| Diclofenac | 0.04 – 0.60 | 0.60 ± 0.00 | 0.60 | 50.00 | ||
| Ibuprofen | 0.07 – 0.60 | 0.60 ± 0.00 | 0.60 | 50.00 | ||
| Paracetamol | ND | NA | NA | 0.00 |
ND – Not Detected; NA – Not Applicable; SD – standard deviation.
3.2. Occurrence of antibiotics in liquid samples
For antibiotics, none of the target analytes were detected in sachet drinking water and municipal waterworks samples (Table 5). The concentrations of antibiotics in leachate samples are shown in (Table 5). Apart from amoxicillin, all target antibiotics were detected in the leachate samples. Antibiotics concentrations ranged between 0.25 mg/L to 1.91 mg/L for ciprofloxacin, 1.78 mg/L to 2.22 mg/L for doxycycline and 2.29 mg/L to 4.66 mg/L for chloramphenicol. Amoxicillin was the antibiotic detected with the highest concentration at 8.76 mg/L in hospital effluents. The concentration of ciprofloxacin was 2.67 mg/L and 2.13 mg/L for doxycycline. The lowest antibiotic concentration recorded was 1.02 mg/L for chloramphenicol (Table 5).
3.3. Occurrence of analgesics in liquid samples
Although caffeine is not an analgesic, it is a common adjuvant in various analgesic formulations. Therefore, caffeine was one of the pharmaceutical residues analyzed. All analgesics were detected in both sachets drinking water and municipal waterworks samples. The analgesic detected in the highest concentration was paracetamol which was found in a sampled sachet water at a concentration of 3.20 mg/L (Table 6). In sachet drinking water, the maximal concentration of both diclofenac and ibuprofen was 0.50 mg/L. The analgesic with the highest detection frequency (67.00%) was diclofenac, with concentrations ranging from 0.10 to 0.50 mg/L. The concentration of ibuprofen in sachet water samples ranged from 0.30 to 0.50 mg/L at a detection frequency of 33.00%. Paracetamol and caffeine were the least frequently detected analytes (17.00%) in sachet water samples, with maximum concentrations of 3.20 and 0.20 mg/L, respectively. All target analgesic analytes were detected in samples from the municipal waterworks (Table 6). Diclofenac and ibuprofen were detected in concentrations ranging from 0.20 mg/L to 0.40 mg/L and, 0.10 mg/L to 1.70 mg/L respectively. The maximum concentration of caffeine was 0.2 mg/L, whereas that of paracetamol was 1.10 mg/L (Table 6). The results for the occurrence of analgesics in leachate samples are shown in Table 6. Ibuprofen, diclofenac, and caffeine were detected at maximal concentrations of 0.20 mg/L, 1.20 mg/L, and 0.20 mg/L, respectively, as shown in Table 6. In hospital effluents, ibuprofen and diclofenac were present in all samples analyzed with maximal concentrations of 0.20.
3.4. Occurrence of antibiotics and analgesics in soil matrices
Amoxicillin was the only antibiotic detected in dumpsite soil samples at a 0.01 mg/kg concentration Table 5. The maximal concentrations of caffeine in both dumpsites soil and municipal waterworks samples were 2.70 mg/kg and 3.00 mg/kg, respectively. Diclofenac was detected with a maximal concentration of 0.90 mg/kg in dumpsite soil and 0.60 mg/kg in municipal waterworks sediment. The concentration of ibuprofen was 1.50 mg/kg in dumpsite soil and 0.60 mg/kg in municipal waterworks soil. The paracetamol concentration was 2.10 mg/kg in dumpsite soil, as shown in Table 6.
3.5. Correlation between pharmaceuticals and physicochemical parameters
Table 7 shows a correlational analysis between basic physicochemical parameters and concentrations of antibiotics and analgesics detected. TDS and EC showed a strong positive correlation (1.00). There was a positive correlation of 0.980 between ciprofloxacin and amoxicillin, doxycycline and chloramphenicol 0.638, chloramphenicol and caffeine 0.492, diclofenac and paracetamol 0.704, diclofenac and caffeine 0.497, caffeine and paracetamol 0.655, and caffeine and ibuprofen 0.608. There was a positive correlation between caffeine and all other analgesics monitored.
Table 7.
