Abstract
Social attachments, the enduring bonds between individuals and groups, are essential to health and well-being. The appropriate formation and maintenance of social relationships depend upon a number of affective processes, including stress regulation, motivation, reward, as well as reciprocal interactions necessary for evaluating the affective state of others. A genetic, molecular, and neural circuit level understanding of social attachments therefore provides a powerful substrate for probing the affective processes associated with social behaviors. Socially monogamous species form long-term pair bonds, allowing us to investigate the mechanisms underlying attachment. Now, molecular genetic tools permit manipulations in monogamous species. Studies using these tools reveal new insights into the genetic and neuroendocrine factors that design and control the neural architecture underlying attachment behavior. We focus this discussion on the prairie vole and oxytocinergic signaling in this and related species as a model of attachment behavior that has been studied in the context of genetic and pharmacological manipulations. We consider developmental processes that impact the demonstration of bonding behavior across genetic backgrounds, the modularity of mechanisms underlying bonding behaviors, and the distributed circuitry supporting these behaviors. Incorporating such theoretical considerations when interpreting reverse genetic studies in the context of the rich ethological and pharmacological data collected in monogamous species provides an important framework for studies of attachment behavior in both animal models and studies of human relationships.
Keywords: Attachment, Oxytocin, Prairie vole, Neural circuit, Genetics
Social attachments, or selective affiliations between individuals, play a central role at all levels of human relationships and represent a key determinant of psychological and physical health (Bowlby & Bowlby, 1982; Holt-Lunstad et al., 2010; O’Connor & Rutter, 2000; Robles et al., 2014; Rutter et al., 1999). Understanding social behavior in general, and attachment in particular, has been of intense interest for those studying affective biology and its neural substrates in recent years. The complexity of such behaviors presents a challenge to constructing cohesive theoretical and experimental frameworks that link observed behaviors with activity and molecular changes in the neural circuits underlying these behaviors (Adolphs, 2009; Anderson & Adolphs, 2014; Goodson, 2013). We aim to provide an introduction to select developmental and neurobiological concepts, namely developmental redundancy, modularity, and distributed circuitry, related to the regulation of attachment behaviors. While these concepts have been elegantly reviewed elsewhere (Anderson & Adolphs, 2014; Anderson, 2016; Hoke et al., 2019), we apply them to understanding the differential effects of specific genetic and neuroendocrine factors (oxytocinergic systems) on defined social attachment behaviors, which may apply not only in animal models but to human behavior as well.
Social attachment is defined by the emotional bonds formed between human infants or young non-human animals and a parent or caregiver as well as bonds between unrelated partners or peers in adulthood (Ainsworth, 1979; Bowlby & Bowlby, 1982; Harlow & Harlow, 1965). Across species, social attachments manifest in complex, but similar, patterns of behaviors, including mate (or pair) bonding, parental behavior, and kin and peer affiliation (Bales et al., 2017; Reichard & Boesch, 2003; Turner et al., 2010; Winslow, Shapiro et al., 1993). Socially monogamous species, representing ~4 to 9% of mammals, allow us to investigate the genetic and neurophysiological mechanisms mediating long-term attachments across the lifespan (Kleiman, 1977; Lukas & Clutton-Brock, 2013). Social attachments are organized around the formation and maintenance of social bonds. One of the most intriguing adult attachments is the enduring bond between mates. Pair bonds are characterized by long-term, preferential mating between two individuals and the active rejection of novel potential mates. Thus, pair bonding represents a rich substrate by which to begin to dissect the mechanisms that mediate and integrate socio-affective processing and behavior.
Robust animal models are essential for human-comparative analysis of attachment behaviors and identification of conserved molecular entry points into the neural circuits for pair bonding and associated affective processes. Studies of prairie voles (Microtus ochrogaster), a socially monogamous rodent species, have been foundational in our understanding of the biology of attachment. Prairie voles were first identified as living in burrows in extended families, and consistent male-female pairs are trapped together in the field (Getz et al., 1981). In the laboratory, prairie voles have been compared to closely related promiscuous species; such studies have provided a basis for understanding the behaviors associated with social monogamy. Prairie voles display long-term social attachments between mates (and peers), as mating partners show an enduring pair bond characterized by preference for spending time in close contact with a partner (Carter & Getz, 1993; DeVries et al., 1996; Getz et al., 1981). This “partner preference” has traditionally been tested in the laboratory using a preference assay, in which a bonded vole is given access to its bonded mate or a novel animal of the opposite sex (Beery, 2021; Carter & Getz, 1993). Bonded prairie voles will spend a majority of time with their partner in such a paradigm. The formation of affiliative bonds dramatically modifies patterns of other innate social behaviors such as aggression and mating, as bonded animals vigorously reject novel potential mates and avoid incest (Carter & Getz, 1993; Resendez et al., 2016; Resendez & Aragona, 2013). Such behaviors are displayed by both sexes and prairie voles show biparental care of offspring (Carter & Getz, 1993; DeVries et al., 1997). Furthermore, separation of bonded mates results in increased anxiety-type behaviors and physiological changes that accompany stress, supporting integrated neural and physiologic mechanisms that facilitate the preservation of such attachments within species (Grippo et al., 2007, 2011; Martin II et al., 2006; Resendez et al., 2016; Resendez & Aragona, 2013).
Oxytocin and Vasopressin Signaling in Pair Bonding
The evolution of varied complex social systems and affiliative behaviors, including social attachment behavior, has intriguingly converged upon the nonapeptide hormones oxytocin (Oxt) and arginine vasopressin (AVP), and their homologues, despite arising in the context of diverse ecological pressures and social constraints (Bielsky & Young, 2004; Carter, 2017; Carter & Perkeybile, 2018; Donaldson & Young, 2008; Feldman, 2017; Insel et al., 1998; Opie et al., 2013; Reichard & Boesch, 2003). Following the initial establishment of Oxt function in the physiology surrounding parturition, namely uterine contraction and milk ejection, investigations of its role in maternal behaviors revealed that Oxtr signaling modulates a range of attachment behaviors across species (Lee et al., 2008; Nishimori et al., 1996; Pinto et al., 1967; Reynolds et al., 1950; Rich et al., 2014; Shapiro & Insel, 1992; Wakerley et al., 1973; Young et al., 1996). These include the initiation of maternal behavior in rodents as well as the quality of maternal-infant interactions in humans (Marlin et al., 2015; Meyer-Lindenberg et al., 2011; Pedersen et al., 1982; Strathearn, 2011). Such investigations eventually lead to its implication in pair bonding (Insel et al., 1998; Shapiro & Insel, 1992).
