Abstract
Background:
Cigarette smoking is the most important preventable cause of several diseases such as malignancies, pulmonary and cardiovascular diseases. Smoking cessation is now supported by both behavioral counseling and medical pharmacotherapy and is the only effective approach for slowing down an accelerated decline in forced expiratory volume in one second (FEV1). Our study aims to examine changes in forced expiratory volume in one second (FEV1) after smoking cessation for smokers attending our smoking cessation clinic their correlation to smokers’ demographic characteristics.
Methods:
114 smokers (48 males and 66 females), with a mean age of 48.36±10.49 years, were enrolled. They were classified in 4 groups, according to their age; <40 years (Group Α), 41-50 years (Group Β), 51-60 years (Group C), >60 years (Group D) and underwent Spirometry on the 1st day of visit, one month (2nd visit) and, 3 months later (3rd visit).
Findings:
Statistically significant increase in FEV1 values at the 2nd and 3rd visit compared to the 1st visit was observed in smokers who quit smoking in Group Α, B and C (p<0.05). In addition, a statistically significant decrease in FEV1 values at the 2nd and 3rd visit compared to the 1st visit was noticed in smokers who continued smoking in Group B, C and D (p<0.05).
Conclusion:
Smoking cessation achieved through smoking cessation support led to the improvement of FEV1 values within 3 months. The greatest benefit was observed in smokers under the age of 60.
Keywords: Forced Expiratory Volume, Smoking Cessation, Tobacco Smoking, Spirometry
Introduction
Cigarette smoking is the most important preventable cause of several diseases such as cancer, pulmonary disorders and cardiovascular diseases.1 It causes inflammation and oxidative stress, leading to vasomotor dysfunction and impaired blood coagulation.2 In addition, smoking is the most significant risk factor for chronic obstructive pulmonary disease (COPD). The main characteristic of COPD is airflow limitation.3 Spirometry is a pulmonary function test used to evaluate airflow limitation, particularly through the calculation of forced expiratory volume in one second (FEV1).4
Low FEV1 has a negative feedback on itself so it leads to more FEV1 drop and develops clinically significant COPD.4 Moreover, low FEV1 predicts correlation between cardiovascular morbidity and mortality.5 More specifically, excess coronary heart disease mortality follows a great decline in FEV1, independently of initially predicted FEV1%, tobacco smoking, or other common coronary heart disease risk factors.5
Decreased FEV1 values have also been associated with mortality in advanced non-small cell lung cancer.6
It is well established that smokers have an average reduction rate of FEV1 that is greater than those who have never smoked7 and it has been mentioned that quitting smoking decreases the rate of FEV1 decline in smokers without respiratory symptoms and in smokers with COPD.8 In addition, patients who quit smoking have less pulmonary symptoms and hyperresponsiveness than people who continue smoking.9
Smoking cessation is now supported by both behavioral counseling and medical pharmacotherapy. Pharmacotherapy for smoking cessation is suggested for all smokers trying to quit, unless there is a contraindication. Through acting by various mechanisms, cessation medications can decrease both withdrawal from nicotine symptoms as well as the immediate effects of nicotine absorbed via tobacco if an individual smokes.10 Tobacco cessation is the only effective strategy for slowing down an accelerated decline in FEV1 and the only intervention able to reduce the progression of COPD. The greatest improvements in pulmonary function and symptoms improvement will develop within the first year after quitting smoking.7
In this research, we aim to investigate the alterations in FEV1 following smoking cessation in smokers that visited our smoking cessation clinic and their correlation to smokers’ demographic characteristics. Similar studies have been conducted in Sweden, the United States, and Japan in healthy smokers, showing important improvement in FEV1 values, following smoking cessation therapy.11-15 In addition, some studies have demonstrated a significant improvement in FEV1 values with smoking cessation therapy in patients with underlying lung disease.16-23
Methods
Study participants and sampling: The design of the present study was prospective. The data collection took place at “Sismanogleio Hospital” from January to April 2019. The study was approved by the relevant Institutional Board (protocol number 7065/7-12-19). The study conformed to the principles of the Declaration of Helsinki (as revised in Edinburgh 2000).