Pearson’s correlation coefficients for antibiotic and analgesic concentrations and basic physicochemical parameters.
| pH | EC | TDS | DOX | AMC | CIP | CHL | DIC | PARA | CAFFEINE | IBUPROFEN | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||
| EC | 0.396 | 1 | |||||||||
| TDS | 0.397 | 1.000** | 1 | ||||||||
| DOX | -0.312 | 0.325 | 0.320 | 1 | |||||||
| AMC | 0.128 | -0.008 | 0-0.010 | 0.150 | 1 | ||||||
| CIP | 0.157 | 0.115 | 0.112 | 0.286 | 0.980** | 1 | |||||
| CHL | -0.390 | -0.022 | -0.025 | 0.638** | 0.128 | 0.152 | 1 | ||||
| DIC | -0.258 | -0.312 | -0.311 | 0.028 | 0-0.172 | 0-0.204 | -0.044 | 1 | |||
| PARA | 0.189 | -0.120 | -0.119 | -0.108 | 0.220 | 0.200 | -0.065 | 0.704** | 1 | ||
| CAFFEINE | -0.119 | -0.171 | -0.168 | 0.147 | -0.108 | -0.129 | 0.492* | 0.497* | 0.655** | 1 | |
| IBUPROFEN | 0.121 | -0.044 | -0.039 | -0.144 | -0.134 | -0.160 | -0.007 | 0.106 | 0.294 | 0.608** | 1 |
* *. Correlation is significant at the 0.01 level (2-tailed). DOX – Doxycycline CHL – Chloramphenicol
CIP – Ciprofloxacin PARA – Paracetamol
Values highlighted in red shows a strong positive correlation between each other
3.6. Risk assessment
The risk quotient (RQ) is used to predict the effect of pharmaceuticals on the lives of some organisms. In this study, ecological risk of the studied antibiotics and analgesic were calculated as the ratio between the pharmaceutical’s maximal environmental concentration, MEC (as obtained from the study) and its predicted no-effect concentration, PNEC (obtained from literature) to assess the possible danger caused by each studied pharmaceutical to environmental species. The risk quotient was estimated on algae (Desmodesmus subspicatus), daphnid (Daphnia magna), crustaceans, and fish (Oncorhynchus mykiss). The lowest ecological PNEC values available in the literature or estimated from the ecological structure activity relationships (ECOSAR) model were assumed for this risk analysis [24].
The results from the ecological risk assessment are presented in Table 8, Table 9 for antibiotics and analgesics, respectively. RQs for each antibiotic is shown in Table 8. The RQ for ciprofloxacin was 777.0492 and 40.9836 for exposure to algae in hospital effluents and leachates, respectively, indicating a high toxicity risk. Doxycycline and amoxicillin also posed high risks to fishes with RQ values of 3.5040 and 1.1720, respectively. The RQs for the antibiotics studied ranged from 0.0002 to 0.4788 and 0.0001–1.172 for daphnia.
Table 8.
Risk assessment of antibiotics in water and soil samples.
| Type | Pharmaceutical | MEC (µg/L) |
Specie | PNEC* (µg/L) |
HQ | Remark | |
|---|---|---|---|---|---|---|---|
| Liquid | Hospital Effluents | Doxycycline | 2.93 × 103 | Algae | 9.80 × 105 | 2.90 × 10−3 | Low |
| 2.93 × 103 | Daphnid | 1.10 × 106 | 2.60 × 10−3 | Low | |||
| 2.93 × 103 | Fishes | 2.50 × 103 | 1.17 | High | |||
| Ciprofloxacin | 4.74 × 103 | Algae | 6.10 | 777.05 | High | ||
| 4.74 × 103 | Daphnid | 9.90 × 103 | 0.48 | Medium | |||
| 4.74 × 103 | Fishes | 2.50 × 106 | 1.90 × 10−3 | Low | |||
| Amoxicillin | 8.76 × 103 | Algae | 1.00 × 104 | 0.88 | Medium | ||
| 8.76 × 103 | Daphnid | 1.10 × 104 | 0.80 | Medium | |||
| 8.76 × 103 | Fishes | 2.50 × 103 | 3.50 | High | |||
| Leachates | Doxycycline | 2.22 × 103 | Algae | 9.80 × 105 | 2.30 × 10−3 | Low | |
| 2.22 × 103 | Daphnid | 1.10 × 106 | 2.00 × 10−3 | Low | |||
| 2.22 × 103 | Fishes | 2.50 × 103 | 0.89 | Medium | |||
| Ciprofloxacin | 2.50 × 102 | Algae | 6.10 | 40.98 | High | ||
| 2.50 × 102 | Daphnid | 9.90 × 103 | 0.03 | Medium | |||
| 2.50 × 102 | Fishes | 2.50 × 106 | 1.00 × 10−4 | Low | |||
| Soil | Municipal Water Works | Amoxicillin | 10.00 | Algae | 1.00 × 104 | 1.00 × 10−3 | Low |
HQ – hazard quotient; MEC -measured environmental concentration; PNEC – predicted no-effect concentration.*PNEC values were adapted from [24]
Table 9.
