Abstract
The high mortality of nosocomial infections caused by Klebsiella spp. has acted as a stimulus to develop immunotherapeutic approaches targeted against surface molecules of these bacteria. Since O-antigen-specific antibodies may add to the protective effect of K antisera, we tested the functional and binding capacity of O-antigen-specific monoclonal antibodies (MAbs) raised against different Klebsiella O antigens. The MAbs tested were specific for the O-polysaccharide partial antigens d-galactan II (MAb Ru-O1), d-galactan I (MAb IV/4-5), or core oligosaccharide (MAb V/9-5) of the Klebsiella serogroup O1 antigen. In enzyme-linked immunosorbent assay binding experiments, we found that all MAbs recognized their epitopes on intact capsule-free bacteria; however, binding to encapsulated wild-type strains belonging to different K-antigen serotypes was significantly reduced. The K2 antigen acted as the strongest penetration barrier, while the K7 and K21 antigens allowed some, though diminished, antibody binding. In vitro phagocytic killing experiments showed that MAb Ru-O1 possessed significant opsonizing activity for nonencapsulated O1 serogroup strains and also, to a much lesser extent, for encapsulated strains belonging to the O1:K7 and O1:K21 serotypes. MAbs or antisera specific for the d-galactan II antigen may thus be the most promising agents for further efforts to develop a second-generation Klebsiella hyperimmune globulin comprising both K- and O-antigen specificities.
Klebsiella pneumoniae is one of the most frequently isolated gram-negative bacterial pathogens in severe nosocomial infections (1, 21, 26). The rapidly progressive clinical course of Klebsiella pneumonia, which is often complicated by multilobular involvement and lung abscesses (3, 22), leaves little time to institute effective antimicrobial treatment. Similarly, other types of nosocomial Klebsiella infection are characterized by a high mortality rate. In addition, an increasing proportion of K. pneumoniae isolates are resistant to multiple antimicrobial agents commonly used in intensive care units (reviewed in reference 20).
An important virulence factor of K. pneumoniae is the capsular polysaccharide (CPS) (35, 40) whose major pathogenic effect is thought to mainly inhibit phagocytosis (11). Specific antibodies against CPS are protective in various animal models of infection (8, 18, 46). There are, however, 77 different serotypes of CPS known in the genus Klebsiella (15). Moreover, there is no significant predominance of certain serotypes (35, 55), although serotypes K2, K21, and K7 have been found more frequently in respiratory and urinary tract infections (6, 9, 33, 34). Apart from CPS, Klebsiella produce lipopolysaccharide (O antigen; LPS) which is an important mediator of septic shock. Since lipid A is the least variable part of LPS within gram-negative bacteria, clinical trials using immunotherapy against lipid A have focused on monoclonal antibodies (MAbs) against this part of LPS but have been unsuccessful so far (4, 53).
Antibodies directed against species-specific O antigens yielded promising results in Escherichia coli (14, 19) and Pseudomonas aeruginosa infection (31, 32, 36). In contrast to other gram-negative bacteria like E. coli which express more than 100 serotypes of O antigens, K. pneumoniae produces only nine different O-antigen serotypes. Four of these, O1, O2ab, O2ac, and O3, account for more than 70% of the O-antigen serotypes found in clinical isolates (45). A specific epitope located in the core oligosaccharide was found in more than 90% of clinical K. pneumoniae and K. oxytoca isolates (51). Since antibodies against LPS were shown to penetrate the capsule of K. pneumoniae (27, 58), MAbs against the O antigen of K. pneumoniae may therefore be more suited as immunotherapy than antibodies against CPS. In this study, we investigated the influence of different capsule serotypes of K. pneumoniae on binding and opsonophagocytic activity of LPS-specific MAbs directed against the O1 partial antigens d-galactan I and d-galactan II as well as against the genus-specific core oligosaccharide antigen of K. pneumoniae.
(This report is part of the M.D. thesis of N. R. M. Jendrike.)
MATERIALS AND METHODS
Bacteria.
The strains used are described in Table 1. The following strains were clinical isolates from the strain collection of one of us: strain 37 (K. pneumoniae subsp. pneumoniae, source not documented), strain 151 (K. oxytoca, source not documented), strain 557 (K. oxytoca, blood culture isolate), and strain 591 (K. pneumoniae subsp. pneumoniae, urinary tract isolate). Decapsulated mutants of these strains were produced by nonmutagenic treatment as described elsewhere (35).
TABLE 1.
Bacterial strains used in this study
| Strain | Serotype | CPS production | Reference or sourcea |
|---|---|---|---|
| K. pneumoniae | |||
| Caroli | O1:K2 | + | 37 |
| Caroli/2 | O1:K− | − | |
| 591 | O1:K2 | + | Clinical isolate |
| 591/1 | O1:K− | − | |
| 37 | O1:K7 | + | 35 |
| 37/2a | O1:K− | − | |
| 58 | O1:K7 | + | 35 |
| 58/5 | O1:K− | − | |
| 151 | O1:K21 | + | Clinical isolate |
| 151/1 | O1:K− | − | |
| 557 | O1:K21 | + | Clinical isolate |
| 557/2 | O1:K− | − | |
| B5055 | O1:K2 | + | SS |
| 1702/49 | O1:K7 | + | SS |
| E. coli | |||
| Bort | O18:K1:H7 | 51 | |
| E701 | O6:K5:H1 | SS |
Clinical isolates are from the laboratory of one of us (R.P.). SS, Statens Seruminstitut, Copenhagen, Denmark. For details, see Materials and Methods.
LPS and CPS.
LPSs from various K. pneumoniae reference strains, prepared in our laboratories by the hot phenol-water method as described previously (54), have been used before (45, 50, 51). CPSs for serotypes K2, K21, and K7 were prepared from strains B5055, 1702/49, and 37, respectively, by precipitation of culture supernatants with cetylammonium bromide (Cetavlon; Merck, Darmstadt, Germany) by the method of Cryz et al. (7). The CPS preparations have been described before (47).
Antibodies.
MAbs Ru-O1 (37), V/9-5 (51), and III/5-1 (46) have been described previously. MAb Ru-O1 is directed against d-galactan II and is a murine immunoglobulin G2b (IgG2b) antibody. MAb V/5-9 is directed against species-specific core oligosaccharide and is a murine IgG2a antibody. MAb III/5-1 is directed against K2 CPS and is a mouse IgM antibody. MAb IV/4-5 was generated by intraperitoneal immunization of 6- to 8-week-old female BALB/c mice with heat-inactivated (60°C, 60 min) bacteria of K. pneumoniae 7380 (O2ab:K−) known to express the d-galactan I antigen (56). Four immunizations using 107 bacteria per injection were performed in 2- to 3-week intervals, and two mice which showed the highest serum antibody response against LPS from K. pneumoniae 7380 were sacrificed 3 days after the last immunization. Fusion of splenic lymphocytes with the mouse myeloma cell line X63-Ag8.653 and cloning of hybridomas were performed as described elsewhere (46, 51). Clones producing specific antibody against d-galactan I were identified by enzyme-linked immunosorbent assay (ELISA) using LPS extracted from K. pneumoniae 7380 as solid-phase antigen. Clone IV/4-5 was selected from three clones producing d-galactan I-reactive MAb on the basis of stable growth during subcloning and persistently high ELISA reactivity of cell culture supernatants. The antibody subclass as determined by ELISA was IgG3.