In this research, adult active smokers who visited the smoking cessation clinic of Sismanogleio Hospital in Athens for smoking cessation support, were enrolled. Smokers <18 years old and pregnant women were excluded. Smokers with a history of pulmonary disease, collagen vascular disease, Pleural disease, neuromuscular disease, or occupational exposure that could probably affect lungs, were also excluded. In addition, none of them had reported lung infections within two weeks before entry and two weeks before each spirometry.
Spirometry and Smoking Cessation Support: Age, gender, height, and weight were recorded prior to the spirometry. Maximal expiratory flow volume was estimated while participants were seated and wore nose clips. An automated spirometer (Cosmed Micro Quark, Italy) was used to measure forced expiratory volume in one second (FEV1). Up to 3 trials were conducted and an average of 2 technically acceptable tests was recorded. The tests had an agreement of 5% to be considered acceptable. FEV1 was measured on the day of the first visit (January 1st -January 15th), one month (February 1st- February 15th) and 3 months later (April 1st-April 15th). All smokers received both behavioral support and treatment with medications including monotherapy with vareniclineor acombinationof nicotine replacement agents. No changes were made to any other previously prescribed medications.
Data Collection:FEV1 values at three visits and thesmoking cessation rates were recorded. Theparticipants were divided to four age groups: <40 years, 41-50 years, 51-60 years and >60 years. Mean FEV1 values were calculated in all age groups of participants who achieved quitting and who continued smoking and were compared between visits in each age group.
Statistical analysis: For statistical analysis, the SPSS version 17.0 for Windows was utilized. Continues variables were checked for normality of distribution with the Kolmogorov-Smirnov test. Descriptive data were presented as mean (±standard deviation) for values with normal distribution. Independent t-test and One Way ANOVA were used for variables with normal distribution. Correlation of characteristics was performed using the Pearson’s chi-square and Linear-by-Linear Association test, as appropriate. Five percent was chosen as the level of statistical significance.
Results
In this research, a total number of 114 smokers (48 males and 66 females, with a mean age of 48.36±10.49 years) were enrolled: Twenty-eight in the age group <40 years (Group A), 34 smokers in the age group 41-50 years (Group B), 44 smokers in the age group 51-60 years (Group C), and 8 smokers in the age group >60 years (Group D). (Table 1).
Table 1. Demographic characteristics of the study population .
| Mean(S.D.) | Median | Range | Min-Max | |
| Age | 48.36(10.49) | 48.00 | 47.00 | 28.00-75.00 |
| N | % | |||
| Gender | Male | 48 | 42.1 | |
| Female | 66 | 57.9 |
* S.D: Standard Deviation
Fourteen smokers in Group A, 8 in Group B, 20 in Group C, and 2 in Group D quit smoking. For smokers who quit smoking the mean value of FEV1 was 2480.00±170.60 ml at the 1st visit, 2545.71±178.52 ml at the 2nd visit (at one month), and 2584.28±192.22 ml at the 3rd visit (at three months) in Group As 2159.75±10.43 ml at the 1st visit, 2259.75±16.74 ml at the 2nd visit, and 2357.50±56.75 ml at the 3rd visit in Group B,s 1901.00±321.42 ml at the 1st visit, 2064.80±284.54 ml at the 2nd visit, and 2193.80±231.90 ml at the 3rd visit in Group C, ands 1560.00±0.00 ml at the 1st visit, 1710.00±0.00 ml at the 2nd visit, and 1890.00±0.00 ml at the 3rd visit in Group D (Table 2).
Table 2. Mean values of FEV1 at 1st visit, at 2nd visit (at one month) and at 3rd visit (at 3 months) in age groups >40 years, 41-50 years, 51-60 years and >60 years in smokers that quit smoking.
| Age group | FEV1 | Mean(S.D) | N |
| <40 years | FEV1 1st Visit | 2480.00(170.60) | 14 |
| FEV1 2nd Visit | 2545.71(178.52) | ||
| FEV1 3rd Visit | 2584.28(192.22) | ||
| 41-50 years | FEV1 1st Visit | 2159.75(10.43) | 8 |
| FEV1 2nd Visit | 2259.75(16.74) | ||
| FEV1 3rd Visit | 2357.50(56.75) | ||
| 51-60 years | FEV1 1st Visit | 1901.00(321.42) | 20 |
| FEV1 2nd Visit | 2064.80(284.54) | ||
| FEV1 3rd Visit | 2193.80(231.90) | ||
| >60 years | FEV1 1st Visit | 1560.00(0.00) | 2 |
| FEV1 2nd Visit | 1710.00(0.00) | ||
| FEV1 3rd Visit | 1890.00(0.00) |
*FEV1: Forced Expiratory Volume in 1 second, S.D: Standard Deviation
There was a statistically significant difference in FEV1 values between 1st and 2nd, 2nd and 3rd, and 1st and 3rd visit of smokers who quit smoking in age groups A, B and C (p<0.05). (Table 3).
