Abstract
Ziziphus abyssinica root bark is widely used in folk medicine to manage liver diseases, particularly, jaundice but its effect on paracetamol-induced liver toxicity (PILT) has not yet been validated. This study explored the ameliorative effect of ethanolic root bark extract of Ziziphus abyssinica (ZAE) against PILT in rats. The flavonoid and phenolic content of ZAE was evaluated using Folin-Ciocalteau and aluminium trichloride colorimetric methods, respectively. Antioxidant activity of ZAE was determined in vitro by evaluating its ferrous reducing antioxidant capacity (FRAC) as well as DPPH and nitic oxide (NO) radicals scavenging activities. Sprague-Dawley rats were assigned to six groups (n = 6) and administered with normal saline (10 mL/kg, p.o.), N-acetylcysteine (50 mg/kg, i.p.) and ZAE (30, 100, and 300 mg/kg, p.o.) respectively for seven days after which they received paracetamol (PCM, 3000 mg/kg, p.o.). Animals were sacrificed 48 h after paracetamol administration under light anaesthesia and assessed for liver toxicity and oxidative stress. Total flavonoid and phenolic contents of ZAE were 1313.425 µg/mL quercetin equivalence and 268.31 µg/mL gallic acid equivalence respectively. ZAE exhibited marked FRAC as well as DPPH and NO radical scavenging activities with IC50s of 80.41 ± 1.56, 67.56 ± 1.11 and 7.11 ± 1.48 μg/mL respectively. ZAE and N-acetylcysteine significantly (p < 0.05) reduced the paracetamol-mediated elevation of serum total bilirubin, proteins and activity of liver enzymes (AST, ALP, and ALT). Similarly, ZAE increased hepatic glutathione, total thiols and catalase activity of the paracetamol intoxicated rats. Morphological changes associated with the paracetamol hepatotoxicity were also ameliorated by ZAE. Overall, the hepatoprotective effect of ZAE may be related to its antioxidant property.
Abbreviations: DPPH, 1,1-diphenyl-2-picrylhydrazyl; DTNB, 5,5-dithiobis-(2-nitrobenzoic acid); TCA, Trichloroacetic acid; CA, Catechin; AA, Ascorbic acid; GA, Gallic acid; DMSO, Dimethyl sulfoxide; EDTA, Ethylenediamine tetraacetic acid; PCM, Paracetamol; NAC, N-acetylcysteine, PILT, Paracetamol-induced liver hepatotoxicity; ZAE, Ethanolic root bark extract of Ziziphus abyssinica; NO, Nitric oxide; GSH, Gluthathione; CAT, Catalase; DRSA, DPPH radical scavenging assay; ALT, alanine aminotransferase; AST, Aspartate aminotransferase; ALP, alkaline phosphatase
Keywords: Hepatoprotection, Rhamnaceae, Acetaminophen, Liver injury, Antioxidant, Ziziphus abyssinica
Graphical Abstract
Highlights
-
•
The ethanolic leaf extract of Ziziphus abyssinica (ZAE) possesses high content of total flavonoids and phenols.
-
•
ZAE exhibited in vitro ferrous reducing activity as well as DPPH and nitic oxide (NO) free radicals scavenging activities.
-
•
ZAE ameliorated paracetamol-induced hepatotoxicity via the attenuation of glutathione, total thiols and catalase activities.
-
•
ZAE reduced paracetamol-mediated elevation of serum total bilirubin, proteins and the activity of liver enzymes in rats.
1. Introduction
One of the largest and most important internal organs of the body which plays a crucial role in metabolism, and regulation of essential biochemical and physiological processes is the liver [1]. It is involved in the synthesis of important biomolecules that are implicated in the fight against infections as well as detoxification of xenobiotics [2]. These processes predispose the liver to damage by hepatotoxic agents which include inflammatory mediators, toxins, and drugs such as paracetamol [1]. These substances contribute to the disruption of the normal architecture of liver, thus leading to a disruption of its functional capacity [3].
Paracetamol (PCM) is an extensively consumed analgesic and antipyretic agent. It is a well-known risk factor for acute liver failure and hepatotoxicity [4]. Paracetamol is also known to be a leading cause of drug-induced liver injury worldwide, and its toxicity is dose-dependent [5], [6]. It accounted for 44% of self-poisoning in the adult population of a hospital’s emergency department [7].
A well-known antioxidant mostly used in attenuating PCM-induced hepatotoxicity is N-acetyl cysteine (NAC) [8]. NAC exerts its protective effects by preventing hepatocellular glutathione (GSH) depletion at the oxidative injury phase, thus inhibiting the production of peroxynitrite and other reactive oxygen species in mitochondria [8]. Treatment with NAC has been associated with nausea, vomiting, and anaphylactoid reactions [7]. Another major limitation of NAC is its ineffectiveness, particularly, when the therapy is initiated eight-hour post PCM poisoning [9]. This, therefore, underscores the need for the pursuit of new drugs as replacement for or alternative to NAC, and plants have been known from time immemorial to be a dependable source of novel therapeutic agents.
Plants are known to have the potential to protect the liver against injuries and hepatotoxicity. Ziziphus abyssinica (Hochst Ex A. Rich) from the Rhamnaceae family is an example of such plants. It is also known in the English language as ‘Catch thorn plant’ and in the Sissala language of Ghana and Burkina Faso as ‘Larukluror’. Traditionally, the plant has been used in treating jaundice and other related live diseases [10]. The plant has also been shown to possess several other pharmacological properties including anti-ulcerogenic [11], anti-inflammatory [12], and anti-nociceptive [13], [14], [15], antioxidant [16], antidiarrheal [17], and antimalarial [18] activities in different experimental studies. In our previous study, we isolated two triterpenoid compounds, polpunonic acid and β-amyrin (BA), from the root bark of the plant and reported their anti-arthritic [19] and analgesic [15] properties. We have also reported on the protective effect of the plant against multi-organ injury induced by phenyl hydrazine in rats [20]. Despite the fact that the plant has been well-documented for its herbal-based therapeutic effect against jaundice and its related liver diseases in traditional medicine [10], its effect on paracetamol-induced liver injury has not yet been validated.
This study aimed at exploring the ameliorative effect of the ethanolic root bark extract of Ziziphus abyssinica in paracetamol-induced liver hepatotoxicity (PILT) in rats. This will afford an evidence-based support for the folkloric use of the plant to treat jaundice and other liver diseases. It will also pave the way for the search for new small molecules that could be alternatives or complementary to NAC in the pharmacological management of paracetamol poisoning.