The concentration of each MAb was determined by a direct ELISA method as described earlier (45). Since antibody dilutions were made from mouse ascites and since there has been evidence that mouse ascites contains a substance which may inhibit complement (43), we used E. coli E701 (a kind gift from J. C. Sadoff, Washington, D.C.), which is sensitive to killing by complement and shares no cross-antigenicity with K. pneumoniae antigen d-galactan I or II, to test antibody preparations for the presence of such a substance. Ten microliters of a bacterial suspension (2 × 108 CFU/ml) was coincubated with 10 μl of complement, 10 μl of ascites to be tested, and 70 μl of Hanks balanced salt solution (HBSS; Servamed, Biochrom KG, Berlin, Germany) at 37°C for 2 h. Killing rates were determined as described below for the phagocytic killing assay and showed no evidence for the presence of complement inhibiting effects (data not shown).
Specific antisera enriched for antibodies against the K7 and K21 capsular antigens, respectively, were obtained as follows. Rabbits were immunized with heat-killed bacteria of strain K. pneumoniae 4140 (O1:K7) (45). Serum was obtained after 4 weeks of immunization and stored at −80°C. A human volunteer was immunized with the 24-valent Klebsiella vaccine Klebvax (13), and the serum was obtained as described elsewhere (17). Specific antibodies in these rabbit and human sera were determined by means of a quantitative ELISA (48). Antibody levels were 100 μg/ml against K7 in the rabbit serum and 64 μg/ml against K21 in the human serum.
Immunoblotting.
Purified LPS preparations were separated by polyacrylamide gel electrophoresis as described earlier (48). Electrophoresed LPS were either visualized directly by the silver stain procedure (39) or transblotted to a 0.45-μm-pore-size nitrocellulose membrane (Millipore, Molsheim, France) as described elsewhere (17). After blocking of nonspecific binding sites with filler buffer (47) for 1 h at room temperature, membranes were reacted overnight with a MAb diluted to a concentration of 10 μg/ml. After washing, bound MAb was visualized by sequential addition of class-specific alkaline phosphatase-conjugated antibodies (Sigma, Deisenhofen, Germany) and developing substrate as described previously (39).
Complement.
Blood was drawn from five healthy volunteers, and serum was obtained by centrifugation. The five sera were pooled and then divided into aliquots, which were absorbed with the decapsulated mutants of O1 strains later used in phagocytic killing experiments. Absorptions were performed exactly as described previously (49). After removal of the bacteria by centrifugation (1,500 × g, 10 min, 4°C), the sera were sterilized through a 0.45-μm-pore-size filter (Millipore, Eschborn, Germany), aliquoted, and stored at −80°C.
The efficacy of antibody absorption was measured by quantitative ELISA as described previously (48), using purified O1 LPS prepared from K. pneumoniae Friedländer 201 as the solid-phase antigen and purified human IgG and IgM (Sigma) as standards. The detection limits of specific antibodies were 0.008 μg/ml for IgG and 0.04 μg/ml for IgM.
Human neutrophils.
Heparinized (50 U/ml) venous blood was obtained from healthy donors. Neutrophils (polymorphonuclear leukocytes [PMN]) were isolated, and residual erythrocytes were lysed as described earlier (5). PMN were adjusted to 2 × 107/ml in HBSS without calcium and magnesium and stored on ice until use. Preliminary experiments showed no variation in the activity of neutrophils from different donors (data not shown).
Inhibition ELISA with supernatants of Klebsiella cultures.
To characterize the different O-antigen epitopes, we used an inhibition ELISA method exactly as described earlier (51). In brief, microtiter plates (Greiner, Nürtingen, Germany) were coated with O1 LPS from various Klebsiella strains (2 μg/ml, 100 μl/well, 4°C, overnight). After blocking with filler buffer (47), plates were washed three times with phosphate-buffered saline (PBS). Klebsiella strains were grown as described previously (51), bacterial suspensions were boiled for 2 h, and bacterial debris was removed by centrifugation. The clear supernatant was added to an equal volume of MAb (10 μg/ml in filler buffer), and the mixture was stored on ice for 2 h, with vortexing every 15 min. One hundred microliters of the mixture was transferred to duplicate wells of LPS-coated microtiter plates and incubated overnight at 4°C. After washing, bound LPS-specific MAb was traced as described elsewhere (51). Controls included irrelevant MAbs of the same Ig subclass (Sigma), incubation of bacteria without MAbs, and a nonreactive E. coli strain (Bort), all of which showed no significant binding.
Binding of MAbs to intact bacteria.
Binding of the MAbs to whole bacteria was determined as described earlier (51). In brief, bacteria were grown overnight at 37°C on Mueller-Hinton agar, washed three times with PBS, and incubated for 8 min at 90°C to destroy intrinsic alkaline phosphatase activity. After another three washes with PBS, CFU counts were adjusted to 4 × 108/ml as described earlier (18), and the bacteria were incubated for 1 h in filler buffer to block nonspecific binding sites. One milliliter of the bacterial suspension was centrifuged for 5 min (13,000 rpm in a no. 13 centrifuge, rotor no. 3743; Heraeus Sepatech, Heraeus, Germany), and the supernatant was discarded. The pellet was resuspended in 0.5 ml of the MAb to be tested (10 μg/ml) and incubated for 1 h at 4°C with vortexing every 15 min. After centrifugation for 5 min (13,000 rpm in a no. 13 centrifuge, rotor no. 3743; Heraeus Sepatech, Heraeus, Germany), the supernatant was discarded, cells were washed as described above, and antibody bound to the bacteria was traced by incubation of the pellet with appropriately diluted, alkaline phosphatase-labeled anti-mouse IgG (Sigma) at 4°C for 1 h with vortexing every 15 min. After a second series of washes, bacterial cells were incubated with p-nitrophenyl phosphate (Sigma; 1 mg/ml in diethanolamine buffer [47]) for 25 min. The reaction was stopped by centrifugation, and 200 μl of the supernatant was transferred to a 96-well flat-bottom microtiter plate (Greiner). The optical density (OD) at 405 nm was read as described above. Controls included irrelevant MAbs of the same Ig subclass (Sigma), incubation of bacteria without primary antibodies, and use of E. coli Bort (Table 1), all of which showed no significant binding.
Capsular swelling reaction and measurement of glucuronic acid.
The swelling reaction on Klebsiella strains Caroli, 591, 37, 58, 151, and 557 was done using monospecific rabbit antisera generated in the laboratory of one of us. The presence of capsular material in the wild-type parent strains and their decapsulated mutants was also assessed by glucuronic acid determination of Zwittergent-extracted bacteria (12) as described previously (2).
Phagocytic killing assay.
