Abstract
Background
The role of transvaginal sonography (TVS) in screening endometrial cancer and hyperplasia is significant in postmenopausal women. The objective of this study is to determine the endometrium thickness (ET) cut-off to distinguish premalignancy and malignancy in asymptomatic postmenopausal women.
Methods
We retrospectively evaluated data of 968 eligible patients among 2537 asymptomatic postmenopausal women with ET ≥ 5 mm examined by TVS who were subjected to hysteroscopy and endometrial biopsy between January 1, 2017, and June 30, 2020 in an urban tertiary specialized hospital in China. The patients were divided into two groups according to the pathology outcomes: benign, and atypical hyperplasia (AH) and endometrial carcinoma (EC). The risk factors and the optimal cut-off of ET for detecting AH and EC were determined by logistic regression analysis and receiver operating characteristic curve.
Results
2537 patients were offered hysteroscopy during a 42-month period. Finally, 968 patients were included for further analysis. Of these, 8 (0.8%) women were diagnosed with EC and 5 (0.5%) women with AH. The mean ET of AH and EC group was substantially higher than that in benign group (10.4 mm vs. 7.7 mm, P < 0.05). ET was significantly correlated with AH and EC shown by logistic regression analysis with an odds ratio (OR) of 1.252 (95% confidence interval [CI] 1.107–1.416, P < 0.001). The optimal cut-off value for AH and EC was found to be 8 mm with the maximum AUC of 0.715 (95% CI 0.686–0.743, P < 0.001), with a sensitivity of 0.846, a specificity of 0.609, positive likelihood ratio (LR+) of 2.164 and negative likelihood ratio (LR−) of 0.253.
Conclusion
An ET cut-off of ≥ 8 mm shows a reasonable performance to detect AH and EC in asymptomatic postmenopausal women, thereby avoiding more invasive endometrial biopsy.
Keywords: Asymptomatic, Endometrial carcinoma, Postmenopausal, Ultrasonography
Introduction
Endometrial carcinoma (EC) is the most common gynecologic cancer in the developed countries with steadily increasing incidence [1, 2]. Although 90% cases with EC present with postmenopausal bleeding [3], 15% cases of EC occur in women without vaginal bleeding [4]. The EC or atypical hyperplasia (AH) rate was reported to be 0.62% to 0.59% among asymptomatic postmenopausal women [5]. Given the fact that the five-year survival for patients with localized EC is 90%, detection of EC is quite pivotal for patients at early stage. American College of Obsetricians and Gynecologists (ACOG) guidelines support the transvaginal sonography (TVS) endometrial threshold of more than 4 mm to screen EC in women with postmenopausal bleeding [6].
TVS is performed in postmenopausal women due to a variety of indications such as pelvic pain, pelvic mass, and physical examination [7, 8]. However, it remains controversial that how to manage an incidental finding of thickened endometrium in asymptomatic postmenopausal women [3, 4, 9–11], consequently leading to a large number of invasive biopsies. Using an endometrial thickness (ET) cut-off value ≥ 4 mm, a prospective study indicated that only 3% of hysteroscopies were useful in the diagnosis of endometrial pre-malignant or malignant lesions [11]. In addition, the hysteroscopy and endometrial sampling could result in surgical risks including uterine perforation and bowel injury, patient anxiety as well as significant healthcare costs [3, 8, 12–14]. A previous study revealed 80% of the women undergoing hysteroscopy had a moderate to severe anxiety state [13]. The average cost of hysteroscopy and dilatation and curettage (D&C) turned out to be $2614 in Australia [14]. Therefore, the ET cut-off for identifying AH and EC in asymptomatic postmenopausal women warrants to be standardized.
In the current study, we sought to review our present practice and determine the ET threshold as well as the risk of EC and AH in asymptomatic postmenopausal women with ET ≥ 5 mm.
Methods
Patients and study design
A total of 2537 patients were provided with inpatient hysteroscopy between January 1, 2017, and June 30, 2020 in International Peace Maternity and Child Health Hospital (IPMCH), an urban tertiary specialized hospital in Shanghai, China. All medical records of these patients were reviewed. Patients who were eligible for both the inclusion criteria and exclusion criteria were included for the study.