2. Method and materials
2.1. Reagents
Reagents used in the study comprised: 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), Potassium ferricyanide, Trichloroacetic acid (TCA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferrous ammonium sulphate, catechin (CA), ascorbic acid (AA), gallic acid (GA), quercetin, Tris-HCl buffer, AlCl3, FeCl3, ammonium molybdate, sodium phosphate, tannic acid, DMSO, acetyl acetone, EDTA, paracetamol (PCM), Folin-Ciocalteu reagent and N-acetylcysteine (NAC). All other chemicals and reagents used in the study were of analytical grade and were procured from Merck KGaA, Darmstadt, Germany.
2.2. Plant collection and preparation
Ziziphus abyssinica root bark was harvested from Ejura (7°23′00.16″N, 1°22′00.00″W) in the Ejura-Sekyedumase Municipality of the Ashanti region in November 2020. The plant sample was authenticated by a botanist in the herbarium unit of the University of Cape Coast, Ghana, with voucher ID. FAA/DR/20/002. The collected plant material was dried under shade for 21 days after it had been washed under running tap water. One [1] kg of the plant sample was milled into a fine powder.
2.3. Plant extraction
Powdered root bark (0.5 kg) was extracted with 1 L of 70% v/v ethanol for 72 h using the cold maceration method. The filtrate obtained was concentrated under reduced temperature (45 °C) and pressure using a rotary evaporator (RotavaporR-215 model, BÜCHI Labortechnik AG, Flawil, Switzerland). The extract obtained was labelled Ziziphus abyssinica extract (ZAE) and it was kept in a desiccator half-filled with activated silica up to the point of use. The percentage yield was 12.75% w/w.
2.4. Total phenolic content (TPC) of ZAE
The TPC of ZAE was quantified according to the Folin-Ciocalteau’s method [21]. The reaction mixture contained 375 μL of 1 in 10 dilution of Folin-Ciocalteu reagent and 125 μL of ZAE (1 mg/mL). After keeping the mixture at 25 °C for 5 min, 375 μL of 7% Na2CO3 was then added. The volume of the reaction mixture was increased to 2.5 mL with distilled water and then kept for 2 h at 25 °C, when a change in colour was observed. The TPC in GA equivalence (GAE) was measured spectrophotometrically (PG T70 Instruments, Leicestershire - UK) at 765 nm optical density, against a reaction blank. A GA standard curve was drawn in the range of 0 – 300 μg/mL. At each concentration, the test was done three times and the average computed.
2.5. Total flavonoid content (TFC) of ZAE
The TFC of ZAE was quantified based on an earlier described protocol [21], [22] using the AlCl3 colorimetric procedure with quercetin being utilised as the standard. Specifically, 200 μL of 1 mg/mL ZAE was added to 50% ethanol (800 mL), followed by addition of 5% sodium nitrite (60 μL), and incubated for 5 min at 25 °C. Subsequently, 60 μL of 10% AlCl3 was added, and kept for 6 min 25 °C before the addition of 1 M NaOH (400 μL). The resulting solution was dissolved in 660 μL of distilled water. A coloured complex generated was measured at 510 nm wavelength using PG T70 Instruments, Leicestershire - UK. The estimated TFC was presented in quercetin equivalence (QE), with varying amounts of quercetin in ethanol as the benchmark.
2.6. In vitro antioxidant assays
2.6.1. DPPH radical scavenging assay (DRSA)
The test is based on the principle that H+ donating ability of test agents with antioxidant properties causes decolourisation of DPPH in methanol solution from purple/violet colour to shades of yellow [23]. The test comprised a reaction mixture containing 2.4 mL of 0.1 mM DPPH in methanol solution mixed with 1.6 mL of ZAE/ascorbic acid in methanol at various concentrations (1.95–500 mg/mL), mixed thoroughly by vortexing and kept at 25 °C for 30 min in the dark. The absorbance of the final product was determined spectrophotometrically (PG T70 Instruments, Leicestershire - UK) at 517 nm. The experiments were conducted in triplicates at each concentration.
The % DRSA of ZAE was estimated using the equation:
In this equation, A0 and A1 are the absorbances of control and tests respectively. From the graph of % DRSA versus concentration, the IC50s were estimated.
2.6.2. Ferrous reducing antioxidant capacity (FRAC)
Rahman's method [23] was used in determining the FRAC of ZAE. In the test, Fe2+ concentration was obtained by measuring the generation of Perl's Prussian blue at 700 nm. The test tubes were filled with 0.25 mL of samples/standard solution in different concentrations (1.95–500 mg/mL), 0.62 mL of potassium buffer (0.2 M) and 0.62 mL of 1% K3Fe (CN)6. The reaction mixture was incubated for 20 min at 50 °C to complete the reaction. Afterwards, to each test tube was added a 0.625% trichloroacetic acid (TCA) solution. After centrifuging the entire mixture for 10 min at 3000 rpm, 1.8 mL of the supernatant was taken and combined with distilled water (1.8 mL) of and 0.1% FeCl3 (0.36 mL). The absorbances of the reaction mixtures were measured at 700 nm (PG T70 Instruments, Leicestershire - UK) against a blank. An identical reaction combination was used in the blank solution, but without the plant extracts or standards. The reducing capacity of the test agent is said to increase when the absorbance of the reaction mixture increases. At every concentration, the experiment was repeated three times.
2.6.3. Nitric oxide scavenging activity (NOSA)
The Griess-Ilosvay reagent was used to evaluate ZAE's nitric oxide scavenging ability [21]. Separate test tubes were prepared with 0.25 mL of various concentrations (1.95–500 g/mL) of ZAE or ascorbic acid. After the addition of sodium nitroprusside (10 mM), 0.125 mL of sodium phosphate buffer (pH 7.4) was added. After incubating the reaction mixture at 25 °C for 180 min, 0.25 mL of sulfanilic acid reagent (0.33% in 20% glacial acetic acid) was added to terminate the diazotization reaction. This was finally accomplished by adding 0.25 mL of 0.1% naphthyl ethylenediamine dihydrochloride to create a pink solution.
The test tubes were incubated at 25 °C for another 30 min after a thorough mixing. The concentration of nitrite was measured at 546 nm against a control setup which had a similar content with the exception of replacing the extract/standard with a buffer. NO scavenging ability of ZAE and ascorbic acid were calculated using the formula below:
2.7. Experimental animals
Male Sprague-Dawley rats (180–250 g) were obtained from the Animal House of the University of Cape Coast. They were given normal feed from Agricare in Kumasi, Ghana, as well as unlimited access to water. Throughout the investigation, the NIH’s Guide for the Care and Use of Laboratory Animals were followed. Ethical approval was obtained from the Research and Ethics Committee of the School of Pharmacy and Pharmaceutical Sciences, University of Cape Coast, on the 25th September, 2020 with the certification number UCCSoPPS/REC/20/011.