Risk Assessment for analgesics in liquid and soil samples.
| Type | Pharmaceutical | MEC (mg/L) |
Specie | PNEC (mg/L) | RQ | Remark | |
|---|---|---|---|---|---|---|---|
| Liquid | Drinking Water | Diclofenac | 0.50 | Crustacean | 30.70 | 0.02 | Medium |
| Ibuprofen | 0.50 | Fish | 0.01 | 50.00 | High | ||
| Paracetamol | 3.20 | Algae | 134.00 | 0.02 | Medium | ||
| Hospital Effluents | Diclofenac | 0.20 | Crustacean | 30.70 | 0.01 | Low | |
| Ibuprofen | 0.30 | Fish | 0.01 | 30.00 | High | ||
| Paracetamol | 1.10 | Fish | 9.20 | 0.12 | Medium | ||
| Leachates | Diclofenac | 1.20 | Fish | 82.00 | 0.02 | Medium | |
| Ibuprofen | 0.20 | Fish | 0.01 | 20.00 | High | ||
| Municipal Water Works | Diclofenac | 0.40 | Crustacean | 30.70 | 0.01 | Medium | |
| Soil | Dumpsite | Diclofenac | 0.90 | Crustacean | 30.70 | 0.03 | Medium |
HQ – hazard quotient; MEC -measured environmental concentration; PNEC – predicted no-effect concentration.
and fishes, respectively. The RQ values measured for analgesics in this study were within medium to high-risk ranges, as depicted in Table 9. The maximum RQ was observed at 170 for the ecological risk of ibuprofen on crustaceans. Most of the RQs were greater than or equal to 0.10 indicating medium risk, and RQs greater than or equal to one indicated high risk. Ibuprofen posed a high risk to fishes in all samples studied.
4. Discussion
pH is one of the most significant water and soil quality parameters. pH values reported from this study were all slightly acidic to slightly basic. Under acidic conditions, analgesic and antibiotics residues can exist as cations, anions, and/or zwitterions, and this can affect their adsorption on soil surfaces as adsorption is pH-dependent [31]. Pharmaceuticals, in their various ionic states, can also interact with surface organic matter. As a result, antibiotics and analgesic residues would remain in the top layer of the soil, making them more susceptible to erosion during downpours [31], [32]. The pH values recorded in this study are also optimum for plant growth and uptake of nutrients and suggest that plant roots may absorb some of these pharmaceuticals and store them in plant tissues [33]. The contaminants could then re-enter the food chain cycle. High conductivity values infer the presence of soluble salts in the soil. Ions that coexist significantly affect the adsorption of antibiotics and analgesics. Ionic strength (IS) can increase or prevent the adsorption of antibiotics and analgesics by inorganic minerals. Monovalent metallic ions such as Na+ and K+ can compete for adsorption sites with cationic/ zero-valence antibiotics (such as sulfathiazole) and analgesics (such iron/persulfate (Fe/Ps)) which extremely impact the adsorption of these pharmaceuticals. Multivalence metal ions like Ca2+, Mg2+, Cu2+, Al3+, and Fe3+ can also influence the adsorption behaviors of antibiotics. These ions can compete with cationic/zero-valence antibiotics for adsorption sites with low pH. Thus they impede adsorption [31]. As the concentration of metal ions (Ca2+, Mg2+) in solution increases, there will be competitive sorption between the antibiotics and metal cations, resulting in a decrease of antibiotic sorption [34]. Competition between ions and PPCPs for sorption sites shows that with the increase of IS, cations (e.g., Ca2+) would be electrostatically bonded to the surface of sediments, and inorganic exchangeable cations will replace the hydrogen ions of acidic groups, thereby reducing the number of initial sorption sites [35]. From the study, high EC values were recorded, which means more soluble salts were in the solution. This could result in low levels of antibiotics and analgesics being detected in the various matrices. TDS measured for dumpsites and hospital effluents were very high, with readings up to 1323.00 and 1121.00 mg/L, respectively. This means that these water samples had vast organic, inorganic, or ionic contaminants in them. Relatively low TDS values were recorded for the water samples from the Sunyani municipal waterworks station and sachet water samples, indicating the absence of a lot of dissolved substances.