We enrolled patients with the following inclusion criteria: (1) amenorrhoea for at least 12 months; (2) the indication of hysteroscopy was ET ≥ 5 mm which was incidentally found by TVS; (3) the ET must be evaluated by TVS in the same hospital, namely IPMCH; (4) patients had no obvious adnexal mass to eliminate the impact of hormone-secreting tumors on the endometrium; and (5) patients had no vaginal bleeding, no vaginal discharge, and no pelvic pain.
Exclusion criteria was as follows: (1) breast cancer patients with unknown information of tamoxifen treatment by reviewing the medical records were excluded; (2) patients with uterine malformation and those who were diagnosed with more than one malignancy were not included.
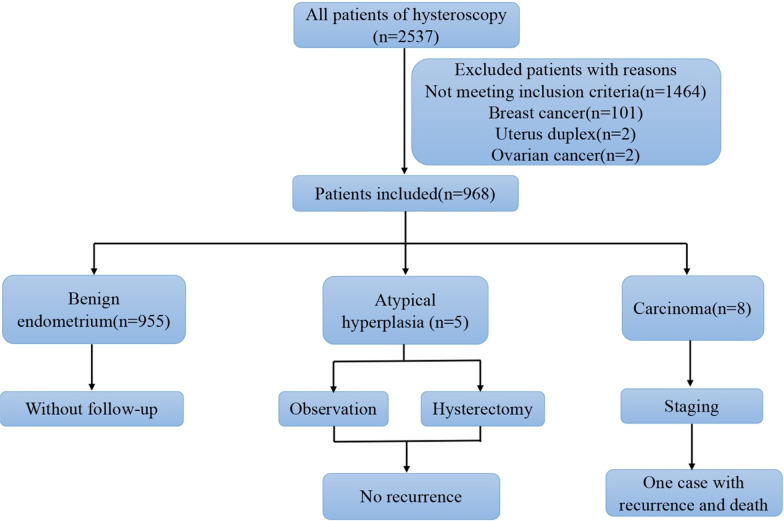
Among the 2537 patients to whom the hysteroscopy was offered during the period, 1464 patients having symptoms were excluded. 101 patients with breast cancer were excluded for the absence of information of tamoxifen therapy in the medical records. Patients diagnosed with uterus duplex (n = 2) and ovarian cancer (n = 2) were not included. Finally, 968 patients were available for further analysis (Fig. 1).
Fig. 1.
Flow diagram of the study
The endometrium of all these women was investigated by TVS in our hospital by two certified radiologists for routine physical examination using a transvaginal probe (GE Voluson scanner, E8, GE-Healthcare, Waukesha, WI, USA). The double wall ET was measured in the longitudinal plane at its thickest point. Hysteroscopies were conducted under total intravenous anesthesia. All the hysteroscopist were experienced with appropriate skill set. Endometrial biopsies were immediately immersed in 10% formaldehyde and sent to a pathology laboratory. The histopathological results were estimated by two pathologists.
Data were collected for all the eligible patients including age, body mass index (BMI), gravidity, parity, hypertension, diabetes, the image findings, histopathology results and outcome of all patients. All the 13 patients with premalignancy and malignancy were followed up until July 2022. All the procedures performed in studies involving human participants were in accordance with the ethical standards of Ethics Committee of IPMCH in Shanghai and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The publication of this study has the permission of the patients.
Statistical analysis
Continuous and nonparametric variables were analyzed using t-test and chi-squared test respectively. Logistic regression analysis was performed to determine risk factors for AH and EC. The optimal cut-off value of ET for AH and EC was determined by receiver operating characteristic (ROC) curve analysis by area under curve (AUC). Statistical analysis were performed with SPSS version 23.0 (IBM Corp., Armonk, NY, USA). A P value of < 0.05 was considered statistically significant.
