Abstract
Background:
Medicinal plants have revealed much attention as an alternative or complementary treatment for opioid withdrawal syndrome. The current review collects all available literature to verify the efficiency of herbal remedies in the management of symptoms associated with opioid withdrawal.
Methods:
A systematic literature search was conducted from January 1990 to May 2021 on four bibliographic databases (Scopus, PubMed, Embase, and Web of Science) using the search terms "medicinal plant", "withdrawal syndrome", "opioid", and all their equivalents. All randomized controlled trials (RCTs), published in the English language were included for data synthesis. The search was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA). The Cochrane risk of bias tool was used to verify the quality of the included clinical trials.
Findings:
A total of 12 RCTs were collected and used for data synthesis. The results of these studies indicated that herbal medicines were effective in treating opioid withdrawal syndrome and could alleviate the withdrawal symptoms, such as abdominal constrictions, diarrhea, bone pain, perspiration, and insomnia, when compared to conventional medications such as buprenorphine, clonidine, and methadone. However, more than 30% of RCTs were found to be at high risk of bias in the areas of selection, performance, detection, attrition, and reporting.
Conclusion:
Although several RCTs have proven that herbal remedies are effective in reducing opioid withdrawal symptoms, the findings need to be viewed more carefully. Further RCTs with more participants, longer duration, and less risk of bias are needed in the claimed cases.
Keywords: Opioid, Opium, Withdrawal syndrome, Herbal therapy
Introduction
Substance withdrawal syndrome is one of the undesired consequences of addictive drug abuse, a multi-organ disorder with various symptoms, most of which are associated with the malfunction of the central nervous system (CNS) and the gastrointestinal tract.1 The severity and frequency of the syndrome vary from person to person, depending on the type of substance consumed, its half-life, its metabolism, and the time of receiving the last dose.2 In general, opioids, cigarettes, alcohol, barbiturates, and sedatives are the most commonly abused substances that have been reported to cause deprivation syndrome.3 Opioid dependence is a compulsion need to use drugs repeatedly. Psychological dependence enhances drug use behavior, while physical dependence leads to drug tolerance and withdrawal syndrome.4 Symptoms of opioid withdrawal include muscle pain, nausea, diarrhea, tachycardia, lacrimation, rhinorrhea, sweating, mood changes, mydriasis, insomnia, and fever.5
Relieving the symptoms of withdrawal syndrome is currently one of the most important concerns for opioid addiction.6 Methadone, buprenorphine, clonidine, naltrexone, and other medications have many side effects that can lead to recurrence.7 Complementary medicine and herbal remedies have recently demonstrated promising efficacy in the treatment of various illnesses. Herbal medicines have long been used for alleviating the symptoms of withdrawal syndrome.8 The mechanism of action of medicinal plants is different. The present study systematically reviews and summarizes the available randomized controlled trials (RCTs) to verify the efficiency of herbal therapies in the management of opioid withdrawal syndrome.
Methods
Study search and inclusion criteria: In the present review, the research question was “whether medicinal plants and herbal therapy can be effective in managing withdrawal syndrome in patients with opium or opioid abuse”. Therefore, from January 1990 to May 2021, a systematic literature search was conducted on PubMed, Scopus, Web of Science, and Embase. The search terms included (Medicinal plant OR herbal remedy OR herbal medicine OR herbal therapy OR traditional medicine OR phytotherapy OR phytomedicine) AND (withdrawal syndrome OR withdraw syndrome OR withdrawal OR withdrawal symptom) AND (opioid OR opium OR morphine OR methadone OR heroin OR buprenorphine OR codeine OR fentanyl OR hydrocodone OR oxycodone OR tramadol OR meperidine). After deleting the duplicates, the search was limited to English articles. Afterward, reviews, editorials, and conference papers were discarded. Finally, irrelevant articles, animal studies, observational studies, case reports, and case series were excluded from further evaluation. Bibliographic searches, article selection, and data extraction were performed independently by the two authors as standard protocols for reporting systematic reviews, as recommended by the 2009 PRISMA Checklist.9 To avoid any misinterpretation, possible discrepancies between the authors were resolved in each step prior to further data processing.
Data synthesis and variables : General information was extracted, including the name of the author, publication date, and demographic information of participants surveyed. Moreover, the measured variables and the reported outcomes were extracted. The data were described qualitatively, and changes in the levels of the variables were compared to the corresponding control groups in each study.
Quality assessment: We checked the quality of the included RCTs by assessing the risk of the following biases using the Cochrane Risk of Bias Tool: random sequence generation, allocation concealment, selective reporting, participants and personnel blindness, blinding of outcome data, incomplete outcome data, and other biases. According to this tool, the rate of these biases is evaluated as “unclear”, “low”, or “high”.10 This tool was used to create a risk of bias graph.