The phagocytic killing assay was performed essentially as described elsewhere (17, 46). In brief, bacteria were grown into early log phase from single-colony isolates in glucose-casein-peptone broth (Unipath Ltd., Hampshire, England) for 3 h at 37°C with shaking. The bacteria were washed three times in sterile physiologic saline, adjusted to a concentration of 2 × 108 CFU/ml (18), and stored on ice until use. In 96-well round-bottom tissue culture plates (Greiner), 10 μl of bacteria, 50 μl of PMN, 10 μl of antibodies in various concentrations, 10 μl of normal human serum as complement source (prepared as described above), and 20 μl of HBSS with calcium and magnesium were incubated for 2 h at 37°C with shaking (300 rpm). Immediately after mixing of the ingredients and at 120 min, samples (10 μl) were taken from each well and placed on ice into a glass tube containing sterile distilled water with 0.1% bovine serum albumin (wt/vol) to lyse the PMN without killing the bacteria. Viable bacterial counts were determined by plating serial dilutions on Mueller-Hinton agar plates. We calculated the percentage of killed bacteria according to the following formula: % phagocytic killing = [(CFU at 0 min − CFU at 120 min)/CFU at 0 min] × 100. Controls in each experiment included bacteria with HBSS alone; bacteria and PMN without antibodies; bacteria, complement, and PMN without antibodies; bacteria, complement, and antibodies without PMN; and bacteria, complement, PMN, and irrelevant MAbs of the relevant subclass (to show that there was no nonspecific serum activity), each of which showed no killing of bacteria.
Statistical analysis.
Differences in the rate of killing between the various MAbs were compared by the Mann-Whitney U test using a software package (Statistics for Windows, version 4.5; StatSoft, Tulsa, Okla.). Since multiple comparisons were made, P ≤ 0.01 was considered significant. All comparisons were two tailed.
RESULTS
Characterization of different O-antigen patterns.
The MAbs bind to different parts of the O1 antigen: MAb IV/4-5 binds to d-galactan I (50), and MAb Ru-O1 binds to d-galactan II (37, 50). MAb V/9-5, however, binds to a species-specific core oligosaccharide epitope of K. pneumoniae which is expressed by all reference strains of Klebsiella except the O serotype 7 reference strain and by 97% of clinical isolates (51). We thus used these antibodies in an inhibition ELISA to characterize the O-antigen pattern of the strains used in this study. The results (Table 2) show that all strains expressed d-galactan I, d-galactan II, and the core oligosaccharide regardless of their CPS serotype as did the reference strain F201, thus characterizing the strains used as LPS serotype O1 strains. To investigate whether the nonmutagenic treatment used for generation of decapsulated mutants caused changes in the composition and antigenicity of the LPS, we analyzed LPS preparations of parent strains and their decapsulated mutants by silver staining and Western blotting with the MAbs. We detected no differences between the LPS before and after nonmutagenic treatment, indicating that the O-antigen pattern of the decapsulated mutants corresponded to that of their encapsulated parent strains (Fig. 1).
TABLE 2.
Characterization of O-antigen composition by inhibition ELISA
| Strain | CPS serotype | Inhibition (ELISA OD) with MAba:
|
O-antigen compositionb
|
||||
|---|---|---|---|---|---|---|---|
| Ru-O1 | IV/4-5 | V/9-5 | d-Galactan II | d-Galactan I | Core oligosaccharide | ||
| K. pneumoniae | |||||||
| Caroli | K2 | 0.00 | 0.00 | 0.00 | + | + | + |
| 591 | K2 | 0.00 | 0.03 | 0.00 | + | + | + |
| 37 | K7 | 0.00 | 0.00 | 0.01 | + | + | + |
| 58 | K7 | 0.00 | 0.00 | 0.00 | + | + | + |
| 151 | K21 | 0.01 | 0.01 | 0.01 | + | + | + |
| 557 | K21 | 0.00 | 0.00 | 0.00 | + | + | + |
| 201c | K− | 0.00 | 0.02 | 0.01 | + | + | + |
| E. coli Bortd | K1 | 0.64 | 0.58 | 0.9 | − | − | − |
Mean of four experiments, each done in duplicate.
+, antigen present; −, antigen not present.
Positive control (K. pneumoniae Friedländer 201, serotype O1:K−).
Negative control (E. coli Bort, serotype O18:H7:K1).
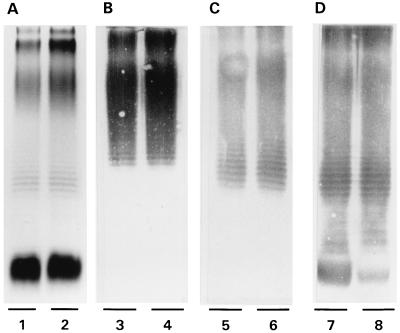
FIG. 1.
Expression of serotype O1 partial antigens by an encapsulated parent Klebsiella strain and its decapsulated mutant. LPS was prepared from strain 591 (lanes 1, 3, 5, and 7) and its decapsulated mutant (lanes 2, 4, 6, and 8). (A) Silver stain; (B) Western blot reacted with MAb Ru-O1; (C) Western blot reacted with MAb IV/4-5; (D) Western blot reacted with MAb V/9-5. Both the parent strain and the decapsulated mutant expressed all three known partial antigens of the O1 serogroup. Similar reactions were obtained with the other strains used in this study.
Binding of MAbs to intact K. pneumoniae.
We next investigated whether capsular material belonging to distinct CPS serotypes has different effects on the binding of the MAbs to their O-antigen epitopes by investigating the binding of the MAbs to intact bacteria. To assess possible inhibitory effects on binding by the capsule, we compared decapsulated mutant strains to their encapsulated parent strains. As detailed in Table 3, the K2 CPS was most effective in reducing the binding of the MAbs to LPS, regardless which of the three MAbs was investigated. K7 CPS and K21 CPS, however, reduced binding of the MAbs to their epitopes to a much lesser, albeit still significant, extent (Table 3). To detect a possible binding of MAb Ru-O1 despite the presence of K2 CPS, we used in separate assays a 10-fold-lower dilution of the detection antibody (1:3,000 instead of 1:30,000). Indeed, the observed OD values increased from 0.049 to 0.742 for strain Caroli. The corresponding values for the irrelevant MAbs of the same IgG subclass were 0.004 for each, indicating that the observed increase in sensitivity was not due to nonspecific binding.
TABLE 3.
Binding of three MAbs recognizing different O-antigen epitopes to encapsulated K. pneumoniae parent strains and their decapsulated mutants
| Strain | CPS serotype | ELISA ODa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MAb
|
Control antibody (subclass)b
|
Anti-CPS-antibodyc | Control | ||||||
| Ru-O1 | IV/4-5 | V/9-5 | IgG2b | IgG3 | IgG2a | ||||
| Caroli | K2 | 0.049 | 0.010 | 0.030 | 0.000 | 0.001 | 0.000 | 3.159 | 0.124 |
| Caroli/2 | K− | 2.452 | 1.575 | 1.433 | 0.000 | 0.000 | 0.000 | 0.063 | 0.014 |
| 591 | K2 | 0.044 | 0.027 | 0.112 | 0.004 | 0.003 | 0.048 | 2.836 | 0.054 |
| 591/1 | K− | 3.511 | 3.560 | 3.362 | 0.003 | 0.022 | 0.058 | 0.328 | 0.000 |
| 37 | K7 | 0.936 | 1.169 | 1.229 | 0.000 | 0.065 | 0.028 | 2.479 | 0.011 |
| 37/2a | K− | 2.781 | 3.056 | 2.762 | 0.000 | 0.085 | 0.068 | 1.951 | 0.000 |
| 58 | K7 | 1.419 | 0.909 | 1.177 | 0.009 | 0.049 | 0.017 | 1.185 | 0.003 |
| 58/5 | K− | 2.932 | 2.205 | 2.661 | 0.002 | 0.021 | 0.016 | 2.419 | 0.000 |
| 151 | K21 | 2.214 | 0.653 | 0.611 | 0.000 | 0.000 | 0.004 | 3.197 | |
| 151/1 | K− | 3.073 | 3.948 | 3.261 | 0.037 | 0.000 | 0.085 | 3.272 | |
| 557 | K21 | 0.452 | 0.423 | 0.635 | 0.017 | 0.012 | 0.032 | 1.580 | |
| 557/2 | K− | 2.610 | 3.316 | 3.420 | 0.010 | 0.004 | 0.026 | 2.033 | |
Mean of four independent experiments, each done in duplicate.
