Abstract
Background
Acute myocarditis has been described as a relatively rare cardiovascular complication of COVID-19 infection. However, data regarding the risk of myocarditis during the post-acute phase of COVID-19 are scant. We assess the risk of incident myocarditis in COVID-19 survivors within 1 year from the index infection by a systematic review and meta-analysis of the available data.
Methods
Data were obtained by searching Medline and Scopus for all studies published at any time up to September 1, 2022, and reporting the long-term risk of incident myocarditis in COVID-19 survivors. Myocarditis risk data were pooled using the Mantel-Haenszel random-effects models with hazard ratio (HR) as the effect measure with 95% confidence interval (CI). Heterogeneity among studies was assessed using the Higgins-Thompson I2 statistic.
Results
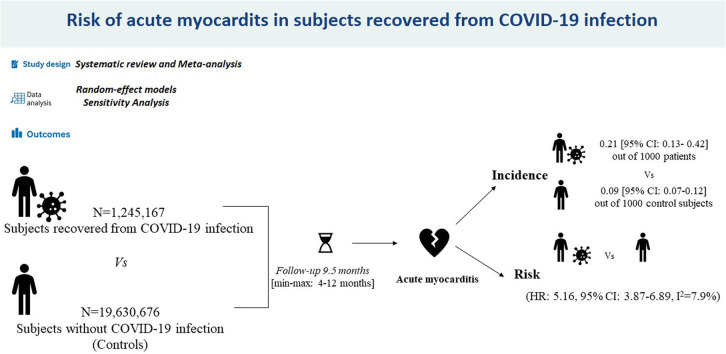
Overall, 20,875,843 patients (mean age 56.1 years, 59.1% male) were included in this analysis. Of them, 1,245,167 experienced (and survived) COVID-19 infection. Over a mean follow-up of 9.5 months, myocarditis occurred to 0.21 (95% CI 0.13-0.42) out of 1000 patients survived to COVID-19 infection compared with 0.09 [95% CI 0.07-0.12) out of 1000 control subjects. Pooled analysis revealed that recovered COVID-19 patients presented an increased risk of incident myocarditis (HR 5.16, 95% CI 3.87-6.89; P < 0.0001; I2 = 7.9%) within 1 year from the index infection. The sensitivity analysis confirmed yielded results.
Conclusions
Our findings suggest that myocarditis represents a relatively rare but important post-acute COVID-19 sequelae.
Graphical abstract
Résumé
Contexte
La myocardite aiguë est une complication cardiovasculaire de l’infection par le virus de la COVID-19 considérée comme étant relativement rare. On dispose toutefois de très peu de données quant au risque de myocardite durant la phase post-aiguë de la COVID-19. Nous avons donc réalisé un examen systématique et une méta-ana-lyse des données disponibles afin d’évaluer le risque de survenue d’une myocardite chez les survivants de la COVID-19 dans l’année suivant l’infection de référence.
Méthodologie
Les données utilisées sont tirées de toutes les études publiées dans les bases de données Medline et Scopus jusqu’au 1er septembre 2022 et rapportant le risque de survenue d’une myocardite chez les survivants de la COVID-19. Les données sur le risque de myocardite ont été regroupées à l’aide de modèles de Mantel-Haenszel à effets aléatoires, le rapport des risques instantanés (RRI) donnant la mesure des effets selon un intervalle de confiance (IC) à 95 %. L’hétérogénéité entre les études a été évaluée au moyen de la statistique I2 de Higgins et Thompson.
Résultats
Au total, 20 875 843 patients (âge moyen : 56,1 ans; hommes : 59,1 %) ont été inclus dans l’analyse. De ce nombre, 1 245 167 ont contracté la COVID-19 (et y ont survécu). Sur une période de suivi d’une durée moyenne de 9,5 mois, on a observé 0,21 (IC à 95 % : 0,13 à 0,42) cas de myocardite pour 1000 patients ayant survécu à la COVID-19, comparativement à 0,09 [IC à 95 % : 0,07 à 0,12) cas pour 1000 sujets témoins. L’analyse des données groupées a révélé que les patients ayant survécu à la COVID-19 étaient exposés à un risque accru de myocardite (RRI de 5,16; IC à 95 % : 3,87 à 6,89; p < 0,0001; I2 = 7,9 %) dans l’année suivant l’infection de référence. L’analyse de sensibilité a confirmé ces résultats.