Numerous therapeutic groups are found in hospital effluents, including antibiotics [36], [37]. The mean concentrations of antibiotics in hospital effluents are presented in Table 5. The concentrations of ciprofloxacin ranged from 0.59 mg/L to 4.74 mg/L in this present study which was higher than the concentrations reported by Azanu and coworkers in hospital effluents in Kumasi (0.01 mg/L to 0.02 mg/L) [24]. The high levels of antibiotics detected in hospital effluents could result from prescription patterns and consumption of antibiotics in the various hospitals sampled. It has been reported that antibiotic prescription in the Sunyani Municipality is one of the highest in Ghana [38]. The high levels of ciprofloxacin reported could be due to the national recommendation of ciprofloxacin for treating urinary tract infection in Ghana, thus, its high rate of usage in hospitals [39]. The high levels of antibiotics could also reflect the disposal patterns of some pharmaceuticals. Most patients excrete antibiotics in their unmetabolized form in urine and feces, and remnants of these wastes could get into hospital effluents [40].The maximal concentration of ciprofloxacin reported in hospital effluents is over 47,000 times higher than the 0. 0001 mg/L reported aiding the development of antimicrobial resistance [41]. The presence of antibiotics in hospital effluents in this study, and those reported elsewhere, could affect the development of resistance in the microbial communities and show that concerns of antimicrobial resistance are a local problem and a worldwide problem. The presence of antibiotics in hospital effluents could also have deleterious effects on humans because runoffs from hospital effluents can enter surface water during a downpour, and these surface waters are used for domestic purposes in many developing countries like Ghana [24], [42]. The consumption of food or water polluted by runoffs or leakages from some of these hospital effluents investigated could expose people to sub-MIC concentrations, leading to antibiotic resistance development [24].
The maximum concentrations of analgesics in hospital effluents in this study were 0.30 mg/L, 0.20 mg/L, and 1.10 mg/L for ibuprofen, diclofenac, and paracetamol, respectively. Thomas et al. (2007) reported concentrations of paracetamol in the effluents of hospitals from Oslo (Norway) as 43.00 µg/L, while ibuprofen and diclofenac were 0.90 µg/L and 0.80 µg/L respectively [43]. Another study by Santos et al. (2013) reported acetaminophen and ibuprofen as part of the analgesics/ anti-inflammatories pharmaceuticals detected in high concentrations in all hospital effluents up to 0.06 mg/L and 0.04 mg/L, respectively [Santos reference]. Analgesics are routinely used in combination with other classes of pharmaceuticals, which could be a reason for their ubiquity in the sampled hospital effluents. Just as in the case of antibiotics, the presence of these analgesics could also reflect their disposal pattern.
The high concentrations of antibiotics recorded in leachate samples could have serious consequences on the environment. Bengtsson-Palme and Larsson (2016) suggested that environmental concentrations beyond 64.00 µg/L (0.064 mg/L) posed a significant risk for resistance selection for most antibiotics [44]. The concentrations of antibiotics detected in this work are way higher than the limit concentration for resistance selection and could tend to cause damage to the environment. The high concentrations of chloramphenicol (4.66 mg/L), ciprofloxacin (0.25 mg/L), and doxycycline (2.22 mg/L) in the leachate samples analyzed in this study could result in a selective pressure which might aid the development of resistant genes among microbes. Of the four analgesics studied, three of them were detected in the leachate samples at maximum concentrations of 0.20, 1.20, and 0.20 mg/L for ibuprofen, diclofenac, and caffeine, respectively. Another study conducted on treated leachate in a landfill in Shanghai, China, recorded 13.40 µg/L and 77.80 µg/L for caffeine and diclofenac, respectively [45]. In comparison, results from this study are higher than those from other studies. Many landfills are constructed to limit runoffs into various surface water bodies. A major concern is the absence of engineering liners and collection systems for most landfills in Ghana and other developing countries. The pollution by landfill leachates and their impact on surface and groundwater could thus be severe. This raises a concern and poses a potential risk to the environment and aquatic organisms.
Studies have reported that antibiotic and analgesic residues can be found in drinking water as a result of runoffs from industrial sewage, hospitals effluents, and leachates which end up in water bodies at concentrations ranging from ng to µg/L [4]. In this study, none of the antibiotics of interest was detected in the sachet water and municipal waterworks samples from the Sunyani municipality. All the analgesics analyzed were detected in the water matrices (sachet water samples and samples from the Sunyani municipal waterworks station) with a maximum concentration of 3.20 mg/L for paracetamol and diclofenac having the highest frequency of detection of 67.00%. Diclofenac concentrations of 0.20 µg/L and 0.50 µg/L have been reported in South Korea and Spain rivers, respectively [46]. Diclofenac concentration in the river water of Karachi in Pakistan was reported to be 0.40 µg/L [47]. The reports from other works compared to this study show the prevalence of diclofenac in the environment, and the Sunyani municipality is no exception. However, these analgesics in the Sunyani Municipality in Ghana are in alarming concentrations, capable of posing health and ecological risks.