Table 3. Mean differences in FEV1 values between 1st and 2nd visit, 2nd and 3rd visit and 1st and 3rd visit in smokers who quit smoking .
| Age group | Difference in FEV1 | Mean (S.D) | p |
| <40 years | FEV1 2nd Visit-FEV1 1st Visit | 65.71(34.58) | 0.000 |
| FEV1 3rd Visit-FEV1 1st Visit | 104.29(80.92) | 0.000 | |
| FEV1 3rd Visit-FEV1 2nd Visit | 38.57(48.49) | 0.011 | |
| 41-50 years | FEV1 2nd Visit-FEV1 1st Visit | 100.00(7.55) | 0.000 |
| FEV1 3rd Visit-FEV1 1st Visit | 197.75(46.41) | 0.000 | |
| FEV1 3rd Visit-FEV1 2nd Visit | 97.75(41.19) | 0.000 | |
| 51-60 years | FEV1 1st Visit - FEV1 2nd Visit | 163.80(58.92) | 0.000 |
| FEV1 3rd Visit-FEV1 1st Visit | 292.80(105.76) | 0.000 | |
| FEV1 3rd Visit-FEV1 2nd Visit | 129.00(68.12) | 0.000 |
*FEV1: Forced Expiratory Volume in 1 second, S.D: Standard Deviation
Figure 1 shows the mean difference between in FEV1 values between the 1st and the 3rd visit of smokers who quit smoking.
Figure 1.
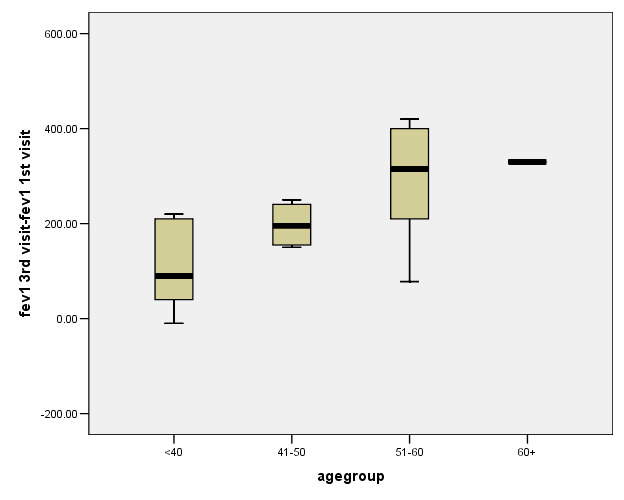
Mean differences in FEV1 values between the 1st and the 3rd visit of smokers who quit smoking
Fourteen smokers reduced smoking in Group A, 26 smokers continued smoking in Group B, 24 smokers continued smoking in Group C, and 6 smokers continued smoking in Group D. The mean value of FEV1 was 2477.14±99.49 ml at the 1st visit, 2449.28±158.12 ml at the 2nd visit, and 2442.85±178.08 ml at the 3rd visit for smokers who continued smoking in Group A, 2326.15±178.73 ml at the 1st visit, 2266.92±230.69 ml at the 2nd visit, and 2239.23±219.98 ml at the 3rd visit in Group B, 1993.33±219.26 ml at the 1st visit, 1928.33±265.97 ml at the 2nd visit, and 1882.50±275.40 ml at the 3rd visit in Group C, and 1586.66±33.86 ml at the 1st visit, 1446.66±121.76 ml at the 2nd visit, and 1350.00±114.19 ml at the 3rd visit in Group D (Table 4).