Results
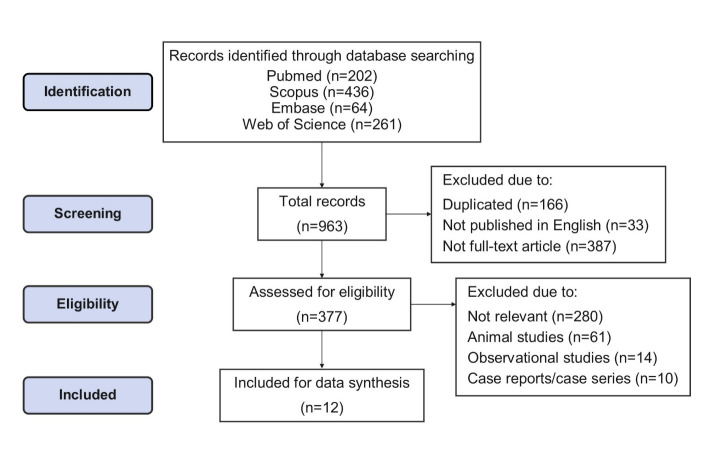
General information: After literature review and considering the inclusion criteria, a total of 963 articles were selected, 202 of which were in PubMed, 436 were in Scopus, 64 were in Embase, and 261 were in Web of Science. After omitting the duplicates, and limiting the records to full-text English articles, 377 articles were retrieved. After the removal of irrelevant documents, animal studies, observational studies, case reports, and case series, 12 RCTs with a total of 1035 participants (mostly male) were selected for data synthesis. The article selection process is shown in Figure 1.
Figure 1.
The article selection process
Study results: A summary of the RCTs is presented in Table 1. These studies compared the effects of herbal therapy with those of methadone,5 lofexidine,11,12 clonidine,13 buprenorphine,14 and placebo alone.4,15-17 The duration of the studies varied from eight days to 12 weeks. The sample sizes ranged from 35 to 225. The clinical trials were limited to Iran, China, and India. Different herbal formulations were investigated in these studies. Five studies examined the effects of a combination of herbal compounds.11,12,14,18,19 In two studies, the effect of saffron on the withdrawal syndrome was investigated,16,17 which in one of them the active constituent of saffron, i.e., crocin, was used as an intervention.17 The main measured outcomes were withdrawal symptoms which primarily included craving, anxiety, diarrhea, abdominal constrictions, watery eyes, insomnia, depression, and muscular pain. In one study, reducing the daily dose of methadone was expressed as a measure of treatment success.16 Withdrawal syndrome was assessed using different questionnaires such as clinical opioid withdrawal scale (COWS), withdrawal symptom rating scale (WSRS), craving rating scale (CRS), craving beliefs questionnaire (CBQ), side effect rating scale (SERS), modified subjective opiate withdrawal scale (MSOWS), objective opiate withdrawal scale (OOWS), and Hamilton anxiety scale (HAMA). The secondary outcomes included retention in treatment, number of opioid-negative urine tests, blood pressure, temperature, pulse rate, and respiratory rate. All studies reported a significant effect of herbal medicine in reducing the symptoms of opioid withdrawal. In the studies that compared the effectiveness of this treatment with methadone, lofexidine, clonidine, and buprenorphine,5,11,12,14,20 no significant difference was observed between the groups, which indicates that the efficacy of herbal medicine is similar to the mentioned drugs. The findings demonstrated that the relief from opioid withdrawal symptoms and craving behaviors were better and faster in the herbal treatment group 10 days after therapy even in comparison with the effects of buprenorphine as the control drug.14 In three studies, the withdrawal symptoms significantly decreased on the third day in the herbal therapy groups.5,11,15 Herbal therapy was also effective in the long-term treatment of opioid dependence as well as infections and the weakness associated with addiction.4 Except for the adverse effects reported in four studies, no side effects were observed when herbal medicine was used. In the study by Abbaszadeh-Mashkani et al., the effect of crocin was examined, and side effects such as headache, nausea, agitation, and urinary incontinence were observed in the intervention group.17 Wang et al. reported dry mouth, dizziness, hypotension, and sinus bradycardia following the use of Fu-Yuan Pellet, a Chinese traditional medicine formula.19 Sayyah et al. detected dizziness, nausea, and abdominal pain in the group that consumed methanolic extract of Zataria multiflora.21 Moosavyzadeh et al. stated the drug side effects as the reason why 10% of the patients receiving Hab-o Shefa dropped out of the study.18
Table 1. Characteristics of the included RCTs .