Conclusions
Les résultats de notre analyse montrent que la myocardite est une séquelle post-aiguë de la COVID-19 relativement rare, mais importante.
Acute myocarditis (AM) has been described as a relatively rare complication of COVID-19 infection.1 Viral infections are a common cause of acute myocarditis, owing to a combination of direct cellular injury and T-cell cytotoxicity pointed at the myocardium, which can be amplified by the cytokine storm syndrome as described in SARS-CoV-2 infection.2, 3, 4 Recent analyses have mainly focused on the potential pathophysiologic mechanisms and occurrence of AM either as a complication of the infection during the acute phase of disease or after the administration of COVID-19 vaccines.5, 6, 7, 8 Conversely, few studies have investigated the risk of myocarditis after the index SARS-CoV-2 infection, and the estimation of potential post-acute COVID-19 myocarditis represents a major knowledge gap to be addressed. Therefore, the aim of the present study was to assess the risk of incident myocarditis in COVID-19 survivors within 1 year from the index infection by a systematic review and meta-analysis of the available data.
Material and Methods
Study design
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplemental Table S1).9 Data were obtained by searching Medline and Scopus for all studies published at any time up to September 1, 2022, reporting the long-term risk of incident myocarditis in COVID-19 survivors diagnosed from 4 months (minimum follow-up length of revised investigations) and a maximum of 12 months after discharge (maximum follow-up length of revised studies) after the index infection. In the revised manuscripts, this group of patients was compared to contemporary cohorts, defined as subjects who did not experience the SARS-CoV-2 infection and developed AM in the same follow-up period. The reviewed investigations identified the occurrence of AM by screening the medical records of enrolled patients with the use of International Classification of Diseases 10th Revision (ICD-10) codes I40 and I51.4.
Data extraction and quality assessment
The selection of studies to be included in our analysis was independently conducted by 2 of the authors (M.Z., C.B.) in a blinded fashion. Any discrepancies in study selection were resolved by consulting a third author (G.R.). The following MeSH terms were used for the search: “Myocarditis” AND “COVID-19 sequelae” OR “myocarditis” AND “COVID-19.” Moreover, we searched the bibliographies of the target studies for additional references. Specifically, inclusion criteria were studies 1) enrolling subjects with previous confirmed COVID-19 infection and 2) providing the hazard ratio (HR) and relative 95% confidence interval (CI) for the risk of incident myocarditis in the long-term period after the index infection compared with contemporary control cohorts. Conversely, case reports, review articles, abstracts, editorials/letters, and case series with fewer than 10 participants were excluded. Data extraction was independently conducted by 2 of the authors (M.Z., G.R). For all reviewed investigations, we extracted, when provided, the number of patients enrolled, the mean age, male sex, prevalence of cardiovascular comorbidities such as arterial hypertension (HT), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), obesity, preexisting HF, and cerebrovascular disease, and length of follow-up. The quality of included studies was graded according to the Newcastle-Ottawa quality assessment scale (NOS).10
Data synthesis and analysis
Continuous variables were expressed as means and categoric variables as numbers and relative percentages. Myocarditis risk data were pooled using the Mantel-Haenszel random-effects models with hazard ratio (HR) as the effect measure with 95% confidence interval (CI). Heterogeneity among studies was assessed by means of the Higgins-Thompson I 2 statistic. Specifically, the I 2 values correspond to the following levels of heterogeneity: low (< 25%), moderate (25%-75%), and high (> 75%). The presence of potential publication bias was verified by visual inspection of the funnel plot. Owing to the low number of included studies (< 10), small-study bias was not examined because our analysis was underpowered to detect such bias. However, a predefined sensitivity analysis (leave-one-out analysis) was performed, removing 1 study at the time, to evaluate the stability of our results regarding the risk of myocarditis. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ, USA).