Table 4. Mean values of FEV1 at 1st visit, at 2nd visit (at one month) and at 3rd visit (at 3 months) in age groups >40 years, 41-50 years, 51-60 years and >60 years in smokers that continued smoking .
| Age group | FEV1 | Mean(S.D) | N |
| <40 years | FEV1 1st Visit | 2477.14(99.49) | 14 |
| FEV1 2nd Visit | 2449.28(158.12) | ||
| FEV1 3rd Visit | 2442.85(178.08) | ||
| 41-50 years | FEV1 1st Visit | 2326.15(178.73) | 26 |
| FEV1 2nd Visit | 2266.92(230.69) | ||
| FEV1 3rd Visit | 2239.23(219.98) | ||
| 51-60 years | FEV1 1st Visit | 1993.33(219.26) | 24 |
| FEV1 2nd Visit | 1928.33(265.97) | ||
| FEV1 3rd Visit | 1882.50(275.40) | ||
| >60 years | FEV1 1st Visit | 1586.66(33.86) | 6 |
| FEV1 2nd Visit | 1446.66(121.76) | ||
| FEV1 3rd Visit | 1350.00(114.19) |
There was a statistically significant difference in FEV1 values between 1st and 2nd, 2nd and 3rd, and 1st and 3rd visit of smokers who continued smoking in age groups B, C and D (p<0.05). (Table 5)
Table 5. Mean differences in FEV1 values between 1st and 2nd visit, 2nd and 3rd visit and 1st and 3rd visit in smokers who continued smoking .
| Age group | Difference in FEV1 | Mean (S.D) | p |
| <40 years | FEV1 2nd Visit-FEV1 1st Visit | -27.85(86.72) | 0.251 |
| FEV1 3rd Visit-FEV1 1st Visit | -34.28(113.25) | 0.278 | |
| FEV1 3rd Visit-FEV1 2nd Visit | -6.42(37.69) | 0.534 | |
| 41-50 years | FEV1 2nd Visit-FEV1 1st Visit | -59.23(69.68) | 0.000 |
| FEV1 3rd Visit-FEV1 1st Visit | -86.92(64.42) | 0.000 | |
| FEV1 3rd Visit-FEV1 2nd Visit | -27.69(46.50) | 0.006 | |
| 51-60 years | FEV1 2nd Visit-FEV1 1st Visit | -65.00(92.82) | 0.002 |
| FEV1 3rd Visit-FEV1 1st Visit | -110.83(110.52) | 0.000 | |
| FEV1 3rd Visit-FEV1 2nd Visit | -45.83(44.61) | 0.000 | |
| >60 years | FEV1 2nd Visit-FEV1 1st Visit | -140.00(88.09) | 0.011 |
| FEV1 3rd Visit-FEV1 1st Visit | -236.66(81.15) | 0.001 | |
| FEV1 3rd Visit-FEV1 2nd Visit | -96.66(13.66) | 0.000 |
*FEV1: Forced Expiratory Volume in 1 second, S.D: Standard Deviation
Discussion
According to our results, there was a statistically significant difference in FEV1 values between sequential visits in age groups <40, 41-50, and 51-60 years for smokers who quit smoking with greatest values at 3rd visit. A statistically significant difference was also observed in age groups 41-50, 51-60 and >60 years for smokers who continued smoking with reduced values at 2nd and 3rd visit. This indicates that smoking cessation support leads to a significant increase of FEV1 values.The influence of smoking cessation on Spirometry values has been studied since 1977 when Bake et al., in a small study of 59 smokers, found that the subjects that remained abstinent for at least five months had a substantial improvement in FEV1.11 Townsend et al. reported that smokers who quit during the first 12 months had smaller declines in FEV1. They also concluded that middle- aged, healthy smokers who stopped smoking permanently, could expect FEV1 values to decrease at a more gradual rate 3-4 years after smoking cessation than those who continue to smoke.12 Burchfiel et al., in a large study of 4.451 smokers, found that smoking cessation led to less steep rates of decline in Spirometry values over a short time period in middle-aged men, as well as in men with confirmed pulmonary impairment.13
In a study of smokers who quit and were followed up for 17 years, Sherrill et al. reported a beneficial effect related to quitting, largest for younger individuals. Better FEV1 values were observed in women compared to men of all ages.14 Iwaoka and Tsuji. studied 81 subjects after 12 weeks of smoking cessation therapy with varenicline and found that spirometric lung age, which is calculated by both height and FEV1, improved in this short period of time.15
The effects of smoking cessation on pulmonary function have also been studied in populations with lung disorders. Chaudhuri et al. in a study of 21 patients with asthma, by six weeks after smoking cessation, described that individuals who quit smoking had achieved significant improvement in lung function, accompanied by decrease in sputum neutrophil counts in comparison with individuals who continued to smoke.16 Jang et al., in a study of asthmatic smokers, found that patients who quit tobacco use showed less airway obstruction, indicating that smoking cessation is a crucial intervention in the management of asthma.17
Scanlon et al. in a large prospective clinical trial studied 3926 smokers with known mild-to-moderate airway obstruction who were randomized to one of two groups of smoking cessation or to a group with no intervention. They concluded that participants who stopped smoking presented with an improvement in FEV1 one year after quitting; and the subsequent rate of FEV1 decline among those who sustained quitting was half of the rate among continuing tobacco users.18. In a randomized, double-blind trial of smokers with mild-to-moderate COPD treated with varenicline, Tashkin et al. found that after one year of cessation, continuous abstinence in comparison with continuous smoking greatly improved FEV1 at week 12 with sustained result thereafter.19 Dhariwal et al. reported that smokers with COPD who quit smoking had a pronounced but transient improvement in FEV1 at 6 weeks, which was sustained until 12 weeks and was only partially present at one year.20
Bohadana et al. studied occupationally exposed workers to respiratory pollutants and found that in males, smoking cessation slowed the annual FEV1 decline.21 In a study by Maci et al., tobacco smoke users were evaluated with spirometry at the beginning and after three months of smoking cessation therapy and a significant increase in FEV1 was observed through the reduction of size of existing lung nodules.22 Pezzuto et al. demonstrated that the combination of anti-EGFR treatment and therapy for smoking cessation was more effective than monotherapy with erlotinib in improving lung function in advanced NSCLC patients having EGFR-mutations.23
To the best of our knowledge, this study is one of the few studies investigating the impact of smoking cessation and reduction in FEV1 in smokers who seek smoking cessation support in Greece. This study has some limitations. This research is based on data from a single center that limits the generalization of the conclusions to a larger population. Thus, larger prospective studies, in multiple smoking cessation clinics in Greece, are needed for better evaluation of the results. Another limitation is that this study did not take into account the number of cigarettes smoked and the severity of smoking which can have a significant effect on the results.
Conclusion
Smoking cessation achieved with smoking cessation support leads to improvement of FEV1 values within 3 months. It is of great importance to decrease smoking-related health complications by educating population about tobacco’s harmful impact, and informing them about tobacco cessation programs, including behavioral counseling and pharmacologic therapy. These policies appear to be effective, as smoking rates are decreasing in countries with developed and developing economy, and can prevent FEV1 decline, especially in smokers younger than 60 years old.
Acknowledgments
None.
Citation: Trakas N, Georgakopoulou VE, Melemeni D, Damaskos C, Konstantinos Mantzouranis K. Association between smoking cessation and alterations in forced expiratory volume in one second (FEV1). A FollowUp Study from a Greek Tobacco Cessation Clinic. Addict Health 2022; 14(2): 87-95.
Footnotes
Conflict of Interests
None.
Authors’ Contribution
Design of the study: VEG, KM, DM, and AL; acquisition of data: CD, NG, AG and PS; analysis and interpretation: PP, SC, DM; drafting of manuscript: VEG and NT; critical revision: NT and XT.