| Study | No. of subjects (male/female), mean age (year), country | Abusedopioid | Intervention (herbal formulation) | Dosage | Control | Duration | Outcome measures | Main result |
| Kianbakht, et al, 2021 15 | 100 (100/-), 40, Iran | Morphine | Sophora alopecuroides L. var. alopecuroides | Three 400-mg capsules once daily | Placebo | 8 days | COWS | Significantly decreased the COWS scores on days 3 and 8 (p < 0.001) |
| Abbaszadeh-Mashkani, et al, 2020 17 | 60 (56/4), 41.3, Iran | Methadone | Crocin (the active constituent of saffron) | 15 mg orally BID | Placebo | 12 weeks | COWS and Craving (DDQ) | Significant improvement in the craving score (p < 0.05) and withdrawal symptoms score (p < 0.05) |
| Moosavyzade, et al 2020 18 | 81 (unclear), 33.9, Iran | Opioid | Hab-o Shefa1 | 500 mg daily, with an increase of 500 mg/day to three capsules per day (eventually after 3 days) | Placebo | 12 weeks | CBQ and HAMA | Significant improvement in craving (p < 0.001), depression (p < 0.05), and anxiety (p < 0.05) |
| Nemat Shahi, 2017 16 | 44 (44/-), 41.5, Iran | Methadone | Saffron | 30-mg capsules of saffron per week | Placebo | 8 weeks | Daily dose of methadone usage | Significantly alleviated the withdrawal symptoms (p < 0.001( |
| Sayyah andRahim, 2017 21 | 40 (40/-), 28, Iran | Opium | Zataria multiflora Boiss. (methanolic extract) | Three 500-mg capsules per day | Methadone (gradual discontinuation) and placebo | 15 days | CINA scale | Significantly decreased the withdrawal symptoms (p < 0.001( |
| Solhi, et al 2013 5 | 81 (81/-), 36.7, Iran | Opium | Rosmarinus officinalis | 16 capsules (300 mg dried leaves of rosemary) (days 1 to 3), 12 capsules (days 4 to 7), and 8 capsules for the next week in divided doses | Methadone and placebo | 4 weeks | COWS | Significant differences on the 3rd and 7th days (p < 0.05) |
| Wang, et al 2009 19 | 225 (unclear), 18-55 (unclear mean), China | Heroin | Fu-Yuan Pellet2 | 6 g TID (days 1 to 3), 8 g TID (days 4 to 6), and 8 g BID (days 7 to 10) | Lofexidine and placebo | 10 days | WSRS and HAMA | Significantly alleviated the symptoms of anxiety and withdrawal syndrome (p < 0.05(, no significant difference between the two groups |
| Sangi, et al, 2008 4 | 35 (35/-), 33.15, India | Opioid | Nigella sativa | 500 mg orally TID | Placebo | 12 weeks | MSOWS and OOWS | Significantly decreased withdrawal effects |
| Shi, et al 2008 12 | 203 (175/28), 30.4, China | Heroin | Jinniu3 | 2.5 g orally TID (days 1 to 3), 2 g TID (days 4 to 5), 1.5 g TID (days 6 to 7), 1.5 g BID (days 8 to 9), and 1-5 g daily (day 10) | Lofexidine | 10 days | OWS and HAMA | Gradually decreased withdrawal symptoms (p < 0.001(, no significant differences between the two groups |
| Kang, et al 2008 11 | 59 (49/10), 30.0, China | Heroin | Tai-Kang-Ning4 | 2 g orally TID (days 1 to 5), 1.5 g TID (days 6 to 7), 1.5 g BID (days 8 to 9), and 1.5 daily (day 10) | Lofexidine and placebo | 10 days | WSRS | Significantly reduced withdrawal symptoms by day 3 (p < 0.001(, no significant difference between the two groups |
| Akhondzadeh, et al 2001 13 | 65 (65/-), 34.8, Iran | Opiates | Passiflora incarnate | 60 drops daily | Clonidine and placebo | 2 weeks | SWS, SOWS, and mental symptoms | Significantly decreased physical symptoms of withdrawal syndrome in both groups, significant improvement over clonidine alone in the management of mental symptoms (p < 0.05) |
|
Hao, et al
2000 14 |
42 (37/5), 26.2, China | Heroin | WeiniCom5 | 8-10 capsules 4-6 TID | Buprenorphine and placebo | 2 weeks | WSRS and CRS | Shorter treatment period to achieve the desired degree of elimination of acute withdrawal symptoms and craving in the WeiniCom group |
RCTs: Randomized controlled trials, BID: two times a day, TID: three times a day, COWS: Clinical opioid withdrawal scale, DDQ: Desire for drug questionnaire, CBQ: craving beliefs questionnaire, HAMA: Hamilton Anxiety Scale, CINA: Clinical Institute Narcotic Assessment scale, SWS: Severity of the opiate withdrawal syndrome, MSOWS: Modified subjective opiate withdrawal scale, OOWS: Objective opiate withdrawal scale, OWS: Opiate Withdrawal Scale, WSRS: Withdrawal symptom rating scale, SOWS: Short opiate withdrawal scale, CRS: craving rating scale.
1 Hab-o Shefa is an herbal formulation in traditional Persian medicine. It consists of four herbs, Datura stramonium L., Zingiber officinale Roscoe, Rheum palmatum L., and Acacia senegal L.
