Abstract
Introduction
Since the beginning of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pandemic an important tool for patients with Coronavirus Disease 2019 (COVID-19) has been the computed tomography (CT) scan, but not always available in some settings The aim was to find a cut-off that can predict worsening in patients with COVID-19 assessed with a computed tomography (CT) scan and to find laboratory, clinical or demographic parameters that may correlate with a higher CT score.
Methods
We performed a multi-center, observational, retrospective study involving seventeen COVID-19 Units in southern Italy, including all 321 adult patients hospitalized with a diagnosis of COVID-19 who underwent at admission a CT evaluated using Pan score.
Results
Considering the clinical outcome and Pan score, the best cut-off point to discriminate a severe outcome was 12.5. High lactate dehydrogenase (LDH) serum value and low PaO2/FiO2 ratio (P/F) resulted independently associated with a high CT score. The Area Under Curve (AUC) analysis showed that the best cut-off point for LDH was 367.5 U/L and for P/F 164.5. Moreover, the patients with LDH> 367.5 U/L and P/F < 164.5 showed more frequently a severe CT score than those with LDH< 367.5 U/L and P/F> 164.5, 83.4%, vs 20%, respectively.
Conclusions
A direct correlation was observed between CT score value and outcome of COVID-19, such as CT score and high LDH levels and low P/F ratio at admission. Clinical or laboratory tools that predict the outcome at admission to hospital are useful to avoiding the overload of hospital facilities.
Keywords: CT score, Pan score, COVID-19, SARS-CoV-2 infection, Severity of disease
Introduction
Since December 2019 a new coronavirus, the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), the causative agent of Coronavirus Disease 2019 (COVID-19), associated with substantial morbidity and mortality [1]. COVID-19 is the clinical manifestation of SARS-CoV-2 infection with a large severity spectrum ranging from asymptomatic to severe manifestation [2], [3].
Considering the impact on the healthcare system [4], the need to identify prognostic factors of severe disease is and has been a priority of the scientific community [5], [6], [7], [8], [9]. Studies showed that the most important clinical factors at admission that can predict severity of COVID-19 were diabetes, hypertension, chronic kidney disease, active oncological disease and dementia [5], [6], [7], [8], and the most common laboratory parameters that can predict COVID-19 prognosis were the alteration of white blood cell counts, elevated neutrophil-to-lymphocyte ratios and platelet-to-lymphocyte, elevated high sensitivity cardiac troponin I, C-reactive proteins, ferritin, lymphocytopenia, elevation of aminotransferases and elevation of lactate dehydrogenase [9], [10], [11], [12], [13].
Since the beginning of the pandemic an important tool for the evaluation of the patient suffering from COVID-19 has been the computed tomography (CT) scan [14], but not always available in some settings. both for economic reasons and for the intensity of traffic on hospital facilities during the waves. The CT feature included ground glass opacity (GGO), consolidation, septal thickening and crazy paving [14]. The predictive power of the CT scan on the outcome was clear [15], [16], [17], [18], [19], [20], [21]. However, to our knowledge, few studies have been carried out on the identification of biochemical factors that predict a worse CT score at admission for COVID-19 [18], [19], [20], [21].
Considering the data available, the aim of the present study was to find a cut-off that can predict a disease worsening in patients evaluated by the semi-quantitative visual CT severity Pan score[22]. In addition, we wanted to find laboratory, clinical or demographic parameters that can correlate with a higher CT score to reserve CT scan for high-risk patients or those suspected of having complications or worsening of the respiratory status.
Materials and methods
Study design and setting
We performed a multicenter, observational, retrospective study involving seventeen COVID-19 Units in eight cities in the Campania region in southern Italy: Naples, Caserta, Salerno, Benevento, Avellino, Pozzuoli, Eboli and Vallo della Lucania. All adult (≥18 years old) patients, hospitalized with a diagnosis of SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) on a naso-oropharyngeal swab, from February 28th 2020 to November 1st 2021 at one of the centers participating in the study, were enrolled in the CoviCamp cohort. Exclusion criteria included minority age (<18 years old), and lack of clinical data and/or of informed consent. No study protocol or guidelines regarding the criteria of hospitalization were shared among the centers involved in the study and the patients were hospitalized following the decision of physicians of each center.
From the CoviCamp cohort, we included all patients for whom a determination at admission of CT score using the Pan et al. score [22] was available. The CT scans were independently evaluated by two radiologists (Al.Re and F.S.) who achieved a common score. The CT scans were evaluated with a semi-quantitative scoring system used to estimate the pulmonary volume involvement. Each of the five lung lobes was visually scored on a scale of 0–5, with 0 indicating no involvement; 1, less than 5% involvement; 2, 5–25% involvement; 3, 26–49% involvement; 4, 50–75% involvement; and 5, more than 75% involvement [18]. The total CT score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement) [18].
The study was approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (n°10877/2020). All procedures performed in this study were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards. Informed consent was obtained from all participants included in the study.
This study was reported following the STROBE recommendations for an observational study (Supplementary Table 1).
Data collection
All demographic and clinical data and therapy details of patients with SARS-CoV2 infection enrolled in the cohort were collected in an electronic database. From this database we extrapolated the data for the present study.
Definitions
The microbiological diagnosis of SARS-CoV-2 infection was defined as a positive RT-PCR test on a naso-oropharyngeal swab. All the sites included used the same RT-PCR kit, Bosphore V3 (Anatolia Genework, Turkey).