Results
TVS showed an ET ≥ 5 mm for all the included patients (n = 968). Pathologic outcomes of biopsy of the endometrium were listed in Table 1. Among the 955 patients with benign results, 696 (71.9%) were reported as endometrial polyps, 26 (2.7%) as hyperplasia, 32 (3.3%) as leiomyoma, 123 (12.7%) as atrophic endometrium, 27 (2.8%) as endometritis and 51 (5.3%) as normal endometrium. 5 (0.5%) cases had AH, and 8 (0.8%) were found to be EC.
Table 1.
Pathologic outcomes in 968 menopausal asymptomatic postmenopausal women with a thickened endometrium ≥ 5 mm
| Pathologic outcomes | n (%) |
|---|---|
| Benign endometrium | 955 (98.7) |
| Endometrial polyps | 696 (71.9) |
| Hyperplasia | 26 (2.7) |
| Leiomyoma | 32 (3.3) |
| Atrophic endometrium | 123 (12.7) |
| Endometritis | 27 (2.8) |
| Normal endometrium | 51 (5.3) |
| Atypical hyperplasia | 5 (0.5) |
| Endometrial carcinoma | 8 (0.8) |
Table 2 shows the demographic and clinical characteristics of patients. Patients were divided into 2 groups according to the pathologic outcomes (Group A, benign endometrium; Group B, AH and EC). The age of the patients varied from 46 to 87 years. The mean age in Group A was 61 years and in Group B it was 63 (P = 0.237). There was no significant difference between the BMI of the two groups (24.1 ± 3.37 vs. 24.2 ± 3.34, P = 0.928). Compared to Group A, it was comparable as with the other risk factors including gravity, parity, duration of menopause, hypertension, diabetes, history of cancer and family history of cancer in Group B. However, a significant difference was observed in the ET between Group A and Group B (7.7 ± 2.50 vs. 10.4 ± 4.50, P = 0.047). No complication was reported for all the included patients.
Table 2.
Demographic and clinical characteristics of patients with different pathologic outcomes
| Group A (n = 955) |
Group B (n = 13) |
P | |
|---|---|---|---|
| Age, yr, mean ± SD | 61 ± 6.5 | 63 ± 6.4 | 0.237 |
| BMI, kg/m2, mean ± SD | 24.1 ± 3.37 | 24.2 ± 3.34 | 0.928 |
| Gravity, mean ± SD | 2 ± 1.2 | 3 ± 1.2 | 0.175 |
| Parity, mean ± SD | 1 ± 0.6 | 1 ± 0.6 | 0.805 |
| Duration of menopause, yr, mean ± SD | 9 ± 6.4 | 12 ± 9.0 | 0.222 |
| Hypertension, n (%) | 373 (39%) | 4 (31%) | 0.543 |
| Diabetes, n (%) | 76 (8%) | 2 (15%) | 0.643 |
| History of cancer, n (%) | 23 (2%) | 1 (8%) | 0.326 |
| Family history of cancer, n (%) | 17 (2%) | 0 (0%) | 1.000 |
| Endometrial thickness, mm, mean ± SD | 7.7 ± 2.50 | 10.4 ± 4.50 | 0.047 |
Logistic regression analysis showed that the ET was the risk factor for the AH and EC in asymptomatic postmenopausal women. The odds ratio (OR) for ET was 1.252 (95% confidence interval [CI] 1.107–1.416, P < 0.001). The diagnostic performance of ET was further analyzed by ROC curve analysis (Fig. 2). The optimal cut-off value for AH and EC was found to be 8 mm with the maximum AUC (0.715, 95% CI 0.686–0.743), with a sensitivity of 0.846, a specificity of 0.609, positive likelihood ratio (LR +) of 2.164 and negative likelihood ratio (LR−) of 0.253. The diagnostic value of ET with different cut-off value in endometrial lesions was compared in Table 3.
Fig. 2.