2 Fu-Yuan pellet (FYP) is an herbal formulation in traditional Chinese medicine for detoxification of opiates. It is made of 10 herbs, including Glabrous Greenbrier Rhizome, Divaricate Saposhnikovia Root, Dried Tangerine peel, Prepared Dried Ginger, Excrementum Pteropi, Eucommia Bark, Hawthorn Fruit, Desert-living Cistanche, Largehead Atractylodes Rhizome, and Tangshen.
3 Jinniu is an herbal formulation containing Radix Ginseng, Corydalis Rhizoma, Hippocampus, and Datura.
4 Tai-Kang-Ning is a polyherbal formulation containing.5 g of an aqueous spray-dried extract of eight herbs including Radix Ginseng, Corydalis Rhizoma, Hippocampus, Flos Daturae, Herba Taraxaci, Small flower milkwort herb with root, Bezoar Bovis, and Margarita.
5 WeiniCom is an herbal compound that composed of extracts of Schisandra chinensis, Astragalus membranaceus, Coptis chinensis rhizome, Angelica sinensis, Terminalia chebula, Glycyrrhiza glabra, Zingiber officinalis, Ganoderma lucidum, Ziziphus jujube, Panax quinquefolius, Nauclea Spp., Magnolia officinalis, Epimedium grandifora, Eleutherococcus senticosus, Cordyceps sinensis, Corydalis yanhusuo, Rhodiola crenulata, Corydalis decumbentis and Sida cordifolia.22
Assessments of 12 clinical trials using the Cochrane Collaboration Review Manager (RevMan version 5.1) are presented in Figures 2 and 3. The studies were randomized, double-blind controlled clinical trials, except for one that was single-blind.4 As shown in Figures 2 and 3, only one study had “low” risk in all five domains of bias (selection, performance, attrition, reporting, and other). Two studies had a“high” risk of selection bias, one due to non-randomization4 and the other due to the lack of allocation concealment.16 Furthermore, because the details were not sufficiently described, 58% (7 of 12) and 75% (9 of 12) of the articles were categorized as “uncertain risk of bias” due to unclear random sequence generation and allocation concealment, respectively. Two studies rated as “high” risk of performance bias due to not blinding the personnel.4,16 One RCT had a “high” risk of attrition bias because a large number of patients dropped out during the two-week period and the authors did not investigate the reason and its effect on the outcome data.13 Additionally, one study yielded ratings of a “high” risk of selective reporting bias due to the inconsistency of the presented results.14 Also, 58% of the papers (7 of 12) rated as “uncertain” risk due to unclear blinding of the participants and personnel, indistinct blinding of outcome assessment, and incomplete outcome data.
Figure 2.
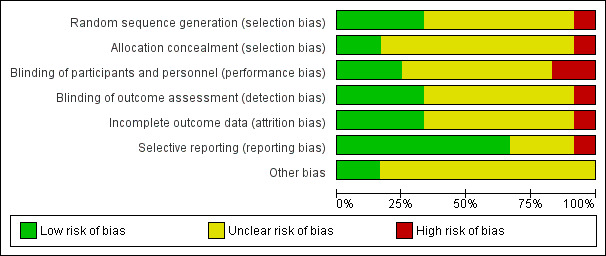
Risk of bias graph: The authors’ judgment about each risk of bias item presented as percentages across all included studies of herbal therapy in opioid withdrawal syndrome.
Figure 3.
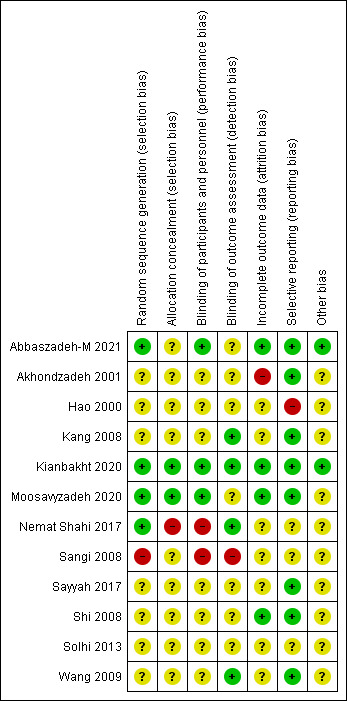
Risk of bias summary: The authors’ judgments about each risk of bias item for each included study of herbal therapy in opioid withdrawal syndrome. +: low risk of bias; -: high risk of bias;?: unclear risk of bias.
In one study, patients received no medication other than the intervention.15In other studies, patients were allowed to take drugs such as alprazolam, which was not analyzed in the study results. Except for four studies,12,15,17,18attrition from the analysis was not identified in the studies. In particular, the inability to tolerate the intervention process could affect the interpretation of the final results. None of the studies had a follow-up period.