Irrelevant MAb of IgG2b subclass for MAb Ru-O1, IgG3 subclass for MAb IV/4-5, or IgG2a subclass for MAb V/9-5.
Anti-K2 CPS antibody was MAb III/5-1, and the control was an irrelevant IgM MAb. Anti-K7 CPS and anti-K21 CPS antibodies were polyclonal rabbit and human IgG antibodies, respectively.
To verify the significant decrease of CPS in decapsulated mutants, we investigated the binding of a MAb against K2 CPS and of polyclonal IgGs against K7 and K21 to whole bacteria. As shown in Table 3, binding of MAb III/5-1 on strain 591/1 was reduced more than eightfold compared to the encapsulated parent strain, 591. However, some binding was still detectable, which indicated residual CPS production of this particular strain. The other decapsulated mutants of the K2 CPS serotype strain Caroli showed virtually no binding of MAb III/5-1, indicating the absence of a capsule (Table 3). Since there were no MAbs against K7 or K21 CPS available, the resulting binding to the encapsulated mutants is most likely due to the polyclonality of the hyperimmune IgG raised against K7 or K21 CPS. We therefore verified the absence of CPS on the mutant strains by two other independent methods. With the capsule swelling reaction, no decapsulated mutant showed a reaction. The content of glucuronic acid was three- to eightfold less in the decapsulated mutants than in the parent strains (Table 4). Thus, there was still some residual production of K7 and K21 CPS but not enough to cause a positive capsule swelling reaction.
TABLE 4.
Glucuronic acid content of encapsulated K. pneumoniae parents strains and their decapsulated mutants
| CPS serotype | Strain | Glucuronic acid (μg/ml)/ 109 bacteriaa
|
|
|---|---|---|---|
| Parent strain | Mutant | ||
| K2 | Caroli | 15.0 | 5.5 |
| 591 | 48.1 | 1.2 | |
| K7 | 37 | 21.0 | 3.3 |
| 58 | 24.0 | 3.3 | |
| K21 | 151 | 4.5 | 1.2 |
| 557 | 5.3 | 2.5 | |
Mean of three independent experiments.
Opsonophagocytic activity of MAbs on encapsulated K. pneumoniae.
Having established the binding of the MAbs to their epitopes on decapsulated mutants as well as on encapsulated parent strains, we investigated whether these MAbs exerted opsonophagocytic effects. With the conditions used for decapsulated mutants (i.e., neutrophil-to-bacteria ratios of 1:2 and 1:8 and concentration of complement of 2.5% [see below]), neither opsonic activity of either of the MAbs on the encapsulated parent strains nor enhancement of phagocytic killing of encapsulated K. pneumoniae of either serotype was observed (data not shown). We therefore aimed to find conditions which gave more than 90% phagocytic killing of the bacteria when antibodies against CPS were used in order to obtain a standard situation in which the MAbs against O-antigen epitopes could be compared to MAbs with known opsonic activity (46). These conditions included a concentration of 10% complement and a ratio of neutrophils to bacteria of 32:1 for CPS serotypes K2 and K7, respectively (i.e., a 64-fold increase in the relative number of neutrophils). For CPS serotype K21, the neutrophil-to-bacteria ratio was 1:2, i.e., a fourfold increase in the relative number of neutrophils.
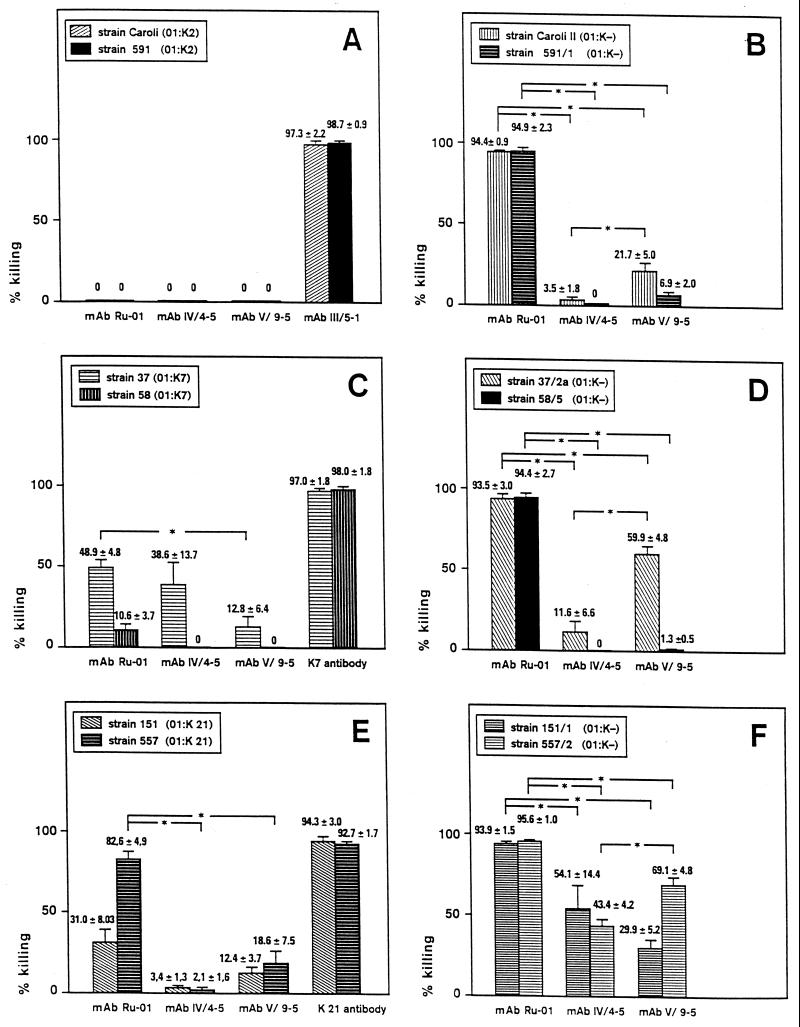
Even with those conditions, there was no opsonic activity of either of the three MAbs tested on strains Caroli and 591 (CPS serotype K2) (Fig. 2A). Addition of a murine IgM antibody directed against K2 CPS (MAb III/5-1), however, resulted in more than 90% killing of the two K2 CPS strains after 120 min (Fig. 2A). With strain 37 (K7 CPS serotype), all three MAbs showed moderate enhancement of phagocytic killing, ranging from 13% with MAb V/9-5 to 49% with MAb Ru-O1. On the other strain of the K7 CPS serotype used, 58, no MAb had significant effects in terms of phagocytic killing (Fig. 2C) despite adequate binding to intact encapsulated bacteria (Table 3). With strains 151 and 557 (both K21 CPS serotype), significant opsonic activity could be demonstrated only for MAb Ru-O1. The other two MAbs, IV/4-5 and V/9-5, respectively, showed no enhancement of phagocytic killing of these encapsulated bacteria (Fig. 2E).