We divided the patients enrolled according to the clinical outcome of COVID-19 during hospitalization in two groups, the first including patients with mild or moderate, the second including severe outcome or death during hospitalization. Precisely, the patients with a mild infection did not need oxygen (O2) therapy and/or had a MEWS score below 3 points during hospitalization. The patients with a moderate infection were hospitalized and required non-invasive O2 therapy (excluding high flow nasal cannula) and/or had a MEWS score equal to or above 3 points (≥3) during hospitalization. The patients with a severe infection needed management in an intensive care unit (ICU) and/or high flow nasal cannula or invasive/non-invasive mechanical ventilation during hospitalization. The patients were followed until SARS-CoV-2-RNA negativity at naso-oropharyngeal swab and/or discharged from hospital or died.
Statistical analysis
For the descriptive analysis, categorical variables were presented as absolute numbers and their relative frequencies. Continuous variables were summarized as mean and standard deviation if normally distributed or as median and interquartile range (Q1-Q3) if not normally distributed. We performed a comparison of patients with mild and moderate disease, severe disease or who died using chi square for categorical variables or Student’s t-test for continuous variables or Mann-Whitney tests for non-parametric independent ordinal variables. Multivariate analysis was performed using binomial logistic regression; this analysis was performed only for parameters resulting statistically significant at univariate analysis. A p-value below 0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve was used to determine the optimum cut-off point for possible effective variables on the patients’ outcome. Analyses were performed by STATA (StataCorp. 2019) [23].
Results
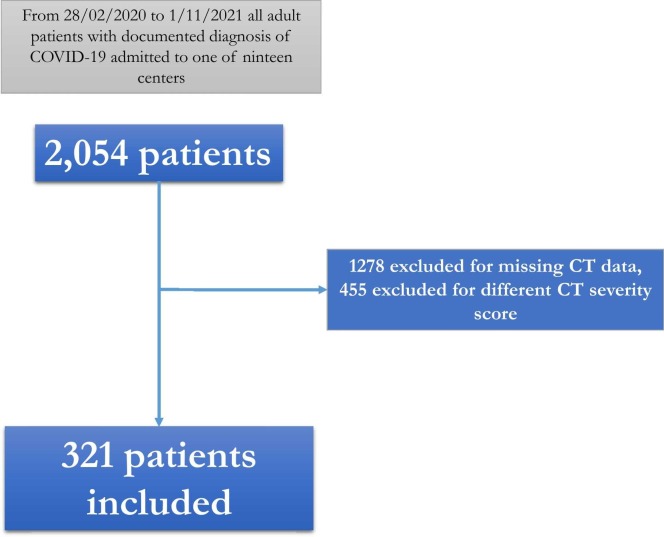
A total of 2054 adult patients were admitted with a documented diagnosis of COVID-19 to one of the nineteen centers from February 20, 2020 to November 1, 2021 and participated in the CoviCamp cohort. Considering the inclusion criteria, 321 patients were included, while 1278 were excluded for missing CT data and 455 for different CT severity scores ( Fig. 1).
Fig. 1.
Flow-chart of patients included in the study.
Considering the 321 patients included, 67.9% were males, with a mean age of 65 years (SD 14.25) and a median of Charlson Comorbidity Index of 3 (1−4), and a median of 6 days (2−9) from first symptoms of COVID-19 to admission to hospital ( Table 1). Only 6 (1.87%) patients had been vaccination against SARS-CoV-2 with full schedule (two doses). The most frequent comorbidities were hypertension (52.2%), cardio-vascular disease (32%) and diabetes (24.4%) (Table 1). As regards the COVID-19-related symptoms, 57.5% of the patients had recent history of fever, while 73.8% had dyspnea and 33.4% cough (Table 1). The patients with a mild or moderate outcome were 57.3% while patients with a severe disease or who died during hospitalization were 42.7%.
Table 1.
Demographic and clinical parameters of patients included in the study.