Results
Search results and included studies
A total of 5235 articles were obtained in our search strategy. After excluding duplicates and preliminary screening, 372 full-text articles were assessed for eligibility. Among them, 368 studies were excluded for not meeting the inclusion criteria, leaving 4 investigations fulfilling the inclusion criteria (Figure 1 ).11, 12, 13, 14
Figure 1.
Flow diagram of selected studies for the meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Characteristics of the population and quality assessment
Overall, 20,875,843 patients (mean age 56.1 years, 59.1% male) were included in this analysis.4, 5, 6, 7 Among them 1,245,167 had confirmed COVID-19 infection. The general characteristics of the included studies are presented in Table 1 . Although the demographic characteristics and concomitant comorbidities were not systematically recorded in all investigations, the cohorts mainly consisted of middle-aged patients. The mean length of follow-up was 9.5 months. Over the follow-up period, myocarditis occurred in 0.21 (95% CI 0.13-0.42) out of 1000 patients surviving COVID-19 infection. Conversely, AM occurred in 0.09 (95% CI 0.07-0.12) out of 1000 control subjects. Quality assessment showed that all studies were of moderate to high quality according to the NOS scale (Table 1).9
Table 1.
General characteristics of the populations reviewed
| Sample size | Age, y | Male | HT | DM | COPD | CKD | Obesity | HF | Cancer | CVD | FU, mo | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohen et al.10 | 2,895,943 | 75.7 | 1,227,545 (42.0) | 2,081,772 (72.0) | 938,043 (32.7) | 578,650 (20)∗ | 528,314 (14.0) | 478,902 (17.0) | 334,654 (12) | 418,700 (14.4) | 364,782 (13.0) | 4 | 8 |
| Wang et al.11 | 2,940,988 | 43.8 | 1,241,483 (42.2) | 440,998 (14.9) | 188,488 (6.4)† | 51,592 (1.7) | 59177 (2.0) | 286,338 (9.7)† | NR | NR | NR | 12 | 7 |
| Xie et al.12 | 5,791,407 | 62.5 | 5,228,431 (90.2) | 1,525,944 (26.3) | 1,321,907 (22.8) | 633,000 (10.9) | 970,057 (16.7) | 2,462.44 (42.5) | NR | 357,192 (6.1) | NR | 12 | 8 |
| Daugherty et al.13 | 9,247,505 | 42.4 | 4,640,393 (50.2) | NR | 521,699 (5.6) | NR | NR | NR | NR | NR | NR | 6 | 6 |
Values in parentheses are %.
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DM, diabetes mellitus; FU, follow-up; HF, heart failure; HT, arterial hypertension; NOS, Newcastle-Ottawa quality assessment scale; NR, not reported.
Defined as chronic pulmonary disease.
Only DM type 2.
One-year risk of incident myocarditis
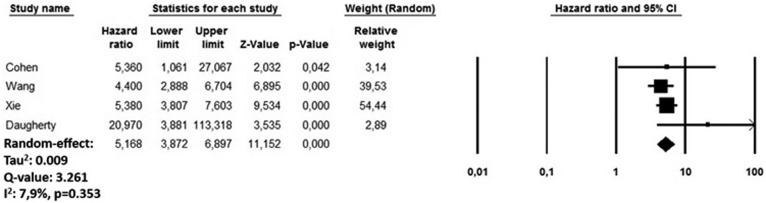
During the follow-up period, recovered COVID-19 patients presented an increased risk of incident myocarditis (HR 5.16, 95% CI 3.87-6.89; P < 0.0001; I 2 = 7.9%) compared with subjects who did not experience COVID-19 infection but developed an AM over the same period (Figure 2 ). The funnel plot disclosed the presence of potential publication bias (Supplemental Figure S1). The sensitivity analysis confirmed an HR ranging from 4.97 (95% CI 3.81-6.47; P < 0.0001, I 2 = 0%) to 5.67 (95% CI 4.06-7.88; P < 0.0001; I 2 = 16.7%), indicating that the obtained results were not driven by any single study.
