Abstract
Background:
As overdoses due to opioids rises, medications for opioid use disorder (MOUD) are underemployed, resulting in the absence of potentially life-saving treatment. Substance use disorders are prevalent in individuals who are incarcerated and are at increased risk for death post-release due to overdose. Few jails and prisons offer MOUD and most limit access. Extended-release buprenorphine (XR-BUP), a novel monthly injectable MOUD formulation, could be uniquely poised to address the circumstances of correctional settings.
Methods:
A retrospective cohort design of statewide datasets were linked to evaluate the real-world use of XR-BUP. Individuals (N=54) who received XR-BUP while incarcerated from January 2019 through February 2022 were included. The study was conducted at the Rhode Island Department of Corrections, the nation’s first comprehensive statewide correctional MOUD program.
Results:
Fifty-four individuals received a combined total of 162 injections during the study period. There was no evidence of tampering with the injection site indicating no attempts to remove, hoard, or divert the medication. Sixty-one percent reported at least one adverse effect after injections were received with an average of 2.8 side effects. Seventy percent of those released on XR-BUP engaged in MOUD after release, 30% continued with XR-BUP.
Conclusions:
XR-BUP is feasible and acceptable in correctional settings. XR-BUP addresses administrative concerns of diversion that obstruct lifesaving MOUD and offer another safe and effective treatment option. Further studies and trials should continue to assess the opportunity for this novel treatment to treat opioid addiction in the correctional setting and upon release to the community.
Keywords: XR-Buprenorphine, Medications for Opioid Use Disorder, Access-to-care, Correctional Facilities
1. Introduction
Individuals with opioid use disorder often continue to use despite the negative health and social consequences, including opioid use that leads to criminal justice involvement (Ingrid A. Binswanger, 2013; Degenhardt et al., 2011b; Strang et al., 2020). More than half of incarcerated individuals have a substance use disorder and approximately 23% have OUD (Brinkley-Rubinstein et al., 2018). Individuals on medication for opioid use disorder (MOUD), often undergo forced withdrawal upon incarceration, a practice shown to decrease treatment retention and increase mortality (Rich et al., 2015). Moreover, stopping or dramatically reducing opioid use, which typically occurs when people with OUD are incarcerated, causes people to lose their opioid tolerance, even during relatively short incarcerations of days or weeks. The reduction in tolerance to opioids causes them more susceptible to overdose upon release. Indeed, risk of overdose spikes within the two-week period post-release, making the expedited transition to MOUD and supported services in the community crucial (Ingrid A Binswanger, Blatchford, Mueller, & Stern, 2013). In one state, these high-risk recently-released patients make up 50% of overall opioid deaths (Saloner et al., 2020).
MOUD is the most effective and evidence-based approach for treating OUD (Connery, 2015; National Academies of Sciences & Medicine, 2019; Volkow & Blanco, 2021), with resultant benefits to correctional populations that include: reductions in post-incarceration illicit opioid use (Mattick, Breen, Kimber, Davoli, & Breen, 2003), criminal behavior (Deck et al., 2009), mortality (Green et al., 2018), overdose risk (Degenhardt et al., 2011a), HIV and HCV risk behaviors (Macarthur et al., 2012); and an increase in treatment engagement (Crits-Christoph, Lundy, Stringer, Gallop, & Gastfriend, 2015; Scott, Dennis, Grella, Mischel, & Carnevale, 2021). Despite high rates of OUDs and strong evidence demonstrating the effectiveness of MOUD in correctional settings, fewer than 12% of US correctional facilities provide MOUD (NIDA, 2020; Project, 2020) and only 5% of people with OUD receive MOUD (National Academies of Sciences & Medicine, 2019).
Among the correctional facilities that offer MOUD, the majority restrict treatment to persons who are pregnant or those who were engaged in treatment prior to incarceration on a taper protocol (Friedmann et al., 2012). In some facilities, the only medication available is injectable extended-release (XR) naltrexone (opioid antagonist), shown to have lesser treatment retention than opioid agonist therapy (OAT) such as buprenorphine (Lee et al., 2018).
However, XR formulations do offer specific benefits to correctional settings. First, the injectable route of administration reduces the risk of diversion for opioid agonist medications, which is a common security concern specific to the correctional facility setting. Oral formulations of controlled substances like buprenorphine and methadone require special procedures to prevent diversion of medication for selling, hoarding, or trading. A recent study reported that incarcerated populations were interested in the practicability and discretion features of XR-BUP (Chappuy et al., 2021), which may then promote treatment engagement. Second, XR formulations reduce the logistical imperatives of once-a-day medications that can disrupt the tight structure and scheduling in a correctional environment and burden medical and security staff by creating long medication lines. XR-buprenorphine (XR-BUP, Sublocade™, Indivior) is the most recently FDA-approved pharmacotherapy for OUD (March 2018) and is a subcutaneous formulation, administered by monthly injection, and delivers sustained therapeutic buprenorphine plasma levels ≥2 ng/mL for most individuals. Studies demonstrate high patient satisfaction with XR-B (Lintzeris et al., 2021), with comparable efficacy of XR-B and sublingual buprenorphine (SL-BUP) in the community (Haight et al., 2019; Lofwall et al., 2018) even among treatment resistant populations (Cotton, Lo, Kurtz, & Waldbauer, 2021). Preliminary data demonstrates similar success in a jail setting (Lee et al., 2021). In addition, predictive modeling techniques have demonstrated that the introduction of XR-BUP in a correctional setting reduces medical and security staff time by 27% and reduces indirect health care costs and security/criminal justice costs by approximately 20%, which potentially offsets any increase in direct cost (Wright et al., 2020).
In 2016, the Rhode Island Department of Corrections (RIDOC) began the first statewide correctional system comprehensive MOUD program in the country. Key components of this program include the following: (1) initiate a comprehensive program to screen for OUD, (2) offer all three FDA approved medications for treating OUD, and (3) initiate or provide continuation of extended, ongoing treatment with MOUD during incarceration, in addition, to facilitate post-release linkages to substance use treatment after release (Clarke, Martin, Gresko, & Rich, 2018; Martin, Alexander-Scott, Wendelken, & Clarke, 2019). As a result of the RIDOC comprehensive program, MOUD therapy has been expanded with linkage to treatment in the community and is associated with a significant drop in statewide overdose deaths post-release (Green et al., 2018). Beginning in January 2019, RIDOC made XR-BUP available to individuals, initially on a limited basis, including for cases of hoarding SL-BUP or methadone. During the Covid-19 pandemic, RIDOC provided all individuals the opportunity to switch to XR-BUP from other forms of MOUD.
This paper provides key descriptive information on the use of XR- BUP and details the reasons for use and discontinuance of XR-BUP in the correctional setting, reported side-effects, and post-release MOUD treatment engagement in the first state-wide comprehensive MOUD program in a unified state jail and prison system. The use of XR-BUP in clinical practice outside of a randomized controlled trial has yet to be examined in the correctional setting in contrast to other MOUDs such as oral methadone, SL-BUP products, and naltrexone. XR-BUP has several potential benefits for the unique circumstances of the correctional setting. To our knowledge, our study is the first in the literature to provide evidence in real-world conditions regarding the use of XR-BUP in clinical practice outside of a randomized controlled trial (whereas previous studies (i.e., Lee et al., 2021) compared SL-BUP vs. XR-BUP in a small randomized controlled trial and medications were provided free of charge) (Lee et al., 2021). Real-world evidence is important because it combines evidence generated from clinical practice and it bridges the gap between clinical research and practice (Corrigan-Curay, Sacks, & Woodcock, 2018; Sherman et al., 2016). Evidence from the natural and uncontrolled environment is key to addressing the ongoing opioid epidemic (Suvarna, 2018).
2. Methods
2.1. Participants & Setting
This study includes individuals if they enrolled in the MOUD program while incarcerated at RIDOC and had a prescription for XR-BUP in the electronic health record. The study protocol was approved by the Brown University Institutional Review Board, the Miriam Hospital Institutional Review Board, the Rhode Island Department of Health Institutional Review Board, the Office for Human Research Protections, and the Medical Research Advisory Group at the Rhode Island Department of Corrections.
RIDOC is the single, statewide unified (prison and jail combined) state-run correctional system with 3,000 men and women housed in six facilities on a single campus, with approximately 13,000 commitments annually, and 30,826 commitments during the study period. RIDOC’s population is 18 years and older and is 94% male, 52% White, 23% Black, and 21% Hispanic. RIDOC’s comprehensive MOUD program screens all individuals for OUD at time of commitment, and initiates and continues individuals on MOUD during incarceration when appropriate. Upon MOUD program referral and upon patient request, the patient meets with a provider who can educate on the benefits and differences between each form of MOUD, including XR-BUP, and discuss why a patient may prefer one over another. The MOUD program also provides counseling and group sessions for individuals and connects individuals to MOUD in the community at release. RIDOC covers all costs of MOUD treatment at rates similar to or below those paid by private and public insurers. (Martin et al., 2019)
The manufacturer of XR-BUP recommends initiating with SL-BUP for a minimum of 7 days, followed by an injection of 300mg monthly for 2 doses and then 100mg monthly thereafter. At RIDOC, providers make the determination of dose as clinically indicated based on the patient presentation. Participants were initially treated with oral or SL-BUP and stabilized on 8mg daily for at least 8 days prior to receipt of XR-BUP, with tapering of methadone for applicable individuals.
2.2. Sources of Data
Data from the RIDOC custody-and-control data (commitment and release dates and demographic data), RIDOC electronic medical record data (OUD screening, MOUD prescribed, start and stop dates, side-effects, and medication discontinuations), the Rhode Island Behavioral Online Database (RI-BHOLD; admissions and discharges to outpatient opioid treatment programs), and the Rhode Island Prescription Drug Monitoring Program (PDMP; prescription records on buprenorphine-containing product prescriptions filled) were linked at the level of the individual. All record linkages were conducted within the Stronghold Research Environment, Brown University’s HIPAA-compliant computing environment, for data compliance.
3. Results
3.1. Results
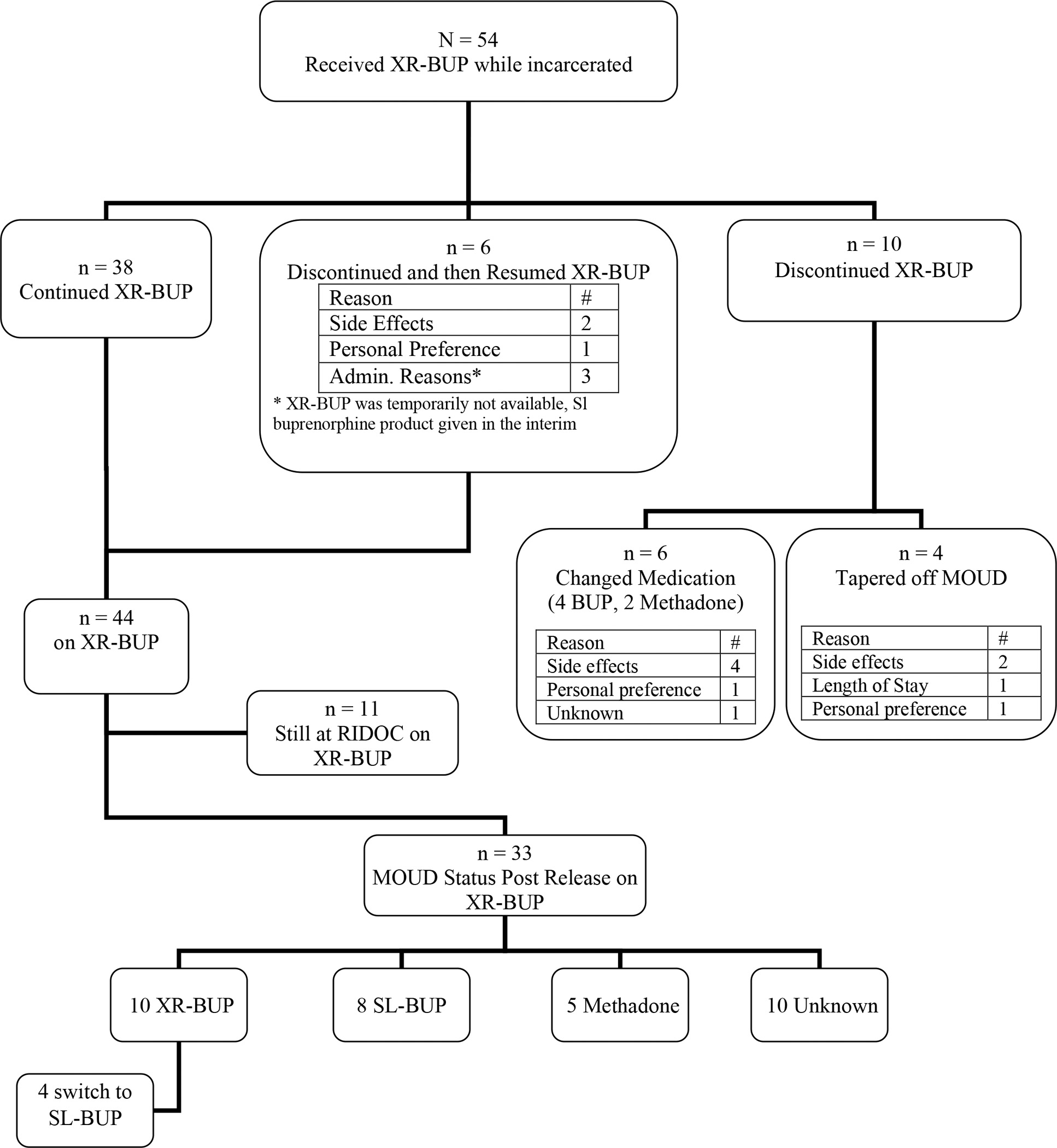
Between January 2019 and February 2022, 2,178 individuals were initiated or continued on MOUD, and of those 2,178 individuals, there were 54 unique individuals who were prescribed and received XR-BUP during incarceration. Table 1 presents demographic characteristics for the study sample. Figure 1 presents data on the number of individuals prescribed XR-BUP, the number who discontinued XR-BUP, the reasons for discontinuance, and post-release MOUD engagement.
Table 1.
Demographic characteristics for 54 incarcerated individuals enrolled in MOUD and received XR-BUP, Rhode Island, 2019–2022.
| Demographics | n/mean | %/SD |
|---|---|---|
| Sex (percent) | ||
| Male | 52 | 96% |
| Female | 2 | 4% |
| Age at Release (years, mean/SD) | 38.7 | 8.8 |
| Race/ethnicity (percent) | ||
| Black | 4 | 7% |
| White | 43 | 80% |
| Hispanic | 7 | 13% |
| Medication at enrollment | ||
| Methadone | 8 | 15% |
| XR-BUP | 4 | 7% |
| Sublingual buprenorphine/naloxone | 42 | 78% |
| MOUD Disposition | ||
| Continued | 22 | 41% |
| Induction | 22 | 41% |
| Prerelease Induction | 10 | 19% |
| Length of time incarcerated (days) | 613 | 540.2 |
MOUD: medications for opioid use disorder. sd = standard deviation.
Figure 1:
Number of individuals prescribed XR-BUP, the number who discontinued XR-BUP, reasons for discontinuance, and post-release MOUD engagement
3.2. Use of XR-BUP
A total of 162 XR-BUP injections (100, 300mg; 6, 100mg) were given during the study period, with an average 3.0 (SD = 2.0) injections per individual (range of 1 to 9) with 15 (28%) individuals receiving only a single injection. Initial attempts to taper the third dose to 100mg often resulted in patients feeling like they needed further medication augmentation for cravings. Through shared decision-making, the vast majority of patients (n=49) were maintained on 300mg with considerations to taper in the community if appropriate or desired. Of the other five participants, two participants received 100mg for all of their doses because they were receiving 100mg in the community prior to incarceration. The remaining three participants received 300mg for their first two doses and 100mg for their third dose. Of those three, one returned to 300mg subsequently because of withdrawal symptoms, one discontinued XR-BUP due to side effects, and one was released after their third dose.
Fifty participants switched from another type of MOUD while incarcerated, and four participants were continued on XR-BUP at the time of commitment (Table 1). Table 2 presents reasons for changing to XR-BUP. Over 50% (n=29) preferred XR-BUP to their prior medications of methadone (n=3 or 10%) or suboxone (n=26 or 90%). Notably, 14 (26%) individuals requested a change to XR-BUP because of concerns of hoarding medication or fear of being accused of hoarding medication.
Table 2.
Reasons for beginning XR-BUP for N= 54 individuals.
| Reason for beginning XR-BUP | # | % |
|---|---|---|
| Personal Preference | 29 | 54% |
| Discipline (hording MOUD) | 9 | 17% |
| Concern about being accused of diversion in the future | 5 | 9% |
| Unknown | 4 | 7% |
| Side Effects Methadone | 1 | 2% |
| Side Effects Sublingual buprenorphine/naloxone | 2 | 4% |
| On XR-BUP at time of commitment | 4 | 7% |
3.3. XR-BUP Discontinuance
Overall, 44 (70%) participants remained on XR-BUP during incarceration, 6 (11%) of participants discontinued XR-BUP but restarted, and 10 (19%) discontinued XR-BUP. Of the 10 (19%) individuals who discontinued XR-BUP, 4 (40%) tapered from MOUD completely and 6 (60%) changed to another form of MOUD.
3.4. Side Effects reported on XR-BUP
Table 3 provides data on the side effects reported during medication-check appointments. After the first injection, 25 (46.3%) individuals reported an adverse effect with an average of 1.9 side effects reported after the first injection. Overall, 33 (61%) individuals reported at least one adverse, with a total average of 2.8 side effects. There was no evidence of tampering with the injection site indicating no attempts to remove, hoard, or divert the medication.
Table 3.
XR-BUP Side effects reported during medication check appointments.
| After First Injection Count (%) |
All Injections Count (%) |
|
|---|---|---|
|
| ||
| Total reporting at least one side effect | Individuals N=25 | Individuals N=33 |
|
| ||
| Gastrointestinal | 9 | 17 |
| Constipation | 2 | 5 |
| Diarrhea | 2 | 5 |
| Nausea | 2 | 9 |
| Weight gain | 2 | 2 |
|
| ||
| General and administration site | 22 | 28 |
| Fatigue | 8 | 10 |
| Injection Site Pain, Discomfort or Bruising | 12 | 16 |
| Sweats | 10 | 13 |
| Withdrawal symptoms | 2 | 7 |
|
| ||
| Nervous system | 6 | 11 |
| Rash | 1 | 4 |
| Dizziness | 0 | 2 |
| Headache | 0 | 1 |
| Insomnia | 5 | 7 |
|
| ||
| Respiratory | 1 | 1 |
| Dyspnea | 1 | 1 |
3.5. MOUD Treatment Retention Post-Release
Of the 33 individuals who were released while on XR-BUP (61% of total XR-BUP participants), 23 (70%) engaged in MOUD post-release. Of these individuals, 10 (30%) received at least one XR-BUP injection post-release, 13 (39%) received another formulation of MOUD post-release, and 10 (30%) are unknown and are assumed to have not receive any MOUD post-release. For those receiving MOUD post-release, the average number of days from release to the first SL-BUP prescription fill date or receipt of methadone was 23.7 (SD = 11.23) and the average number of days from the last XR-BUP injection was 34.7 (SD = 14.38). Of the participants who were released on XR-BUP 7 of the 33 (21%) were reincarcerated during the study period. Of the seven reincarcerated participants, one received XR-BUP, one received both XR-BUP and SL-BUP, two received SL-BUP, three received methadone.
4. Discussion
4.1. Discussion
This study demonstrates that XR-BUP represents a potential long-acting injectable option to the challenges of providing MOUD in the correctional setting, whereas previously only injectable naltrexone was able to provide. In the community, opioid agonist therapy is often preferred; two large clinical trials seek to compare XR-naltrexone to XR-B in correctional settings (Gordon et al., 2021; Waddell et al., 2021). Our research shows the use of XR-BUP as part of a comprehensive MOUD program in the correctional setting is acceptable with no identified attempt at diverting the medication. Importantly, this study examines use of XR-BUP under standard clinical practice conditions and not in the controlled conditions of a research trial.
In correctional settings, XR-BUP has the added benefit of an easier initiation process because there is no need for patients to go through a lengthy medically supervised withdrawal as compared to XR-naltrexone. All individuals who transitioned from methadone to XR-BUP tolerated the taper and began XR-BUP, indicating that individuals can switch medications quickly and with no treatment dropout.
Although the proportion of individuals receiving MOUD after release was 61%, only 30% continued on XR-BUP post release, which is lower than the single randomized controlled trial analyzing injectable buprenorphine in a jail setting, that demonstrated 69% of individuals received at least one injection of XR-BUP after release. (Lee et al., 2021) This reduction in treatment may be because in the randomized controlled trial, the correctional facility and participants received XR-BUP at no cost and participants did not have to coordinate insurance or appointments to receive post-release doses. Further, larger community randomized controlled trials demonstrate approximately 19% of individuals with non-severe injection site side effects (Lofwall et al., 2018), similar to the 22% of study subjects who reported injection site discomfort or other mild side effects observed here.
4.2. Limitations
This observational study had several limitations that are typical of novel observational studies including small sample size, a single correctional facility site, and non-randomized treatment arms. Individuals receiving XR-BUP in the RIDOC setting were offered the medication in very specific circumstances, typically in response to personal preference or confirmed episodes of diversion or hoarding medication. This study used datasets specific to the state of Rhode Island. Individuals lost to follow-up may have continued treatment in another state and thus were not identified in the RI-BHOLD or RI PDMP dataset. Previously, those treated with XR-BUP had fewer positive urine screens over the course of treatment compared to injectable naltrexone (Haight et al., 2019; Lee et al., 2018). We were not able to examine urine toxicology screens, which is a limitation of the study. Other treatment outcomes (including reported illicit drug use, urine toxicology screens, overdose deaths, and longer-term treatment retention) are clearly important as further study of the implementation of XR-BUP in other correctional settings.
Conclusions
XR-BUP can address barriers that obstruct lifesaving MOUD access in correctional facilities. By addressing administrative concerns of diversion, patient preferences of avoiding daily med lines, and by offering another safe and effective treatment option for OUD, XR-BUP has the potential to increase treatment among a high-risk patient population with criminal justice exposure. Further studies and trials should continue to assess the opportunity for this novel treatment to treat opioid addiction in the correctional setting and upon release to the community.
Funding/Support:
This work was supported by the Rhode Island General Fund through the Rhode Island Department of Corrections and by the National Institutes of Health (NIH) through the NIH HEAL Initiative under award number U01 DA050442-01 (Multiple PIs: Rosemarie Martin, Lauren Brinkley-Rubinstein, Damaris Rohsenow). Drs Rich and Berk were supported by NIH grant P20GM125507. Dr. Berk was also supported by NIH Grant K23DA055695.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: Nothing declared.
Author Credit
RM JC JR and JB conceptualized the study. RM and JF did data collection, analyses and interpretation of the data. RM, JB, and JF drafted the first version of manuscript. JC, AK, and JR accessed and verified the underlying study data. All authors had access to the data and had responsibility for the decision to submit for publication. All authors helped to execute the study and contributed to the writing.
References
- Binswanger IA (2013). Mortality After Prison Release: Opioid Overdose and Other Causes of Death, Risk Factors, and Time Trends From 1999 to 2009. Annals of Internal Medicine, 159(9), 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, & Stern MF (2013). Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine, 159(9), 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley-Rubinstein L, McKenzie M, Macmadu A, Larney S, Zaller N, Dauria E, & Rich J (2018). A randomized, open label trial of methadone continuation versus forced withdrawal in a combined US prison and jail: Findings at 12 months post-release. Drug and Alcohol Dependence, 184, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuy M, Meroueh F, Trojak B, Bachellier J, Bendimerad P, Kosim M, . . . Rolland B (2021). Factors of Interest in Extended-Release Buprenorphine: Comparisons Between Incarcerated and Non-Incarcerated Patients with Opioid Use Disorder. Patient Preference and Adherence, Volume 15, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JG, Martin RA, Gresko SA, & Rich JD (2018). The First Comprehensive Program for Opioid Use Disorder in a US Statewide Correctional System. American Journal of Public Health, 108(10), 1323–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry, 23(2), 63–75. [DOI] [PubMed] [Google Scholar]
- Corrigan-Curay J, Sacks L, & Woodcock J (2018). Real-World Evidence and Real-World Data for Evaluating Drug Safety and Effectiveness. JAMA, 320(9), 867. [DOI] [PubMed] [Google Scholar]
- Cotton AJ, Lo K, Kurtz FB, & Waldbauer L (2021). Extended-release buprenorphine outcomes among treatment resistant veterans. The American Journal of Drug and Alcohol Abuse, 1–4. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Lundy C, Stringer M, Gallop R, & Gastfriend DR (2015). Extended-Release Naltrexone for Alcohol and Opioid Problems in Missouri Parolees and Probationers. J Subst Abuse Treat, 56, 54–60. [DOI] [PubMed] [Google Scholar]
- Deck D, Wiitala W, McFarland B, Campbell K, Mullooly J, Krupski A, & McCarty D (2009). Medicaid Coverage, Methadone Maintenance, and Felony Arrests: Outcomes of Opiate Treatment in Two States. Journal of Addictive Diseases, 28(2), 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, & McLaren J (2011a). Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction, 106(1), 32–51. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, & McLaren J (2011b). Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction, 106(1), 32–51. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Gordon M, Schwartz R, Kinlock T, Knight K, . . . For The Mat Working Group Of, C.-D. (2012). Medication-Assisted Treatment in Criminal Justice Agencies Affiliated with the Criminal Justice-Drug Abuse Treatment Studies (CJ-DATS): Availability, Barriers, and Intentions. Substance Abuse, 33(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Mitchell SG, Blue TR, Vocci FJ, Fishman MJ, Murphy SM, . . . Jarvis DK (2021). A clinical protocol of a comparative effectiveness trial of extended-release naltrexone versus extended-release buprenorphine with individuals leaving jail. Journal of Substance Abuse Treatment, 128, 108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, Marshall BDL, Alexander-Scott N, Boss R, & Rich JD (2018). Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psychiatry, 75(4), 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, . . . Wiest KL (2019). Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 393(10173), 778–790. [DOI] [PubMed] [Google Scholar]
- Lee JD, Malone M, McDonald R, Cheng A, Vasudevan K, Tofighi B, . . . Macdonald R (2021). Comparison of Treatment Retention of Adults With Opioid Addiction Managed With Extended-Release Buprenorphine vs Daily Sublingual Buprenorphine-Naloxone at Time of Release From Jail. JAMA Network Open, 4(9), e2123032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, . . . Rotrosen J (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. The Lancet, 391(10118), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Dunlop AJ, Haber PS, Lubman DI, Graham R, Hutchinson S, . . . Tiberg (2021). Patient-Reported Outcomes of Treatment of Opioid Dependence With Weekly and Monthly Subcutaneous Depot vs Daily Sublingual Buprenorphine. JAMA Network Open, 4(5), e219041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Nunes EV, Bailey GL, Sigmon SC, Kampman KM, . . . Kim S (2018). Weekly and Monthly Subcutaneous Buprenorphine Depot Formulations vs Daily Sublingual Buprenorphine With Naloxone for Treatment of Opioid Use Disorder. JAMA Internal Medicine, 178(6), 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, . . . Hickman M (2012). Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ, 345(oct03 3), e5945–e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RA, Alexander-Scott N, Wendelken J, & Clarke JG (2019). Collaborating to Address Substance Use Disorder in Correctional Settings: The Rhode Island Experience. In A Public Health Guide to Ending the Opioid Epidemic: Oxford University Press [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, & Breen R (2003). Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, E., & Medicine. (2019). Medications for Opioid Use Disorder Save Lives. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- NIDA. (2020). Criminal Justice DrugFacts. Retrieved from: https://nida.nih.gov/publications/drugfacts/criminal-justice. (Accessed. October 1 2021)
- Project J. a. P. O. (2020). Medication for Opioid Use Disorder and the Criminal Justice System Retrieved from: www.PrisonOpioidProject.org. (Accessed July 30 2021 ).
- Rich JD, McKenzie M, Larney S, Wong JB, Tran L, Clarke J, . . . Zaller N (2015). Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. The Lancet, 386(9991), 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Chang H-Y, Krawczyk N, Ferris L, Eisenberg M, Richards T, . . . Weiner JP (2020). Predictive Modeling of Opioid Overdose Using Linked Statewide Medical and Criminal Justice Data. JAMA Psychiatry, 77(11), 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Grella CE, Mischel AF, & Carnevale J (2021). The impact of the opioid crisis on U.S. state prison systems. Health & Justice, 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, . . . Califf RM (2016). Real-World Evidence — What Is It and What Can It Tell Us? New England Journal of Medicine, 375(23), 2293–2297. [DOI] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, . . . Walsh SL (2020). Opioid use disorder. Nature Reviews Disease Primers, 6(1). [DOI] [PubMed] [Google Scholar]
- Suvarna VR (2018). Real world evidence (RWE) - Are we (RWE) ready? Perspectives in clinical research, 9(2), 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Blanco C (2021). The changing opioid crisis: development, challenges and opportunities. Molecular Psychiatry, 26(1), 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell EN, Springer SA, Marsch LA, Farabee D, Schwartz RP, Nyaku A, . . . Lee JD (2021). Long-acting buprenorphine vs. naltrexone opioid treatments in CJS-involved adults (EXIT-CJS). Journal of Substance Abuse Treatment, 128, 108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N, Hard J, Fearns C, Gilman M, Littlewood R, Clegg R, . . . Alam F (2020). OUD Care Service Improvement with Prolonged-release Buprenorphine in Prisons: Cost Estimation Analysis ClinicoEconomics and Outcomes Research, Volume 12, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]