| Number of patients with data available | ||
|---|---|---|
| Males, n° (%) | 321 | 218(67.9) |
| Age, years, mean (SD) | 321 | 65(14.25) |
| Days from symptoms to admission, median (Q1-Q3) | 213 | 6(2–9) |
| Charlson comorbidity index, mean (SD) | 317 | 3(1–4) |
| n° (%) with hypertension | 316 | 165(52.2) |
| n° (%) with cardio-vascular disease | 316 | 101(32) |
| n° (%) with diabetes | 316 | 77(24.4) |
| n° (%) with chronic kidney disease | 317 | 24(7.6) |
| n° (%) with chronic obstructive pulmonary disease | 316 | 26(8.2) |
| n° (%) with chronic hepatopathy | 316 | 13(4.0) |
| n° (%) with malignancy | 315 | 22(6.9) |
| n° (%) with dementia | 316 | 12(3.7) |
| n° (%) with fever during recent history | 320 | 184(57.5) |
| n° (%) with dyspnea during recent history | 320 | 236(73.8) |
| n° (%) with astheny during recent history | 320 | 97(30.3) |
| n° (%) with cough during recent history | 320 | 107(33.4) |
| n° (%) with ageusia/dysgeusia during recent history | 319 | 4(1.3) |
| n° (%) with anosmia/hyposmia during recent history | 319 | 5(1.6) |
| n° (%°) with diarrhoea during recent history | 319 | 9(2.8) |
| n° (%) with skin lesions during recent history | 318 | 0(0) |
| Days from admission to discharge*, mean (SD) | 318 | 12(7–17) |
| Patients with mild or moderate outcome, n° (%) | 321 | 184(57.3) |
| Patients with severe outcome or who died during hospitalization, n° (%) | 321 | 137(42.7) |
| n° (%) patients who died during hospitalization | 321 | 48(15) |
| CT score, mean (SD) | 321 | 13.05(4.79) |
or who died during hospitalization
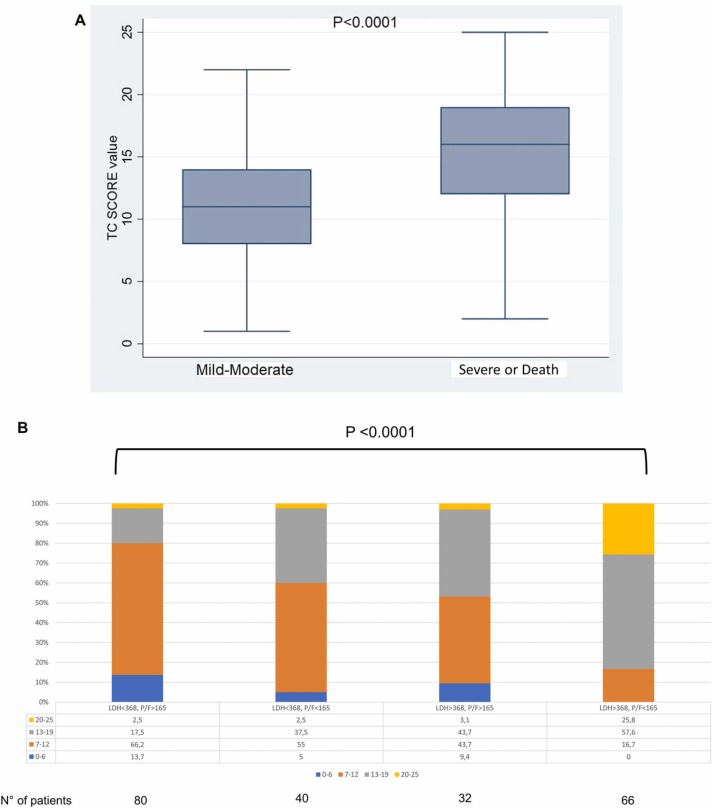
The CT score mean was 13.5 (SD 4.79) (Table 1 ). Dividing the patients in two groups, with a mild or moderate outcome or a severe outcome or who died during hospitalization, the difference in the CT severity score was statistically significant (mean 11.23 (SD: 4) vs mean 15.49(SD: 4.7), p < 0.008) ( Fig. 2 A). We calculated the AUC for the CT severity score and the result was 0.749 (95%CI: 0.695–0.803, p < 0.0001) (Supplementary Figure 1a) with the best cut-off point of 12.5 (sensitivity: 71.5%, specificity: 66.3%).
Fig. 2.
: A:Box plot of CT score value at admission in patients who did not need ICU care and patients who needed it. B: Stacked column graph considering CT score and LDH/PF value at admission (data in percentage).
For the second aim (to find laboratory, clinical or demographic parameters correlated with a higher CT score), we divided the patients in two groups the first included the 161 patients with less than 13 of CT severity score (Group 1), the second included the 160 patients with more than or equal to 13 of CT severity score (Group 2). Considering the two groups, no difference was found in the demographic or clinical history at admission, excluding the presence of dyspnea (65.6% vs 81.9%, p < 0.001) ( Table 2). Considering laboratory parameters at admission we found a statistical significance between Group 1 and Group 2 evaluating the count of white blood cells [median 7300 cells/uL (6000−10,400) vs 8100 (6200−11,640) (p = 0.041)], ALT serum concentration [median 27 U/L (22−43) vs 38.5 U/L (23.5–59.5) (p = 0.002)], AST serum concentration [median 29.5 U/L (21−44) vs 41.5 U/L (29−59) (p < 0.001)], LDH serum concentration [median 293 U/L (235−563) vs 412 U/L (330−563)(p < 0.001)] and PaO2/FiO2 ratio (P/F) [median 213 (146−294) vs 133 (98–186.5) (p < 0.001)] (Table 2). In order to identify the factors independently associated with a higher CT score, we performed a multivariate binomial logistic regression including AST, dyspnea, P/F, LDH, and white cell count (Table 2); since AST and ALT proved correlated (p = 0.635), we excluded ALT serum value from this analysis. The only factors associated with a higher CT score were LDH (OR 1.005; 95% CI: 1.003–1.008) and low P/F ratio (OR 0.993; 95%CI: 0.989–0.997) (Table 2).
Table 2.