Figure 2.
Forest plots investigating the long-term risk of incident myocarditis after COVID-19 infection. CI, confidence interval.
Discussion
Our findings, based on a large population of more than 20 million people, demonstrated that myocarditis occurred in about 0.2 out of 1000 patients who experienced and survived COVID-19 infection. Moreover, after COVID-19 recovery, subjects had a significantly higher risk of myocarditis within 1 year from the index infection. To the best of our knowledge, the present analysis represents the first attempt to comprehensively assess the risk of incident myocarditis in the post-acute phase of COVID-19 subjects. Currently, long COVID represents a worldwide epidemic, caused by long-lasting multiorgan involvement, including the cardiovascular system, that endures for weeks or months after the index SARS-CoV-2 infection has already subsided.15 Our results demonstrate that the incidence rate of myocarditis among survivors of COVID-19 is 2-fold higher than that observed in nonvaccinated subjects with COVID-related myocarditis in a recent study by Barda et al. (21 vs 11 cases per 100,000 individuals).16 Of note, in that analysis, 2 contemporary series of subjects (vaccinated and nonvaccinated) were followed for 42 days after the administration of the first dose of mRNA vaccine against SARS-CoV-2 infection.
AM also has been recognised as a rare complication of COVID-19 mRNA vaccinations, especially in young adult and adolescent males, with an estimated incidence of about 12.6 cases per million doses of second-dose mRNA vaccine.17 mRNA vaccines contain nucleoside-modified mRNA, encoding the viral spike glycoprotein of SARS-CoV-2. However, selected RNA molecules can be immunogenic and stimulate the innate immune system, destroying the mRNA before it reaches target cells, preventing the spike protein and neutralizing antibody production, promoting the activation of an aberrant innate and acquired immune response that may lead to the activation of proinflammatory cascades and immunologic pathways triggering AM.18 , 19 However, the benefit-risk assessment for COVID-19 vaccination shows a favourable balance for all age and sex groups, and COVID-19 vaccination is currently recommended for everyone 12 years of age or older.17
Findings from the present study point toward a mild increase in the incidence of myocarditis in the first year compared with that observed in the first 1 to 2 months following the index SARS-CoV-2 infection. Moreover, the risk of AM was higher compared with other subjects who did not experience the infection in the same period. Available studies did not systematically report data regarding the risk of incident myocarditis according to age, sex, preexistence of any cardiovascular conditions, and hospitalisation for COVID-19 infection; therefore, dedicated subanalyses on these were not feasible. However, a higher risk of incident myocarditis after COVID-19 recovery has been reported in patients aged < 44 years5 , 6 as well as in subjects with cardiovascular disease before SARS-CoV-2 exposure.7 , 8 Moreover, available data regarding the risk of AM during the follow-up period are controversial; Daugherty et al.14 observed a higher risk among subjects who were not hospitalised for the COVID-19 infection, and Xie et al.13 reported an increased risk in patients managed in the intensive care unit at the time of the index infection. No significant sex differences were observed regarding AM.