From Table 7, there was a very strong significant correlation between TDS and EC at a 99% confidence interval. This means more dissolved solids were present, which contributed to the high conductivity. Again, there is a strong correlation between the pain killers, paracetamol, diclofenac, and caffeine, which suggests these pain killers are administered together. Caffeine is correlated with other analgesics because it has been added to a large number of analgesics for years which showed an enhanced analgesic effect when combined [48]. There was a strong correlation between chloramphenicol and doxycycline (0.638). Also, amoxicillin and ciprofloxacin showed a very strong correlation (0.980). The correlations between the antibiotics could be because they may be complementary drugs or provide an improved therapy when used together.
Maximal environmental concentrations (MEC) of antibiotics in hospital effluents and leachates collected from the Sunyani municipality were used to calculate the risk quotient (RQ). PNEC values and RQs for each antibiotic are shown in Table 8. The RQ for ciprofloxacin was 777.05 and 40.98 for exposure to algae in hospital effluents and leachates, respectively, indicating a high toxicity risk. Doxycycline and amoxicillin also posed a high risk to fishes with RQ values of 3.50 and 1.17, respectively. The RQs for the antibiotics studied ranged from 2.00 × 10−4 to 0.48 and 1.00 × 10−4 to 1.17 for daphnia and fishes, respectively. Largely, the antibiotics posed medium to no risk to daphnia and fishes, indicating a low risk of toxicity. Comparatively, a study that focused on the occurrence of pharmaceuticals in water from Pego-Oliva Marsh, Spain, computed RQ for ciprofloxacin to be 6.90 for algae [49]. In the Azanu study in Kumasi, Ghana, the RQ for ciprofloxacin was found to be 0.13, which could pose a medium risk to algae in the aquatic environment [24]. This indicates that the presence of ciprofloxacin at high concentrations in the environment is of great concern. This is because it poses a risk to organisms in the aquatic environment, especially to algae which are vital in the ecosystem and may lead to the destruction of the food chain. Ciprofloxacin inhibits or kills algae's cell growth, which may lead to their death and hence affect the ecosystem [50].
Risks towards some of the targeted aquatic organisms (algae, fishes, and crustaceans) for analgesics were also assessed in both water and soil matrices within the Sunyani Municipality. Averagely, the RQ values measured in the study area were within medium to high-risk ranges as represented in Table 9, with a maximum RQ of 170.00. Most of the RQs were greater than or equal to 0.10 indicating medium risk, and RQs greater than or equal to one indicating a high risk. Ibuprofen poses a high risk to fishes with an RQ > 1, which can impact fish reproduction by male fish feminization. Male fish feminization can reduce fish population and, hence, an economic impact. Studies have shown that RQ > 1 for diclofenac can lead to endocrine disruption by acting on the prostaglandin pathway in rodents and human cells due to hindrances in prostaglandin synthesis [51].
5. Conclusion
The occurrence of five antibiotics and four analgesics in the Sunyani municipality was analyzed in both soil and water samples. The high concentrations of antibiotics and analgesics found in almost all samples analyzed indicated considerable pollution of these samples in the Sunyani municipality, Ghana, with antibiotics and analgesics. Ciprofloxacin, doxycycline, diclofenac, and ibuprofen were the most frequently detected antibiotics in all samples. Hence, they could be considered part of the possible dangerous compounds from an environmental risk point of view in Ghana. It was observed that all analgesics could be found in both soil and water matrices in varying concentrations. Risk quotients computed for both antibiotics and analgesics in the various environmental compartments for algae, crustaceans, and fish were in most instances low and medium risk, with few cases of high risk. Based on the risk assessment calculated, ciprofloxacin posed a high risk to aquatic organisms. The antibiotic concentrations found in hospital effluents and leachates samples in this study pose a high risk to antibiotic resistance development in the environment. The high concentrations of antibiotics and analgesics recorded in this study implies that the presence of antibiotics and analgesics in the Sunyani municipality can pose a great ecological risk to organisms and possibly other members of their ecosystem with time. The high concentrations of pharmaceuticals in the environment can be reduced by adopting proper disposal methods for pharmaceuticals. Regular monitoring of pharmaceutical residues and implementation of remediation protocols are recommended.