Laboratory parameters of patients included in the study.
| Number of patients with data available | ||
|---|---|---|
| Median (Q1-Q3) white blood cells (WBC) at admission (cells/uL) | 269 | 7900(6100–10700) |
| Mean (SD) International Normalized Ratio (INR) at admission | 230 | 1.10(0.29) |
| Median (Q1-Q3) Blood creatinine at admission (mg/dl) | 266 | 0.9(0.75–1.17) |
| Median (Q1-Q3) creatine phosphokinase (CPK) at admission (U/L) | 148 | 100.5(54–189.5) |
| Median (Q1-Q3) lactate dehydrogenase (LDH) at admission (U/I) | 243 | 347(267–461) |
| Median (Q1-Q3) PaO2/FiO2 Ratio (P/F) at admission | 273 | 164(115–251) |
| Median (Q1-Q3) ALT at admission (U/L) | 242 | 32(22–52) |
| Median (Q1-Q3) AST at admission (U/L) | 262 | 36.5(25–50) |
| Median (Q1-Q3) Bilirubin at admission(mg/dl) | 204 | 0.64(0.495–0.975) |
| Median (Q1-Q3) procalcitonin at admission (ng/ml) | 162 | 0.09(0.05–0.24) |
Considering the data above, we calculated the AUC for LDH considering the severe outcome group or patients who died as the status variable and the result was 0.748 (95%CI: 0.686–0.810, p < 0.0001) (Supplementary Figure 1b), with the best cut-off point of 367.5 U/L (sensitivity: 66.7%, specificity: 72.5%), showing a direct correlation between the severity of disease and increase in LDH; instead, the AUC for P/F considering the mild or moderate group as the status variable was 0.733 (95%CI: 0.675–0.792, p < 0.0001) (Supplementary Figure 1c), with the best cut-off point of 164.5 (sensitivity: 67.9%, specificity: 68.4%), showing a better CT score in patients with a higher P/F. Moreover, considering the cut-offs of these two parameters, the 66 patients with both LDH> 367.5 U/L and P/F < 164.5 more frequently showed a severe CT-score than those with both LDH< 367.5 U/L and P/F> 164.5, and those with only one severe parameter (P/F<164.5 or LDH >367.5 U/L): 83.4%, 20%, 40% and 48%, respectively (p < 0.0001) (Fig. 2B). Considering the data above we calculated a positive (PPV) and a negative predictive value (NPV). In patients who presented LDH< 367.5 and P/F > 164.5 the PPV was 80% and NPV was 37.7% to predict a CT score < 12.5; in patients who presented LDH> 367.5 U/L and P/F> 164.5 the PPV was 71.4% and the NPV was 73.3% to predict a CT score > 12.5; in patients who had P/F< 164.5 and LDH< 367.5 U/L the PPV was 67% and NPV was 72.3% to predict a CT score > 12.5; in patients who had LDH> 364.5 U/L and P/F< 164.5 the PPV was 83.3% and the NPV was 69.1% to predict a CT score > 12.5.
Discussion
Given the important impact that the COVID-19 pandemic has determined all over the globe [1], despite the scientific advances made in the last 2 years with the introduction of vaccines, monoclonal antibodies and antivirals for prevention, it still appears a healthcare emergency linked to the spread of infection. Clinical or laboratory tools that predict the outcome at admission to hospital are useful to reduce the need for hospitalization and avoid overloading hospital facilities. The utility of the CT scan to stage COVID-19 pneumonia is well known but, considering the overload and the availability of machinery, it is not always possible to perform, especially in some geographical areas.
In the present observational retrospective study performed in 19 COVID-19 units in southern Italy enrolling 321 patients, we confirmed a correlation between the CT score according to Pan and disease progression, as found in previously published studies [15], [16], highlighting that the best cut-off to predict a worse outcome (severe or death) was 12.5. The data available in the literature on this point are few identifying the best cut-off to predict ICU admission with Pan CT scores [15], [16], ranging from 11 [16] to 12.5 [15]. In particular, Aziz-Ahari et al. [15] including 148 patients with a high mortality rate (37%) found the best CT score cut-off for discriminating severe patients was 12.5 with 68.3% sensitivity and 72.7% specificity. Shayganfar et al., including 176 patients with a 21.5% mortality rate found a CT score cut-off point of about 11.
Moreover, we found a direct correlation between the CT score value and LDH levels and an indirect correlation between the CT score value and P/F at admission. In particular, we demonstrated that an LDH value higher than 367.5 U/L and a P/F ratio less than 164.5 at admission were associated with a severe CT score (>12.5). In addition, our data showed than when LDH was less than 367.5 U/L and P/F was more than 164.5, the PPV to predict a CT score less than 12.5 was 80%; instead, if LDH was > 367.5 U/L and P/F < 164.5 the PPV to predict a CT score > 12.5 was 83.3%. Some studies showed that LDH usually increase in COVID-19 patients and it’s increase could predict severity [24], [25]. LDH is a cytoplasmatic enzymes highly expressed in the lung, liver, hearth, kidney and skeletal muscle, generally release in blood after cell death: thus, the lung damage, frequently founded in COVID-19 postmortem pathology [26], could increase LDH blood levels [27]. The P/F, a parameters widely used to define the severity of Acute Respiratory Distress Syndrome [28], has already been shown to be able to predict outcome in patients with COVID-19 [29], [30]; moreover, one study highlighted its inverse correlation with extension of the pulmonary inflammatory process on CT [31].