2.8. Experimental design
The experiment was conducted using an earlier described protocol [24]. Six groups of rats (n = 6) were fasted for 24 h before treatment was initiated. The animals were subsequently treated once a day for seven days. Group I (naïve control) and Group II (negative control) received normal saline (NS 10 mL/kg, p.o.), Group III (positive control) received N-acetylcysteine (NAC) 50 mg/kg, i.p., Groups IV, V, and VI received 30, 100, and 300 mg/kg, p.o. of ZAE respectively. With the exception of Group I animals which received additional normal saline (10 mL/kg. p.o.), the rest of the animals were additionally dosed with 3000 mg/kg p.o. of paracetamol (PCM) 3 h after the last dose of ZAE or NAC had been administered on the 7th day. All the animals were subsequently fasted for 48 h. after PCM administration. Afterwards, the animals were administered with a light anaesthesia (pentobarbitone 50 mg/kg, i.p.) and blood was collected by cardiac puncture into gel-separator vacutainer tubes for biochemical analysis. The animals were then humanely sacrificed by cervical dislocation and their liver harvested for further investigation. The treatment schedule is presented in Table 1.
Table 1.
Treatment schedule of the various groups.
| Groups | Description | Pre-treatment for 7 days | Induction of hepatotoxicity |
|---|---|---|---|
| I | Naïve Control | NS (10 mL/kg, p.o.) | NS (10 mL/kg, p.o.) |
| II | Negative Control | NS (10 mL/kg, p.o.) | PCM (3000 mg/kg, p.o.) |
| III | Positive Control | NAC (50 mg/kg, i.p.) | PCM (3000 mg/kg, p.o.) |
| IV | Low Dose | ZAE (30 mg/kg, p.o.) | PCM (3000 mg/kg, p.o.) |
| V | Medium Dose | ZAE (100 mg/kg, p.o.) | PCM (3000 mg/kg, p.o.) |
| VI | High Dose | ZAE (300 mg/kg, p.o.) | PCM (3000 mg/kg, p.o.) |
The lethal dose of the extract has been found to be beyond 5000 mg/kg according to our previous study [15]. This is because, no adverse effect or dearth was recorded at doses as high as 5000 mg/kg of the extract. Also, selection of the doses for NAC, PCM and ZAE were based on previous studies [15], [20], [24] as well as preliminary investigations in our laboratories.
2.8.1. Evaluation of serum biochemical markers
Blood samples were collected by cardiac puncture into gel separator tubes for biochemical analysis. The biochemical parameters that were evaluated in both the control and test groups were total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP).
2.8.2. Histopathology of liver tissues
The rats were sacrificed under light anaesthesia, dissected, and the liver was harvested for gross necropsy and histopathological examination. The liver organs harvested from the rats were divided into two and one-half of each liver was fixed in 10% neutral buffered formalin (NBF) immediately after collection for 24 h. The liver was trimmed into cassettes and re-fixed. The tissue cassettes were processed for routine histopathology in a series of graded ethanol and xylene. The tissues were embedded in paraffin wax and a semi-automated rotary microtome (Bright 5040, Japan) was used to cut sections of 5 µm thickness from the tissue blocks. The sections mounted on glass slides were processed for routine haematoxylin and eosin staining. The tissue slides were observed using a binocular light microscope (Olympus, Japan) connected with a digital camera, Amscope (MD500, USA), and a computer. Further analysis was carried out on the tissue micrographs that were taken.
2.8.3. Estimation of liver antioxidant activity
2.8.3.1. Preparation of tissue homogenate and supernatant
The remaining one-half of each liver organ removed from the sacrificed rats were individually washed with 0.9% normal ice-cold saline, weighed, and stored at − 80 °C. The tissues were homogenised using a homogenizer (WiseTis® HG-15D, China). The homogenate was centrifuged for 30 min at 4 °C at 800 g. The supernatant containing fractions of the liver was used for the antioxidant assay.
2.8.3.2. Total tissue protein
The total protein content of 10% liver homogenate was estimated using the modified Lowry’s method [25].
2.8.3.3. Catalase (CAT)
Catalase activity, was determined using a previously described method [26]. Catalase activity represents the amount of enzyme required to remove 1 µmol of H2O2 per unit per gram of tissue. To do this, 0.1 mL of the liver homogenate was added to 1 mL of 4% ammonium molybdate and 2 mL of 0.03% H2O2 solution. The reaction mixture was measured spectrophotometrically (Lasany, China) at 410 nm.
2.8.3.4. Glutathione (GSH)
Glutathione levels were determined by precipitating proteins with 50% TCA. The mixture was then centrifuged for 15 min at 3000 rpm. Supernatant from the centrifugation was mixed with 4.0 mL of 0.4 M Tris buffer solution (pH=8.0) and 0.1 mL of DTNB. Subsequently, the new reaction mixture was incubated for 10 min. This was followed by the addition of DNTB and within 5 min, the absorbance was recorded at 412 nm [27].
2.8.3.5. Total thiol (TSH)
To estimate total thiols levels in the liver tissues of the rats, the procedure outlined by Sedlack and Lindsay [27] was used. To 0.5 mL of tissue homogenate, 0.1 mL of 0.01 M DTNB and 1.5 mL of 0.2 M Tris buffer (pH=8.2) were added mixed together. Absolute methanol (7.9 mL) was added to the mixture to obtain 10 mL. An absorbance measurement was taken at 412 nm between the mixture and appropriate blanks after 10 min of incubation. By using ε = 13,600 cm/M −1 the amount of TSH in the reaction mixture was computed and analysed.
2.9. Statistical analysis
Group means ± SEM (standard error of the mean) were used in presenting the results. For all tests, statistical significance of p < 0.05 was considered. Graphpad Prisms® version 8.0 for Windows® 10 (GraphPad Software, San Diego, CA, USA) was used to plot all graphs. To determine the differences between treatment groups, one-way ANOVA plus a Dunnet’s post hoc test was used.
3. Results
3.1. Total flavonoid and phenol content
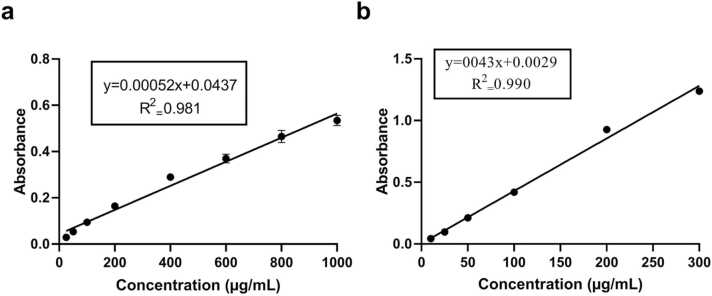
Using the standard graphs as shown in Fig. 1a and Fig. 1b respectively, the total flavonoid content of ZAE in QE was found to be 1313.425 µg/mL, whereas the total phenolic content in GAE was found to be 268.31 µg/mL.