Funding Statement
This work was supported by a Kwame Nkrumah University of Science and Technology (KNUST) KNUST Research Fund (2019) grant awarded to Lawrence Sheringham Borquaye and Godfred Darko.
CRediT authorship contribution statement
Bernice Araba Otoo: Methodology, Investigation, Data curation, Writing − original draft preparation, Writing − review & editing. Ivy Anima Amoabeng: Methodology, Investigation, Data curation, Writing − original draft preparation. Godfred Darko: Data curation, Writing − review & editing, Formal analysis, Funding acquisition, Supervision. Lawrence Sheringham Borquaye: Conceptualization, Data curation, Writing − review & editing, Formal analysis, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Department of Chemistry and the KNUST Central Laboratory are acknowledged for using their facilities for this study. The authors are grateful to Mr. Daniel Nimako Ampratwum of the Central Laboratory, KNUST, and Mr. Joseph Nana Gyesi of the Department of Chemistry, KNUST, for technical support.
Availability of data and materials
All data obtained or analysed during this study are included in this published article.
Contributor Information
Bernice Araba Otoo, Email: bernicearaba45@gmail.com.
Ivy Anima Amoabeng, Email: ivy.amoabeng22@gmail.com.
Godfred Darko, Email: gdarko.sci@knust.edu.gh.
Lawrence Sheringham Borquaye, Email: lsborquaye.sci@knust.edu.gh, slborquaye@yahoo.com.
References
- 1.Soran M.L., Lung I., Opriş O., Floare-Avram V., Coman C. Determination of antibiotics in surface water by solid-phase extraction and high-performance liquid chromatography with diode array and mass spectrometry detection. Anal. Lett. 2017;50(7):1209–1218. [Google Scholar]
- 2.Donkor E.S., Tetteh-Quarcoo P.B., Nartey P., Agyeman I.O. Self-medication practices with antibiotics among tertiary level students in Accra, Ghana: a cross-sectional study. Int J Environ Res Public Health. 2012;9(10):3519–3529. doi: 10.3390/ijerph9103519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerbech A.M., Opintan J.A., Bekoe S.O., Ahiabu M.A., Tersbøl B.P., Hansen M., et al. Antibiotic exposure in a low-income country: screening urine samples for presence of antibiotics and antibiotic resistance in coagulase negative staphylococcal contaminants. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milmo S. Pharmaceuticals in the environment. Pharm. Technol. 2018;42(8) [Google Scholar]
- 5.Kümmerer K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J. Environ. Manag. 2009;90(8):2354–2366. doi: 10.1016/j.jenvman.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Singh G. Gastrointestinal complications of prescription and over-the-counter nonsteroidal anti-inflammatory drugs: a view from the ARAMIS database. Arthritis, Rheumatism, and Aging Medical Information System. Am. J. Ther. 2000;7(2):115–121. doi: 10.1097/00045391-200007020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120(3):594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 8.Polianciuc S.I., Gurzău A.E., Kiss B., Ştefan M.G., Loghin F. Antibiotics in the environment: causes and consequences. Med Pharm. Rep. 2020;93(3):231. doi: 10.15386/mpr-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueno M.M., Gomez M.J., Herrera S., Hernando M.D., Agüera A., Fernández-Alba A.R. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: two years pilot survey monitoring. Environ. Pollut. 2012;164:267–273. doi: 10.1016/j.envpol.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Molnar E., Maasz G., Pirger Z. Environmental risk assessment of pharmaceuticals at a seasonal holiday destination in the largest freshwater shallow lake in Central Europe. Environ. Sci. Pollut. Res. 2021;28(42):59233–59243. doi: 10.1007/s11356-020-09747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puckowski A., Mioduszewska K., Lukaszewicz P., Borecka M., Caban M., Maszkowska J., et al. Bioaccumulation and analytics of pharmaceutical residues in the environment: A review. J. Pharm. Biomed. Anal. 2016;127:232–255. doi: 10.1016/j.jpba.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Kümmerer K. Antibiotics in the aquatic environment–A review–Part II. Chemosphere. 