Discussion
In this study, the effect of herbal therapy in the management of opioid withdrawal syndrome was investigated through a systematic review of 12 published RCTs. Various medications have been developed for opioid withdrawal symptoms, but since their side effects can affect many organs, particularly the gastrointestinal system and the CNS, there is a wide range of withdrawal symptoms that may not be all managed by a single medication. Medicinal plants have great capabilities with various applications. Due to their numerous biologically active components, some medicinal plants can be used to treat various gastrointestinal and CNS symptoms.23 These herbal compounds have also shown promising effects in the management of pain and opioid physical dependence.24 Herbal medication leads to faster effectiveness as well as good safety and tolerability. Moreover, a shorter treatment period is required to eliminate the withdrawal symptoms and craving behaviors when this treatment is used.14,15 These properties make herbal medications an effective and promising alternative for relieving anxiety symptoms and facilitate drug switches, for example, from methadone to buprenorphine/naloxone.25 Animal studies have demonstrated that the effects of herbal medications are dose-dependent. Moreover, extracts of different plants have been shown to have different effects, indicating that a specific component is responsible for the therapeutic effects in alleviating the withdrawal syndrome.26,27
Opioid receptors belong to the G protein-coupled receptors family, and their stimulation inhibits adenylate cyclase and calcium channels and activates potassium channels as well as several kinase cascades. Activation of opioid receptors including μ, δ, κ, and nociceptin/orphanin FQ (NOP) leads to analgesia. The μ-opioid receptor (MOP) plays a major role in drug addiction and its activation causes euphoria, analgesia, constipation, and respiratory depression. Stimulation of the κ-opioid receptor (KOP), despite having an analgesic effect, can lead to anxiety and dysphoria. The δ-opioid receptor (DOP) agonists are used to treat anxiety and chronic pain. The most abundant opioid receptor in the brain is NOP, which modulates the effects of μ-opioid receptors.28 The similarity between the effects of herbal therapies and conventional drugs, such as clonidine, in reducing the manifestations of opioid withdrawal syndrome suggests that the mechanistic pathways of these medications, which are thought to be mediated through the protein kinase A pathway, may be similar.29 The antinociceptive effect of medicinal plants may also be mediated by opioidergic and adrenergic systems. Phytochemical studies have indicated that the extract of medicinal plants such as Rosmarinus officinalis and Sophora alopecuroides var. alopecuroides has an alkaloid component that is responsible for opioids similar effects.7,30,31 Animal studies have demonstrated that some plant extracts can reduce withdrawal symptoms and pain stimulation by affecting the CNS and the potentiation of GABAergic system.32 It has been shown that anti-anxiety and pain-relieving properties of Passiflora incarnate can be related to its effect on opioid receptors and the synergistic action of gamma-aminobutyric acid (GABA) A and B.33
The dopaminergic system also plays an important role in substance dependence. Dopamine D2 receptor (D2R) is also a member of the G protein-coupled receptor family, and its activation leads to the inhibition of adenylate cyclase and the reduction of cyclic adenosine monophosphate (cAMP), resulting in the release of intracellular Ca+2. In various parts of the brain, including the ventral tegmental area (VTA) and the nucleus accumbens (NAc), deep interactions between opioid and dopamine receptors can lead to addiction through reward processing. Sudden withdrawal of opioids reduces dopamine in the NAc and the mesolimbic dopamine system.34,35 On the other hand, long-term use of opioids causes several changes in the brain, especially in the dopaminergic system. These changes include decreased levels of dopamine transporter (DAT), D2R, and tyrosine hydroxylase (TH), which is the main enzyme in dopamine synthesis, and dopamine neurons in the VTA. It also increases sensitivity to dopamine antagonists and decreases glutamate release from the VTA. These changes, which persist for a long time after opioid withdrawal, can be some important factors in recurrence.36,37 Animal studies have demonstrated that treatment with An-jun-ning, a plant-based medication, effectively interdicted DAT, D2R, and TH reduction induced by morphine. Furthermore, it could restore the levels of DAT, D2R, and TH to the normal values, indicating that the ameliorating effect of plant-based therapies on withdrawal symptoms may be modulated by the dopaminergic system.38 Modulation of monoaminergic neurotransmitters, modification of the nitric oxide (NO) pathway, and possible interactions with inhibitory neurotransmitters in the CNS are other mechanisms by which medicinal plants may manage opiate withdrawal symptoms and anxiety.26,39
In the present study, the efficacy of herbal therapy in the treatment of opioid withdrawal syndrome was evaluated through a systematic review of 12 randomized clinical trials. In these RCTs, type herbal formulation, study method, and outcome evaluation measurements were different. The herbal formulations included Sophora alopecuroides, saffron, Zataria multiflora, Rosmarinus officinalis, Nigella sativa, Passiflora incarnate, and five combination formulas. The effect of Sophora alopecuroides on the suppression of withdrawal symptoms can be due to its cytisine and matrine alkaloids which have nicotinic receptor agonistic activity.31 Crocin is a carotenoid chemical compound that is found in saffron and has an interaction with dopaminergic pathways in the VTA and the dopaminergic system in the CNS, and it can be responsible for the alleviation of withdrawal symptoms.17 Hosseinzadeh et al., found that Zataria multiflora Boiss. extract exhibits analgesic properties in mice by acting on opioid receptors.40 It was demonstrated that the extract of Rosmarinus officinalis could modulate the withdrawal syndrome via an increase in the GABA content in the brain.30 Studies have suggested that the effect of Nigella sativa in reducing withdrawal symptoms may be due to its major active compound, i.e., thymoquinone, which is a calcium channel blocking agent.20 Evidence has shown that the β-carboline compounds of harman, harmalin, and norharman, which have been found in Passiflora incarnate, are involved in reducing withdrawal symptoms via the modulation of the dopaminergic pathway and the inhibition of dopamine reuptake.41,42
In all of these studies, herbal remedies showed a significant difference in reducing the symptoms of opioid withdrawal syndrome compared to placebo. In addition, their effects were similar to those of methadone, lofexidine, clonidine, and buprenorphine.