In the literature, to our knowledge, few studies evaluated the clinical and laboratory parameters that can predict a worse CT score at admission [18], [19], [20], [21]. The study by Francone et al., including 130 symptomatic COVID-19 patients showed that the Pan CT score was significantly correlated with C-reaction protein (CRP) (p < 0.0001) and D-dimer (p < 0.0001) levels [18]. The paper by Yadzi et al. [19] is the largest study, to our knowledge, including 478 participants, that evaluated the impact of different laboratory, clinical and demographic parameters to predict a CT score, with a score 0–25 based, similar to the Pan score. They found that anosmia, respiratory rate, CRP (with a cut-off of 90), WBC (with a cut-off of 10.000) and SpO2 (with a cut-off point of 93) was associated with a higher chest CT score. In the study by Man et al. [20], they found that the neutrophil-to-lymphocyte ratio and platelets-to-lymphocyte rate correlated positive with CT scan severity [20]. However, the inhomogeneity observed in these studies was certainly due to the demographic, clinical and laboratory differences in the patients included, the period of inclusion, the different mortality, response to the therapy applied and routine examinations performed during admission.
Our study shows some limits: first, the retrospective nature of the study; second, we evaluated only hospitalized patients and hospital mortality; third, some data are missing considering the studies published; fourth, nevertheless the impact of vaccination and viral variants on the clinical presentation and clinical outcome of COVID-19 [32], [33] the data of viral variants were not available and the number of patients enrolled in the present study with full vaccination was very low. Table 3.
Table 3.
Demographic, clinical and laboratory data at admission according to the CT score.
|
Patients with CT score less than 12.5 n° 161 (50.1%) |
Patients with CT score more than 12.5 n° 160 (49.9%) |
p value |
Multivariate analysis Binomial Logistic Regression |
||
|---|---|---|---|---|---|
| OR (95% CI) | P value | ||||
| Males, n° (%) | 108 (67.1%) | 110 (68.8%) | 0.749a | – | – |
| Age, years, mean (SD) | 64.73 (14.08) | 66.83 (14.383) | 0.188b | – | – |
| Days from symptoms to admission, median (Q1-Q3) | 5(2–10) | 6(2–9) | 0.56c | – | – |
| Charlson comorbidity index, median (Q1-Q3) | 2.5(1–4) | 3(1–4) | 0.32c | – | – |
| n° (%) with hypertension | 76 (47.2%) | 89 (55.6%) | 0.114a | – | – |
| n° (%) with cardio-vascular disease | 50 (31.1%) | 51 (31.9%) | 0.843a | – | – |
| n° (%) with diabetes | 36 (22.4%) | 41 (25.6%) | 0.472a | – | – |
| n° (%) with chronic kidney disease | 10 (6.2%) | 14 (8.8%) | 0.369a | – | – |
| n° (%) with chronic obstructive pulmonary disease | 15 (9.3%) | 11 (6.9%) | 0.432a | – | – |
| n° (%) with liver cirrhosis | 6 (3.7%) | 7 (4.4%) | 0.759a | – | – |
| n° (%) with malignancy | 12 (7.5%) | 10 (6.3%) | 0.692a | – | – |
| n° (%) with dementia | 9 (5.6%) | 3 (1.9%) | 0.81a | – | – |
| n ° (%) with fever during recent history | 98(61.3) | 86(53.8) | 0.213a | – | – |
| n° (%) with dyspnea during recent history | 105(65.6) | 131(81.9) | 0.001a | 0.755 (0.353–1.615) |
0.468 |
| n° (%) with astheny during recent history | 54(33.8) | 43(26.9) | 0.224a | – | – |
| n° (%) with cough during recent history | 52(32.5) | 55(34.4) | 0.813a | – | – |
| n° (%) with ageusia/dysgeusia during recent history | 3(1.9) | 1(0.6) | 0.371a | – | – |
| n° (%) with anosmia/hyposmia during recent history | 4(2.5) | 1(0.6) | 0.214a | – | – |
| n° (%) with diarrhea during recent history | 5(3.1) | 4(2.5) | 0.750a | – | – |
| n° (%) with skin lesion during recent history | 0(0) | 0(0) | ND | – | – |
| Median (Q1-Q3) white blood cells (WBC) | 7300(6000–10400) | 8100(6200–11640) | 0.041b | 1.000 (1.000–1.000) |
0.859 |
| Mean (SD) International Normalized Ratio (INR) | 1.12 (0.352) | 1.09 (0.201) | 0.463b | – | – |
| Median (Q1-Q3) Blood creatinine | 0.9(0.77–1.13) | 0.915(0.725–1.22) | 0.299b | – | – |
| Median (Q1-Q3) creatine phosphokinase (CPK) | 91(53–163) | 105(68–268) | 0.125b | – | – |
| Median (Q1-Q3) lactate dehydrogenase (LDH) | 293(235–360) | 412(330–563) | 0.001b | 1.005(1.003–1.008) | 0.0001 |
| Median (Q1-Q3) PaO2/FiO2 Ratio (P/F) | 213(146–294) | 133(98–186.5) | 0.001b | 0.993(0.989–0.997) | 0.001 |
| Mean(SD) ALT | 27(22–43) | 38.5(23.5–59.5) | 0.002b | – | -d |
| Median (Q1-Q3) AST | 29.5(21–44) | 41.5(29–59) | 0.001b | 1.003(0.986–1.019) | 0.752 |
| Median (Q1-Q3) Bilirubin | 0.6(0.47–0.91) | 0.7(0.5–1) | 0.069b | – | – |
| Median (Q1-Q3) Procalcitonin | 0.08(0.04–0.18) | 0.11(0.07–0.36) | 0.083b | – | – |
a, Chi-square test; b, Student-T test; c, Wilcoxon rank-sum (Mann-Whitney) test.; d: Not included in multivariate due to the high correlation with AST (0.635)
The strengths of our study were the multicenter nature of the design and the size of the population; moreover, this study highlights that two simple biochemical markers generally carried out at admission to hospital can predict the CT score value, thus allowing to discriminate, in a moment of greater affluence to the healthcare facilities, any priorities on the execution of CT.