2009;75(4):435–441. doi: 10.1016/j.chemosphere.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Green R.E., Taggart M.A., Senacha K.R., Raghavan B., Pain D.J., Jhala Y., et al. Rate of decline of the oriental white-backed vulture population in India estimated from a survey of diclofenac residues in carcasses of ungulates. PLoS One. 2007;2(8) doi: 10.1371/journal.pone.0000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borquaye L.S., Ekuadzi E., Darko G., Ahor H.S., Nsiah S.T., Lartey J.A., et al. Occurrence of antibiotics and antibiotic-resistant bacteria in landfill sites in Kumasi. Ghana. J. Chem. 2019;2019 [Google Scholar]
- 15.Santos L.H., Gros M., Rodriguez-Mozaz S., Delerue-Matos C., Pena A., Barceló D., et al. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013;461:302–316. doi: 10.1016/j.scitotenv.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 16.Gyesi J.N., Nyaaba B.A., Darko G., Mills-Robertson F.C., Miezah K., Acheampong N.A., et al. Occurrence of Pharmaceutical Residues and Antibiotic-Resistant Bacteria in Water and Sediments from Major Reservoirs (Owabi and Barekese Dams) in Ghana. J. Chem. 2022 [Google Scholar]
- 17.Ebele A.J., Abou-Elwafa Abdallah M., Harrad S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017;3(1):1–16. [Google Scholar]
- 18.Mahmood A.R., Al-Haideri H.H., Hassan F.M. Detection of antibiotics in drinking water treatment plants in Baghdad City, Iraq. Adv. Public Health. 2019:2019. [Google Scholar]
- 19.Borquaye L.S., Ekuadzi E., Esseku Y., Ahor H.S., Nsiah S.T., Lartey J.A., et al. Disposal of unused and expired medicines in Ghana. West Afr. J. Pharm. 2018;29(1):83–91. [Google Scholar]
- 20.Bashaar M., Thawani V., Hassali M.A., Saleem F. Disposal practices of unused and expired pharmaceuticals among general public in Kabul. BMC Public Health. 2017;17(1):1–8. doi: 10.1186/s12889-016-3975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong A.Y., Peake B.M., Braund R. Disposal practices for unused medications around the world. Environ. Int. 2011;37(1):292–298. doi: 10.1016/j.envint.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Amoabeng I.A., Otoo B.A., Darko G., Borquaye L.S. Disposal of Unused and Expired Medicines within the Sunyani Municipality of Ghana: A Cross-Sectional Survey. J. Environ. Public Health. 2022 26;2022 doi: 10.1155/2022/6113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bediako-Bowan A.A., Owusu E., Labi A.K., Obeng-Nkrumah N., Sunkwa-Mills G., Bjerrum S., et al. Antibiotic use in surgical units of selected hospitals in Ghana: a multi-centre point prevalence survey. BMC Public Health. 2019;19(1):1–10. doi: 10.1186/s12889-019-7162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azanu D., Styrishave B., Darko G., Weisser J.J., Abaidoo R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018;622:293–305. doi: 10.1016/j.scitotenv.2017.11.287. [DOI] [PubMed] [Google Scholar]
- 25.Darko G., Borquaye L.S., Acheampong A., Oppong K. Veterinary antibiotics in dairy products from Kumasi, Ghana. Cogent Chem. 2017;3(1):1343636. [Google Scholar]
- 26.Mingle C.L., Darko G., Borquaye L.S., Asare-Donkor N.K., Woode E., Koranteng F. Veterinary Drug Residues in Beef, Chicken, and Egg from Ghana. Chem. Afr. 2021;4(2):339–348. [Google Scholar]
- 27.Poku M.K., Seidu O., Fayorsey C.K. 2010 Population and Housing Census, Regional Analytical Report - Brong Ahafo. Ghana: Ghana Statistical Service; 2013 p. 152.
- 28.Akanchise T., Boakye S., Borquaye L.S., Dodd M., Darko G. Distribution of heavy metals in soils from abandoned dump sites in Kumasi. Ghana. Sci. Afr. 2020;10 [Google Scholar]
- 29.Konwuruk N., Borquaye L.S., Darko G. Dodd M. Distribution, bioaccessibility and human health risks of toxic metals in peri-urban topsoils of the Kumasi Metropolis. Sci. Afr. 2021;11 [Google Scholar]
- 30.Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency. Environmental risk assessment of medicinal products for human use [Internet]. European Medicines Agency; 2006 [cited 2022 Jan 10] p. 1–12. Report No.: EMEA/CHMP/SWP/4447/00. Available from: https://www.ema.europa.eu/en/environmental-risk-assessment-medicinal-products-human-use.