Different recovery settings, reflecting illness severity, may have influenced such results. Indeed, subjects with a mild/moderate COVID-19 infection, generally treated at home, should have received a lower dosage or immunomodulatory treatment, such as corticosteroids, while those admitted into intensive care unit, owing to more severe disease, may have received a higher dose of immunomodulatory drugs. Therefore, the different systemic corticosteroid regimens, or more generally the administration of immunomodulatory drugs, related to the severity of the infection, may have influenced the patient’s immunosuppression and as a consequence the cytocidal effect of the virus on cardiac muscle, which has a critical role in the genesis of myocarditis.18 , 19 Several pathophysiologic mechanisms have been suggested to explain the cardiac involvement, including direct damage to the myocardium by the virus, microthrombotic damage to vessels or endothelium, and persistent systemic inflammation.20 Intriguingly, our results demonstrate that the incidence and risk of myocarditis after hospital discharge is much lower compared with the in-hospital incidence and relative risk observed during the acute phase of COVID-19 infection.21 Unfortunately, to date, specific markers able to identify and guide managing physicians in the treatment of long COVID and its cardiovascular sequelae have not yet been identified. Dedicated studies are urgently required in the future to identify the profiles of subjects at higher risk of post–acute COVID sequelae and strategies to minimise their cardiovascular risk. In this regard, future investigation assessing the risk of post–COVID-19 sequelae will have to evaluate whether current criteria for vaccination against COVID-19 should be revised or enriched, especially in subjects with previous cardiovascular disease or just having some cardiovascular risk factors.22
Study limitations
The present study has several limitations related to the observational nature of the studies reviewed and their own limitations with all inherited bias. Potential underestimation could derive from detection bias, considering that most of the articles reviewed identified the occurrence of incident myocarditis from larger medical records data sets by means of the relevant ICD-10 codes; therefore, the investigators did not perform a clinical follow-up and we cannot exclude that miscoding may have biased our results. Moreover, we cannot exclude potential overestimation of our results due to the presence of competing risks. Nevertheless, the sample size analysed, the sensitivity analysis, and the very low heterogeneity level observed confirmed the robustness of our results. Unfortunately, the revised studies did not systematically report data regarding potential risk factors for AM nor data related to the characteristics of observed events. Moreover, sampling bias by the competing risk of death could be another potential source of biases. Furthermore, available data from the included studies did not allow us to provide information on the diagnostic criteria adopted (noninvasive or invasive) and the type of AM, the type and number of vaccinations against SARS-CoV-2, or the proportion of patients having COVID-related myocarditis during the index infection. Finally, the reviewed data may have underestimated the real impact of myocarditis after COVID-19 recovery, especially during the early phase of the pandemic, owing to the presence of undiagnosed cases and patients lost during the follow-up period. Unfortunately, no data were provided regarding the demographic and clinical characteristics of subjects experiencing acute myocarditis after the COVID-19 infection, limiting potential subanalyses.
Conclusion
Myocarditis represents a relatively rare post-acute COVID-19 sequela within 1 year after the index infection. Physicians must be aware of this potential sequela, allowing its prompt recognition and treatment.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See editorial by Réa and Luk, pages 845-848 of this issue.
See page 844 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2022.12.003.
Supplementary Material
References
- 1.Ammirati E., Lupi L., Palazzini M., et al. Prevalence, characteristics, and outcomes of COVID-19–associated acute myocarditis. Circulation. 2022;145:1123–1139. doi: 10.1161/CIRCULATIONAHA.121.056817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yajima T., Knowlton K.U. Viral myocarditis. Circulation. 2009;119:2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- 3.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinney J., Connelly K.A., Dorian P., et al. COVID-19–myocarditis and return to play: reflections and recommendations from a Canadian working group. Can J Cardiol. 2021;37:1165–1174. doi: 10.1016/j.cjca.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haussner W., DeRosa A.P., Haussner D., et al. COVID-19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150–155. doi: 10.1016/j.ajem.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castiello T., Georgiopoulos G., Finocchiaro G., et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27:251–261. doi: 10.1007/s10741-021-10087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simone A., Herald J., Chen A., et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G.A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses 2012. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at:
- 11.Cohen K., Ren S., Heath K., et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Wang C.Y., Wang S.I., Wei J.C. Long-term cardiovascular outcomes in COVID-19 survivors among nonvaccinated population: a retrospective cohort study from the TriNetX US collabourative networks. EClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daugherty S.E., Guo Y., Heath K., et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyöngyösi M., Alcaide P., Asselbergs F.W., et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc Res. 2023;119:336–356. doi: 10.1093/cvr/cvac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Caso F., Costa L., Ruscitti P., et al. Could SARS-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siripanthong B., Asatryan B., Hanff T.C., et al. The pathogenesis and long-term consequences of COVID-19 cardiac injury. JACC Basic Transl Sci. 2022;7:294–308. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priyadarshni S., Westra J., Kuo Y.F., et al. COVID-19 infection and incidence of myocarditis: a multi-site population-based propensity score–matched analysis. Cureus. 2022;14 doi: 10.7759/cureus.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.