In conclusion, our study suggests that the best CT score to predict a severe outcome or death during hospitalization in patients with COVID-19 was 12.5 (sensitivity: 71.5%, specificity: 66.3%) and that LDH and P/F values at admission correlated with the CT score. Moreover, the data suggest that the patients with a low LDH and high P/F ratio at admission have a low probability of having a severe CT score and, thus, they may undergo CT scan with less urgency, while those with both high LDH and low P/F ratio more frequently have a severe CT score and, thus, should undergo CT scan immediately. However, studies on larger cohorts of patients with the analysis of all the biochemical parameters are needed to confirm these data.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (n°10877/2020, May 11, 2020).
Funding
“POR Campania FESR 2014–2020-Avviso per l’acquisizione di manifestazioni di interesse per la realizzazione di servizi di ricerca e sviluppo per la lotta contro il Covid-19 (DGR n. 140 del 17 marzo 2020), Project: ”IDENTIFICAZIONE DEI FATTORI DEMOGRAFICI, CLINICI, VIROLOGICI, GENETICI, IMMUNOLOGICI E SIEROLOGICI ASSOCIATI AD OUTCOME SFAVOREVOLE NEI SOGGETTI CON COVID-19”, Regione Campania, Italy, and POR FESR Campania 2014 – 2020- Avviso per l’acquisizione di manifestazioni di interesse da parte degli Organismi di Ricerca per la realizzazione di servizi di ricerca, sviluppo e innovazione per la lotta contro il Covid-19 (DGR n. 504 del 10.11.2021)-Regione Campania, Italy; Project: Impatto delle nuove varianti, l’uso di terapie antivirali precoci e stato vaccinale sulla presentazione clinica del COVID-19: studio restrospettivo/prospettico multicentrico.
CRediT authorship contribution statement
NC, AR, MP and AlRe were involved in study concept and design, drafting of the manuscript,: AlRe, FS, PM, IG, FGN, VS, VE, RP, RoPa, GC, EM, AM, ASM, GDA, GR, MG, AP, CR and IDL, were involved in critical revision of the manuscript for important intellectual content; FS, PM, IG, FGN, VS, VE, RP, RoPa, AlRe, GC, EM, AM, ASM, GDA, GR, MG, AP, CR and IDL were involved in acquisition of data, analysis and interpretation of data and in critical revision of the manuscript; CoviCamp (Campania COVID-19 group) was involved in the enrolment of the patients. All authors contributing to data analysis, drafting or revising the article have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
Campania COVID-19 network:
Nicola Coppola, Caterina Monari, Caterina Sagnelli, Paolo Maggi, Vincenzo Sangiovanni, Fabio Giuliano Numis, Ivan Gentile, Alfonso Masullo, Carolina Rescigno, Giosuele Calabria, Angelo Salomone Megna, Michele Gambardella, Elio Manzillo, Grazia Russo, Vincenzo Esposito, Giuseppina Dell’Aquila, Roberto Parrella, Rodolfo Punzi, Antonio Ponticiello, Mariantonietta Pisaturo, Enrico Allegorico, Raffaella Pisapia, Giovanni Porta, Margherita Macera, Federica Calò, Annamaria Rossomando, Mariana Di Lorenzo, Ferdinando Calabria, Nicola Schiano Moriello, Antonio Russo, Giorgio Bosso, Claudia Serra, Ferdinando Dello Vicario, Valentina Minerva, Giulia De Angelis, Stefania De Pascalis, Giovanni Di Caprio, Addolorata Masiello, Domenica Di Costanzo, Mariano Mazza, Vincenzo Bianco, Valeria Gentile, Antonio Riccardo Buonomo, Biagio Pinchera, Riccardo Scotto.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.12.009.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
References
- 1.WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data, https://covid19.who.int/, [Accessed: 2022-05–26].