- 31.Wang S., Wang H. Adsorption behavior of antibiotic in soil environment: a critical review. Front Environ. Sci. Eng. 2015;9(4):565–574. [Google Scholar]
- 32.Atugoda T., Vithanage M., Wijesekara H., Bolan N., Sarmah A.K., Bank M.S., et al. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021;149 doi: 10.1016/j.envint.2020.106367. [DOI] [PubMed] [Google Scholar]
- 33.Gentili R., Ambrosini R., Montagnani C., Caronni S., Citterio S. Effect of soil pH on the growth, reproductive investment and pollen allergenicity of Ambrosia artemisiifolia L. Front Plant Sci. 2018;9:1335. doi: 10.3389/fpls.2018.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S., Zhang Y., Shen G., Zhang H., Yuan Z., Zhang W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil Tillage Res. 2019;186:233–241. [Google Scholar]
- 35.Pavlović D.M., Ćurković L., Grčić I., Šimić I. Župan J. Isotherm, kinetic, and thermodynamic study of ciprofloxacin sorption on sediments. Environ. Sci. Pollut. Res. 2017;24(11):10091–10106. doi: 10.1007/s11356-017-8461-3. [DOI] [PubMed] [Google Scholar]
- 36.Verlicchi P., Al Aukidy M., Galletti A., Petrovic M., Barceló D. Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012;430:109–118. doi: 10.1016/j.scitotenv.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 37.Khan N.A., Ahmed S., Farooqi I.H., Ali I., Vambol V., Changani F., et al. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: a critical review. TrAC Trends Anal. Chem. 2020;129 [Google Scholar]
- 38.Opoku M.M. Assessment of Antibiotics Prescribing Practices at the Sunyani Municipal Hospital In 2015 [Masters Thesis]. [Legon, Ghana]: University of Ghana; 2017.
- 39.Kamberi M., Tsutsumi K., Kotegawa T., Kawano K., Nakamura K., Niki Y., et al. Influences of urinary pH on ciprofloxacin pharmacokinetics in humans and antimicrobial activity in vitro versus those of sparfloxacin. Antimicrob. Agents Chemother. 1999;43(3):525–529. doi: 10.1128/aac.43.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finley R.L., Collignon P., Larsson D.J., McEwen S.A., Li X.Z., Gaze W.H., et al. The scourge of antibiotic resistance: the important role of the environment. Clin. Infect. Dis. 2013;57(5):704–710. doi: 10.1093/cid/cit355. [DOI] [PubMed] [Google Scholar]
- 41.Gullberg E., Cao S., Berg O.G., Ilbäck C., Sandegren L., Hughes D., et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7(7) doi: 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obiri-Danso K., Weobong C.A.A., Jones K. Aspects of health-related microbiology of the Subin, an urban river in Kumasi, Ghana. J. Water Health. 2005;3(1):69–76. [PubMed] [Google Scholar]
- 43.Thomas K.V., Dye C., Schlabach M., Langford K.H. Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J. Environ. Monit. 2007;9(12):1410–1418. doi: 10.1039/b709745j. [DOI] [PubMed] [Google Scholar]
- 44.Bengtsson-Palme J., Larsson D.J. Concentrations of antibiotics predicted to select for resistant bacteria: proposed limits for environmental regulation. Environ. Int. 2016;86:140–149. doi: 10.1016/j.envint.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Yu X., Sui Q., Lyu S., Zhao W., Liu J., Cai Z., et al. Municipal solid waste landfills: An underestimated source of pharmaceutical and personal care products in the water environment. Environ. Sci. Technol. 2020;54(16):9757–9768. doi: 10.1021/acs.est.0c00565. [DOI] [PubMed] [Google Scholar]
- 46.Gros M., Petrović M., Ginebreda A., Barceló D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010;36(1):15–26. doi: 10.1016/j.envint.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 47.López-Serna R., Petrović M., Barceló D. Development of a fast instrumental method for the analysis of pharmaceuticals in environmental and wastewaters based on ultra high performance liquid chromatography (UHPLC)–tandem mass spectrometry (MS/MS) Chemosphere. 2011;85(8):1390–1399. doi: 10.1016/j.chemosphere.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 48.Derry C.J., Derry S., Moore R.A. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst. Rev. 2014;(12)) doi: 10.1002/14651858.CD009281.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-Roig P., Andreu V., Blasco C., Picó Y. Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego-Oliva Marshlands (Valencia, eastern Spain) Sci. Total Environ. 2012;440:24–32. doi: 10.1016/j.scitotenv.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 50.Wilson B.A., Smith V.H., deNoyelles F., Larive C.K. Effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. Environ. Sci. Technol. 2003;37(9):1713–1719. doi: 10.1021/es0259741. [DOI] [PubMed] [Google Scholar]
- 51.Peake B.M., Braund R., Tong A., Tremblay L. The Life-cycle of Pharmaceuticals in the Environment. 1st ed. Elsevier; 2015. 109–152 p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data obtained or analysed during this study are included in this published article.