Fig. 1.
Standard graphs of (a) quercetin for the estimation of the flavonoid content of ZAE and (b) gallic acid for the estimation of phenolic content of ZAE.
3.2. In vitro antioxidant assay
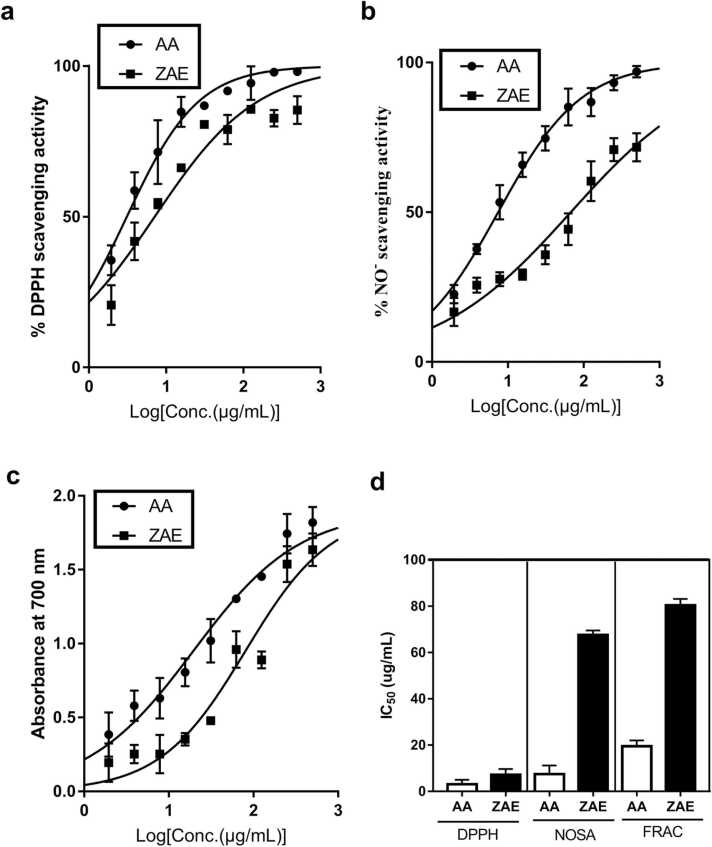
ZAE exhibited a notable DPPH radical scavenging activity with an IC50 of 7.11 ± 1.48 μg/mL, which was comparable to the IC50 of 3.10 ± 1.10 μg/mL for ascorbic acid used as a reference drug (Fig. 2a and d). Again, ZAE produced an NO radical scavenging activity with an IC50 of 67.56 ± 1.11 μg/mL whereas ascorbic acid, used as a standard reference drug, produced an IC50 value of 7.45 ± 2.14 μg/mL (Fig. 2b and d). With respect to ferrous reducing antioxidant capacity, ZAE produced a ferric reducing power with an IC50 of 80.41 ± 1.56 μg/mL as compared to 19.48 ± 1.45 μg/mL for the ascorbic acid used as the positive control drug (Fig. 2c and d).
Fig. 2.
Graphs depicting ZAE and ascorbic acid's (AA) (a) DPPH scavenging capacity, (b) nitric oxide scavenging activity (NOSA), and (c) ferrous reducing antioxidant capacity (FRAC). The IC50s of the various assays are depicted graphically in panel (d).
3.3. Effect of ZAE on ALT, AST, ALP, and TB
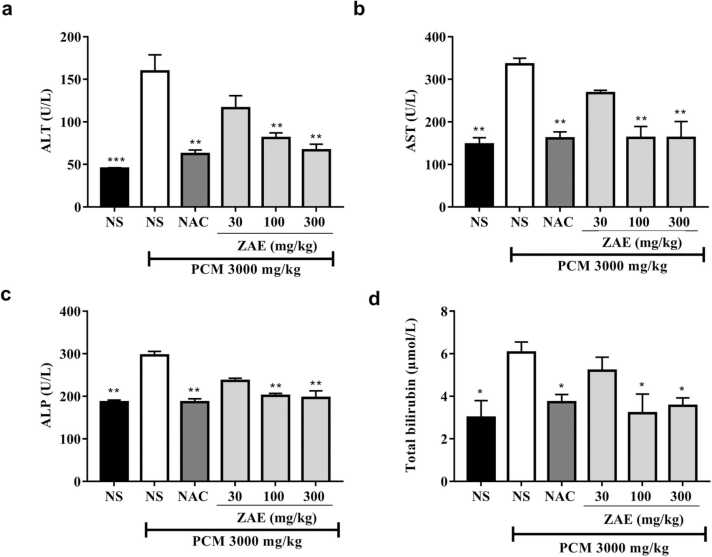
Oral administration of PCM (3000 mg/kg) caused significant (p < 0.01) elevations in serum biochemical marker activities such as ALP, ALT, and AST, as well as increased total bilirubin levels compared to naïve control groups. However, rats that received additional treatments with ZAE (100 and 300 mg/kg) or NAC (50 mg/kg) recorded significant (p < 0.05) decreases in biochemical marker levels (Fig. 3).
Fig. 3.
Effect of ZAE on serum biochemical parameters in paracetamol-induced hepatotoxicity in rats. The results are presented as mean ± SEM. **p < 00.01 and *p < 0.05 compared to the PCM +NS treatment group. One-way ANOVA followed by Dunnet’s post hoc test.