- 2.Sagnelli C., Celia B., Monari C., Cirillo S., De Angelis G., Bianco A., Coppola N. Management of SARS-CoV-2 pneumonia. J Med Virol. 2021;93(3):1276–1287. doi: 10.1002/jmv.26470. Epub 2020 Oct 10. PMID: 32856728; PMCID: PMC7461283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macera M., De Angelis G., Sagnelli C., Coppola N. Vanvitelli Covid-Group. Clinical presentation of COVID-19: case series and review of the literature. Int J Environ Res Public Health. 2020;17(14):5062. doi: 10.3390/ijerph17145062. PMID: 32674450; PMCID: PMC7399865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheinson D.M., Wong W.B., Solon C.E., Cheng M.M., Shah A., Elsea D., Meng Y. Estimated impact of public and private sector COVID-19 diagnostics and treatments on US healthcare resource utilization. Adv Ther. 2021;38(2):1212–1226. doi: 10.1007/s12325-020-01597-3. Epub 2020 Dec 26. PMID: 33367984; PMCID: PMC7765700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisaturo M., Calò F., Russo A., Camaioni C., Giaccone A., Pinchera B., Gentile I., Simeone F., Iodice A., Maggi P., Coppola N. Dementia as risk factor for severe coronavirus disease 2019: a case-control study. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.698184. PMID: 34267649; PMCID: PMC8276052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monari C., Sagnelli C., Maggi P., Sangiovanni V., Numis F.G., Gentile I., Masullo A., Rescigno C., Calabria G., Megna A.S., Gambardella M., Manzillo E., Russo G., Esposito V., Camaioni C., Messina V., Pisaturo M., Allegorico E., Pinchera B., Pisapia R., Catalano M., Salzillo A., Porta G., Signoriello G., Coppola N. More severe COVID-19 in patients with active cancer: results of a multicenter cohort study. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.662746. PMID: 34026639; PMCID: PMC8139554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sardu C., D'Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., Maggi P., Coppola N., Paolisso G., Marfella R. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. Epub 2020 May 19. PMID: 32430456; PMCID: PMC7305003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., Salazar-Mather T.P., Dumenco L., Savaria M.C., Aung S.N., Flanigan T., Michelow I.C. Predictors of COVID-19 severity: A literature review. Rev Med Virol. 2021 Jan;31(1):1–10. doi: 10.1002/rmv.2146. Epub 2020 Jul 30. PMID: 32845042; PMCID: PMC7855377. [DOI] [PMC free article] [PubMed]
- 9.Russo A., Pisaturo M., Palladino R., Maggi P., Numis F.G., Gentile I., Sangiovanni V., Esposito V., Punzi R., Calabria G., Rescigno C., Salomone Megna A., Masullo A., Manzillo E., Russo G., Parrella R., Dell’Aquila G., Gambardella M., Ponticiello A., Coppola N., on behalf of CoviCam Group Prognostic value of transaminases and bilirubin levels at admission to hospital on disease progression and mortality in patients with COVID-19—an observational retrospective study. Pathogens. 2022;11(6):652. doi: 10.3390/pathogens11060652. https://doi.org/10.3390/pathogens11060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini R.K., Saini N., Ram S., Soni S.L., Suri V., Malhotra P., Kaur J., Verma I., Sharma S., Zohmangaihi D. COVID-19 associated variations in liver function parameters: a retrospective study. Post Med J. 2022;98(1156):91–97. doi: 10.1136/postgradmedj-2020-138930. Epub 2020 Nov 12. [DOI] [PubMed] [Google Scholar]
- 11.Tripon S., Bilbault P., Fabacher T., Lefebvre N., Lescuyer S., Andres E., Schmitt E., Garnier-Kepka S., Borgne P.L., Muller J., Merdji H., Chaffraix F., Mutter D., Baumert T.F., Meziani F., Doffoel M. Abnormal liver tests and non-alcoholic fatty liver disease predict disease progression and outcome of patients with Covid-19. Clin Res Hepatol Gastroenterol. 2022 doi: 10.1016/j.clinre.2022.101894. Epub ahead of print. PMID: 35227956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu X., Zhang G.F., Zheng Y.K., Zhong Y.G., Wen L., Zeng P., Fu C.Y., Tong X.L., Long Y.F., Li J., Liu Y.L., Chang Z.G., Xi H. Clinical features and risk factors of severely and critically ill patients with COVID-19. World J Clin Cases. 2022;10(3):840–855. doi: 10.12998/wjcc.v10.i3.840. PMID: 35127900; PMCID: PMC8790448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C.H., Lin S.W., Shen C.F., Hsieh K.S., Cheng C.M. Biomarkers during COVID-19: Mechanisms of Change and Implications for Patient Outcomes. Diagn (Basel) 2022;12(2):509. doi: 10.3390/diagnostics12020509. PMID: 35204599; PMCID: PMC8870804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Z.Y., Yan X.C., You X.D., Zhang X.W. CT imaging research progress in COVID-19. Curr Med Imaging. 2022;18(3):267–274. doi: 10.2174/1573405617666210816091217. PMID: 34465280; PMCID: PMC8972255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziz-Ahari A., Keyhanian M., Mamishi S., Mahmoudi S., Bastani E.E., Asadi F., Khaleghi M., Chest C.T. severity score: assessment of COVID‑19 severity and short-term prognosis in hospitalized Iranian patients. Wien Med Wochenschr. 2022;172(3–4):77–83. doi: 10.1007/s10354-022-00914-5. Epub 2022 Feb 8. PMID: 35133531; PMCID: PMC8824536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shayganfar A., Sami R., Sadeghi S., Dehghan M., Khademi N., Rikhtehgaran R., Basiratnia R., Ferdosi F., Hajiahmadi S. Risk factors associated with intensive care unit (ICU) admission and in-hospital death among adults hospitalized with COVID-19: a two-center retrospective observational study in tertiary care hospitals. Emerg Radiol. 