FIG. 2.
Opsonophagocytic activity on encapsulated parent strains of K. pneumoniae (A, C, and E) and their decapsulated mutants (B, D, and F) of MAbs directed against different O1 antigen epitopes of K. pneumoniae LPS. The assay conditions for encapsulated bacteria included a ratio of neutrophils to bacteria of 32:1 (A and C) or 1:2 (E); the concentration of complement was 10% in all experiments. For decapsulated bacteria, the assay conditions included a ratio of neutrophils to bacteria of 1:2 (B and D) or 1:8 (F); the concentration of complement was 2.5% in all experiments (B, D, and F). The MAbs were used at a concentration of 5 μg/ml in each experiment. Values are given as mean ± standard deviation and are from three (A, C, and E) and four (B, D, and F) independent experiments, each done in duplicate. Controls in each experiment included bacteria with HBSS alone; bacteria and PMN without antibodies; bacteria, complement, and PMN without antibodies; bacteria, complement, and antibodies without PMN; and bacteria, complement, PMN, and irrelevant MAbs of the relevant subclass (to show that there was no nonspecific serum activity), each of which showed no killing of bacteria. For statistical analysis, we compared the percentage of killing for each bacterial strain separately by the Mann-Whitney U test. Thus, six comparisons were made for each panel, and statistically significant results (P < 0.01) are indicated by asterisks. For panels A, C, and E, comparison of MAbs with control antibodies was statistically significant (P < 0.01) for each strain and each comparison and hence is not noted explicitly.
Opsonophagocytic activity of MAbs on decapsulated mutants of K. pneumoniae.
Initial assay conditions included a neutrophil/bacteria ratio of 1:2 and a complement concentration of 10%. Since under these conditions antibodies in the serum which was used as a complement source induced phagocytic killing of strain 37/2a even without additional MAbs (80% phagocytic killing at 120 min in the controls containing neutrophils, bacteria, and complement only), the concentration of complement had to be lowered to 2.5%. With this low complement concentration, there was no killing of decapsulated mutants of the K2 and K7 CPS serotype without addition of MAbs in the various negative controls. Using the decapsulated mutants of the K21 CPS serotype, however, we found that there was still a significant phagocytic killing. We thus changed the ratio of neutrophils to bacteria for these two mutants (151/1 and 557/2, respectively) from 1:2 to 1:8.
With these conditions, MAb Ru-O1 showed the most pronounced opsonizing activity (Fig. 2B, D, and F). With mutants of all CPS serotypes tested, virtually all bacteria were killed by 120 min (Fig. 2B, D, and F). MAbs IV/4-5 and V/9-5, however, had only minimal effects on the killing rates of mutants Caroli/2 and 591/1 (K2 CPS serotype), respectively. MAb V/9-5, however, showed a higher activity on one of the two mutants with the K7 and K21 CPS serotype. MAb IV/4-5 had moderate opsonic activity for mutants derived from K21 CPS serotype strains but not for all other strains investigated (Fig. 2B, D, and F).
We have demonstrated previously that human serum contains antibodies against LPS from various gram-negative bacteria (48). To investigate whether the differences in opsonic activity of the respective MAbs might be caused by antibodies in the pooled human serum used as complement source, we measured the content of specific antibodies against K. pneumoniae O1 LPS by a quantitative ELISA. Before absorption, the pooled human serum contained 6 μg of O1 LPS-specific IgG antibodies per ml and 16 μg of O1 LPS-specific IgM antibodies per ml. This was in the range of the concentration of naturally occurring antibodies against this antigen which we have found previously in human Ig preparations (48). After thorough absorption with decapsulated O1 strains, the concentration of O1-specific IgG antibodies decreased to 0.11 μg/ml (absorption with strain 37/2a), 0.04 μg/ml (absorption with strain Caroli/2), and 0.06 μg/ml (absorption with strain 151/1). The concentration of O1-specific IgM antibodies decreased to 0.07 μg/ml (absorption with strain 37/2a), <0.04 μg/ml (absorption with strain Caroli/2), and <0.04 μg/ml (absorption with strain 151/1). Since controls using PMN, bacteria, and complement without antibodies showed no killing of the bacteria, and since the concentrations of O1-specific antibodies (IgG as well as IgM) in the complement source are 50- to 100-fold lower than the concentration of the MAbs (5 μg/ml) after absorption with decapsulated mutants, enhancement of opsonic activity by the complement source is unlikely.
DISCUSSION
CPS of K. pneumoniae has been shown to behave as a partial barrier against O-specific antibodies (9, 23). In the present study, we describe the binding of MAbs against different regions of the O antigen of K. pneumoniae to their epitopes on intact bacterial cells and the effects of various capsule serotypes on this binding and on subsequent functional activity of these MAbs. Our results show that all three MAbs bound to the intact bacteria when decapsulated mutants were studied. MAb Ru-O1 showed the strongest binding both to encapsulated strains as well as to decapsulated mutants (Table 3). This was reflected by the results of the phagocytosis studies in which MAb Ru-O1 had the most pronounced opsonic activity against all six decapsulated mutants and against three of the six encapsulated parent strains studied (Fig. 2) compared to the other MAbs, IV/4-5 and V/9-5.
Although MAbs IV/4-5 and V/9-5 bound equally well to intact encapsulated and decapsulated bacteria (Table 3), there was only minimal opsonic activity against all strains and mutants studied except the decapsulated mutants 37/2a, 151/1, and 557/2 (Fig. 2). The differences in relative efficacy between the three MAbs studied may be explained in part by the fact that those MAbs belong to different IgG subclasses (IV/4-5 being mouse IgG3, V/9-5 being mouse IgG2a, and Ru-O1 being mouse IgG2b). Indeed, MAb Ru-O1 (IgG2b) showed enhancement of opsonophagocytic killing in all six decapsulated mutants, MAb V/9-5 (IgG2a) showed enhancement of opsonophagocytic killing in four of six decapsulated mutants (Caroli/2, 37/2a, 151/1, and 557/2), whereas MAb IV/4-5 (IgG3) enhanced phagocytosis in only two out of six decapsulated mutants (151/1 and 557/2) (Fig. 2B, D, and F). These data suggest a role for the Ig isotype with regard to the relative efficacy of the MAbs (Ru-O1 > V/9-5 > IV/4-5). However, the epitope to which MAb V/9-5 binds (core oligosaccharide) is the most internal epitope, whereas d-galactan I, the binding epitope of MAb IV/4-5 is located more distant from the bacterial cell surface. The outermost epitope is d-galactan II, which is covalently bound to the outermost end of d-galactan I (52). Thus, deducting from the epitope location, and hence from the accessibility for binding of the MAbs to the epitopes, the expected order of relative efficacy of the MAbs studied would be Ru-O1 > IV/4-5 > V/9-5. As shown in Fig. 2B, D, and F, however, the efficacy of MAb IV/4-5 is inferior to that of MAb V/9-5 in terms of phagocytic killing.