Although, as mentioned earlier, the high risk of bias in more than 30% of these studies jeopardizes their internal validity. Moreover, in a significant number of studies, the risk of bias was unclear in most domains due to the lack of sufficient detailed description. On the other hand, extensive variation in the type of herbal formulations, the intervention period, the amount and method of use, and the outcome evaluation measures make it difficult to compare and interpret the results.
This study was limited to English language articles. Therefore, it was not possible to review and compare similar studies reported in other languages.
Conclusion
Based on the results of the included literature, it can be concluded that some plant extracts and herbal formulas can attenuate the symptoms of opioid withdrawal syndrome. Hence, herbal therapy could be considered as a complementary treatment in alleviating the symptoms of opioid withdrawal. However, further studies with more participants, longer duration, and less risk of bias in the five mentioned domains are needed in the claimed cases.
Acknowledgments
This study was the result of a research project approved by the Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran (No: 99000927) with the financial support of the Vice Chancellor for Research and Technology of this university.
Citation: Nematollahi MH, Ahmadianmoghadam MA, Mehrabani M, Moghadari M, Ghorani-Azam A, Mehrbani M. Herbal therapy in opioid withdrawal syndrome: A systematic review of randomized clinical trials. Addict Health 2022; 14(2): 152-63.
Footnotes
Conflict of Interests
The authors declare no conflicts of interest.
Authors’ Contribution
Contributed to the design of the manuscript: MM2; carried out the search based on the keywords and data extraction: MM1 and MM3; checked the data extraction and wrote the last report: MM3 and MHN. Contributed to the drafting of the manuscript: MAA and AGA. All authors approved the last version of the manuscript.
References
- 1.Hodding GC, Jann M, Ackerman IP. Drug withdrawal syndromes-- a literature review. West J Med. 1980;133(5):383–91. [PMC free article] [PubMed] [Google Scholar]
- 2.Farrell M. Opiate withdrawal. Addiction. 1994;89(11):1471–5. doi: 10.1111/j.1360-0443.1994.tb03745.x. [DOI] [PubMed] [Google Scholar]
- 3.Riahi-Zanjani B, Balali-Mood M, Asoodeh A, Es’haghi Z, Ghorani-Azam A. Potential application of amino acids in analytical toxicology. Talanta. 2019;197:168–74. doi: 10.1016/j.talanta.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Sangi S, Ahmed SP, Channa MA, Ashfaq M, Mastoi SM. A new and novel treatment of opioid dependence: Nigella sativa 500 mg. J Ayub Med Coll Abbottabad. 2008;20(2):118–24. [PubMed] [Google Scholar]
- 5.Solhi H, Salehi B, Alimoradian A, Pazouki S, Taghizadeh M, Saleh AM, et al. Beneficial Effects of Rosmarinus Officinalis for Treatment of Opium Withdrawal Syndrome during Addiction Treatment Programs: A Clinical Trial. Addict Health. 2013;5(3-4):90–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Kosten TR, Baxter LE. Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am J Addict. 2019;28(2):55–62. doi: 10.1111/ajad.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motaghinejad M, Bangash MY, Hosseini P, Karimian SM, Motaghinejad O. Attenuation of morphine withdrawal syndrome by various dosages of curcumin in comparison with clonidine in mouse: possible mechanism. Iran J Med Sci. 2015;40(2):125–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Kamali M, Tajadini H, Mehrabani M, Moghadari M. Consequences of Opioid Abuse and their Treatments in Persian Medicine: A Review Study. Addict Health. 2020;12(1):46–57. doi: 10.22122/ahj.v12i1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang L, Li B, Gao L, Li S, Wang D, Hu M, et al. Tai-Kang-Ning, a Chinese herbal medicine formula, alleviates acute heroin withdrawal. Am J Drug Alcohol Abuse. 2008;34(3):269–76. doi: 10.1080/00952990802013409. [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Xu GZ, Liu TT, Wang X, Shen LY, Li J, et al. A comparative clinical study of the effects of the traditional Chinese medicine Jinniu capsules and lofexidine on acute heroin withdrawal symptoms. Am J Drug Alcohol Abuse. 2008;34(6):792–800. doi: 10.1080/00952990802491563. [DOI] [PubMed] [Google Scholar]