2021;28(4):691–697. doi: 10.1007/s10140-021-01903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laino M.E., Ammirabile A., Lofino L., Lundon D.J., Chiti A., Francone M., Savevski V. Prognostic findings for ICU admission in patients with COVID-19 pneumonia: baseline and follow-up chest CT and the added value of artificial intelligence. Emerg Radiol. 2022;29(2):243–262. doi: 10.1007/s10140-021-02008-y. Epub 2022 Jan 20. PMID: 35048222; PMCID: PMC8769787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L., Panebianco V., Andreoli C., Colaiacomo M.C., Zingaropoli M.A., Ciardi M.R., Mastroianni C.M., Pugliese F., Alessandri F., Turriziani O., Ricci P., Catalano C. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808–6817. doi: 10.1007/s00330-020-07033-y. Epub 2020 Jul 4. PMID: 32623505; PMCID: PMC7334627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdi N.A., Ghadery A.H., SeyedAlinaghi S., Jafari F., Jafari S., Hasannezad M., Koochak H.E., Salehi M., Manshadi S.A.D., Meidani M., Hajiabdolbaghi M., Ahmadinejad Z., Khalili H., Mehrabi Nejad M.M., Abbasian L. Predictors of the chest CT score in COVID-19 patients: a cross-sectional study. Virol J. 2021;18(1):225. doi: 10.1186/s12985-021-01699-6. Erratum in: Virol J. 2021 Dec 6;18(1):241. PMID: 34794467; PMCID: PMC8600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Man M.A., Rajnoveanu R.M., Motoc N.S., Bondor C.I., Chis A.F., Lesan A., Puiu R., Lucaciu S.R., Dantes E., Gergely-Domokos B., Fira-Mladinescu O. Neutrophil-to-lymphocyte ratio, platelets-to-lymphocyte ratio, and eosinophils correlation with high-resolution computer tomography severity score in COVID-19 patients. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252599. PMID: 34181675; PMCID: PMC8238190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canovi S., Besutti G., Bonelli E., Iotti V., Ottone M., Albertazzi L., Zerbini A., Pattacini P., Giorgi Rossi P., Colla R., Fasano T. Reggio Emilia COVID-19 Working Group;. The association between clinical laboratory data and chest CT findings explains disease severity in a large Italian cohort of COVID-19 patients. BMC Infect Dis. 2021;21(1):157. doi: 10.1186/s12879-021-05855-9. PMID: 33557778; PMCID: PMC7868898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L., Zheng C. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. Epub 2020 Feb 13. PMID: 32053470; PMCID: PMC7233367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.
- 24.Fialek B., Pruc M., Smereka J., Jas R., Rahnama-Hezavah M., Denegri A., Szarpak A., Jaguszewski M.J., Peacock F.W., Szarpak L. Diagnostic value of lactate dehydrogenase in COVID-19: a systematic review and meta-analysis. Cardiol J. 2022 doi: 10.5603/CJ.a2022.0056. Epub ahead of print. PMID: 35762075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M.Y., Yao L., Wang Y., Zhu X.Y., Wang X.F., Tang P.J., Chen C. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir Res. 2020;21(1):171. doi: 10.1186/s12931-020-01427-8. PMID: 32631317; PMCID: PMC7336103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammoud H., Bendari A., Bendari T., Bougmiza I. Histopathological findings in COVID-19 cases: a systematic review. Cureus. 2022;14(6) doi: 10.7759/cureus.25573. PMID: 35784976; PMCID: PMC9249248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drent M., Cobben N.A., Henderson R.F., Wouters E.F., van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9(8):1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 28.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526–33. doi: 10.1001/jama.2012.5669. PMID: 22797452. [DOI] [PubMed]
- 29.Vedovati M.C., Barbieri G., Urbini C., D'Agostini E., Vanni S., Papalini C., Pucci G., Cimini L.A., Valentino A., Ghiadoni L., Becattini C. Clinical prediction models in hospitalized patients with COVID-19: A multicenter cohort study. Respir Med. 2022;202 doi: 10.1016/j.rmed.2022.106954. Epub ahead of print. PMID: 36057141; PMCID: PMC9392655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartini S., Massobrio L., Cutuli O., Campodonico P., Bernini C., Sartini M., Cristina M.L., Castellani L., Ceschi L., Spadaro M., Gratarola A., Barbera P. Role of SatO2, PaO2/FiO2 Ratio and PaO2 to Predict Adverse Outcome in COVID-19: A Retrospective, Cohort Study. Int J Environ Res Public Health. 2021;18(21):11534. doi: 10.3390/ijerph182111534. PMID: 34770046; PMCID: PMC8582831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turcato G., Panebianco L., Zaboli A., Scheurer C., Ausserhofer D., Wieser A., Pfeifer N. Correlation between arterial blood gas and CT volumetry in patients with SARS-CoV-2 in the emergency department. Int J Infect Dis. 2020;97:233–235. doi: 10.1016/j.ijid.2020.06.033. Epub 2020 Jun 15. PMID: 32553834; PMCID: PMC7295461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baradaran H.R., Dehghanbanadaki H., Moradpour F., Eshrati B., Moradi G., Azami M., Haji Ghadery A., Mehrabi Nejad M.M., Moradi Y. The effect of COVID-19 mRNA vaccines against postvaccination laboratory-confirmed SARS-CoV-2 infection, symptomatic COVID-19 infection, hospitalization, and mortality rate: a systematic review and meta-analysis. Expert Rev Vaccin. 2022:1–10. doi: 10.1080/14760584.2022.2102001. Epub ahead of print. PMID: 35830883. [DOI] [PubMed] [Google Scholar]
- 33.Mendiola-Pastrana I.R., López-Ortiz E., Río de la Loza-Zamora J.G., González J., Gómez-García A., López-Ortiz G. SARS-CoV-2 variants and clinical outcomes: a systematic review. Life (Basel) 2022;12(2):170. doi: 10.3390/life12020170. PMID: 35207458; PMCID: PMC8879159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material