3.4. Antioxidant activity
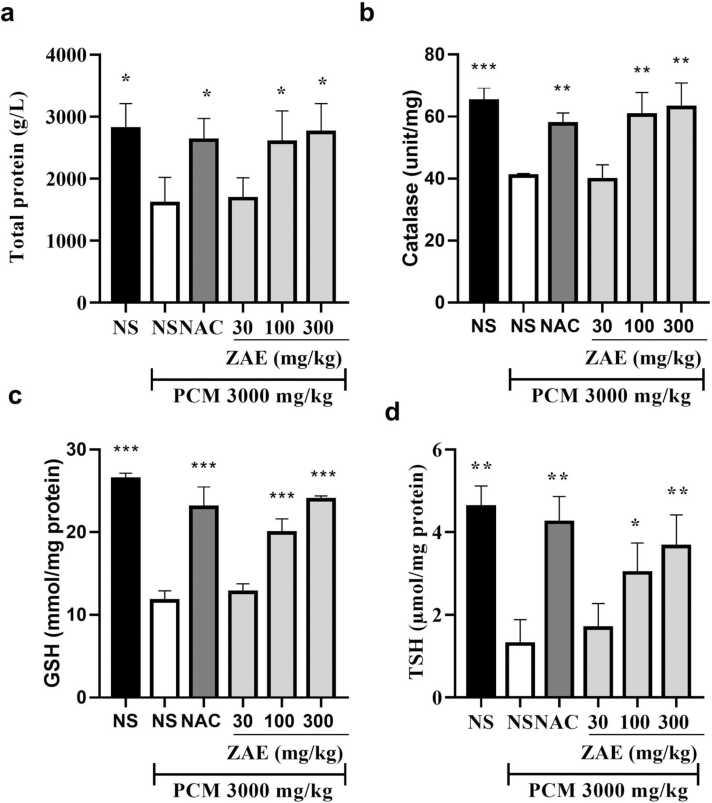
The activities of in vivo antioxidant enzymes and total proteins following the administration of a toxic dose of paracetamol and subsequent treatment with ZAE or NAC. Total protein, GSH, CAT, and TSH levels increased significantly (p < 0.05) in the treatment groups compared to rats treated with paracetamol only (Fig. 4).
Fig. 4.
Effect of ZAE on protein (a), CAT (b), GSH (c) and TSH (d) in paracetamol-induced liver toxicity in rats. ***p < 0.001, **p < 0.01 and *p < 0.05 compared to the PCM group. One-way ANOVA followed by a Dunnet’s post hoc test.
3.5. Histopathological observation
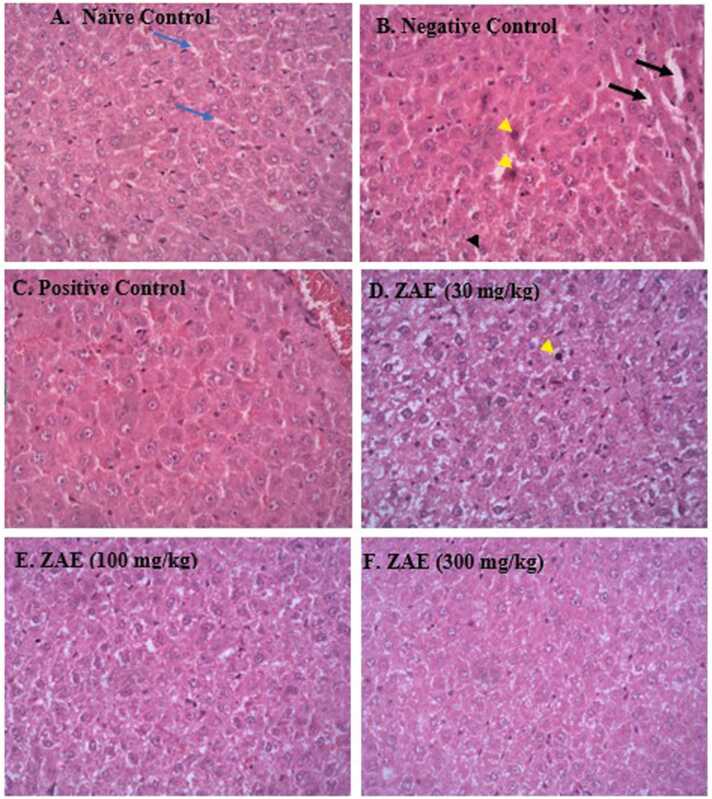
Fig. 5 shows micrographs of the liver sections of paracetamol-induced toxicity in rats. A photomicrograph of the naive control group (Fig. 5 A) showed a normal parenchymal structure of the liver. However, in the negative control group (Fig. 5B) that was treated with only PCM (3000 mg/kg), single-cell hepatocellular necrosis (yellow arrowhead) characterised by a condensed, dark stained nucleus with some degeneration in the cytoplasm (ballooning degeneration) were observed. Also, the hepatocytes’ sinusoidal dilatations were observed in the negative control group more than in normal hepatocytes as observed in the naïve control group. However, reduced hepatocellular damage was observed in the groups treated with ZAE 30 mg/kg (5D), 100 mg/kg (5E) and 300 mg/kg (5 F) as well as the group treated with NAC. Also, uniform and normal sinusoidal spaces were observed in the groups treated with the extract and NAC.
Fig. 5.
(A-F) shows photomicrographs of the liver tissue of all the treated and control groups at a magnification of x 400 (H & E). Dilatable sinusoidal spaces (black arrow in 5B), normal sinusoidal spaces (blue arrows in A), hepatocellular necrosis (yellow arrowhead in B), ballooning degeneration of hepatocytes (black arrowhead).
4. Discussion
Paracetamol is a popular over-the-counter medication used in the management of pyrexia and pain. It is also known to cause liver toxicities when taken above the normal therapeutic dose [28]. Paracetamol is converted to a reactive metabolite called N-acetyl-para-benzoquinone immine (NAPQI) by the liver isoform enzymes cytochrome P450 CYP2E1 and CYP2A6 [29]. Higher levels of NAPQI causes oxidative stress and cellular damage in the liver. The resultant effect is the accumulation of reactive oxygen species that affect the membrane of hepatocytes, induce lipid peroxidation, and cause liver necrosis [24].
The first part of the study was conducted to determine ZAE's antioxidant and radical scavenging potential in light of the role free radicals and reactive oxygen species play in PCM-induced liver injury as described earlier [30], and the importance of antioxidant restoration in attenuating PCM-induced hepatotoxicity. To determine the antioxidant capacity of test substances, it’s been established that a single assay approach is insufficient as different antioxidant tests differ in terms of assay principle and experimental outcomes [31]. For instance, DPPH as uses organic radical generators whereas the FRAC assay technique uses metallic ions for oxidation. In this study, ZAE exhibited remarkable antioxidant properties which included a high DPPH scavenging activity, nitric oxide reducing property, and ferrous reducing antioxidant capacity (Fig. 2).
It is important to mention that antioxidant capacity of extracts has been shown to be correlated with their total phenolic [32] and flavonoid [21] capacities. The phenolics and flavonoids have been documented to possess hepatoprotective properties [33], [34] and previous study indicated [15] the presence of these phytochemicals in Ziziphus abysinnica, thus confirming the potential of ZAE to reverse liver injury induced by phenylhydrazine. In this study, ZAE was found to have high levels of phenolic and flavonoid content. Plants that contain high levels of flavonoids and phenolic compounds have been shown to contain high exogenous antioxidant properties and are naturally beneficial in the prevention of liver diseases [35].
Since oxidative stress plays a key role in the development of liver diseases [8], any substance with antioxidative potential should also possess protective effects against liver damage. On the basis of this assertion, it might also be plausible to suggest that ZAE may exhibit in vivo antioxidant and hepatoprotective properties hence the need to investigate these effects using PCM-induced hepatotoxicity in rats. Notably, an essential event in PCM-induced hepatotoxicity is mitochondrial oxidative stress [8]. As a result, research into treatment techniques for PCM-induced hepatotoxicity has mostly focused on ways to mitigate this occurrence. Based on the foregoing, several natural substances have been identified to have the ability to cure PCM-induced liver impairment via antioxidant pathways [31]. The antioxidant activities of these natural compounds were reported to be attributable to increased levels of endogenous enzymes superoxide dismutase, GSH, catalase, as well as their exogenous antioxidant properties as discussed earlier.
One of the most important endogenous antioxidant that is involved in hepatoprotection is Glutathione (GSH). It is one of the most abundant non-enzyme free radical scavengers produced by the liver. It eliminates free radicals like hydrogen peroxides, superoxide radicals, and alkoxy radicals, while also stabilising membrane proteins. GSH also serves as a substrate for glutathione peroxidase [36]. In this study, the administration of PCM caused a significant reduction in the levels of liver GSH. However, treatment of rats with ZAE and NAC caused a significant elevations in the levels of GSH as observed in Fig. 4b.
Catalase (CAT), on the other hand, is an essential superoxide and hydrogen peroxide scavenger found in animal tissues, including the liver and red blood cells. It protects cells from oxidative stress caused by the generation of hydroxyl radicals in cells [37]. In this study, the concentration of CAT decreased in the liver tissues of the PCM only treated group, indicating an increase in the production of free radicals due to the activities of paracetamol metabolic product, NAPQI. However, the administration of ZAE, as well as NAC, ameliorated the paracetamol-induced hepatotoxicity by increasing the levels of CAT. This agrees with previous studies that reported on the hepatoprotective effects of gallic acid [38], 6-gingerol [39], and methanol extract of Fagonia olivieri DC [40] in rodent models through amelioration of oxidative stress. An elevated CAT levels resulting from ZAE administration indicates that superoxide radicals generated from PCM were be effectively disabled.
In this study, it was also realised that reduced levels of total thiols (-SH group) caused by PCM-induced toxicity was reversed by both ZAE and NAC as observed in Fig. 4c. It is important to note that antioxidant defense of cells relies heavily on total thiols for the detoxification of extracellular and intracellular reactive oxygen species. Protein thiols involved in DNA synthesis and repair depend on total thiols concentration for maintaining redox equilibrium [41].
A prerequisite for the synthesis of serum proteins is a non-compromised liver. This study found hypoproteinemia in the PCM-treated group compared to the nave control. This is possibly due to decreased protein synthesis, which is a common sign of hepatotoxicity [42]. However, treatment of the animals with ZAE and NAC reversed the hypoproteinemia. This emphasizes the hepatoprotective effect of the extract and the NAC.
Through lipid peroxidation, the reactive species (NAPQI) produced by paracetamol overdose causes damages to hepatic cells, resulting in elevated serum levels of ALT and AST. ALT, in particular, is an important biomarker for hepatotoxicity. This is because the liver has the highest amount of the enzyme compared to the heart, brain, and skeletal muscles, hence making ALT. On the other hand, due to its presence in other organs, AST has a lower specificity for liver damage despite the fact that its importance in hepatotoxicity cannot be overlooked. As such, when the liver is stressed, these enzymes leak into the blood in direct proportions to the extent of liver damage [43], [44]. The evaluation of the levels of ALT and AST in the liver is, therefore, indispensable especially in the assessment of paracetamol toxicity. In the present study, the administration of 3000 mg/kg of PCM caused a marked increase in serum ALT and AST levels as observed in Fig. 3. This was expected as similar results were obtained in earlier studies that used animal models to explore paracetamol-induced hepatotoxicity [45], [46], [47]. It is critical to note that injured hepatocytes can be identified based on the alterations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes in plasma. However, the pre-treatment of the rats with ZAE (100 and 300 mg/kg) produced a significant reduction in AST and ALT. This gives an indication that ZAE may reduce transaminase enzyme activity while producing regeneration and stabilisation of the membranes of liver cells.
Another liver enzyme worth investigating is the alkaline phosphatase (ALP). ALP is a hydrolysable enzyme produced in the membranes that line the bile duct and canaliculi, and are found in high concentrations in the blood, especially, in the presence of hepatobiliary damage [21], [42]. Hepatotoxicity is known to cause biliary congestion, thus leading to the inability of the body to excrete ALP, resulting in its elevation as seen in the PCM group (Fig. 3). However, treatment of the animals with ZAE reversed the ALP levels to normal. This suggests that ZAE exhibited hepatoprotective properties possibly via the reduction in the obstruction of the bile duct.
Also, low serum levels of total bilirubin was reversed to normal. The level of total bilirubin could be attributed to the ability of ZAE to reverse the injury caused by PCM. Again, the groups pre-treated with ZAE saw a marked increase in hepatocyte regeneration and a decreased cytoplasmic degeneration. This is supported by a previous study conducted by Henneh et al. [19] in which ZAE offered protection against multi-organ injury induced by phenyl hydrazine administration in rats.
The alterations in liver enzyme levels following paracetamol administration were confirmed by histopathological changes observed in liver tissues. Administration of paracetamol overdose caused pathological changes in the liver such as the presence of degeneration of hepatocytes, haemorrhagic necrosis with widened and congested sinusoids, which is consistent with previous reports [1], [28]. The presence of necrosis could be attributed to the stimulation of the innate immune response that recruits neutrophils and other inflammatory cells through the release of mediators that trigger the pro-inflammatory cytokine pathway by Kupffer cells, resulting in their accumulation in the sinusoids [48]. The extract ameliorated this effect possibly through its membrane stabilising and inhibitory effect against protein denaturation, neutrophil degranulation and pro-inflammatory cytokines expression [12], [15].
5. Conclusion
The study demonstrates that ethanolic root bark extract of Ziziphus abyssinica ameliorates paracetamol-induced hepatic injury. These protective effects may, at least in part, be attributed to the extract's antioxidant properties. This is due to the fact that exogenously, ZAE exhibited marked ferrous reducing antioxidant capacity as well as DPPH and NO radical scavenging activities in a similar manner as ascorbic acid. Compared to the standard reference drug, NAC, the extract was able to significantly increase the levels of endogenous antioxidants such as catalase, gluthathione and total thiols. ZAE and N-acetylcysteine significantly reduced the paracetamol-mediated elevation of serum total bilirubin, proteins and activity of liver enzymes (AST, ALP, and ALT).
Ethics approval and consent to participate
Ethical approval was obtained from the Research and Ethics Committee of the School of Pharmacy and Pharmaceutical Sciences, University of Cape Coast, with the certification number UCCSoPPS/REC/20/011.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors are grateful to the staff of the Department of Biomedical Sciences and the Department of Biochemistry, University of Cape Coast for their support.
Authors’ contribution
FAA and ITH conceived the idea, supervised the project, contributed to study design, data interpretation, and review of the manuscript. WA, AK, MAA, and JA contributed to bench work, data analyses, and writing of the draft manuscript. ME and MBA contributed to study design, data interpretation, and review of the manuscript. BKA contributed to the antioxidant as well as phenolic and flavonoid content assays. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Data availability
All data generated or analysed during this study are included in this published article.
Handling Editor: Dr. L.H. Lash
Contributor Information
Martins Ekor, Email: martins.ekor@ucc.edu.gh.
Francis Ackah Armah, Email: francis.armah@ucc.edu.gh.
Data availability
Data will be made available on request.
References
- 1.Mishra G., Khosa R., Singh P., Jha K. Hepatoprotective potential of ethanolic extract of Pandanus odoratissimus root against paracetamol-induced hepatotoxicity in rats. J. Pharm. Bioallied Sci. 2015;7(1):45–48. doi: 10.4103/0975-7406.148776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamed Saleem T.S., Madhusudhana Chetty C., Ramkanth S., Rajan V.S.T., Mahesh Kumar K., Gauthaman K. Hepatoprotective herbs–a review. Int. J. Res. Pharm. Sci. 2010;1(1):1–5. [Google Scholar]
- 3.Nema A.K., Agarwal A., Kashaw V. Hepatoprotective activity of Leptadenia reticulata stems against carbon tetrachloride-induced hepatotoxicity in rats. Indian J. Pharmacol. 2011;43(3):254. doi: 10.4103/0253-7613.81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong H.Y., Medrano N., Borobia A.M., Ruiz J.A., Martínez A.M., Martín J., et al. Hepatotoxicity induced by acute and chronic paracetamol overdose in children: where do we stand? World J. Pediatr. 2017;13(1):76–83. doi: 10.1007/s12519-016-0046-6. [DOI] [PubMed] [Google Scholar]
- 5.Bao Y., Wang P., Shao X., Zhu J., Xiao J., Shi J., et al. Acetaminophen-induced liver injury alters expression and activities of cytochrome P450 enzymes in an age-dependent manner in mouse liver. Drug Metab. Dispos. 2020;48(5):326–336. doi: 10.1124/dmd.119.089557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunchorntavakul C., Reddy K.R. Acetaminophen-related hepatotoxicity. Clin. Liver Dis. 2013;17(4):587–607. doi: 10.1016/j.cld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Wong A., Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin. Toxicol. 2017;55(8):879–892. doi: 10.1080/15563650.2017.1317349. [DOI] [PubMed] [Google Scholar]
- 8.Yan M., Huo Y., Yin S., Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274–283. doi: 10.1016/j.redox.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyte A.J., Kehrl T., Brooks D.E., Katz K.D., Sokolowski D. Safety and effectiveness of acetadote for acetaminophen toxicity. J. Emerg. Med. 2010;39(5):607–611. doi: 10.1016/j.jemermed.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J. Ethnopharmacol. 2009;124:69–78. doi: 10.1016/j.jep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Ugwah M.O., Etuk E.U., Bello S.O., Aliero A.A., Ugwah-Oguejiofor C.J. Comparative studies of anti-ulcerogenic activities of three Nigerian medicinal plants: a preliminary evaluation. J. Med. Plants Res. 2013;7(9):490–495. [Google Scholar]
- 12.Henneh I.T., Ameyaw E.O., Biney R.P., Armah F.A., Obese E., Konjah D., et al. Ziziphus abyssinica hydro-ethanolic root bark extract attenuates acute inflammation possibly through membrane stabilization and inhibition of protein denaturation and neutrophil degranulation. West Afr. J. Pharm. 2018;29(2):81–94. [Google Scholar]
- 13.Boakye-Gyasi E., Henneh I.T., Abotsi W.K.M., Ameyaw E.O., Woode E. Hydro-ethanolic leaf extract of Ziziphus abyssinica Hochst Ex A. Rich (Rhamnaceae) exhibits anti-nociceptive effects in murine models. BMC Complement. Altern. Med. 2017;17(1):1–12. doi: 10.1186/s12906-017-1750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boakye-gyasi E., Henneh I.T., Kofi W., Abotsi M., Ameyaw O., Woode E., et al. Possible mechanisms involved in the anti- nociceptive effects of hydro-ethanolic leaf extract of Ziziphus abyssinica. Pharm. Biol. 2017;0(0):1962–1971. doi: 10.1080/13880209.2017.1355927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henneh I.T., Armah F.A., Ameyaw E.O., Biney R.P., Obese E., Boakye-Gyasi E., et al. Analgesic effect of Ziziphus abyssinica involves inhibition of inflammatory mediators and modulation of KATP channels, opioidergic and nitrergic pathways. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.714722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyaberi M.O., Onyango C.A., Mathooko F.M., Maina J.M., Makobe M., Mwaura F. Evaluation of phytochemical, antioxidant and antibacterial activity of edible fruit extracts of Ziziphus abyssinica A. Rich. 2010:623–629. [Google Scholar]
- 17.Ugwah-Oguejiofor J.C., Alkali I.Y., Ugwah M.O., Abubakar K. Antidiarrhoeal potential of the aqueous root extract of Ziziphus abyssinica a. Rich. Sch. Acad. J. Pharm. 2013;2:419–423. [Google Scholar]
- 18.Muthaura C.N., Keriko J.M., Mutai C., Yenesew A., Gathirwa J.W., Irungu B.N., et al. Antiplasmodial potential of traditional phytotherapy of some remedies used in treatment of malaria in Meru–Tharaka Nithi County of Kenya. J. Ethnopharmacol. 2015;175:315–323. doi: 10.1016/j.jep.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Henneh I.T., Huang B., Musayev F.N., Al Hashimi R., Safo M.K., Armah F.A., et al. Structural elucidation and in vivo anti-arthritic activity of β-amyrin and polpunonic acid isolated from the root bark of Ziziphus abyssinica HochstEx. A Rich (Rhamnaceae) Bioorg. Chem. 2020;98 doi: 10.1016/j.bioorg.2020.103744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henneh I.T., Agyei P.E.O., Obese E., Biney R.P., Antwi-Adjei M., Yahaya E.S., et al. Leaf and root bark extracts of Ziziphus abyssinica Hochst ex. A. Rich (Rhamnaceae) ameliorate hepatic, renal and splenic injuries induced by phenylhydrazine in rats. J. Basic Clin. Physiol. Pharmacol. 2021;32(1):20200111. doi: 10.1515/jbcpp-2020-0111. [DOI] [PubMed] [Google Scholar]
- 21.Armah F.A., Henneh I.T., Alake J., Ahlidja W., Amoani B., Ofori E.G., et al. In: Helegbe G.K., editor. Vol. 2021. 2021. Allanblackia floribunda seed extract attenuates the ethanol-induced gastric ulcer in rats via the inhibition of TNF-α and INF-γ levels and modulation in the expression of Ki67 protein; p. 6694572. (Biomed Res Int). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 23.Rahman M.M., Islam M.B., Biswas M., Alam A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015;8(1):1–9. doi: 10.1186/s13104-015-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmood N.D., Mamat S.S., Kamisan F.H., Yahya F., Kamarolzaman M.F.F., Nasir N., et al. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. Biomed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/695678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Prabhakar K.R., Veerapur V.P., Parihar K.V., Priyadarsini K.I. Evaluation and optimization of radioprotective activity of Coronopus didymus Linn. in c -irradiated mice. Int. J. Radiat. Biol. 2006;82(8):525–536. doi: 10.1080/09553000600876686. [DOI] [PubMed] [Google Scholar]
- 27.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 28.Prescott L.F., Roscoe P., Wright N., Brown S.S. Plasma-paracetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet. 1971;297(7698):519–522. doi: 10.1016/s0140-6736(71)91125-1. [DOI] [PubMed] [Google Scholar]
- 29.Tittarelli R., Pellegrini M., Scarpellini M.G., Marinelli E., Bruti V., Di Luca N.M., et al. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017;21(1):95–101. [PubMed] [Google Scholar]
- 30.Zakaria Z.A., Kamisan F.H., Kek T.L., Salleh M.Z. Hepatoprotective and antioxidant activities of Dicranopteris linearis leaf extract against paracetamol-induced liver intoxication in rats. Pharm. Biol. 2020;58(1):478–489. doi: 10.1080/13880209.2020.1764058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rašković A., Milanović I., Pavlović N., Ćebović T., Vukmirović S., Mikov M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014;14(1):1–9. doi: 10.1186/1472-6882-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng J., Yu X., Maninder M., Xu B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018;21(1):1524–1540. [Google Scholar]
- 33.Wang H., Liu Y.M., Qi Z.M., Wang S.Y., Liu S.X., Li X., et al. An overview on natural polysaccharides with antioxidant properties. Curr. Med. Chem. 2013;20(23):2899–9913. doi: 10.2174/0929867311320230006. [DOI] [PubMed] [Google Scholar]
- 34.Fu L., Xu B.-T., Xu X.-R., Gan R.-Y., Zhang Y., Xia E.-Q., et al. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129(2):345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed A., Islam T., Uddin M.E., Chowdhury A.U., Rahman M., Habib R., et al. In Vivo Antidiarrheal and Cytotoxic Potential of Different Fractions of Pandanus Foetidus Leaves. 1937;5(3):208–216. [Google Scholar]
- 36.Han D., Hanawa N., Saberi B., Kaplowitz N. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am. J. Physiol. Liver Physiol. 2006;291(1):G1–G7. doi: 10.1152/ajpgi.00001.2006. [DOI] [PubMed] [Google Scholar]
- 37.Scott M.D., Lubin B.H., Zuo L., Kuypers F.A. Erythrocyte defense against hydrogen peroxide: preeminent importance of catalase. J. Lab. Clin. Med. 1991;118(1):7–16. [PubMed] [Google Scholar]
- 38.Rasool M.K., Sabina E.P., Ramya S.R., Preety P., Patel S., Mandal N., et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010;62(5):638–643. doi: 10.1211/jpp.62.05.0012. [DOI] [PubMed] [Google Scholar]
- 39.Sabina E.P., Pragasam S.J., Kumar S., Rasool M. 6-gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. Zhong xi yi jie he xue bao. J. Chin. Integr. Med. 2011;9(11):1264–1269. doi: 10.3736/jcim20111116. [DOI] [PubMed] [Google Scholar]
- 40.Rashid U., Khan M.R., Sajid M. Hepatoprotective potential of Fagonia olivieri DC. against acetaminophen induced toxicity in rat. BMC Complement. Altern. Med. 2016;16(1):1–18. doi: 10.1186/s12906-016-1445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahajan L., Verma P.K., Raina R., Pankaj N.K., Sood S., Singh M. Alteration in thiols homeostasis, protein and lipid peroxidation in renal tissue following subacute oral exposure of imidacloprid and arsenic in Wistar rats. Toxicol. Rep. 2018;3(5):1114. doi: 10.1016/j.toxrep.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islam M.T., Quispe C., Islam M.A., Ali E.S., Saha S., Asha U.H., et al. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomed. Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111732. [DOI] [PubMed] [Google Scholar]
- 43.Hall P., Cash J. What is the real function of the liver ‘function’tests? Ulst. Med. J. 2012;81(1):30. [PMC free article] [PubMed] [Google Scholar]
- 44.Gowda S., Desai P.B., Hull V.V., Math A.A.K., Sonal N. A review on laboratory liver function tests. Pan Afr. Med. J. 2009;3:17. [PMC free article] [PubMed] [Google Scholar]
- 45.El Morsy E.M., Kamel R. Protective effect of artichoke leaf extract against paracetamol-induced hepatotoxicity in rats. Pharm. Biol. 2015;53(2):167–173. doi: 10.3109/13880209.2014.913066. [DOI] [PubMed] [Google Scholar]
- 46.Parmar S.R., Vashrambhai P.H., Kalia K. Hepatoprotective activity of some plants extract against paracetamol induced hepatotoxicity in rats. J. Herb. Med. Toxicol. 2010;4(2):101–106. [Google Scholar]
- 47.El-Bakry H.A., El-Sherif G., Rostom R.M. Therapeutic dose of green tea extract provokes liver damage and exacerbates paracetamol-induced hepatotoxicity in rats through oxidative stress and caspase 3-dependent apoptosis. Biomed. Pharmacother. 2017;96:798–811. doi: 10.1016/j.biopha.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 48.Jaeschke H. How relevant are neutrophils for acetaminophen hepatotoxicity? Hepatology. 2006;43(6):1191–1194. doi: 10.1002/hep.21246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
All data generated or analysed during this study are included in this published article.