- 13.Akhondzadeh S, Kashani L, Mobaseri M, Hosseini SH, Nikzad S, Khani M. Passionflower in the treatment of opiates withdrawal: a double-blind randomized controlled trial. J Clin Pharm Ther. 2001;26(5):369–73. doi: 10.1046/j.1365-2710.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Hao W, Zhao M. A comparative clinical study of the effect of WeiniCom, a Chinese herbal compound, on alleviation of withdrawal symptoms and craving for heroin in detoxification treatment. J Psychoactive Drugs. 2000;32(3):277–84. doi: 10.1080/02791072.2000.10400450. [DOI] [PubMed] [Google Scholar]
- 15.Kianbakht S, Hajiaghaee R, Akhondzadeh S. Efficacy and safety of Sophora alopecuroides var alopecuroides seed extract for opioid detoxification: A randomized, double-blind, and placebo-controlled clinical trial. Phytother Res. 2020;34(5):1108–113. doi: 10.1002/ptr.6578. [DOI] [PubMed] [Google Scholar]
- 16.Nemat Shahi M, Asadi A, Behnam Talab E. The Impact of Saffron on Symptoms of Withdrawal Syndrome in Patients Undergoing Maintenance Treatment for Opioid Addiction in Sabzevar Parish in 2017. Adv Med. 2017;2017:1079132. doi: 10.1155/2017/1079132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbaszadeh-Mashkani S, Hoque SS, Banafshe HR, Ghaderi A. The effect of crocin (the main active saffron constituent) on the cognitive functions, craving, and withdrawal syndrome in opioid patients under methadone maintenance treatment. Phytother Res. 2021;35(3):1486–94. doi: 10.1002/ptr.6913. [DOI] [PubMed] [Google Scholar]
- 18.Moosavyzadeh A, Mokri A, Ghaffari F, Faghihzadeh S, Azizi H, Jafari Hajati R, et al. Hab-o Shefa, a persian medicine compound for maintenance treatment of opioid dependence: randomized placebo-controlled clinical trial. J Altern Complement Med. 2020;26(5):376–83. doi: 10.1089/acm.2019.0390. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Li J, Huang M, Kang L, Hu M. A study on Fu-Yuan Pellet, a traditional chinese medicine formula for detoxification of heroin addictions. Am J Drug Alcohol Abuse. 2009;35(6):408–11. doi: 10.3109/00952990903377146. [DOI] [PubMed] [Google Scholar]
- 20.Adnan LH, Bakar NHA, Mohamad N. Opioid dependence and substitution therapy: thymoquinone as potential novel supplement therapy for better outcome for methadone maintenance therapy substitution therapy. Iran J Basic Med Sci. 2014;17(12):926–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Sayyah M, Rahim ASF. The role of alcoholic extract of Zataria multiflora Boiss in controlling opium withdrawal symptoms: a randomized, double-blind study. Archives of Psychiatry and Psychotherapy. 2017;3:27–33. [Google Scholar]
- 22.Doosti F, Dashti S, Tabatabai SM, Hosseinzadeh H. Traditional Chinese and Indian medicine in the treatment of opioid-dependence: a review. Avicenna J Phytomed. 2013;3(3):205–15. [PMC free article] [PubMed] [Google Scholar]
- 23.Sepahi S, Ghorani-Azam A, Asoodeh A, Rostami S. In Vitro Study to Evaluate Antibacterial and Non-haemolytic Activities of Four Iranian Medicinal Plants. West Indian Med J. 2014;63(4):289–93. doi: 10.7727/wimj.2013.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson LL, Harris HM, Eans SO, Brice-Tutt AC, Cirino TJ, Stacy HM, et al. Lyophilized Kratom Tea as a Therapeutic Option for Opioid Dependence. Drug Alcohol Depend. 2020;216:108310. doi: 10.1016/j.drugalcdep.2020.108310. [DOI] [PubMed] [Google Scholar]
- 25.Yu KC, Wei H-T, Yeh YH, Hsu CH. Traditional Chinese medicine-facilitated treatments may relieve anxiety symptoms during drug switching from methadone to buprenorphine/naloxone for treating opioid dependence. BMJ Case Rep. 2017;2017:bcr2017220815. doi: 10.1136/bcr-2017-220815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharifzadeh M, Hadjiakhoondi A, Khanavi M, Susanabadi M. Effects of aqueous, methanolic and chloroform extracts of rhizome and aerial parts of Valeriana officinalis L on naloxone-induced jumping in morphine-dependent mice. Addict Biol. 2006;11(2):145–51. doi: 10.1111/j.1369-1600.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramezani M, Hosseinzadeh H, Mojtahedi K. Effects of Ferula gummosa Boiss fractions on morphine dependence in mice. J Ethnopharmacol. 