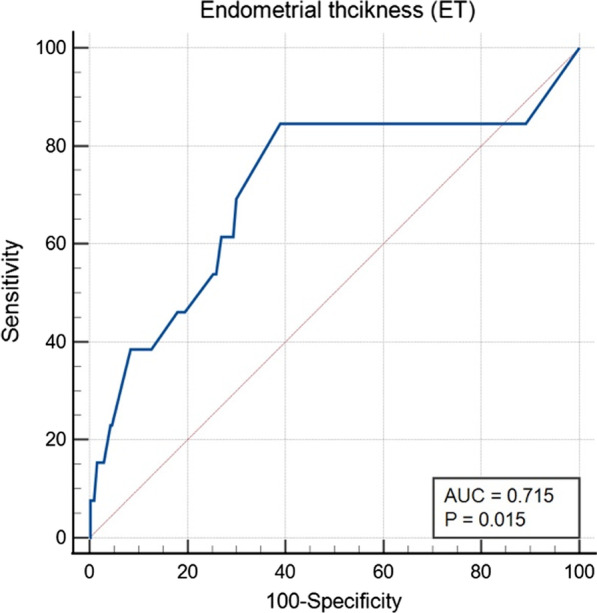
Effects of endometrial thickness detected using TVS in predicting the results of endometrial sampling
Table 3.
Comparison of diagnostic value of endometrial thickness with different cut-off value in endometrial lesions
| Cut-off (mm) |
Sensitivity (% (95% CI)) |
Specificity (% (95% CI)) |
LR + (% (95% CI)) |
LR- (% (95% CI)) |
|---|---|---|---|---|
| ≧ 7.0 | 84.6 (57.8–95.7) | 43.5 (40.3–46.6) | 1.50 (0.97–1.79) | 0.35 (0.09–0.44) |
| ≧ 8.0 | 84.6 (57.8–95.7) | 60.9 (57.8–64.0) | 2.16 (1.37–2.66) | 0.25 (0.07–0.44) |
| ≧ 9.0 | 53.8 (29.1–76.8) | 74.8 (71.9–77.4) | 2.13 (1.05–3.40) | 0.62 (0.30–0.92) |
| ≧ 10.0 | 46.2 (23.2–70.9) | 82.0 (79.4–84.3) | 2.57 (1.13–4.52) | 0.66 (0.35–1.08) |
| ≧ 11.0 | 38.5 (17.7–64.5) | 88.1 (85.9–90.0) | 3.24 (1.26–6.45) | 0.70 (0.39–1.28) |
| ≧ 12.0 | 38.5 (17.7–64.5) | 91.6 (89.7–93.2) | 4.58 (1.72–9.49) | 0.67 (0.38–1.28) |
Amongst women with the diagnosis of AH, 4 underwent hysterectomy, with one for observation. All 8 women with EC underwent staging surgery, with 3 at stage IA (grade 1, G1), 2 at stage IA (G2), 1 at stage IA (G3), 1 at stage IB and 1 at stage II (Fig. 3). 75% cases were found to be at stage IA. Follow-up information was available for all the 13 patients with AH and EC. With a follow-up period of between 2 and 5 years, one case of EC aging 70 years at stage IA (G2) exhibited recurrence and died 2 years after the staging surgery. The other 12 patients were alive without recurrence until July 2022.
Fig. 3.
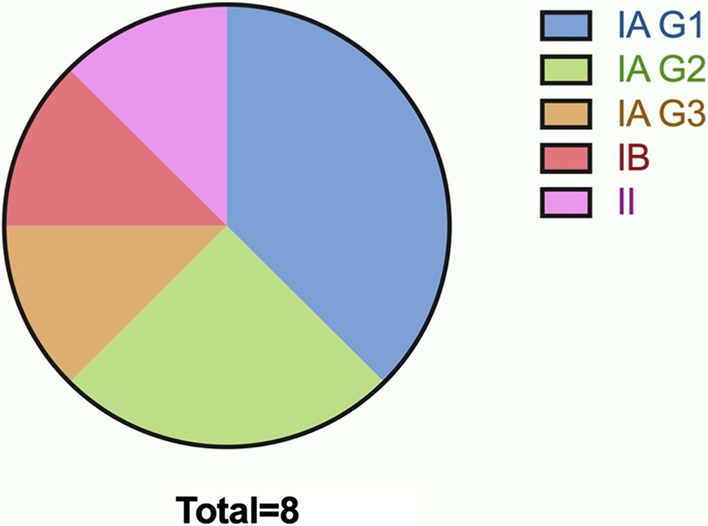
Distribution of pathologic outcomes among EC patients
Discussion
The current retrospective study included 968 eligible cases and revealed that ET was the risk factor for the endometrial premalignancy and malignancy in asymptomatic postmenopausal women. The 8-mm ET was identified as the optimal threshold to detect AH and EC in asymptomatic postmenopausal women.
The strength of this study is that a large number of patients were included. But there are several limitations to our study. First, the analysis was limited due to the low prevalence of endometrial AH and EC as well as the retrospective property, which could result in bias. Second, it is a drawback that our analysis has not included the risk factors such as the use of hormone therapy and tamoxifen due to incomplete medical records, so the findings cannot be extrapolated to the whole population of asymptomatic postmenopausal women. Although it was revealed that there was no significant association between endometrial cancer and postmenopausal hormone usage [11, 15], the use of hormone therapy is necessary to be considered. Third, all the patients with benign conditions were not followed up. It remains unknown what effects the threshold would pose on the outcomes of these patients.
EC ranks fourth in female cancers, the management of which is of great concern. The surgical complications were reported especially in patients treated with laparotomy [15–17]. Given the fact that patients with late stage were more frequently subject to laparotomy, early identification of EC is quite important. The current study indicates that an incidentally-found ET of more than 8 mm in asymptomatic postmenopausal women might be recommended for further endometrial biopsy. Although different ET cut-off values had been investigated by various groups, there is no consensus as with the recommended ET to distinguish abnormal endometrium in postmenopausal woman without vaginal bleeding. The identified thresholds ranged 8–15 mm to distinguish EC or AH in asymptomatic women [3, 11, 18, 19]. Hysteroscopy was recommended to perform at a measurement of ET ≥ 8 mm in asymptomatic postmenopausal women in a study in Italy [11], while the threshold of 10 mm was suggested by several studies [3, 9, 18, 20]. The ET of 11 mm was identified as the optimal diagnostic threshold for atypical endometrium and malignancies [3, 4, 21, 22].Of note, the reported cut-offs were mostly based on AUC ROC method, which could be different from the ideal threshold when all the risk factors and patient outcomes are prospectively considered. Thus, prospective study with more samples will provide a more favorable threshold and diagnostic model for asymptomatic postmenopausal women in the future.
The present study also provides evidence for the fact that the incidence of malignancies or premalignancy is minimal in asymptomatic postmenopausal women. The prevalence of AH and/or EC was reported to be 0.62–9.9% [3, 5, 7, 10, 22–24]. As the endometrial condition is not routinely screened among asymptomatic women, the prevalence of EC and AH is difficult to estimate in this population. Thus, whether screening EC and AH is necessary warrants further investigation among postmenopausal women without symptoms. Many studies reported a significant association between EC and obesity [17, 25]. However, the benign and EC and AH groups showed no difference as with the BMI in our study, which may result from the small sample size in EC and AH group. Our study and previous studies consistently found that most cases were at stage I in postmenopausal women with no symptoms [3, 21, 26]. It was reported that there was no significant difference in rates of high-grade histology between asymptomatic women and women with abnormal uterine bleeding [26]. However, whether the survival rate benefits from the detection of EC in asymptomatic postmenopausal women compared to that in symptomatic postmenopausal women remains to be investigated. A further study including all the EC patients will provide more detailed information for pathological outcomes and survival rates.
This study has practice implications for clinicians to help provide with a threshold for management of asymptomatic postmenopausal women with endometrial thickening. However, the findings require confirmation in future clinical trials. The prospective study underlying the potential cut-off for endometrial premalignancy and malignancy desiderates to be further investigated and will shed light on the management of asymptomatic postmenopausal women with thickened endometrium.
Conclusion
The mean ET of women with AH and EC is higher than that of patients with benign endometrium. An ET cut-off of ≥ 8 mm shows a reasonable performance to detect AH and EC in asymptomatic postmenopausal women, thereby avoiding more invasive endometrial biopsy.
Acknowledgements
Not applicable.