Elegant work applying behavioral, pharmacological, and viral approaches to understand attachments in prairie voles revealed a role for these same neuroendocrine mediators, (Carter et al., 2008; Cho et al., 1999; Insel et al., 1998; Insel & Hulihan, 1995; Keebaugh et al., 2015; Shapiro & Insel, 1992; Winslow, Hastings et al., 1993). Pioneering studies identified interspecies variation in the patterns of expression of Oxtr and the vasopressin 1a receptors (V1aR) that correlates with the potential for pair bonding between closely related vole species (Carter et al., 1995; Shapiro & Insel, 1992; Wang & Young, 1997; Winslow, Hastings et al., 1993, Winslow, Shapiro et al., 1993). Pharmacological inhibition of Oxt and AVP signaling, either applied systemically or localized to brain regions enriched for receptor binding in prairie voles, was sufficient to disrupt pair bonding following cohabitation. Consistently, exogenous administration of these hormones promotes pair bonding under conditions that do not typically result in pair bond formation (Carter et al., 2008; Cho et al., 1999; Insel & Hulihan, 1995; Lim & Young, 2004; Liu et al., 2001; Wang et al., 1998; Winslow, Hastings et al., 1993, Winslow, Shapiro et al., 1993). In line with these observations, viral manipulations that increase or decrease Oxtr expression in specific brain regions mirror pharmacologic agonism or antagonism of its signaling, respectively (Keebaugh et al., 2015; Keebaugh & Young, 2011; Ross et al., 2009). Such responses to manipulation suggested the primacy of oxytocin, and presumably its signaling via Oxtr, in mediating the formation and behavioral expression of partner preference (Keebaugh & Young, 2011; Numan & Young, 2016; Fig. 1A). However, our understanding of the genetic and neural control of attachment behaviors is incomplete. For example, the full extent of the neural circuits affected by Oxt modulation is not fully understood, nor are the compensatory contributions of the AVP system, the genetic regulation of Oxtr expression across development, and the action of Oxt at the synapse, among others. A large gap in our understanding of the genetic and neural substrates of attachment behaviors stems from a previous absence of tools to manipulate the prairie vole genome constitutively throughout development and with spatial and temporal control.
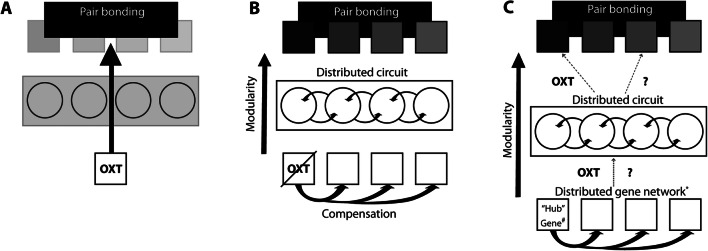
Fig. 1.
Models of oxytocin function within circuits encoding pair bond behavior. A Common model based on historical studies suggesting oxytocin acts as a primary genetic mediator of pair bonding behavior, whether through its action on local circuitry in select brain regions or on nodes integrated within a distributed circuitry. This is also in contrast to its potential modular action on specific subcomponents of pair bonding behavior, where other genes or gene networks may independently regulate distinct components of pair bonding (both shown in gray). B Model depicting three hypotheses: one in which the loss of Oxt/Oxtr is compensated for by other genes, another proposing a modular structure of genetic and neural circuit architecture, and finally, the potential for distributed but coordinated circuitry mediating distinct domains of pair bonding behavior (gray squares). C A third model depicting the hypotheses in B, but indicating a neural structure that is not specified by Oxt/Oxtr, but by either another regulatory “hub” gene (#) or a distributed gene network (*). In this model, Oxt signaling and/or other molecular mediators may act to control aspects of gene or circuit function (dashed lines) without being necessary for development of the underlying circuitry for pair bond behaviors. Parallel or compensatory processes may play a role in mediating specific behavioral outputs at any of the genetic or neural levels of regulation depicted
Molecular Genetics Applied to Attachment Behavior
Modern molecular genetic tools will help clarify our understanding of how neuroendocrine factors mediate bonding and attachment behaviors and related affective states as we implement them in new species. Interference with a gene’s function is commonly used to elucidate its role in a biological process (Baker et al., 2001; Konopka & Benzer, 1971; Nüsslein-Volhard & Wieschaus, 1980; Vitaterna et al., 1994). Recent developments in the tools for generating such alterations to gene expression have made it possible to implement these techniques in a wide variety of species with high efficiency and relatively low barriers to entry. Such tools generally rely on knockdown, or the reduction in expression of a given gene, or knockout, the complete loss of expression of a targeted gene. Given the central role of oxytocin signaling in affiliative and parental behaviors across taxa, we and other groups adapted clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-based molecular genetic tools to generate prairie voles that lack the oxytocin receptor (Oxtr; Horie et al., 2019; Berendzen et al., 2022). We examined partner preference following cohabitation for one week with an opposite sex partner using the established assay described above (Beery, 2021; Williams et al., 1992). We made the surprising finding that Oxtr is genetically dispensable for pair bond formation, as partner preference was maintained in Oxtr knockout animals (Berendzen et al., 2022). Horie et al., 2019 examined a different subset of behaviors commonly attributed to oxytocinergic regulation, including pup vocalization, anxiety-like behavior, alloparental behavior (parental behavior exhibited by individuals towards non-descendant young), and sociability. They found no difference in Oxtr knockout animals compared to wild type in most of these behaviors, although did find mild increases in repetitive behavior, indicating anxiety-like behavioral differences, and impairment in preference for social novelty (Horie et al., 2019). These surprising findings suggest a differential requirement for oxytocin signaling across distinct domains of affective, attachment, and other social behaviors in prairie voles.
Pharmacological and viral-based interventions have been used to examine oxytocin signaling primarily in adulthood and have demonstrated a role for oxytocin in controlling pair bond formation. One initial interpretation of the differential results between pharmacological knockdown and constitutive genetic knockout is that the drugs themselves are non-specific and act at other sites to influence behavior (Busnelli et al., 2013; Manning et al., 2012). While promiscuous action of pharmacological agents should be considered and examined in interpreting these seemingly disparate results, other developmental and neurobiological mechanisms may be at play. Incorporating frameworks from developmental biology will aid in interpreting gene-modifying studies and broadly apply when considering directed molecular genetic approaches in the context of ethological and pharmacological studies of behavior.
Below, we discuss potential hypotheses derived from common concepts in the evolutionary-developmental literature for understanding the differential effects of constitutive, whole-organism, loss of function alleles of specific genes, such as in knockout models, in comparison to later manipulations of expression or activity in adulthood, as seen with knockdown of expression or pharmacological studies. We first address the developmental timing of intervention and the potential for invoking compensatory or parallel mechanisms with constitutive loss of Oxtr signaling, as compared to selective inhibition postnatally or in adulthood. Next, we consider the evidence for modularity in the genetic and neural systems underlying attachment behaviors, the study of which is facilitated by targeted genetic approaches. Finally, we examine the impact of constitutive deletion of the receptor in all tissues and cell types vs. regional or restricted depletion of Oxtr signaling, and discuss the differential impacts on activity across distributed circuits that may underlie pair bonding behaviors. For each discussion, we provide evidence from various species and systems, followed by support for similar processes in pair bond behavior and their potential regulation by oxytocinergic signaling. These proposed mechanisms are not mutually exclusive and any or all of these processes may be integrated at various levels to produce the pair bonded state and influence associated behaviors (Fig. 1B, C).
Importantly, the conceptual approaches we discuss may also have implications for our understanding of affective processing and the underlying developmental and neural circuit impacts on human attachment behavior. Patterns of relationships transform over the life course, and attachment with parental figures during early development may powerfully impact the quality and style of attachments in adulthood (Ainsworth, 1979; O’Connor & Rutter, 2000; Rutter et al., 1999). This suggests strong developmental regulation of the neural mechanisms underlying attachment behaviors. The formation and persistence of social bonds require coordination of a multitude of affective and cognitive domains, including social motivation, learning and memory, reward and valence processing, and threat detection. These domains are integrated to guide the display of prosocial and agonistic behaviors in the appropriate ethological context (Gustison & Phelps, 2022; Insel & Young, 2001; Krach, 2010; Resendez & Aragona, 2013). Each of these domains and related behaviors has been attributed to specific regions and circuits in the brain. Local neural circuits are also integrated into functionally connected networks that are interrelated and mediate multiple affective domains that influence attachment behaviors (Gustison & Phelps, 2022; Seeley et al., 2007). For example, activity in the amygdala is thought to encode valence, the positive or negative affective response to a stimuli, as well as other affective domains (Lanska, 2018; Tye, 2018). However, this area is part of a distributed network of anatomically and functionally connected neural circuits implicated in the processing of social information, e.g., the salience network (Seeley et al., 2007). Activity within and across these networks may contribute to both the development of positive valence associated with an attachment figure and the effects of healthy attachments on adaptive buffering of emotional response to strongly positively or negatively valenced stimuli (DeWall et al., 2012; Gillath et al., 2005; Kubzansky et al., 2009; Perry et al., 2017; Vrtička et al., 2012). While numerous theoretical frameworks have been applied to the understanding of human attachment and the underlying neurobiology, the application of concepts from fields like developmental and evolutionary biology will aid in deepening and testing our understanding of such models and the molecular, neural, behavioral, and psychological processes involved.
Developmental Factors may Buffer Variation in Oxytocinergic Systems to Preserve Pair Bonding
Previous studies in prairie voles described above primarily employed experimental paradigms in which Oxtr signaling is manipulated in adulthood, immediately prior to specific social interactions or assays of behavior, but is present and active throughout development. Manipulations of Oxtr function in the wild-type adult brain may have distinct effects on attachment behaviors when compared to the constitutive, organismal absence of its function throughout development. This dichotomy is well articulated in developmental biology, whereby genetic contributions to behavior directly affect developmental processes, thus “organizing” or specifying the process. Alternatively, genetic manipulations may contribute to adult function of the gene product itself, having “activational” effects in adulthood (Baker et al., 2001; Hammock, 2014). Across species, compensatory mechanisms may thus arise in the context of genetic perturbations during development resulting in distinct phenotypes to those due to impairment of gene function later in life (Daude et al., 2012; De Souza et al., 2006; El-Brolosy & Stainier, 2017; Rossi et al., 2015; Smart & Riley, 2013; White et al., 2013).
Canalization is the tendency for development of a specific phenotype to follow the same trajectory under different conditions, such as different environments or different genetic backgrounds (Hoke et al., 2019; Waddington, 1959). The presence of mechanisms, whether compensatory for or parallel to Oxtr function, that support the preservation of pair bonding in voles provides evidence for the canalization of attachment behaviors in this species (Bergman & Siegal, 2003; Cadigan et al., 1994; El-Brolosy & Stainier, 2017; Rossi et al., 2015; Tautz, 1992; Teng et al., 2013). Canalization by mechanisms such as those detailed below may indicate strong evolutionary pressures to maintain certain behavioral phenotypes. The persistence of attachment behaviors in the absence of Oxtr function, despite their regulation by Oxtr signaling in the context of wild-type development, suggests a species-specific selection towards the transition to monogamous mating strategies (Waddington, 1959). This is consistent with observations that loss of function mutations in genes with pleiotropic, or diverse, functions nevertheless often result in largely normal anatomy and function (Waddington, 1942; Wagner et al., 1997). Oxt and Oxtr display an enormous degree of such pleiotropy, affecting not only aspects of social behavior and maternal physiology, but also having widespread effects on feeding behavior and peripheral autonomic physiology (Carter, 2014; Dölen, 2015; Jurek & Neumann, 2018; Lawson, 2017; McCormack et al., 2020, p. 20). A number of theoretical and experimental studies demonstrate that such functional pleiotropy may be buffered evolutionarily both by genetic redundancy, often accomplished through gene duplications, and/or changes to regulatory mechanisms controlling tissue and context-specific expression levels of the gene. Either of these processes may compensate for gene loss (Cadigan et al., 1994; Carroll et al., 2006; Kafri et al., 2009; Tautz, 1992). We first discuss evidence in other systems for differential compensatory responses to genetic manipulation and then apply these principles to the potential for developmental compensation in oxytocinergic systems and pair bonding.
Diverse species exhibit evidence for developmental compensation with genetic manipulation. In zebrafish, knockdown of genes associated with vascular function, for example egl-7 or vegfaa, results in severe vascular deficits, while constitutive loss (knockout) of these genes throughout development reveals developmental compensation, as no obvious phenotype is observed (Rossi et al., 2015). Further studies reveal upregulation of compensatory gene networks that buffer against deleterious mutations in egl-7, which is not observed after translational or transcriptional knockdown later in development (Rossi et al., 2015). Transcriptional and phenotypic comparisons of knockout vs. knockdown of genes involved in diverse physiological processes in mammals, including metabolism, neural function, and vascular development (Ppara, PrP-like Sprn, and thymosin beta-4 respectively), also reveal distinct compensatory responses. Mice-bearing null mutations for these genes lack the phenotypic effects seen in the context of knockdown of gene expression, in otherwise wild-type animals (Daude et al., 2012; De Souza et al., 2006; Smart & Riley, 2013).
The abundance and dynamic nature of oxytocin peptide and receptor expression during early development suggests a possible role in organizing the neural circuits for certain behaviors (Hammock, 2014; Newmaster et al., 2020). Expression of Oxtr mRNA, measured by quantitative PCR, is seen in rats and mice by embryonic day 12 (Chen et al., 2000; Tamborski et al., 2016). Oxt continues to be expressed in the developing brain, and mice show a steady increase in oxytocinergic cells in the hypothalamus and amygdala at postnatal days 1–8. In prairie voles, the number of Oxt expressing cells steadily increases from postnatal day 1 to 21 in males and females (Vaidyanathan & Hammock, 2017; Yamamoto et al., 2004). Oxytocin and its receptor show dynamic, species-specific changes in expression over the course of embryonic development and early postnatal life, impacting the development of cortical and subcortical circuitry (Grinevich et al., 2015; Newmaster et al., 2020; Yamamoto et al., 2004). Developmental manipulations as well as regulation by gonadal hormones of oxytocinergic signaling have sex-specific effects on cellular expression of the peptide and receptor and on behavior in adulthood (Champagne et al., 2001; Hammock, 2014). In mice, early postnatal injection of Oxt leads to an increase in Oxt-expressing cells specifically in adult females (Yamamoto et al., 2004). In prairie voles, neonatal treatment with Oxt increases cFos immunoreactivity, a marker of neuronal activity, in Oxt-producing cells in male pups, while decreasing oxytocinergic signaling increased cFos in the same regions in females (Cushing et al., 2003). Interestingly, male prairie voles given a single perinatal injection of oxytocin showed increased partner preference in adulthood, while those given an oxytocin antagonist perinatally show no change from wild-type animals in partner preference during adulthood (K. Bales et al., 2004; Bales & Carter, 2003). Such time, dose- and sex-dependent effects on social behavior after exogenous manipulation of oxytocin suggests a high degree of plasticity in response to perturbations in oxytocinergic signaling during development.
While it is unknown what specific molecular and neural mechanisms facilitate attachment in the absence of Oxtr, the vasopressin neuropeptide system is an obvious candidate as an alternative and potentially compensatory mechanism of regulation on pair bonding behavior (Bosch & Neumann, 2008; Cho et al., 1999; Paré et al., 2016; Song et al., 2014; Yamamoto et al., 2004; Young et al., 1998). Evidence for overlap in these systems comes partially from evolution of the peptides themselves, which are homologs and likely resulted from a duplication of the gene for AVP (Borie et al., 2021; Grinevich et al., 2015; Theofanopoulou et al., 2021). It is also well-established that vasopressin receptors in multiple mammalian species bind Oxt, having clear functional consequences (Chini & Fanelli, 2000; Kelly & Goodson, 2014; Kesteren & Geraerts, 1998). Oxt displays high affinity for V1aR, and V1aR has been strongly implicated in mediating oxytocin driven effects on physiology and social behaviors (Chini & Fanelli, 2000; Everett et al., 2018; Stoop, 2012). For example, V1aR antagonists inhibit the ability of exogenous Oxt to induce flank marking, a form of social communication, in Syrian hamsters (Song et al., 2014). Additionally, in studies of methamphetamine seeking behavior in rats, co-administration of a V1aR antagonist significantly reduced the effects of Oxt on methamphetamine seeking, suggesting V1aR signaling mediates Oxt-dependent effects on reward behavior (Everett et al., 2018). Consistent with this model, paternal care by prairie voles is reduced only when both Oxt and AVP signaling are blocked (Bales et al., 2004). Studies aimed at rigorously determining changes in AVP or V1aR signaling in animals lacking Oxtr will likely elucidate if such mechanisms are at play. As an alternative to the evolution of compensatory mechanisms, parallel oxytocin-independent pathways may exist that support attachment behaviors and allow for the preservation of pair bonding despite absence of Oxtr function throughout development. In this case, Oxtr is not necessary for the development and patterning of the neural substrates for partner preference formation, but may later act on these substrates in a context and experience dependent manner to control their behavioral and affective output (Fig. 1C).
Modularity of the Genetic and Neural Architecture Shapes Behaviors Supporting Pair Bonding
The selective nature of behavioral deficits with genetic loss of Oxtr suggests modularity in the genetic and neural encoding of pair bonding behaviors. In developmental terms, modularity can be defined as the division of a biological process into distinct units, each of which can develop or be regulated largely independent of other units (Hoke et al., 2019; Streelman et al., 2007; Xu et al., 2012). This concept has traditionally been applied to the action of genes during development to drive morphological phenotypes that are related but independently regulated by separable gene networks (Atchley & Hall, 1991; Hallgrímsson et al., 2002; Wagner, 2005). In this case, genetic modularity results in phenotypic modules. This concept can also be applied to understanding genetic regulation of behavioral phenotypes. As genes specify the development of local and interconnected neural circuits and thus influence the activity within these pathways, which in turn drives behavior, modularity is reflected at multiple neurobiological levels and in the behaviors that result. We present evidence for the modular structure of various social and non-social behaviors across species, and we discuss the potential for modular encoding of pair bond behaviors by oxytocinergic signaling (Greenwood et al., 2013; Levine et al., 2002; Weber et al., 2013; Weissbourd et al., 2021; Xu et al., 2012; Yang & Shah, 2014).
Some of the most striking evidence for modularity in behavior has come from studies of circadian behaviors as well as sexual behavior and associated sexually dimorphic regions within the brain (Anderson, 2016; Chan et al., 2002; Konopka & Benzer, 1971; Levine et al., 2002; Shah et al., 2004; Villella & Hall, 2008; Vitaterna et al., 1994; Xu et al., 2012). Studies in species ranging from fruit flies to mice have demonstrated that disruptions in individual genes that comprise the “molecular clock” result in specific changes in circadian patterns of behavior (Chan et al., 2002; Konopka & Benzer, 1971; Vitaterna et al., 1994). Similarly, studies examining sexually dimorphic gene expression in the hypothalamus and amygdala in mice identified sex-biased gene expression signatures in these regions. Targeted single gene disruptions within a subset of genes led to highly specific deficits in sex-typical behaviors while leaving other components intact, demonstrating separable genetic programs directing select behavioral subdomains (Xu et al., 2012). Such genetically separable modules have also been found to mediate social behaviors. Work in stickleback fish, Astyanax, examined schooling, a group behavior in which fish swim in a synchronized and polarized manner. Deconstruction of this dynamic social behavior identified separable genetic signatures that underly distinct components or modules of schooling behavior, such as tendency to school and body position (Greenwood et al., 2013). Studies of complex social behaviors like mating and aggression in fruit flies and mammals identified not only distinct genetically identified populations separably controlling these behaviors, but also distinct heterogenous neural populations in which both mating and aggression are influenced by functional changes in these neurons (Anderson, 2016; Asahina et al., 2014; Bayless et al., 2016; Certel et al., 2010; Koganezawa et al., 2016; Yang & Shah, 2014). These separable heterogenous populations may be functionally differentiated such that unique sets of genes regulate different neural modules and thus encode distinct behaviors reflecting this modular structure.
Studies in Oxtr knockout animals, including voles, mice, and rats, have demonstrated selective and species-specific deficits in behavioral and affective phenotypes suggesting modularity in the genetic and neural structures underlying attachment behaviors. Prairie voles bearing mutations in Oxtr demonstrated decreased affinity for social novelty in a three-chamber sociability test, without more general effects on sociality or anxiety-like behavior (Horie et al., 2019). Oxtr knockout mice and rats show decreased social recognition, without general deficits in sensory processing or generalized impairment of learning or memory, in addition to deficits in lactation and maternal nursing and reduced infant ultrasonic vocalizations (USVs; Numan, 1988; Pedersen et al., 1982; Takayanagi et al., 2005; Winslow & Insel, 2002). Oxtr knockout vole pups, in contrast, do not show decreased USVs when separated from parents (Horie et al., 2019). Disruption of species-specific subdomains of social and attachment behavior with loss of Oxtr suggests modularity in the pair bonding phenotypes regulated by Oxtr that may map onto specific neural circuit substrates. In Oxtr knockout mice, many brain regions, including the olfactory bulbs, lateral septum, piriform cortex, and dorsal lateral septum, show similar levels of activity (reflected by cFos induction) as wild-type animals after a brief social encounter, while less such activity is observed in the medial amygdala (MeA) and bed nucleus of the stria terminalis (BNST; Ferguson et al., 2001). The differential sensitivity across species in specific components of social behaviors, such as recognition, motivation or communication, to disruption of Oxtr may reflect not only separable genetic and neural modules, but differential selective pressure acting on these distinct modules in the evolution of social and attachment behaviors.
While further experiments are necessary to delineate the behavioral domains affected by loss of Oxtr and the genetic and neural substrates affected, examining social behaviors in the absence of Oxtr begins to reveal the modular structure and component behaviors that comprise attachment. The species-specific sensitivity of particular behaviors to loss of Oxtr signaling, and the potential selectivity of the brain regions involved provide further clues regarding the structural and functional building blocks of the systems supporting attachment behaviors. We can now combine molecular genetic techniques with, for example, transcriptomic and chromatin-profiling approaches and in vivo Ca2+ imaging to analyze gene expression and regulatory signatures, as well as patterns of neural activity across behavioral conditions and between species. These approaches will allow us to differentiate the genetically delineated components underlying pair bonding and their developmental origins (Gegenhuber et al., 2020). Understanding this modular structure may also have particular relevance for the integration of affective or internal states with sensory and environmental cues.
Distributed Structure of Neural Circuits Affects the Demonstration of Pair Bonding
The striking observation that pair bonding occurs in the absence of Oxtr function may suggest that, like other neuromodulators, Oxt and Oxtr modulate specific behaviors not through binary, all-or-nothing action on isolated brain regions, but rather via coordinated, selective control of activity across a network comprised of multiple circuits. Such distributed encoding is observed in numerous neural systems underlying behavior across species, including olfactory processing, associative learning and motivated behavior, motor function, as well as mating and aggression and social behaviors more broadly (Anderson, 2016; Bargmann, 2012; Cachope et al., 2012; Cohn et al., 2015; Newman, 1999; Woolley et al., 2014; Yang & Shah, 2014). Complex behaviors may therefore be mediated by activity across a distributed network of circuits that each subserve distinct aspects of behavior, cognition, and affective components. We present evidence that neuromodulators, including Oxt, coordinate circuit activity across the network for a particular ethological context, developmental stage, and based on experience. We then discuss how complex attachment behaviors like pair bonding may similarly involve the coordination of a repertoire of closely interrelated, neuroendocrine-regulated circuits that mediate component behaviors.
Circuit organization characterized by parallel processing and functional “switches” between parallel streams allows for a distributed but interconnected circuit structure. Such a structure permits variability in specific components that comprise larger behavioral routines in response to genetic or environmental factors, and has been suggested as a mechanism by which the nervous system achieves behavioral flexibility (Bargmann, 2012; Chan et al., 2002; Falkner et al., 2014; Hashikawa et al., 2016; Mets et al., 2021; Ragozzino, 2007). Across such distributed networks, neuromodulators and hormones coordinate circuit engagement, i.e., levels or patterns of activity within local circuits or the influence of their output (Bargmann, 2012; Cohn et al., 2015; Marder, 2012). This is exemplified in the case of dopaminergic control of the neural pathways underlying motor behavior. Differential motor outputs are generated by functionally distinct parallel processing streams in the dorsal striatum. These streams, known as the direct and indirect pathways, are associated with specific neuronal populations defined by expression of distinct dopamine receptor subtypes. (Gittis & Kreitzer, 2012; Kravitz et al., 2010, 2012; Wiltschko et al., 2020). Dopaminergic signaling is also implicated in the reinforcement of specific aspects of animal behavior by signaling through distinct populations of cells in the basal ganglia (Graybiel et al., 1994; Kreitzer & Malenka, 2008; Tritsch & Sabatini, 2012). Similarly, in Drosophila, dopaminergic signaling in the mushroom body, a structure in the insect brain important for olfactory learning and memory, influences the flow of sensory information to direct specific circuit states that result in the appropriate enactment of innate and learned behaviors in response to olfactory cues (Aso et al., 2014; Cohn et al., 2015).
Studies across species suggest that oxytocin also functions as a neuromodulator across circuits recruited under specific ethological contexts. The invertebrate homologue of oxytocin, nematocin, coordinates circuit states in the context of mating behavior in the invertebrate species C. elegans (Garrison et al., 2012). Males lacking nematocin engage in mating attempts but exhibit fragmented sequencing of copulatory behaviors. This suggests that neuromodulator input across multiple local circuits involved in mating modulates the function and processing within these populations such that the outputs of these distributed networks are coordinated into coherent reproductive behavior (Bargmann, 2012; Garrison et al., 2012). That such functional compartmentalization of a distributed circuit occurs even in the absence of a centralized nervous system may reveal an organizing principle of neural circuits and systems. In rodents, the recruitment of specific circuits is mediated, in part, by the organization of Oxt production and function. Oxt is produced by two distinct cell types in the hypothalamus. These cells are anatomically segregated and project to distinct neural populations or release Oxt peripherally. The release of Oxt from multiple neuronal sites, including axons, soma, and dendrites, and its action on neuronal and non-neuronal cells throughout the brain and periphery may account for the functional division of behaviors and physiological processes linked to oxytocin signaling (Chini et al., 2017; Dölen, 2015; Dölen et al., 2013). Studies of long-range projections of oxytocin-producing neurons in the hypothalamus using mouse lines labeling oxytocin-expressing cells reveals widespread projection to diverse targets throughout the brain. Oxytocin fibers were identified in 29 brain regions in which Oxtr is expressed, including the islands of Calleja, frontal association cortex, shell of the nucleus accumbens (NAc), lateral septal nucleus, BNST, and MeA (Mitre et al., 2016). Furthermore, the correlation between fiber density and receptor expression is highly dynamic, beginning low in virgin females but increasing significantly in lactating females in both mice and rats, suggesting conserved, context-specific changes to circuitry through which oxytocin signals (Grinevich et al., 2016; Mitre et al., 2016).
Oxytocinergic signaling may act during pair bonding in prairie voles to coordinate the function of parallel and distributed circuits mediating bonding. Changes to activity across neurons projecting from the medial prefrontal cortex to the NAc within the context of pair bonding causally accelerates female affiliation towards naïve, opposite sex voles (Amadei et al., 2017). While the role for oxytocin in mediating the dynamic response of social behavior to corticostriatal activity is unknown, the coordination of activity across localized circuits to produce specific behavioral responses appropriate to distinct social settings and experience supports a role for context-specific control of distributed circuitry in mediating attachment behavior.
The nervous system thus balances developmental stability and flexibility to environmental stimuli using redundant or distributed processes comprised of genetically defined functional modules expressed in specific contexts (Cohn et al., 2015; Fig. 1B, C). Local Oxtr signaling may modulate the behavioral expression of these modules only in the context of regional circuit activity that occurs during specific social behaviors (Dölen et al., 2013; Marlin et al., 2015). Alternatively, it might act to coordinate the activity across a subset of circuits and influence aspects such as the latency, likelihood, intensity, or duration of behaviors and associated affective states. In the case of systemic delivery of pharmacologic antagonists, differential or incomplete inhibition of signaling may disrupt coordinated activity to interfere with specific displays of behavior that manifest as global disruptions to pair bonding. Nevertheless, when development occurs in the absence of Oxtr, as with genetic knockout models, whether compensatory mechanisms exist or not, the innate coordinated activity of distributed circuits during salient social interactions, such as mating, may be sufficient to facilitate pair bonding. The transition in behavioral or affective states, manifested as attachment, may thus be encoded by an interaction between patterns of circuit activity and neuromodulation, not just by the modulators themselves.
Conclusions and Future Directions
Numerous reviews and studies over the last decade have expressed caution in attributing widespread, functional necessity to oxytocinergic signaling in social behavior (Fink et al., 2006; Goodson, 2013; Guastella & Hickie, 2016). The availability of molecular genetic tools, in combination with pharmacologic and viral-mediated modulation, now allow us to genetically interrogate the neural and genetic substrates of attachment and other social behaviors with a new level of precision. We therefore believe that these results present an opportunity for reviewing and reconciling ideas about the modularity of genetic control of behaviors and the distributed function of neural circuits, providing a more comprehensive understanding of the role of oxytocin in social behavior and as an intervention in neuropsychiatric disorders of attachment.
The genetic and developmental perspectives offered here have implications for the intensive interest in and efforts to use oxytocin as a therapeutic agent for a host of clinical disorders in humans. Based on observations in prairie voles and other mammals including humans, clinical trials have sought to use exogenous Oxt and AVP or small molecule ligands to their receptors to ameliorate the deficits in social attachment and cognition experienced by patients with diverse neuropsychiatric conditions, yielding mixed results (Di Simplicio & Harmer, 2016; Feifel et al., 2010; Green & Hollander, 2010; Guastella & Hickie, 2016; Heinrichs et al., 2009; Hendaus et al., 2019; Leppanen et al., 2017; Parker et al., 2019; Rubin et al., 2010; Sikich et al., 2021). The initial pharmacological studies in voles and other species which sought to identify mechanisms underlying attachment behavior elucidated the first molecular candidates and their neural substrates. The advent of genetic and neural tools to monitor activity across distributed circuits in vivo, longitudinally label neural populations in response to activity, and capture the full range of molecular signatures associated with distinct behavioral states and defined cellular populations now facilitates an unbiased and comprehensive survey of the genetic, molecular, and neural landscape mediating attachment behavior.
The approaches to understanding neural mechanisms in behavior outlined here may also influence our understanding of affective processes that support attachment behaviors across species. Modularity in affective-related constructs like valence processing, reward, fear or stress response, and motivation, as well as a distributed circuitry across brain regions for encoding these affective states, is reflected in behavioral domains as well as endophenotypes within clinical syndromes (Anderson & Adolphs, 2014; Braff, 2015; Campbell, 2015; Jeste & Geschwind, 2014). Using the framework outlined, which strives to incorporate manipulations to behavioral systems at various developmental timepoints with context-specific perturbations to defined components of the circuitry, we can begin to address whether affective substrates become associated with behavioral states like pair bonding only with experience or whether they are fundamental to the developmental specification of attachment circuitry. Having genetic and neural entry points into the systems subserving attachment behaviors, derived from unbiased and comprehensive profiling of the molecular and circuit mediators of attachment behavior, will be essential as non-human studies are translated to work in humans, with careful consideration of the conservation of the structure and function of the systems and behaviors under examination.
Acknowledgments
The authors would like to acknowledge all members of the Manoli lab, especially Gina Williams and Nerissa Hoglen, for helpful comments and feedback on the manuscript.
Additional Information
Funding Information
This work was funded by NIH R01MH123513, National Science Foundation grant 1556974, Burroughs Wellcome Fund 1015667, Whitehall Foundation Grant 2018-08-83, NIH R25MH060482, AP Giannini Foundation Fellowship, and a Larry Hillblom Foundation Fellowship #2020-A023-FEL.
Conflict of Interest
The authors declare no competing interests.
Data Availability
Not applicable.
Author Contributions
All authors contributed significantly to the article conceptualization, interpretation, writing and editing of the manuscript. All authors have approved the final version of the submitted manuscript.
Ethics Approval
All work was performed to ethical standards and prior ethics board approval was not necessary for this manuscript.
Informed Consent for Human Participants
Not applicable.
Open Practices Statement:
(1) No additional data and/or materials exist for this manuscript.
(2) No experiments were conducted or preregistered.
References
- Adolphs R. The social brain: Neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MS. Infant–mother attachment. American Psychologist. 1979;34(10):932–937. doi: 10.1037/0003-066X.34.10.932. [DOI] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Ryan SJ, Walum H, Rainnie DG, Young LJ, Liu RC. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature. 2017;546(7657):297–301. doi: 10.1038/nature22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nature Reviews. Neuroscience. 2016;17(11):692–704. doi: 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157(1):187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, Eyjólfsdóttir EA, Perona P, Anderson DJ. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell. 2014;156(1–2):221–235. doi: 10.1016/j.cell.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo T-TB, Dionne H, Abbott LF, Axel R, Tanimoto H, Rubin GM. The neuronal architecture of the mushroom body provides a logic for associative learning. ELife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biological Reviews of the Cambridge Philosophical Society. 1991;66(2):101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Baker BS, Taylor BJ, Hall JC. Are complex behaviors specified by dedicated regulatory genes? Reasoning from Drosophila. Cell. 2001;105(1):13–24. doi: 10.1016/S0092-8674(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Bales K, Abdelnabi M, Cushing B, Ottinger M, Carter C. Effects of neonatal oxytocin manipulations on male reproductive potential in prairie voles. Physiology & Behavior. 2004;81(3):519–526. doi: 10.1016/j.physbeh.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Bales KL, Arias Del Razo R, Conklin QA, Hartman S, Mayer HS, Rogers FD, Simmons TC, Smith LK, Williams A, Williams DR, Witczak LR, Wright EC. Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. The Yale Journal of Biology and Medicine. 2017;90(3):373–387. [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2003;117(4):854. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: How neuromodulators shape neural circuits. BioEssays. 2012;34(6):458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Shah NM, Bayless DW. Genetic dissection of neural circuits underlying sexually dimorphic social behaviours. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2016;371(1688):20150109. doi: 10.1098/rstb.2015.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK. Familiarity and mate preference assessment with the partner preference test. Current Protocols. 2021;1(6):e173. doi: 10.1002/cpz1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendzen, K. M., Sharma, R., Mandujano, M. A., Wei, Y., Rogers, F. D., Simmons, T. C., et al. (2022). Oxytocin receptor is not required for social attachment in prairie voles. bioRxiv. 2022.07.22.501192. 10.1101/2022.07.22.501192 [DOI] [PMC free article] [PubMed]
- Bergman A, Siegal ML. Evolutionary capacitance as a general feature of complex gene networks. Nature. 2003;424(6948):549–552. doi: 10.1038/nature01765. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25(9):1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Borie, A. M., Theofanopoulou, C., & Andari, E. (2021). The promiscuity of the oxytocin–vasopressin systems and their involvement in autism spectrum disorder. In Handbook of Clinical Neurology (Vol. 182, pp. 121–140). Elsevier. 10.1016/B978-0-12-819973-2.00009-5 [DOI] [PMC free article] [PubMed]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proceedings of the National Academy of Sciences. 2008;105(44):17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby, J., & Bowlby, J. (1982). Attachment (2. ed). Basic Books.
- Braff DL. The importance of endophenotypes in schizophrenia research. Schizophrenia Research. 2015;163(1–3):1–8. doi: 10.1016/j.schres.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. Journal of Pharmacology and Experimental Therapeutics. 2013;346(2):318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Reports. 2012;2(1):33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Grossniklaus U, Gehring WJ. Functional redundancy: The respective roles of the two sloppy paired genes in Drosophila segmentation. Proceedings of the National Academy of Sciences. 1994;91(14):6324–6328. doi: 10.1073/pnas.91.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB. Genetic investigation of autism-related social communication deficits. The American Journal of Psychiatry. 2015;172(3):212–213. doi: 10.1176/appi.ajp.2014.14121503. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nature Genetics. 2006;38(11):1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annual Review of Psychology. 2014;65(1):17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Carter CS. The oxytocin-vasopressin pathway in the context of love and fear. Frontiers in Endocrinology. 2017;8:356. doi: 10.3389/fendo.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-H. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Scientific American. 1993;268(6):100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Progress in Brain Research. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perkeybile AM. The monogamy paradox: What do love and sex have to do with it? Frontiers in Ecology and Evolution. 2018;6:202. doi: 10.3389/fevo.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel SJ, Leung A, Lin C-Y, Perez P, Chiang A-S, Kravitz EA. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE. 2010;5(10):e13248. doi: 10.1371/journal.pone.0013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Villella A, Funes P, Hall JC. Courtship and other behaviors affected by a heat-sensitive, molecularly novel mutation in the cacophony calcium-channel gene of Drosophila. Genetics. 2002;162(1):135–153. doi: 10.1093/genetics/162.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Schreiber SS, Brinton RD. Vasopressin and oxytocin receptor mRNA expression during rat telencephalon development. Neuropeptides. 2000;34(3–4):173–180. doi: 10.1054/npep.2000.0809. [DOI] [PubMed] [Google Scholar]
- Chini B, Fanelli F. Molecular basis of ligand binding and receptor activation in the oxytocin and vasopressin receptor family. Experimental Physiology. 2000;85(s1):59s–66s. doi: 10.1111/j.1469-445X.2000.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Chini B, Verhage M, Grinevich V. The action radius of oxytocin release in the mammalian CNS: From single vesicles to behavior. Trends in Pharmacological Sciences. 2017;38(11):982–991. doi: 10.1016/j.tips.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113(5):1071–1079. doi: 10.1037/0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cohn R, Morantte I, Ruta V. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015;163(7):1742–1755. doi: 10.1016/j.cell.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Yamamoto Y, Hoffman GE, Carter CS. Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Developmental Brain Research. 2003;143(2):129–136. doi: 10.1016/S0165-3806(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Daude N, Wohlgemuth S, Brown R, Pitstick R, Gapeshina H, Yang J, Carlson GA, Westaway D. Knockout of the prion protein (PrP)-like Sprn gene does not produce embryonic lethality in combination with PrPC-deficiency. Proceedings of the National Academy of Sciences. 2012;109(23):9035–9040. doi: 10.1073/pnas.1202130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza AT, Dai X, Spencer AG, Reppen T, Menzie A, Roesch PL, He Y, Caguyong MJ, Bloomer S, Herweijer H, Wolff JA, Hagstrom JE, Lewis DL, Linsley PS, Ulrich RG. Transcriptional and phenotypic comparisons of Ppara knockout and siRNA knockdown mice. Nucleic Acids Research. 2006;34(16):4486–4494. doi: 10.1093/nar/gkl609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences. 1996;93(21):11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster) Canadian Journal of Zoology. 1997;75(2):295–301. doi: 10.1139/z97-037. [DOI] [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7(2):184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Harmer CJ. Oxytocin and emotion processing. Journal of Psychopharmacology (Oxford, England) 2016;30(11):1156–1159. doi: 10.1177/0269881116641872. [DOI] [PubMed] [Google Scholar]
- Dölen G. Oxytocin: Parallel processing in the social brain? Journal of Neuroendocrinology. 2015;27(6):516–535. doi: 10.1111/jne.12284. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science (New York, N.Y.) 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- El-Brolosy MA, Stainier DYR. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genetics. 2017;13(7):e1006780. doi: 10.1371/journal.pgen.1006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett NA, McGregor IS, Baracz SJ, Cornish JL. The role of the vasopressin V1A receptor in oxytocin modulation of methamphetamine primed reinstatement. Neuropharmacology. 2018;133:1–11. doi: 10.1016/j.neuropharm.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Falkner AL, Dollar P, Perona P, Anderson DJ, Lin D. Decoding ventromedial hypothalamic neural activity during male mouse aggression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(17):5971–5984. doi: 10.1523/JNEUROSCI.5109-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry. 2010;68(7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Feldman R. The neurobiology of human attachments. Trends in Cognitive Sciences. 2017;21(2):80–99. doi: 10.1016/j.tics.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of Neuroscience. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S, Excoffier L, Heckel G. Mammalian monogamy is not controlled by a single gene. Proceedings of the National Academy of Sciences. 2006;103(29):10956–10960. doi: 10.1073/pnas.0602380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338(6106):540–543. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenhuber, B., Wu, M. V., Bronstein, R., & Tollkuhn, J. (2020). Regulation of neural gene expression by estrogen receptor alpha [Preprint]. Neuroscience.10.1101/2020.10.21.349290
- Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology. 1981;8(3):189–194. doi: 10.1007/BF00299829. [DOI] [Google Scholar]
- Gillath O, Bunge SA, Shaver PR, Wendelken C, Mikulincer M. Attachment-style differences in the ability to suppress negative thoughts: Exploring the neural correlates. NeuroImage. 2005;28(4):835–847. doi: 10.1016/j.neuroimage.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Kreitzer AC. Striatal microcircuitry and movement disorders. Trends in Neurosciences. 2012;35(9):557–564. doi: 10.1016/j.tins.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology. 2013;38(4):465–478. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science (New York, N.Y.) 1994;265(5180):1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Green JJ, Hollander E. Autism and oxytocin: New developments in translational approaches to therapeutics. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2010;7(3):250–257. doi: 10.1016/j.nurt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Yoshida K, Peichel CL. Genetic and neural modularity underlie the evolution of schooling behavior in threespine sticklebacks. Current Biology. 2013;23(19):1884–1888. doi: 10.1016/j.cub.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Desarménien MG, Chini B, Tauber M, Muscatelli F. Ontogenesis of oxytocin pathways in the mammalian brain: Late maturation and psychosocial disorders. Frontiers in Neuroanatomy. 2015;8:164. doi: 10.3389/fnana.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biological Psychiatry. 2016;79(3):155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, Larocca MA, Bates SL, Porges SW. 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosomatic Medicine. 2011;73(1):59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69(2):149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB. Oxytocin treatment, circuitry, and autism: A critical review of the literature placing oxytocin into the autism context. Biological Psychiatry. 2016;79(3):234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Gustison ML, Phelps SM. Individual differences in social attachment: A multi-disciplinary perspective. Genes, Brain and Behavior. 2022;21(3):e12792. doi: 10.1111/gbb.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. American Journal of Physical Anthropology, Suppl. 2002;35:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology. 2014;40(10):24–42. doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The effect of rearing conditions on behavior. International Journal of Psychiatry. 1965;1:43–51. [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Falkner A, Lin D. The neural circuits of mating and fighting in male mice. Current Opinion in Neurobiology. 2016;38:27–37. doi: 10.1016/j.conb.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hendaus MA, Jomha FA, Alhammadi AH. Vasopressin in the amelioration of social functioning in autism spectrum disorder. Journal of Clinical Medicine. 2019;8(7):1061. doi: 10.3390/jcm8071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Adkins-Regan E, Bass AH, McCune AR, Wolfner MF. Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity. Journal of Experimental Biology. 2019;222(8):jeb190058. doi: 10.1242/jeb.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue K, Suzuki S, Adachi S, Yada S, Hirayama T, Hidema S, Young LJ, Nishimori K. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Hormones and Behavior. 2019;111:60–69. doi: 10.1016/j.yhbeh.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behavioral Neuroscience. 1995;109(4):782–789. doi: 10.1037/0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Advances in Experimental Medicine and Biology. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews. Neuroscience. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nature Reviews. Neurology. 2014;10(2):74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Neumann ID. The oxytocin receptor: From intracellular signaling to behavior. Physiological Reviews. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- Kafri R, Springer M, Pilpel Y. