Abstract
Background
Interferon (IFN) regulatory factors (IRFs) is a kind of transcription factors, which play an important role in regulating the expression of type I IFN and related genes. In mammals, IRF6 is not relevant with IFN expression, while zebrafish IRF6 was reported to be a positive regulator of IFN expression and could be phosphorylated by both MyD88 and TBK1. However, the role of IRF6 in the immune response and IFN transcription of common carp is unknown.
Results
In the present study, the cDNA of IRF6 gene (CcIRF6) was cloned from common carp using RACE technique, with a total length of 1905 bp, encoding 471 amino acid residues, which possesses two functional domains of DBD and IAD. Similarity analysis showed that CcIRF6 had more than 50% similarity with IRFs of other vertebrates, and had the highest similarity with grass carp and zebrafish, among which the DBD domain was much more conserved. The phylogenetic analysis showed that CcIRF6 is in the branch of Osteichthyes and has the closest relationship with grass carp. In healthy common carp, the CcIRF6 was expressed in all the examined tissues, with the highest level in the oral epithelium, and the lowest level in the head kidney. After intraperitoneal injection of poly(I:C) or Aeromonas hydrophila, the expression of CcIRF6 increased in spleen, head kidney, foregut and hindgut of common carp. Moreover, poly(I:C), LPS, PGN and flagellin induced the expression of CcIRF6 in peripheral leukocytes and head kidney leukocytes of common carp in vitro. In EPC cells, CcIRF6 inhibited the expression of some IFN-related genes and pro-inflammatory cytokines, and dual luciferase reporter assay showed that CcIRF6 reduced the activity of IFN and NF-κB reporter genes.
Conclusions
The present study suggests that CcIRF6 is involved in the antiviral and antibacterial immune response of common carp, and negatively regulate the expression of IFN and NF-κB signalling pathways, which provides a theoretical basis for the study and prevention of fish disease pathogenesis.
Keywords: Interferon regulatory factor 6 (IRF6), Poly(I:C), Aeromonas hydrophila, Interferon, NF-κB, Common carp (Cyprinus carpio L.)
Introduction
Interferon (IFN) regulatory factors (IRFs) is a kind of transcription factors regulating the expression of type I IFN and related genes, and play an important role in immune regulation and inflammatory response [1–3]. Moreover, IRFs also participate in the regulation of cell growth, differentiation and apoptosis, as well as tumorigenesis and metabolism [2, 4–8]. The first member of the IRF family, IRF1, was found in 1988; and till now, there are nine IRF members identified in humans and mice. In addition, IRF10 exists in chickens and fishes, while IRF11 is unique to fishes [9–14]. The N-terminal of IRF is a DNA-binding domain (DBD), which consists of about 120 amino acids. The DBD contains five or six conserved tryptophan repeat regions forming a helix-turn-helix motif, which recognizes and binds the IFN-stimulated response elements (ISRE) to regulate the expression of multiple immune related genes [4, 15, 16]. Compared with the DBD, the C-terminal IRF-related domain (IAD) is more diversiform and critical for the interaction of IRFs with other transcription factors or cofactors, which determines the different transcriptional activities and biological functions of IRFs [2, 17, 18].
Studies in mammals have shown that IRF6 regulates epidermal proliferation and differentiation [19, 20], and mutations in the IRF6 gene can lead to Van der Woude syndrome (VWS) and popliteal pterygium syndrome (PPS) [21], but is not relevant with IFN production. At present, fish IRF6 is rarely studied and has only been reported in zebrafish (Danio rerio), half-smooth tongue sole (Cynoglossus semilaevis), mandarin fish (Siniperca chuatsi), Atlantic cod (Gadus morhua) and blunt snout bream (Megalobrama amblycephala) [22–27]. The expression level of IRF6 was the highest in the muscle of half-smooth tongue sole, and increased in the spleen, head kidney or liver after stimulation with Vibrio harveyi, Edwardsiella tarda or megalocytivirus [23]. In mandarin fish, IRF6 had a higher expression level in intestine, and significant increase was observed in skin and intestine at 6 h following the stimulation of poly(I:C), and had an increase at a later stage of infection from 120 hpi in spleen and head-kidney [25]. The IRF6 in blunt snout bream was highly expressed in liver, intestine and gills, and increased in the intestine, liver, spleen, kidney and gills after infection with A. hydrophila [27]. However, the transcript of IRF6 in Atlantic cod appeared to have little or no expression in several important immune related tissues such as the spleen and blood, and was non-responsive to LPS as well as poly(I:C) [26]. Furthermore, overexpression of zebrafish IRF6 enhanced the IFN promoter activity and activated the transcription of ISG15, RIG-I and MAVS. In addition, zebrafish IRF6 can be phosphorylated by MyD88 and TBK1, and plays a positive regulatory role in the transcription of IFN [24], which is different from mammalian IRF6.
Common carp (Cyprinus carpio L.) is an important freshwater fish cultured in more than one hundred countries, especially in China and many other Asiatic and European countries, which accounts for up to 10% of freshwater aquaculture production worldwide [28, 29]. As common carp is suffering from outbreaks of a wide range of infectious diseases, it is necessary to elucidate the innate immune mechanism of common carp. To date, IRF1, IRF2, IRF3, IRF4, IRF5, IRF7, IRF9 and IRF10 have been reported in common carp, which performed pivotal antiviral and antibacterial immune functions through the regulation of IFN or NF-kappaB signalling pathways [30–36]. In the present study, the full-length IRF6 cDNA sequence (CcIRF6) was cloned from common carp and its amino acid sequence was analyzed. In vivo and in vitro experiments were performed to investigate its antiviral and antibacterial effects. Meanwhile, the regulatory role of CcIRF6 in IFN and NF-κB signaling pathway was clarified by gene overexpression experiment and dual luciflucase reporter gene assay. The present study will reveal the antiviral and antibacterial immune function of IRF6 in common carp, and provide a theoretical basis for the prevention and control of fish infecious diseases.
Materials and methods
Experimental challenges of common carp and sampling
The fish used in the present study were obtained from a fish farm (Jinan, Shandong, PR China), which weigh about 200 g per fish and were feeded at 20 °C in a fish feeding system (Aiwen) with purified water in the lab. Six fish were maintained in each 150 L tank and fed daily with commercial fish feed. One week later, four healthy fish were anesthetized and the liver, spleen, head kidney, gills, skin, foregut, hindgut, buccal epithelium and muscle were collected and homogenized using the automatic grinding instrument (Shanghai Jingxin), and total RNAs were extracted using TRIzol reagent (Tiangen). For experimental challenges, forty-eight fish were divided into two groups: twenty-four fish were anesthetized in 100 mg/L MS222 solution (Sigma) and injected intraperitoneally (i.p.) with 500 μl of poly(I:C) solution (2.6 mg/ml, Sigma); twenty-four were also anesthetized but injected i.p. with 500 μl of A. hydrophila suspension containing 2.0 × 108 formalin-killed bacteria [34, 37, 38]. At different time points after injection, three fish per group were anesthetized and some immune-related tissues were collected to extract total RNAs. In the challenge experiments, the fish without treatment were as control groups, and denoted by 0 h in the figures. The quality of these RNAs was analyzed by NanoDrop One (Thermo Scientific), and the cDNAs were synthesized using a FastQuant RT Kit (Tiangen). The protocol used in this study referenced our previously published articles [31, 34, 35].
Cells preparation and culture
The leukocytes of peripheral blood or head kidney (PBLs and HKLs) were isolated from common carp according to the previous studies [34, 39]. In brief, the single-cell suspensions derived from peripheral blood or head kidney were loaded onto the 34 to 51% Percoll density gradients (Sigma) and then centrifugate at 650 g for 30 min. The separated cells were cultivated in the Leibovitz’s L-15 medium at 25 °C. The epithelioma papulosum cyprini (EPC) cells and 293 T cells were cultivated in M199 medium (HyClone) at 25 °C or in DMEM medium (HyClone) at 37 °C. All these medium above were supplemented with 10% foetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco).
Cloning and expression analysis of the CcIRF6
The full-length cDNA sequence of CcIRF6 was obtained using the reverse transcription PCR and 3′- and 5′-Full RACE (rapid amplification of cDNA ends) method (TaKaRa). The IRF6 sequences of different species were aligned and the phylogenetic tree was constructed using the method of ClustalW and Neighbor-Joining respectively in MEGA 6.0 software, and the similarity analysis was performed using the Megalign program in DNAstar 7.0 software. The GenBank accession numbers of these sequences were listed in Table 1. The domains composition of CcIRF6 protein was analysed using the SMART software (http://smart.embl-heidelberg.de). The expression levels of CcIRF6 in different tissues of common carp were detected by real-time PCR in a Rotor-Gene Q PCR instrument (Qiagen) with TransStart Tip Green qPCR SuperMix (TransGen). The relative expression of all genes was calculated using the 2(−∆∆Ct) method, with the ribosomal protein S11 or β-actin as reference gene [31, 34, 35]. The primers used in the present study are listed in the Table 2.
Table 1.
Protein length and GenBank accession numbers of the IRF6 family members
| Species | Protein length | GenBank accession No. |
|---|---|---|
| Ctenopharyngodon idella | 472 | AMT92196 |
| Danio rerio | 492 | AAX57954 |
| Miichthys miiuy | 491 | AHB59739 |
| Corvus cornix | 460 | XP_010402606 |
| Xenopus laevis | 460 | NP_001085345 |
| Mus musculus | 467 | AAH08515 |
| Homo sapiens | 367 | AEL89176 |
| Branchiostoma belcheri tsingtauense | 267 | AJA02101 |
Table 2.
Primers used in the present study
| Primers | Sequences(5′-3′) | Use |
|---|---|---|
| IRF6-F | CATCTTTAAGGCCTGGGC | partial sequence amplifying |
| IRF6-R | GTACAGCTGCTTCAGGTG | |
| IRF6-5Rout | CTCGCGGCTCTTGTTGAGGGCGCACC | 5′ RACE |
| IRF6-5Rin | CATCCACGCCTTCCTGATACTTCCCCG | |
| IRF6-3Fout | CTCATCATGGTGCAGGTGGTGCC | 3′ RACE |
| IRF6-3Fin | GCAGCGTGCGGCTGCAGATCTCC | |
| CcIRF6-Frt | GAAGTATCAGGAAGGCGTGGATG | Real-time PCR |
| CcIRF6-Rrt | CCGTCGTAGATGAGGTTGAACTC | |
| CcS11-F | CCGTGGGTGACATCGTTACA | |
| CcS11-R | TCAGGACATTGAACCTCACTGTCT | |
| CcIFN-F | TCAATCTCATGGATGCCTCAGAGC | |
| CcIFN-R | TGGTATTGGGCCACGCATTCTT | |
| ccTNFα-F | ACAGGTGATGGTGTCGAGGAGGA | |
| CcTNFα-R | TCTGAGACTTGTTGAGCGTGAAG | |
| CcISG15-F | GTGAGCGGTGAAGCCACAGTTG | |
| CcISG15-R | GCGAACCGTTATCGGCAGACAG | |
| CcViperin-F | GAGAGCCCTTCCTTCACGAGAGAG | |
| CcViperin-R | ACTGCCATTGCTAACGATGCTGAC | |
| CcPKR-F | AGGCTTGATCCACAGAGACCTGAA | |
| CcPKR-R | CGTTCCAGAAGTTGCACGTCATTG |
Construction and transfection of recombinant vectors
The coding sequences of CcIRF6 or CcTRIF were amplified by PCR using Phusion High-Fidelity DNA polymerase (PrimeSTAR), and ligated into the pcDNA3.1-EGFP or pEGFP-N1 vectors to obtain the recombinant vectors pcDNA3.1-EGFP-CcIRF6 or pEGFP-N1-CcTRIF, which were extracted from positive clones using an endotoxin-free plasmid isolation kit (Tiangen) and verified by sequencing. The EPC cells were transfected with pcDNA3.1-EGFP-CcIRF6 and/or pEGFP-N1-CcTRIF using the X-tremeGENE HP DNA Transfection Reagent (Roche) [31, 34, 35].
Dual-luciferase reporter assays
The effects of CcIRF6 on the activation of IFN promoters and NF-κB were performed using Dual-luciferase reporter assays [31, 34, 35]. Briefly, 293 T cells were transfected with reporter gene plasmids pGL-IFN1/2/3-luc or pGL-NF-κB-luc and the recombinant vectors pcDNA3.1-EGFP-CcIRF6 or pEGFP-N1-CcTRIF using Lipofectamine 2000 (Invitrogen). The pGL-Renilla-luc plasmids were transfected together as control. Forty-eight hours later, the Dual-Glo® Luciferase Reagent (Promega) was used to measure the firefly and Renilla luciferase activity according to the manufacturer’s instructions.
Statistical analysis
The differences significance analysis was performed using t-test in GraphPad Prism 6.0, and P < 0.05 was considered as significative.
Results
Identification of the IRF6 cDNA sequence in common carp (CcIRF6)
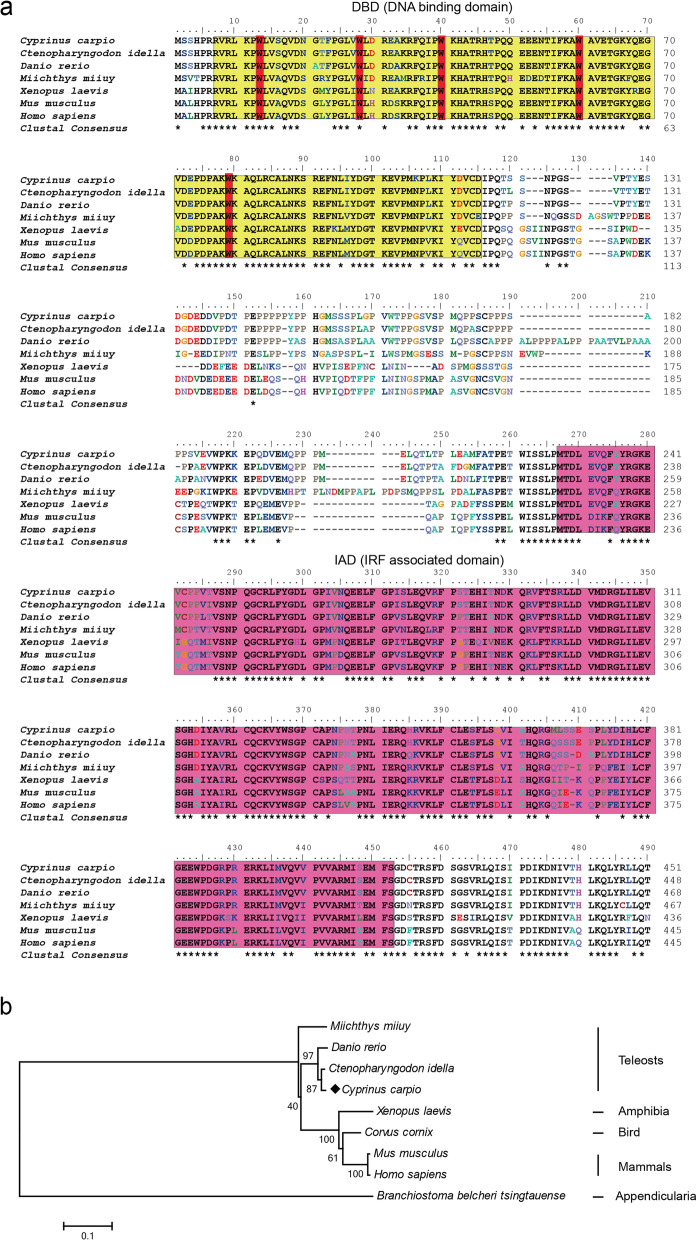
The conserved region of IRF6 genes was identified by multi-species sequence alignment, and the primers IRF6-F and IRF6-R designed according to the sequence of DrIRF6 were used to obtain a partial sequence of CcIRF6, with a length of 1138 bp. The 5′ and 3′ RACE were used to amplify the full length of CcIRF6, which was 1905 bp and includes 260 bp 5 ‘UTR, 1416 bp ORF and 229 bp 3’ UTR. The CcIRF6 protein contained 471 amino acids and was composed of a DBD (R7-D115) and an IAD (M228-S413) predicted by SMART software. The DBD domain contained five conserved tryptophan residues, including Trp13, Trp28, Trp40, Trp60 and Trp79 (Fig. 1).
Fig. 1.
Multiple alignments (a) and phylogenetic analysis (b) of IRF6 protein sequences in different species. Identical residues are indicated by (*). CcIRF6 is marked with solid diamond (◆). The GenBank accession numbers of the genes are listed in Table 1
Amino acid sequence alignment and phylogenetic analysis
Multiple sequence alignment of the IRF6 protein sequences in various species was performed in the present study. The similarity of CcIRF6 with CiIFR6 and DrIRF6 was the highest, up to 94.2 and 90.2% respectively (Table 3). Except for BbIRF6, the DBD domains in all other IRF6 have five tryptophan residues, while the conservation of IAD domains in different species was lower (Fig. 1a). The phylogenetic tree was constructed using the neighbor-joining method, and the results showed that CcIRF6 is in the branch of Osteichthyes, and has the closest relationship with grass carp (Fig. 1b).
Table 3.
Amino acid identities of CcIRF6 to other IRF6 proteins
| Species | Identities (%) |
|---|---|
| B. belcheri | 24.7 |
| C. idella | 94.2 |
| D. rerio | 90.2 |
| M. miiuy | 78.4 |
| X. laevis | 69.4 |
| C. cornix | 71.4 |
| M. musculus | 69.4 |
| H. sapiens | 68.7 |
Tissue-specific expression pattern of CcIRF6
In order to examined the tissue-specific expression pattern of IRF6 gene in common carp, the expression of CcIRF6 mRNA in nine tissues of healthy common carp was detected by real-time PCR, which is relatively high in oral epithelium, followed by skin, gills, hindgut, foregut and liver, and very low in spleen, muscle and head kidney (Fig. 2).
Fig. 2.
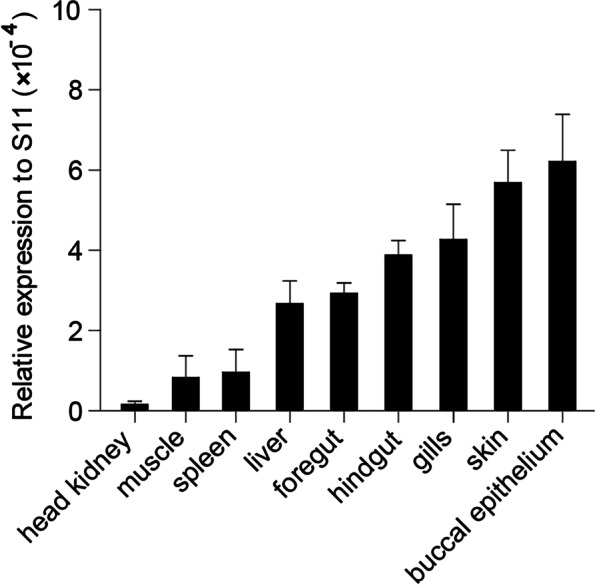
Tissue specific expression of CcIRF6 under normal physiological condition. CcIRF6 expressions in the liver, spleen, head kidney, gills, skin, foregut, hindgut, buccal epithelium, and muscle were determined by Real-time PCR. The expression levels were normalized using the 40S ribosomal protein S11 mRNA. (n = 4, mean ± SD)
The expression of CcIRF6 in response to poly(I:C) and A. hydrophila stimulation in vivo
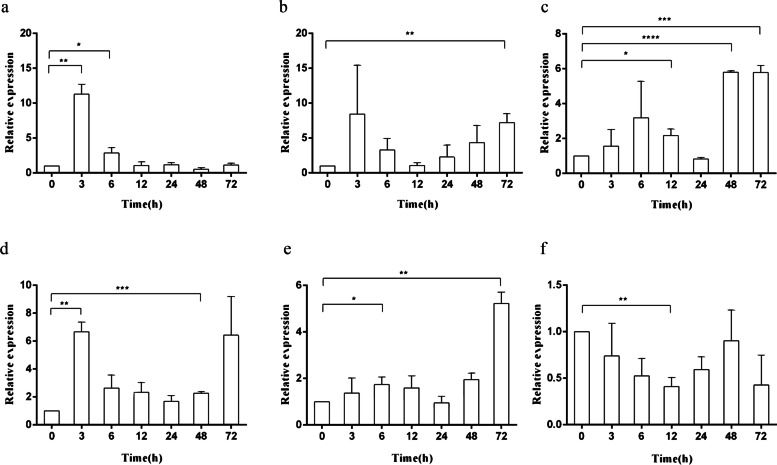
Three hours post intraperitoneal injection (hpi) of poly(I:C), the expression levels of CcIRF6 mRNA in liver and skin of common carp were increased by 11.5 and 6.5 times as much as those in the control group, respectively. The CcIRF6 expression increased to 7.2, 5.5 and 9.5 times of the control group in the spleen, head kidney and foregut at 72 hpi, respectively. However, the expression of CcIRF6 in the hindgut decreased to 0.4 times at 12 hpi (Fig. 3). After i.p. injection of Aeromonas hydrophila, the expression of CcIRF6 mRNA in the head kidney and hindgut of common carp reached the maximum value at 6 hpi, up to 2.3 times and 4.7 times of that in the control group, respectively, and increased to 3.7 times in the foregut at 3 hpi. However, the expression of CcIRF6 mRNA in spleen decreased by 0.17 times at 12 hpi (Fig. 4).
Fig. 3.
Expression analysis of CcIRF6 in response to poly(I:C) challenge in vivo. Total RNA was extracted from liver (a), spleen (b), head kidney (c), skin (d), foregut (e) and hindgut (f) at 0, 3, 6, 12, 24, 48 and 72 h post injection for Real-time PCR. The expression was normalized to the 40S ribosomal protein S11. (n = 3, mean ± SD, *P < 0.05)
Fig. 4.
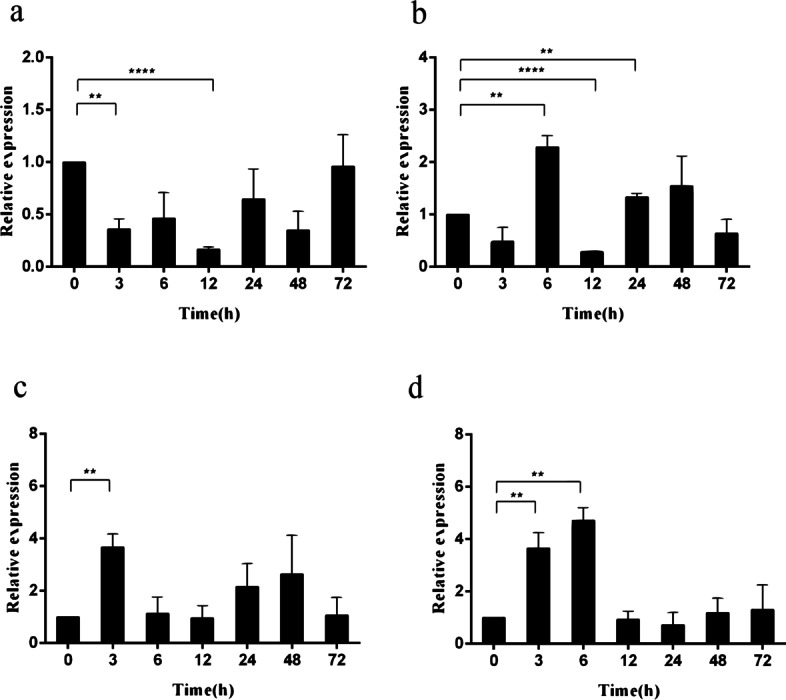
Expression analysis of CcIRF6 in response to A. hydrophila challenge in vivo. Total RNA was extracted from spleen (a), head kidney (b), foregut (c) and hindgut (d) at 0, 3, 6, 12, 24, 48 and 72 h post injection for Real-time PCR. The expression was normalized to the 40S ribosomal protein S11. (n = 3, mean ± SD, *P < 0.05)
Expression of CcIRF6 upon different stimulation in vitro
Twenty-four or 6 hours post poly(I:C) or LPS stimulation, the expression of CcIRF6 mRNA in PBLs of common carp increased 1.5 or 4.6 times as much as that in the control group. 12 h after PGN and flagellin stimulation, the expression levels of CcIRF6 increased to 1.3 and 3.8 times, respectively (Fig. 5). At 24 h after stimulation with poly(I:C), LPS, PGN or flagellin, the CcIRF6 mRNA expression in HKLs of common carp were increased to the maximum with 2.6 times, 3.1 times, 4.4 times and 3.7 times, respectively (Fig. 6).
Fig. 5.
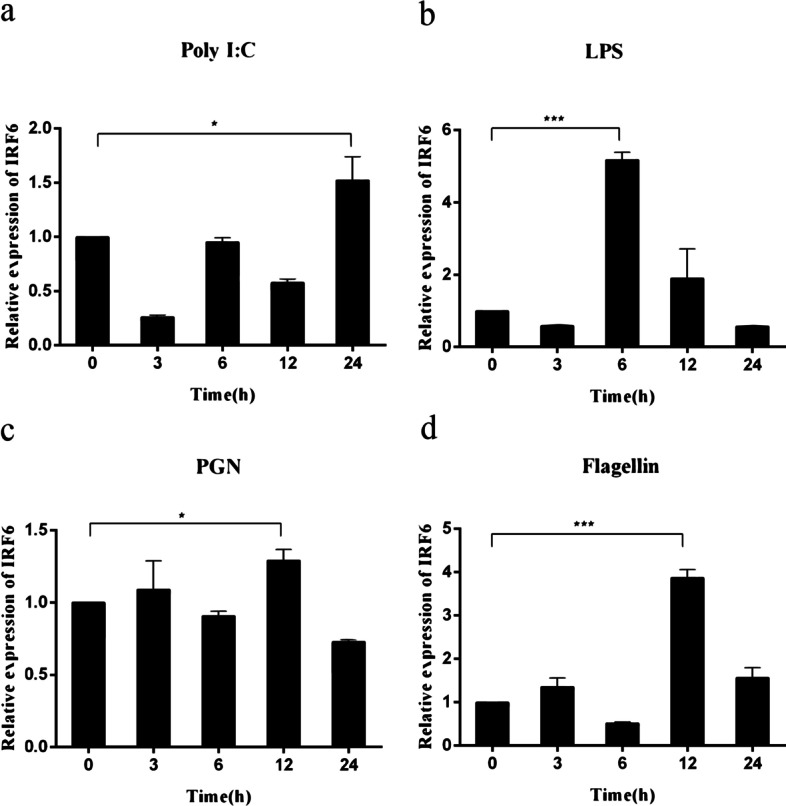
Expression levels of CcIRF6 in the PBLs induced by poly(I:C), LPS, PGN and flagellin. The expression was normalized using the 40S ribosomal protein S11. (n = 3, mean ± SD, *P < 0.05)
Fig. 6.
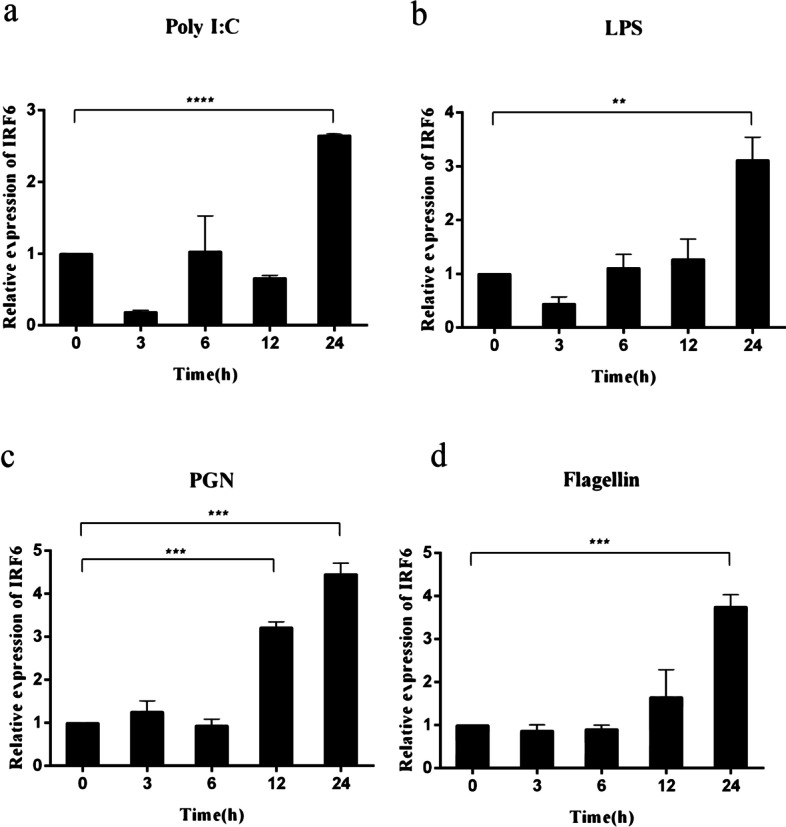
Expression levels of CcIRF6 in the HKLs induced by poly(I:C), LPS, PGN and flagellin. The expression was normalized using the 40S ribosomal protein S11. (n = 3, mean ± SD, *P < 0.05)
Regulation of IFN and NF-κB signalling pathways by CcIRF6
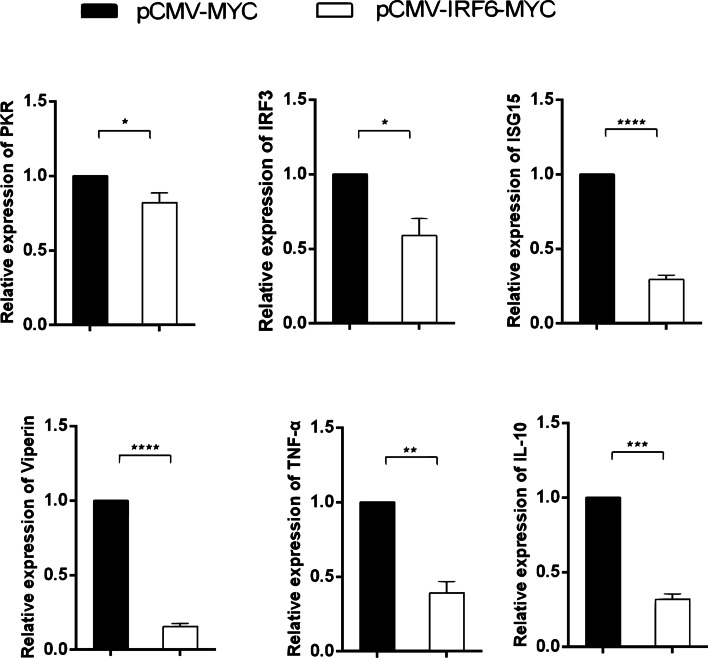
To investigate the role of CcIRF6 in the IFN and NF-κB signalling pathway, the gene expression of PKR, ISG15, Viperin, IRF3, TNF-α and IL-10 was detected after overexpression of CcIRF6 in EPC cells. The results showed that the expression levels of these genes were significantly reduced (Fig. 7). Furthermore, dual-luciferase reporter assays performed in 293 T cells showed that TRIF could activate IFN1, IFN2 and IFN3 promoters, and CcIRF6 reduced the activity of TRIF-induced IFN promoters (Fig. 8A-C). Meanwhile, CcIRF6 also inhibited TRIF-induced NF-κB activity (Fig. 8D).
Fig. 7.
Effect of CcIRF6 on the expression of IFN-stimulated genes and inflammatory cytokines. The expression levels of the PKR (a), ISG15 (b), Viperin (c), IRF3 (d), TNF-α (e) and IL-10 (f) genes in CcIRF6-overexpressed EPC cells were detected by real-time PCR and normalized to β-actin (n = 3, mean ± SD, *P < 0.05)
Fig. 8.
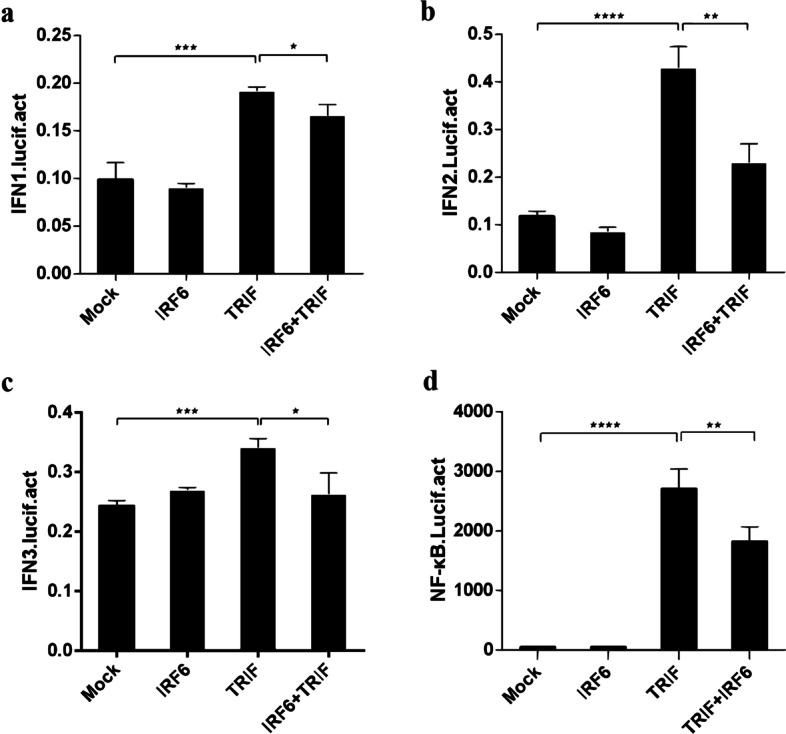
CcIRF6 negatively regulates the IFN and NF-κB pathways. 293 T cells were cotransfected with three IFNs or an NF-κB reporter gene, CcIRF6 and/or TRIF expression plasmids. Luciferase activity was measured after 48 h and determined against Renilla luciferase activity (n = 3, mean ± SD, *P < 0.05)
Discussion
Most of the organisms control the pathogens invasion by a series of inflammatory factors in the early stage of infection. IRFs were first discovered as transcription factors that regulate IFN expression, and in recent years, studies have shown that IRFs has multiple functions. In addition to the important role in innate and adaptive immunity, IRFs also participates in regulating the development and maturation of the immune system, as well as cell growth [2, 4, 40, 41]. With the rapid development of fishery economy, there are more and more studies on IRFs in teleost fish.
Both mammalian and fish IRFs have DBD domains, which are responsible for binding ISRE/IRF-E sequences on target genes [42]. In this study, the N-terminal region of CcIRF6 has a DBD domain, which is highly similar to the DBD of other vertebrate IRFs, suggesting that the function of IRFs may be relatively conserved in evolution. The DBD domain of IRF2 has six tryptophan residues, while IRF4, IRF9, IRF10 and IRF6 all have five tryptophan residues [31, 34–36]. Although IRF10 and IRF6 are not the same family, their tryptophan residues are all located in Trp13, Trp28, Trp40, Trp60 and Trp79. The C-terminal of IRFs have two types [6]: IAD1 was originally found in IRF8 and is present in all members of the IRF family except IRF1 and IRF2; IAD2 exists only in IRF1 and IRF 2[6]. In this study, CcIRF6 have an IAD1 domain.
In this study, the IRF6 amino acid sequences similarity between common carp and other species up to more than 70%, which indicates the evolutionary conservatism of vertebrate IRFs and reflects the essential role of IRF family members in organisms. Phylogenetic analysis showed that the 11 members of IRF family were divided into four subfamilies, namely IRF1 subfamily, including IRF1, IRF2 and IRF11; IRF3 subfamily, including IRF3 and IRF7; IRF4 subfamily, including IRF4, IRF8, IRF9 and IRF10; IRF5 subfamily, including IRF5 and IRF6. CcIRF6 belongs to the IRF5 subfamily, on the same branch as grass carp and zebrafish.
Mammalian IRF6 is both a transcriptional regulator in keratinocyte differentiation and can also control the transformation from proliferation to differentiation by activating differentiation-related genes [43, 44]. IRF6 is expressed in a variety of human organs and plays an important role in the expression of TLR2-mediated inflammatory cytokines in human oral epithelial cells [45]. The present study found that the expression of CcIRF6 is highest in the oral epithelium of healthy common carp, which is the first line of defense against pathogen invasion. Therefore, IRF6 may play a key role in the immune system of mammals and common carp. In addition, the expression level of IRF6 in muscles of half-smooth tongue sole is the highest, implied that IRF6 may be involved in the regulatory process of tissue connecter formation of fish [23].
Poly(I:C) is an inducer of fish IFN, ISGs and a variety of IRFs, and can also induce the expression of TLR3 and TLR22 [46–48]. A. hydrophila is a pathogen of many aquatic organisms, which is widely distributed in natural water and can induce the expression of PRR, IFN and IRFs. In this study, the temporal changes of CcIRF6 mRNA expression in immune-related tissues of commom carp were investigated after stimulation with poly(I:C) and A. hydrophila. After poly(I:C) stimulation, the expression of CcIRF6 is increased in various immune-related tissues, including liver, spleen, head kidney, skin and foregut. After A. hydrophila infection, the expression of CcIRF6 increased in the head kidney, foregut and hindgut. Similarly, in liver, spleen and head kidney of half-smooth tongue sole, the expression of IRF6 increased after stimulation with E. tarda and megalocytivirus [23], indicating that IRF6 is involved in the process of antiviral and anti-bacterial immune responses in fish.
The immune system of bony fish is in a special position in evolution, with the differentiation of natural and adaptive immunity. T and B lymphocytes appear in peripheral blood of bony fish, which play an important role in the immune process [49–52]. Meanwhile, the head kidney is an important immune organ of fish, rich in a variety of immune cells. Therefore, in this study, the peripheral blood leukocytes (PBLs) and head kidney leukocytes (HKLs) were isolated from common carp and stimulated by different PAMPs, such as poly(I:C), LPS, PGN and flagellin. The results showed that the expression of CcIRF6 were significantly increased, indicating that CcIRF6 was involved in the immune response process of antiviral and anti-bacterial in common carp.
As transcription factors, various IRFs play an important role in the regulation of IFN and ISGs expression. However, mammalian IRF6 has nothing to do with the production of IFN, but is involved in the formation of tissue ligand and controls the development of lingual dorsal filamental papillae [53], and its gene mutation can lead to congenital chromosomal dominant genetic disease VWS or PPS [21]. Studies in zebrafish suggest that IRF6 plays a positive regulatory role in the expression of IFN. Overexpression of IRF6 in zebrafish can enhance IFN promoter activity and activate transcription of ISG15, RIG-I and MAVS in host cells [22]. Differently from IRF6 of zebrafish, the expression levels of PKR, ISG15, Viperin, IRF3, TNF-α and IL-10 were significantly reduced after overexpression of CcIRF6, and CcIRF6 reduced the activity of TRIF-induced IFN promoters and NF-κB. Therefore, the function of IRF6 is not conserved in the evolution of lower vertebrates to mammals. Although mammalian IRF6 is unrelated to the production of IFN, fish IRF6 participates in the innate immune process and plays a regulatory role in the transcription and phosphorylation of IFN.
Conclusions
In the present study, the full-length IRF6 cDNA sequence (CcIRF6) was cloned from common carp and its amino acid sequence was analyzed. We found that various bacterial and viral PAMPs induced the expression of CcIRF6 in vivo and in vitro. Furthermore, CcIRF6 inhibited the expression of some IFN-stimulated genes and inflammatory cytokines, and reduced the activity of IFN promoter and NF-κB. Taken together, these results suggest that CcIRF6 is involved in the antiviral and antibacterial immune response of common carp, and negatively regulate the IFN and NF-κB signalling pathways. On the one hand, this study is helpful to understand the evolution of immune system from lower fish to higher mammals; on the other hand, it provides a theoretical basis in the prevention and control of fish infecious diseases, for example, through providing drug targets or new vaccine research strategies.
Acknowledgments
Not applicable.
Abbreviations
- IRF
Interferon regulatory factor
- DBD
DNA-binding domain
- IAD
IRF associated domain
- ISRE
IFN-stimulated response element
- VWS
Van der Woude syndrome
- PPS
Popliteal pterygium syndrome
- ISG15
IFN-stimulated gene 15
- RIG-I
Retinoic acid-inducible gene I
- MAVS
Mitochondrial antiviral signaling protein
- MyD88
Myeloid differentiation primary response gene 88
- TBK1
TANK binding kinase 1
- i.p.
Intraperitoneally
- PBL
Peripheral blood leucocyte
- HKL
Head kidney leukocyte
- EPC
Epithelioma papulosum cyprinid
- RACE
Rapid amplification of the cDNA ends
- SMART
Simple modular architecture research tool
- PAMP
Pathogen associated molecular pattern
- NF-κB
Nuclear factor kappa B
Authors’ contributions
H.L. and G.Y. designed the study; Y.L., R.L., J.Z. and Y.C. performed the experiments; Y.Z. and S.S. analyzed the data; H.L. and Y.L. wrote the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32002419, 31972828) and the National Key R&D Program of China (2018YFD0900302–8), which were not involved in the design, performance and analysis of all the experiments, or in the writing of the manuscript.
Availability of data and materials
The accession number of CcIRF6 in this article is ON256314, which is available in the GenBank (https://www.ncbi.nlm.nih.gov/nuccore/ON256314).
Declarations
Ethics approval and consent to participate
All animal experiments in this article were performed in accordance with relevant guidelines, which was approved by the Animal Experimental Ethics Committee of Shandong Normal University. The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaxin Liang and Rongrong Liu contributed equally to this work.
Contributor Information
Guiwen Yang, Email: yanggw@sdnu.edu.cn.
Hua Li, Email: lihua@sdnu.edu.cn.
References
- 1.Fujita T, Sakakibara J, Sudo Y, Miyamoto M, Kimura Y, Taniguchi T. Evidence for a nuclear factor(s), IRF-1, mediating induction and silencing properties to human IFN-beta gene regulatory elements. EMBO J. 1988;7(11):3397–3405. doi: 10.1002/j.1460-2075.1988.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 3.Honda K, Taniguchi T. IRFs: master regulators of signalling by toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 5.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59(4):489–510. doi: 10.1007/s00262-009-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaoka A, Tamura T, Taniguchi T. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci. 2008;99(3):467–478. doi: 10.1111/j.1349-7006.2007.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battistini A. Interferon regulatory factors in hematopoietic cell differentiation and immune regulation. J Interferon Cytokine Res. 2009;29(12):765–780. doi: 10.1089/jir.2009.0030. [DOI] [PubMed] [Google Scholar]
- 8.Zhao GN, Jiang DS, Li H. Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim Biophys Acta. 2015;1852(2):365–378. doi: 10.1016/j.bbadis.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Nehyba J, Hrdlickova R, Burnside J, Bose HR., Jr A novel interferon regulatory factor (IRF), IRF-10, has a unique role in immune defense and is induced by the v-Rel oncoprotein. Mol Cell Biol. 2002;22(11):3942–3957. doi: 10.1128/MCB.22.11.3942-3957.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Jia QQ, Liang Y, Huang WS, Nie P. Interferon regulatory factor 10 (IRF10): cloning in orange spotted grouper, Epinephelus coioides, and evolutionary analysis in vertebrates. Fish Shellfish Immunol. 2015;46(2):669–677. doi: 10.1016/j.fsi.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Inkpen SM, Hori TS, Gamperl AK, Nash GW, Rise ML. Characterization and expression analyses of five interferon regulatory factor transcripts (Irf4a, Irf4b, Irf7, Irf8, Irf10) in Atlantic cod (Gadus morhua) Fish Shellfish Immunol. 2015;44(1):365–381. doi: 10.1016/j.fsi.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Yasuike M, Kondo H, Aoki T, Hirono I. Molecular cloning and expression analysis of interferon regulatory factor 10 (IRF10) in Japanese flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2011;30(1):67–76. doi: 10.1016/j.fsi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Jiang Y, Wangkahart E, Zou J, Chang M, Yang D, Secombes CJ, Nie P, Wang T. Sequence and expression analysis of interferon regulatory factor 10 (IRF10) in three diverse teleost fish reveals its role in antiviral defense. PLoS One. 2016;11(1):e0147181. doi: 10.1371/journal.pone.0147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu QQ, Yang DQ, Tuo R, Wan J, Chang MX, Nie P. Gene cloning and induced expression pattern of IRF4 and IRF10 in the Asian swamp eel (Monopterus albus) Dong wu xue yan jiu = Zoological Res. 2014;35(5):380–388. doi: 10.13918/j.issn.2095-8137.2014.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veals SA, Schindler C, Leonard D, Fu XY, Aebersold R, Darnell JE, Jr, Levy DE. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992;12(8):3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 17.Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cell. 2007;129(6):1111–1123. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Royer WE., Jr Structural insights into interferon regulatory factor activation. Cell Signal. 2010;22(6):883–887. doi: 10.1016/j.cellsig.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingraham CR, Kinoshita A, Kondo S, Yang BL, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38(11):1335–1340. doi: 10.1038/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38(11):1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- 21.Leslie EJ, Standley J, Compton J, Bale S, Schutte BC, Murray JC. Comparative analysis of IRF6 variants in families with Van der Woude syndrome and popliteal pterygium syndrome using public whole-exome databases. Genet Med. 2013;15(5):338–344. doi: 10.1038/gim.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben J, Jabs EW, Chong SS. Genomic, cDNA and embryonic expression analysis of zebrafish IRF6, the gene mutated in the human oral clefting disorders Van der Woude and popliteal pterygium syndromes. Gene Expr Patterns. 2005;5(5):629–638. doi: 10.1016/j.modgep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Li YX, Hu YH. Molecular characterization and expression analysis of eleven interferon regulatory factors in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2015;44(1):272–282. doi: 10.1016/j.fsi.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Lu LF, Wang ZX, Chen DD, Zhang YA. Fish IRF6 is a positive regulator of IFN expression and involved in both of the MyD88 and TBK1 pathways. Fish Shellfish Immun. 2016;57:262–268. doi: 10.1016/j.fsi.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 25.Laghari ZA, Li L, Chen SN, Huo HJ, Huang B, Zhou Y, Nie P. Composition and transcription of all interferon regulatory factors (IRFs), IRF1-11 in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev Comp Immunol. 2018;81:127–140. doi: 10.1016/j.dci.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Inkpen SM, Solbakken MH, Jentoft S, Eslamloo K, Rise ML. Full characterization and transcript expression profiling of the interferon regulatory factor (IRF) gene family in Atlantic cod (Gadus morhua) Dev Comp Immunol. 2019;98:166–180. doi: 10.1016/j.dci.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Zhan FB, Jakovlic I, Wang WM. Identification, characterization and expression in response to Aeromonas hydrophila challenge of five interferon regulatory factors in Megalobrama amblycephala. Fish Shellfish Immunol. 2019;86:204–212. doi: 10.1016/j.fsi.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Xu P, Zhang X, Wang X, Li J, Liu G, Kuang Y, Xu J, Zheng X, Ren L, Wang G, et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat Genet. 2014;46(11):1212–1219. doi: 10.1038/ng.3098. [DOI] [PubMed] [Google Scholar]