References
- 1. Sealock T, Sharma S. Smoking Cessation Treasure Island (FL): StatPearls Publishing; 2022. [PubMed]
- 2.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Zarghami M, Taghizadeh F, Sharifpour A, Alipour A. Efficacy of Smoking Cessation on Stress, Anxiety, and Depression in Smokers with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Clinical Trial. Addict Health. 2018;10(3):137–47. doi: 10.22122/ahj.v10i3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng YJ, Chen ZG, Li ZY, Mei WY, Bi WT, Luo DL. Longitudinal change in lung function and subsequent risks of cardiovascular events: evidence from four prospective cohort studies. BMC Medicine. 2021;19(1):153. doi: 10.1186/s12916-021-02023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Song EM, Sim YS, Ryu YJ, Chang JH. Forced expiratory volume in one second as a prognostic factor in advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(2):305–9. doi: 10.1097/JTO.0b013e318201884b. [DOI] [PubMed] [Google Scholar]
- 7.Richard P, Gilles H, Alavi Z, Christine L, Maryline LB, Ronan G, et al. Screening for Chronic Obstructive Pulmonary Disease in Smoking Cessation Clinic in France. Addict Health. 2016;8(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Willemse BW, Postma DS, Timens W, ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23(3):464–76. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 9.Lapperre TS, Postma DS, Gosman MM, Snoeck-Stroband JB, ten Hacken NH, Hiemstra PS, et al. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax. 2006;61(2):115–21. doi: 10.1136/thx.2005.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prochaska JJ, Benowitz NL. Smoking cessation and the cardiovascular patient. Curr Opin Cardiol. 2015;30(5):506–11. doi: 10.1097/HCO.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bake B, Oxhöj H, Sixt R, Wilhelmsen L. Ventilatory lung function following two years of tobacco abstinence. Scand J Respir Dis. 1977;58(6):311–8. [PubMed] [Google Scholar]
- 12.Townsend MC, DuChene AG, Morgan J, Browner WS. Pulmonary function in relation to cigarette smoking and smoking cessation MRFIT Research Group. Prev Med. 1991;20(5):621–37. doi: 10.1016/0091-7435(91)90059-d. [DOI] [PubMed] [Google Scholar]
- 13.Burchfiel CM, Marcus EB, Curb JD, Maclean CJ, Vollmer WM, Johnson LR, et al. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med. 1995;151(6):177885. doi: 10.1164/ajrccm.151.6.7767520. [DOI] [PubMed] [Google Scholar]
- 14.Sherrill DL, Holberg CJ, Enright PL, Lebowitz MD, Burrows B. Longitudinal analysis of the effects of smoking onset and cessation on pulmonary function. Am J Respir Crit Care Med. 1994;149(3 Pt 1):591–7. doi: 10.1164/ajrccm.149.3.8118623. [DOI] [PubMed] [Google Scholar]
- 15.Iwaoka M, Tsuji T. Twelve Weeks of Successful Smoking Cessation Therapy with Varenicline Reduces Spirometric Lung Age. Intern Med. 2016;55(17):2387–92. doi: 10.2169/internalmedicine.55.6844. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, Spears M, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174(2):127–33. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]
- 17.Jang AS, Park SW, Kim DJ, Uh S, Kim YH, Whang HG, et al. Effects of smoking cessation on airflow obstruction and quality of life in asthmatic smokers. Allergy Asthma Immunol Res. 2010;2(4):254–9. doi: 10.4168/aair.2010.2.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS, et al. Lung Health Study Research Group Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381–90. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 19.Tashkin DP, Rennard S, Taylor Hays J, Lawrence D, Marton JP, et al. Lung function and respiratory symptoms in a 1-year randomized smoking cessation trial of varenicline in COPD patients. Respir Med. 2011;105(11):1682–90. doi: 10.1016/j.rmed.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Dhariwal J, Tennant RC, Hansell DM, Westwick J, Walker C, Ward SP, et al. Smoking cessation in COPD causes a transient improvement in spirometry and decreases micronodules on high-resolution CT imaging. Chest. 2014;145(5):1006–15. doi: 10.1378/chest.13-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohadana AB, Nilsson F, Westin A, Martinet N, Martinet Y. Smoking cessation--but not smoking reduction--improves the annual decline in FEV1 in occupationally exposed workers. Respir Med. 2006;100(8):1423–30. doi: 10.1016/j.rmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Maci E, Comito F, Frezza AM, Tonini G, Pezzuto A. Lung nodule and functional changes in smokers after smoking cessation short-term treatment. Cancer Invest. 2014;32(8):388–93. doi: 10.3109/07357907.2014.919308. [DOI] [PubMed] [Google Scholar]
- 23.Pezzuto A, Stumbo L, Russano M, Crucitti P, Scarlata S, Caricato M, et al. Impact of Smoking Cessation Treatment" on Lung Function and Response Rate in EGFR Mutated Patients: A Short-Term Cohort Study. Recent Pat Anticancer Drug Discov. 2015;10(3):342–51. doi: 10.2174/157489281066615080611101. [DOI] [PubMed] [Google Scholar]