With E. coli and P. aeruginosa, there have been differences in functional activity between the murine IgG subclasses, mostly at low antibody concentrations (30, 36, 38). There, IgG2a demonstrated the most pronounced bacterial killing activity second only to IgM (30). Furthermore, IgG3 has been shown to even block protective effects of IgG1 and IgG2a MAb's in lethal cryptococcal infections (29), but it could be made protective when switched to a different isotype (60). Here, IgG2 was more protective and opsonic than IgG1; however, in contrast to bacterial infections studied (30), IgG2b was superior to IgG2a (60). Other studies on functional differences of various murine IgG subclasses in K. pneumoniae are lacking. Taken together, the data indicate that both epitope location and isotype likely contribute to the relative efficacy of the antibodies studied. Isotype-switch variants of those MAbs (e.g., generating an IgG2a isotype switch of MAb IV/4-5) may help differentiate the relative role of isotype versus epitope location. In addition, since d-galactan I displays structural heterogeneity on living bacteria (44), this may also contribute to the lower activity of MAb IV/4-5 than of MAb Ru-O1.
CPS of K. pneumoniae has been shown to behave as a partial barrier against O-specific antibodies (9, 23). This property, however, depends on the capsular serotype since CPS serotype K1 acts as a complete barrier (23) whereas CPS serotype K2 permits partial penetration of anti-O-antigen antibodies (9, 27). With encapsulated strains, no opsonophagocytic killing could be observed when assay conditions similar to those of decapsulated mutants were used (data not shown). Facilitating the opsonophagocytic killing conditions by increasing the relative number of neutrophils and the concentration of complement, however, led to significant killing of encapsulated strains of the CPS serotypes K7 and K21 but not of CPS serotype K2 (Fig. 2). This finding correlated with the enhanced binding of all three MAbs to encapsulated K7 and K21 serotype strains compared to strains Caroli and 591 (CPS serotype K2) (Table 3).
In contrast, there were no significant differences in binding of the three MAbs to encapsulated strains or decapsulated mutants of CPS serotype K7 versus K21 (Table 3). With regard to phagocytic killing, however, strains 37 and 58 (CPS serotype K7) and their decapsulated mutants were less susceptible to opsonization by MAb IV/4-5 and V/9-5 than their counterparts of CPS serotype K21 (Fig. 2). This was also reflected by the assay conditions, where the relative number of neutrophils had to be 64-fold higher with strains of CPS serotype K7 than with strains of CPS serotype K21 and 4-fold higher with the decapsulated mutants 37/2a and 58/5 than with mutants 151/1 and 557/2.
The susceptibility for opsonophagocytic killing mediated by O-antigen-specific MAbs in this study correlated with the capsular serotype (K2 < K7 < K21), indicating a specific effect of different CPS compositions. The expanse of the capsule, however, may be equally important, since the virulence of K. pneumoniae correlates with the amount of CPS production (10). Antibodies against O antigens were shown to penetrate through the capsule of K. pneumoniae but to be covered by CPS without being able to exert opsonic effects (27, 40, 58). To test this possibility, we measured the content of glucuronic acid as a parameter for the amount of CPS production. Although strain Caroli (K2 CPS) produced less glucuronic acid than did strain 37 (K7 CPS) (Table 4), the latter strain was killed more efficiently in the presence of either MAb (Fig. 2). Likewise, MAb IV/4-5 enhanced phagocytic killing of strain 37 (K7 CPS) significantly more than it did with strains 151 and 557 (K21 CPS), respectively (Fig. 2), despite the former producing four- to fivefold more glucuronic acid (Table 4). On the other hand, we found differences in the effectivity of one MAb on enhancement of phagocytic killing between strains of identical CPS serotype and similar production of glucuronic acid (Table 4; Fig. 2). Thus, the different outcomes may be attributable not only to variances in CPS production but to the composition of CPS as well. CPS of serotypes K2, K7, and K21 has been shown to be permeable for O-antigen-specific antibodies in whole-cell binding experiments, but the functional relevance of this observation has not been studied (28). Also, our results confirmed previous studies showing that the production of K2 CPS led to enhanced hydrophilic properties of the bacterial surface (35) and to enhanced resistance against phagocytic killing compared to the production of K7 CPS (13, 35).
Antibodies specific for O antigens increased phagocytosis of K. pneumoniae in vitro (56, 59) and protected in vivo against experimental bacterial challenge (37), even though they had to be given in a 200-fold-higher dose in order to be as protective as antibodies against CPS (37). In addition, MAbs against the core oligosaccharide of K. pneumoniae were protective in both lethal endotoxemia and experimental intraperitoneal infection (25). Since different growth conditions as well as antimicrobial agents change the production and composition of CPS (16, 24, 57), combination therapy with MAbs against O-antigen epitopes and antibiotics may be effective even in the presence of extensive CPS production.
Specifically, the in vitro phagocytosis data obtained with MAb Ru-O1, which show killing of the decapsulated strain K. pneumoniae Caroli/2 but not of the encapsulated parent strain K. pneumoniae Caroli, seem to contrast with previous in vivo data from our laboratories demonstrating protective efficacy of MAb Ru-O1 against the encapsulated strain K. pneumoniae Caroli in mice (37). However, during multiplication in vivo, significant subpopulations of encapsulated organisms may have a thinner capsule or even lack the capsule, as suggested by data for E. coli (14). Furthermore, in addition to promoting phagocytosis, O-antigen-specific MAbs may also exert protection by neutralizing circulating free LPS and thereby reduce activation of proinflammatory cytokines (14). Indeed, Straus et al. showed that the release of soluble LPS plays a significant role in the pathogenesis of Klebsiella-induced lung injury (41, 42).
In conclusion, our results confirm that MAb against O antigens can penetrate through capsules belonging to different CPS serotypes and demonstrate the functional significance of this observation by showing that they exert opsonic activity depending on the CPS serotype. In this respect, antibodies against partial O antigens which are located on the outer regions of the LPS seem to be more effective than those directed against epitopes located next to the bacterial cell wall. Together with CPS-specific antibodies, they might provide a basis for a second-generation O-K passive immunotherapy against K. pneumoniae infection as has been shown for E. coli (14). Experimental and clinical studies to test this approach are clearly needed.
ACKNOWLEDGMENTS
We thank A. Cross for supplying E. coli Bort, E. coli E701, and control antibodies, as well as for helpful discussion. We are grateful to A. Möricke for skillful technical assistance.
REFERENCES
- 1.Bartlett J G, O'Keefe P, Tally F P, Louie T J, Gorbach S L. Bacteriology of hospital-acquired pneumonia. Arch Intern Med. 1986;146:868–871. [PubMed] [Google Scholar]
- 2.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter J L. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev Infect Dis. 1990;12:672–682. doi: 10.1093/clinids/12.4.672. [DOI] [PubMed] [Google Scholar]
- 4.Cross A S. Antiendotoxin antibodies: a dead end? Ann Intern Med. 1994;121:58–60. doi: 10.7326/0003-4819-121-1-199407010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Cross A S, Lowell G H, Palmblad J, Sadoff J C, Young L, Berger M. Mechanism of priming of human neutrophils by a soluble lymphoblastoid cell factor. J Immunol. 1985;135:2074–2083. [PubMed] [Google Scholar]
- 6.Cryz S J., Jr Progress in immunization against Pseudomonas aeruginosa and Klebsiella spp. Pathol Immunopathol Res. 1987;6:147–152. doi: 10.1159/000157056. [DOI] [PubMed] [Google Scholar]
- 7.Cryz S J, Jr, Fürer E, Germanier R. Purification and vaccine potential of Klebsiella capsular polysaccharides. Infect Immun. 1985;50:225–230. doi: 10.1128/iai.50.1.225-230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryz S J, Jr, Fürer E, Germanier R. Immunization against fatal experimental Klebsiella pneumoniae pneumonia. Infect Immun. 1986;54:403–407. doi: 10.1128/iai.54.2.403-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryz S J, Jr, Mortimer P M, Mansfield V, Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986;23:687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenico P, Johanson W G, Jr, Straus D C. Lobar pneumonia in rats produced by clinical isolates of Klebsiella pneumoniae. Infect Immun. 1982;37:327–335. doi: 10.1128/iai.37.1.327-335.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domenico P, Salo R J, Cross A S, Cunha B A. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella pneumoniae. Infect Immun. 1994;62:4495–4499. doi: 10.1128/iai.62.10.4495-4499.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domenico P, Schwartz S, Cunha B A. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donta S T, Peduzzi P, Cross A S, Sadoff J, Haakenson C, Cryz S J, Jr, Kauffman C, Bradley S, Gafford G, Elliston D, Beam T R, Jr, John J F, Jr, Ribner B, Cantey R, Welsh C H, Ellison III R T, Young E J, Hamill R J, Leaf H, Schein R M H, Mulligan M, Johnson C, Abrutyn E, Griffiss J M, Hamadeh R, Eliasson A H, McClain J B, Melcher G P, Kelly J W, Byrne W R, Wallace M, Amundson D, Gumpert B, Slagle D The Federal Hyperimmune Immunoglobuline Trial Study Group. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosa infections. J Infect Dis. 1996;174:537–543. doi: 10.1093/infdis/174.3.537. [DOI] [PubMed] [Google Scholar]
- 14.Frasa H, Benaissa-Trouw B, Tavares L, van Kessel K, Poppelier M, Kraaijeveld K, Verhoef J. Enhanced protection by use of a combination of anticapsule and antilipopolysaccharide monoclonal antibodies against lethal Escherichia coli O18K5 infection in mice. Infect Immun. 1996;64:775–781. doi: 10.1128/iai.64.3.775-781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen D S, Mestre F, Albertí S, Hernández-Alléz S, Álvarez D, Doménech-Sánchez A, Gil J, Merino S, Tomás J M, Benedí V J. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J Clin Microbiol. 1999;37:56–62. doi: 10.1128/jcm.37.1.56-62.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Held T K, Adamczik C, Trautmann M, Cross A S. Effects of MICs and sub-MICs of antibiotics on production of capsular polysaccharide of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1995;39:1093–1096. doi: 10.1128/aac.39.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Held T K, Mielke M E A, Chedid M, Unger M, Trautmann M, Huhn D, Cross A S. Granulocyte colony-stimulating factor worsens the outcome of experimental Klebsiella pneumoniae pneumonia through direct interaction with the bacteria. Blood. 1998;91:2525–2535. [PubMed] [Google Scholar]
- 18.Held T K, Trautmann M, Mielke M E A, Neudeck H, Cryz S J, Jr, Cross A S. Monoclonal antibody against Klebsiella capsular polysaccharide reduces severity and hematogenic spread of experimental Klebsiella pneumoniae pneumonia. Infect Immun. 1992;60:1771–1778. doi: 10.1128/iai.60.5.1771-1778.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman W D, Pollack M, Banks S M, Koev L A, Solomon M A, Danner R L, Koles N, Guelde G, Yatsiv I, Mouginis T, Elin R J, Hosseini J M, Bacher J, Porter J C, Natanson C. Distinct functional activities in canine septic shock of monoclonal antibodies specific for the O polysaccharide and core regions of Escherichia coli lipopolysaccharide. J Infect Dis. 1994;169:553–561. doi: 10.1093/infdis/169.3.553. [DOI] [PubMed] [Google Scholar]
- 20.Jacoby G A. Antimicrobial-resistant pathogens in the 1990s. Annu Rev Med. 1996;47:169–179. doi: 10.1146/annurev.med.47.1.169. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis W R, Munn V P, Highsmith A K, Culver D H, Hughes J M. The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect Control. 1985;6:68–74. doi: 10.1017/s0195941700062639. [DOI] [PubMed] [Google Scholar]
- 22.Jay S J. Nosocomial pneumonia. Nosocomial Infect. 1983;74:221–235. doi: 10.1080/00325481.1983.11698392. [DOI] [PubMed] [Google Scholar]
- 23.Jong G-M, Hsiue T-R, Chen C-R, Chang H-Y, Chen C-W. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107:214–217. doi: 10.1378/chest.107.1.214. [DOI] [PubMed] [Google Scholar]
- 24.Kadurugamuwa J L, Anwar H, Brown M R W, Zak O. Effect of subinhibitory concentrations of cephalosporins on surface properties and siderophore production in iron-depleted Klebsiella pneumoniae. Antimicrob Agents Chemother. 1985;27:220–223. doi: 10.1128/aac.27.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandine E, Salles M-F, Zalisz R, Guenounou M, Smets P. Murine monoclonal antibodies to Klebsiella pneumoniae protect against lethal endotoxemia and experimental infection with capsulated K. pneumoniae. Infect Immun. 1990;58:2828–2833. doi: 10.1128/iai.58.9.2828-2833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGowan J E., Jr Changing etiology of nosocomial bacteremia and fungemia and other hospital-acquired infections. Rev Infect Dis. 1985;7(Suppl. 3):S357–S370. doi: 10.1093/clinids/7.supplement_3.s357. [DOI] [PubMed] [Google Scholar]
- 27.Meno Y, Amano K. Morphological evidence for penetration of anti-O antibody through the capsule of Klebsiella pneumoniae. Infect Immun. 1990;58:1421–1428. doi: 10.1128/iai.58.5.1421-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merino S, Camprubi S, Alberti S, Benedí V J, Tomas J M. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun. 1992;60:2529–2535. doi: 10.1128/iai.60.6.2529-2535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nussbaum G, Yuan R R, Casadevall A, Scharff M D. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J Exp Med. 1996;183:1905–1909. doi: 10.1084/jem.183.4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oishi K, Koles N L, Guelde G, Pollack M. Antibacterial and protective properties of monoclonal antibodies reactive with Escherichia coli O111.B4 lipopolysaccharide: relation to antibody isotype and complement-fixing activity. J Infect Dis. 1992;165:34–45. doi: 10.1093/infdis/165.1.34. [DOI] [PubMed] [Google Scholar]
- 31.Pennington J E, Pier G B, Sadoff J C, Small G J. Active and passive immunization strategies for Pseudomonas aeruginosa pneumonia. Rev Infect Dis. 1986;8(Suppl. 4):S426–S433. doi: 10.1093/clinids/8.supplement_4.s426. [DOI] [PubMed] [Google Scholar]
- 32.Pier G B, Meluleni G, Goldberg J B. Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun. 1995;63:2818–2825. doi: 10.1128/iai.63.8.2818-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podschun R, Heineken P, Ullmann U, Sonntag H-G. Comparative investigations of Klebsiella species of clinical origin: plasmid patterns, biochemical reactions, antibiotic resistances and serotypes. Zentbl Bakteriol Hyg A. 1986;262:335–345. doi: 10.1016/s0176-6724(86)80006-2. [DOI] [PubMed] [Google Scholar]
- 34.Podschun R, Sievers D, Fischer A, Ullmann U. Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J Infect Dis. 1993;168:1415–1421. doi: 10.1093/infdis/168.6.1415. [DOI] [PubMed] [Google Scholar]
- 35.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollack M, Koles N L, Preston M J, Brown B J, Pier G B. Functional properties of isotype-switched immunoglobulin M (IgM) and IgG monoclonal antibodies to Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1995;63:4481–4488. doi: 10.1128/iai.63.11.4481-4488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rukavina T, Ticac B, Susa M, Jendrike N, Jonjic S, Lucin P, Marre R, Doric M, Trautmann M. Protective effect of antilipopolysaccharide monoclonal antibody in experimental Klebsiella infection. Infect Immun. 1997;65:1754–1760. doi: 10.1128/iai.65.5.1754-1760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber J R, Cooper L J N, Diehn S, Dahlhauser P A, Tosi M F, Glass D D, Patawaran M, Greenspan N S. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J Infect Dis. 1993;167:221–226. doi: 10.1093/infdis/167.1.221. [DOI] [PubMed] [Google Scholar]
- 39.Sidberry H, Kaufman B, Wright D C, Sadoff J. Immunochemical analysis by monoclonal antibodies of bacterial lipopolysaccharides after transfer to nitrocellulose. J Immunol Methods. 1985;76:299–305. doi: 10.1016/0022-1759(85)90307-2. [DOI] [PubMed] [Google Scholar]
- 40.Simoons-Smit A M, Verweij-van Vught A M, Maclaren D M. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986;21:133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- 41.Straus D C. Production of an extracellular toxic complex by various strains of Klebsiella pneumoniae. Infect Immun. 1987;55:44–48. doi: 10.1128/iai.55.1.44-48.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straus D C, Atkisson D L, Garner C W. Importance of a lipopolysaccharide-containing extracellular toxic complex in infections produced by Klebsiella pneumoniae. Infect Immun. 1985;50:787–795. doi: 10.1128/iai.50.3.787-795.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarkkanen A-M, Allen B L, Williams P H, Kauppi M, Haahtela K, Siitonen A, Ørskov I, Ørskov F, Clegg S, Korhonen T K. Fimbriation, capsulation, and iron-scavenging systems of Klebsiella strains associated with human urinary tract infection. Infect Immun. 1992;60:1187–1192. doi: 10.1128/iai.60.3.1187-1192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomás J M, Benedí V J, Ciurana B, Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986;54:85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trautmann M, Cross A S, Reich G, Held T K, Podschun R, Marre R. Evaluation of a competitive ELISA method for the determination of Klebsiella O antigens. J Med Microbiol. 1996;44:44–51. doi: 10.1099/00222615-44-1-44. [DOI] [PubMed] [Google Scholar]
- 46.Trautmann M, Cryz S J, Jr, Sadoff J C, Cross A S. A murine monoclonal antibody against Klebsiella capsular polysaccharide is opsonic in vitro and protects against experimental Klebsiella pneumoniae infection. Microb Pathog. 1988;5:177–187. doi: 10.1016/0882-4010(88)90020-4. [DOI] [PubMed] [Google Scholar]
- 47.Trautmann M, Ghandchi A, Held T, Cryz S J, Jr, Cross A S. An enzyme-linked immunosorbent assay for the detection of soluble Klebsiella pneumoniae capsular polysaccharide. J Microbiol Methods. 1991;13:305–313. [Google Scholar]
- 48.Trautmann M, Held T K, Susa M, Karajan M A, Wulf A, Cross A S, Marre R. Bacterial lipopolysaccharide (LPS)-specific antibodies in commercial human immunoglobulin preparations: superior antibody content of an IgM-enriched product. Clin Exp Immunol. 1998;111:81–90. doi: 10.1046/j.1365-2249.1998.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trautmann M, Müller-Leutloff Y, Hofstaetter T, Seiler F R, Hahn H. In vitro and in vivo activities of different immunoglobulin G preparations from the rabbit against enterobacteriaceae. Vox Sang. 1985;49:267–276. doi: 10.1111/j.1423-0410.1985.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 50.Trautmann M, Ruhnke M, Rukavina T, Held T K, Cross A S, Marre R, Whitfield C. O-antigen seroepidemiology of Klebsiella clinical isolates and implications for immunoprophylaxis of Klebsiella infections. Clin Diagn Lab Immunol. 1997;4:550–555. doi: 10.1128/cdli.4.5.550-555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trautmann M, Vogt K, Hammack C, Cross A S. A murine monoclonal antibody defines a unique epitope shared by Klebsiella lipopolysaccharides. Infect Immun. 1994;62:1282–1288. doi: 10.1128/iai.62.4.1282-1288.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ullmann U. The distribution of Klebsiella pneumoniae serotypes from different sources and their sensitivity to cephalosporins. Infection. 1983;11(Suppl. 1):S28–S31. doi: 10.1007/BF01641102. [DOI] [PubMed] [Google Scholar]
- 53.Warren H S, Danner R L, Munford R S. Anti-endotoxin monoclonal antibodies. N Engl J Med. 1992;326:1153–1157. doi: 10.1056/NEJM199204233261711. [DOI] [PubMed] [Google Scholar]
- 54.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 55.Whitfield C, Perry M B, MacLean L L, Yu S H. Structural analysis of the O-antigen side chain polysaccharides in the lipopolysaccharides of Klebsiella serotypes O2(2a), O2(2a,2b), and O2(2a,2c) J Bacteriol. 1992;174:4913–4919. doi: 10.1128/jb.174.15.4913-4919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitfield C, Richards J C, Perry M B, Clarke B R, MacLean L L. Expression of two structurally distinct d-galactan O antigens in the lipopolysaccharide of Klebsiella pneumoniae serotype O1. J Bacteriol. 1991;173:1420–1431. doi: 10.1128/jb.173.4.1420-1431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams P. Sub-MICs of cefuroxime and ciprofloxacin influence interaction of complement and immunoglobulins with Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987;31:758–762. doi: 10.1128/aac.31.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams P, Lambert P A, Brown M R W. Penetration of immunoglobulins through the Klebsiella capsule and their effect on cell-surface hydrophobicity. J Med Microbiol. 1988;26:29–35. doi: 10.1099/00222615-26-1-29. [DOI] [PubMed] [Google Scholar]
- 59.Williams P, Lambert P A, Brown M R W, Jones R J. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J Gen Microbiol. 1983;129:2181–2191. doi: 10.1099/00221287-129-7-2181. [DOI] [PubMed] [Google Scholar]
- 60.Yuan R R, Spira G, Oh J, Paizi M, Casadevall A, Scharff M D. Isotype switching increases efficacy of antibody production against Cryptococcus neoformans infection in mice. Infect Immun. 1998;66:1057–1062. doi: 10.1128/iai.66.3.1057-1062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]