2001;77(1):71–5. doi: 10.1016/s0378-8741(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 28.Cahill CM, Walwyn W, Taylor AMW, Pradhan AAA, Evans CJ. Allostatic Mechanisms of Opioid Tolerance Beyond Desensitization and Downregulation. Trends Pharmacol Sci. 2016;37(11):963–76. doi: 10.1016/j.tips.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moayeri A, Azimi M, Karimi E, Aidy A, Abbasi N. Attenuation of Morphine Withdrawal Syndrome by Prosopis Farcta Extract and Its Bioactive Component Luteolin in Comparison with Clonidine in Rats. Med Sci Monit Basic Res. 2018;24:151–8. doi: 10.12659/MSMBR.909930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosseinzadeh H, Nourbakhsh M. Effect of Rosmarinus officinalis L Effect of Rosmarinus officinalis L aerial parts extract on morphine withdrawal syndrome in mice. Phytother Res. 2003;17(8):938–41. doi: 10.1002/ptr.1311. [DOI] [PubMed] [Google Scholar]
- 31.Kianbakht S, Hashem Dabaghian F. Sophora alopecuroides L var alopecuroides alleviates morphine withdrawal syndrome in mice: involvement of alkaloid fraction and matrine. Iran J Basic Med Sci. 2016;19(10):1090–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Roome T, Dar A, Naqvi S, Choudhary MI. Evaluation of antinociceptive effect of Aegiceras corniculatum stems extracts and its possible mechanism of action in rodents. J Ethnopharmacol. 2011;135(2):351–8. doi: 10.1016/j.jep.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Nemat-Shahi M, Mir Mohammadi SM, Soroosh D, Asadi A, Nakhaee S, Mehrpour M. Comparison of the Effects of Passiflora Incarnata and Piroxicam in opioids withdrawal-Induced Myalgia and Anxiety: A randomized Clinical Trial. Indian Journal of Forensic Medicine & Toxicology. 2020;14(2):1766–70. doi: 10.37506/ijfmt.v14i2.3192. [DOI] [Google Scholar]
- 34.Gómez-A A, Shnitko TA, Barefoot HM, Brightbill EL, Sombers LA, Nicola SM, et al. Local μ-Opioid Receptor Antagonism Blunts Evoked Phasic Dopamine Release in the Nucleus Accumbens of Rats. ACS Chem Neurosci. 2019;10(4):1935–1940. doi: 10.1021/acschemneuro.8b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasudevan L, Borroto-Escuela DO, Huysentruyt J, Fuxe K, Saini DK, Stove C. Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D(2) Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor. Biomolecules. 2019;9(8):368. doi: 10.3390/biom9080368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu S, Gao J, Liu J, Zhang J, Huang Y, Xu S, et al. Effects of Jitai tablet, a traditional Chinese medicine, on spontaneous withdrawal symptoms and modulation of dopaminergic functions in morphine-dependent rats. Phytother Res. 2015;29(5):687–94. doi: 10.1002/ptr.5300. [DOI] [PubMed] [Google Scholar]
- 37.Koob GF. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry. 2020;87(1):44–53. doi: 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Gao JL, Tu SA, Liu J, Zhang JM, Huang Y, Han M, et al. An-jun-ning, a traditional herbal formula, attenuates spontaneous withdrawal symptoms via modulation of the dopamine system in morphine-dependent rats. BMC Complement Altern Med. 2014;14:308. doi: 10.1186/1472-6882-14-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C, Nong Z, Huang J, Chen Z, Zhang S, Jiao Y, et al. Yulangsan polysaccharide attenuates withdrawal symptoms and regulates the NO pathway in morphine-dependent rats. Neurosci Lett. 2014;570:63–8. doi: 10.1016/j.neulet.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73(3):379–85. doi: 10.1016/s0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- 41.Drucker G, Raikoff K, Neafsey EJ, Collins MA. Dopamine uptake inhibitory capacities of beta-carboline and 3,4-dihydro-beta-carboline analogs of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) oxidation products. Brain Res. 1990;509(1):125–33. doi: 10.1016/0006-8993(90)90318-6. [DOI] [PubMed] [Google Scholar]
- 42.Farzin D, Haghparast A, Motaman S, Baryar F, Mansouri N. Effects of harmane and other β-carbolines on apomorphine-induced licking behavior in rat. Pharmacol Biochem Behav. 2011;98(2):215–9. doi: 10.1016/j.pbb.2011.01.001. [DOI] [PubMed] [Google Scholar]