- 29.Xu P, Xu J, Liu G, Chen L, Zhou Z, Peng W, Jiang Y, Zhao Z, Jia Z, Sun Y, et al. The allotetraploid origin and asymmetrical genome evolution of the common carp Cyprinus carpio. Nat Commun. 2019;10(1):4625. doi: 10.1038/s41467-019-12644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan S, Qi C, Zhu Y, Li H, An L, Yang G. Expression profile of carp IFN correlate with the up-regulation of interferon regulatory factor-1 (IRF-1) in vivo and in vitro: the pivotal molecules in antiviral defense. Fish Shellfish Immunol. 2016;52:94–102. doi: 10.1016/j.fsi.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Chen X, Zhu Y, Liu R, Zheng L, Shan S, Zhang F, An L, Yang G. Molecular characterization and immune functional analysis of IRF2 in common carp (Cyprinus carpio L.): different regulatory role in the IFN and NF-kappaB signalling pathway. BMC Vet Res. 2021;17(1):303. doi: 10.1186/s12917-021-03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng H, Liu H, Kong R, Wang L, Wang Y, Hu W, Guo Q. Expression profiles of carp IRF-3/−7 correlate with the up-regulation of RIG-I/MAVS/TRAF3/TBK1, four pivotal molecules in RIG-I signaling pathway. Fish Shellfish Immunol. 2011;30(4–5):1159–1169. doi: 10.1016/j.fsi.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhu YY, Qi CC, Shan SJ, Zhang FM, Li H, An LG, et al. Characterization of common carp (Cyprinus carpio L.) interferon regulatory factor 5 (IRF5) and its expression in response to viral and bacterial challenges. BMC Vet Res. 2016;12:127. [DOI] [PMC free article] [PubMed]
- 34.Zhu YY, Shan SJ, Feng HX, Jiang L, An LG, Yang GW, Li H. Molecular characterization and functional analysis of interferon regulatory factor 9 (irf9) in common carp Cyprinus carpio: a pivotal molecule in the Ifn response against pathogens. J Fish Biol. 2019;95(2):510–519. doi: 10.1111/jfb.14000. [DOI] [PubMed] [Google Scholar]
- 35.Zhu YY, Shan SJ, Zhao HP, Liu RR, Wang H, Chen XP, et al. Identification of an IRF10 gene in common carp (Cyprinus carpio L.) and analysis of its function in the antiviral and antibacterial immune response. BMC Vet Res. 2020;16(1):450. [DOI] [PMC free article] [PubMed]
- 36.Zhu Y, Yang G. Molecular identification and functional characterization of IRF4 from common carp (Cyprinus carpio. L) in immune response: a negative regulator in the IFN and NF-kappaB signalling pathways. BMC Vet Res. 2022;18(1):106. doi: 10.1186/s12917-022-03205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan SJ, Liu DZ, Wang L, Zhu YY, Zhang FM, Li T, An LG, Yang GW. Identification and expression analysis of irak1 gene in common carp Cyprinus carpio L.: indications for a role of antibacterial and antiviral immunity. J Fish Biol. 2015;87(2):241–255. doi: 10.1111/jfb.12714. [DOI] [PubMed] [Google Scholar]
- 38.Shan S, Wang L, Zhang F, Zhu Y, An L, Yang G. Characterization and expression analysis of toll-interacting protein in common carp, Cyprinus carpio L., responding to bacterial and viral challenge. SpringerPlus. 2016;5:639. doi: 10.1186/s40064-016-2293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu YY, Xing WX, Shan SJ, Zhang SQ, Li YQ, Li T, An L, Yang GW. Characterization and immune response expression of the rig-I-like receptor mda5 in common carp Cyprinus carpio. J Fish Biol. 2016;88(6):2188–2202. doi: 10.1111/jfb.12981. [DOI] [PubMed] [Google Scholar]
- 40.Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. J Interferon Cytokine Res. 2002;22(1):59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- 41.Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007;37(2):306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- 42.Paun A, Pitha PM. The IRF family, revisited. Biochimie. 2007;89(6–7):744–753. doi: 10.1016/j.biochi.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwa MQ, Huynh J, Aw J, Zhang L, Nguyen T, Reynolds EC, Sweet MJ, Hamilton JA, Scholz GM. Receptor-interacting protein kinase 4 and interferon regulatory factor 6 function as a signaling axis to regulate keratinocyte differentiation. J Biol Chem. 2014;289(45):31077–31087. doi: 10.1074/jbc.M114.589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de la Garza G, Schleiffarth JR, Dunnwald M, Mankad A, Weirather JL, Bonde G, Butcher S, Mansour TA, Kousa YA, Fukazawa CF, et al. Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of Grainyhead-like 3. J Investig Dermatol. 2013;133(1):68–77. doi: 10.1038/jid.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwa MQ, Nguyen T, Huynh J, Ramnath D, De Nardo D, Lam PY, Reynolds EC, Hamilton JA, Sweet MJ, Scholz GM. Interferon regulatory factor 6 differentially regulates toll-like receptor 2-dependent chemokine gene expression in epithelial cells. J Biol Chem. 2014;289(28):19758–19768. doi: 10.1074/jbc.M114.584540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holland JW, Bird S, Williamson B, Woudstra C, Mustafa A, Wang T, Zou J, Blaney SC, Collet B, Secombes CJ. Molecular characterization of IRF3 and IRF7 in rainbow trout, Oncorhynchus mykiss: functional analysis and transcriptional modulation. Mol Immunol. 2008;46(2):269–285. doi: 10.1016/j.molimm.2008.08.265. [DOI] [PubMed] [Google Scholar]
- 47.Collet B, Hovens GC, Mazzoni D, Hirono I, Aoki T, Secombes CJ. Cloning and expression analysis of rainbow trout Oncorhynchus mykiss interferon regulatory factor 1 and 2 (IRF-1 and IRF-2) Dev Comp Immunol. 2003;27(2):111–126. doi: 10.1016/S0145-305X(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 48.Zou J, Carrington A, Collet B, Dijkstra JM, Yoshiura Y, Bols N, Secombes C. Identification and bioactivities of IFN-gamma in rainbow trout Oncorhynchus mykiss: the first Th1-type cytokine characterized functionally in fish. J Immunol. 2005;175(4):2484–2494. doi: 10.4049/jimmunol.175.4.2484. [DOI] [PubMed] [Google Scholar]
- 49.Fischer U, Utke K, Somamoto T, Kollner B, Ototake M, Nakanishi T. Cytotoxic activities of fish leucocytes. Fish Shellfish Immunol. 2006;20(2):209–226. doi: 10.1016/j.fsi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Nakanishi T, Shibasaki Y, Matsuura Y. T cells in fish. Biology. 2015;4(4):640–663. doi: 10.3390/biology4040640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scapigliati G. Functional aspects of fish lymphocytes. Dev Comp Immunol. 2013;41(2):200–208. doi: 10.1016/j.dci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Laing KJ, Hansen JD. Fish T cells: recent advances through genomics. Dev Comp Immunol. 2011;35(12):1282–1295. doi: 10.1016/j.dci.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Kawasaki M, Kawasaki K, Oommen S, Blackburn J, Watanabe M, Nagai T, Kitamura A, Maeda T, Liu B, Schmidt-Ullrich R, et al. Regional regulation of Filiform tongue papillae development by Ikkalpha/Irf6. Dev Dynamics. 2016;245(9):937–946. doi: 10.1002/dvdy.24427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The accession number of CcIRF6 in this article is ON256314, which is available in the GenBank (https://www.ncbi.nlm.nih.gov/nuccore/ON256314).