Abbreviations
- EC
Endometrial carcinoma
- AH
Atypical hyperplasia
- ET
Endometrial thickness
- TVS
Transvaginal sonography
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- OR
Odds ratio
- CI
Confidence interval
- LR+
Positive likelihood ratio
- LR−
Negative likelihood ratio
- BMI
Body mass index
Author contributions
LNZ: data collection, data analysis, YG: data analysis, manuscript writing, GXQ: data collection, TS and HX: supervision, manuscript editing. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (NO. 2020YFC2002804), the National Natural Science Foundation of China (NO. 82071622, 81771551 and 82001622), and Shanghai Sailing Program (20YF1453700).
Availability of data and materials
The data analyzed during the study is available from the corresponding author upon reasonable request.
Declarations
Ethical approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and approved by Ethic Committee of the International Peace Maternity and Child Health Hospital in Shanghai. All the patients signed the informed consent.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Linna Zhang and Ying Guo contributed equally to this work
Contributor Information
Tao Su, Email: sutaodoctor@126.com.
Hong Xu, Email: xuhong1558@sjtu.edu.cn.
References
- 1.Siegal R, Miller KD, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of adulthood overweight, obesity, and cancer risk in the women's health initiative: a longitudinal study from the United States. PLoS Med. 2016;13(8):e1002081. doi: 10.1371/journal.pmed.1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li JX, Chan F, Johansson C. Can a higher endometrial thickness threshold exclude endometrial cancer and atypical hyperplasia in asymptomatic postmenopausal women? A systematic review. Aust N Z J Obstet Gynaecol. 2022;62(2):190–197. doi: 10.1111/ajo.13472. [DOI] [PubMed] [Google Scholar]
- 4.Smith-Bindman R, Weiss E, Feldstein V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet Gynecol. 2004;24(5):558–565. doi: 10.1002/uog.1704. [DOI] [PubMed] [Google Scholar]
- 5.Breijer M, Peeters J, Opmeer B, Clark TJ, Verheijen R, Mol B, et al. Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic postmenopausal women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2012;40(6):621–629. doi: 10.1002/uog.12306. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice ACOG committee opinion. No. 336: tamoxifen and uterine cancer. Obstet Gynecol. 2006;107(6):1475–1478. doi: 10.1097/00006250-200606000-00057. [DOI] [PubMed] [Google Scholar]
- 7.Famuyide AO, Breitkopf DM, Hopkins MR, Laughlin-Tommaso SK. Asymptomatic thickened endometrium in postmenopausal women: malignancy risk. J Minim Invasive Gynecol. 2014;21(5):782–786. doi: 10.1016/j.jmig.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Laiyemo R, Dudill W, Jones SE, Browne H. Do postmenopausal women with thickened endometrium on trans-vaginal ultrasound in the absence of vaginal bleeding need hysteroscopic assessment? A Pilot Study J Obstet Gynaecol. 2016;36(2):223–226. doi: 10.3109/01443615.2015.1050649. [DOI] [PubMed] [Google Scholar]
- 9.Ghoubara A, Emovon E, Sundar S, Ewies A. Thickened endometrium in asymptomatic postmenopausal women - determining an optimum threshold for prediction of atypical hyperplasia and cancer. J Obstet Gynaecol. 2018;38(8):1146–1149. doi: 10.1080/01443615.2018.1458081. [DOI] [PubMed] [Google Scholar]
- 10.Bracco Suarez MB, Benetti-Pinto CL, Gibran L, Yela DA. Asymptomatic postmenopausal women: What are the risk factors for endometrial malignancies? A Multicent Retrosp Study Gynecol Endocrinol. 2021;37(9):853–856. doi: 10.1080/09513590.2020.1843621. [DOI] [PubMed] [Google Scholar]
- 11.Giannella L, Mfuta K, Setti T, Boselli F, Bergamini E, Cerami LB. Diagnostic accuracy of endometrial thickness for the detection of intra-uterine pathologies and appropriateness of performed hysteroscopies among asymptomatic postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2014;177:29–33. doi: 10.1016/j.ejogrb.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Gambadauro P, Navaratnarajah R, Carli V. Anxiety at outpatient hysteroscopy. Gynecol Surg. 2015;12(3):189–196. doi: 10.1007/s10397-015-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carta G, Palermo P, Marinangeli F, Piroli A, Necozione S, De Lellis V, et al. Waiting time and pain during office hysteroscopy. J Minim Invasive Gynecol. 2012;19(3):360–364. doi: 10.1016/j.jmig.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Aston B, Weaver E. Risks and benefits of hysteroscopy and endometrial sampling as a standard procedure for assessing serendipitous findings of endometrial thickening in postmenopausal women. Aust N Z J Obstet Gynaecol. 2014;54(6):597–599. doi: 10.1111/ajo.12259. [DOI] [PubMed] [Google Scholar]
- 15.Giannini A, Di Donato V, Schiavi MC, May J, Panici PB, Congiu MA. Predictors of postoperative overall and severe complications after surgical treatment for endometrial cancer: the role of the fragility index. Int J Gynaecol Obstet. 2020;148(2):174–180. doi: 10.1002/ijgo.13020. [DOI] [PubMed] [Google Scholar]
- 16.Buzzaccarini G, Torok P, Vitagliano A, Petousis S, Noventa M, et al. Predictors of pain development after laparoscopic adnexectomy: a still open challenge. J Invest Surg. 2022;35(6):1392–1393. doi: 10.1080/08941939.2022.2056274. [DOI] [PubMed] [Google Scholar]
- 17.Di Donato V, D'Oria O, Giannini A, Bogani G, Fischetti M, Santangelo G, et al. Age-adjusted charlson comorbidity index predicts survival in endometrial cancer patients. Gynecol Obstet Invest. 2022;87(3–4):191–199. doi: 10.1159/000525405. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal A, Hatti A, Tirumuru SS, Nair SS. Management of asymptomatic postmenopausal women referred to outpatient hysteroscopy service with incidental finding of thickened endometrium–a UK district general hospital experience. J Minim Invasive Gynecol. 2021;28(10):1725–1729. doi: 10.1016/j.jmig.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Louie M, Canavan TP, Mansuria S. Threshold for endometrial sampling among postmenopausal patients without vaginal bleeding. Int J Gynaecol Obstet. 2016;132(3):314–317. doi: 10.1016/j.ijgo.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Ai F, Wang Y, Wang Y, Wang J, Zhou L, Wang S. Clinicopathological features of endometrial lesions in asymptomatic postmenopausal women with thickened endometrium. Menopause. 2022;29(8):952–956. doi: 10.1097/GME.0000000000001993. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Li L. Risk of malignancies among asymptomatic postmenopausal women with thickened endometrium: a cohort study. Medicine (Baltimore) 2019;98(6):e14464. doi: 10.1097/MD.0000000000014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcázar JL, Bonilla L, Marucco J, Padilla AI, Chacón E, et al. Risk of endometrial cancer and endometrial hyperplasia with atypia in asymptomatic postmenopausal women with endometrial thickness≥ 11 mm: a systematic review and meta-analysis. J Clin Ultrasound. 2018;46(9):565–570. doi: 10.1002/jcu.22631. [DOI] [PubMed] [Google Scholar]
- 23.Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(5):1197–1205. doi: 10.1097/AOG.0b013e3181f74864. [DOI] [PubMed] [Google Scholar]
- 24.Archer DF, McIntyre-Seltman K, Wilborn WW, Dowling EA, Cone F, Creasy GW, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–320. doi: 10.1016/0002-9378(91)90084-5. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Cheung ECW, Lao TT. Obesity increases endometrial cancer risk in Chinese women with postmenopausal bleeding. Menopause. 2021;28(10):1093–1098. doi: 10.1097/GME.0000000000001822. [DOI] [PubMed] [Google Scholar]
- 26.Segev Y, Dain-Sagi L, Lavie O, Sagi S, Gemer O. Is there a survival advantage in diagnosing endometrial cancer in asymptomatic patients? A systemic review and meta-analysis. J Obstet Gynaecol Can. 2020;42(4):481–487. doi: 10.1016/j.jogc.2019.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during the study is available from the corresponding author upon reasonable request.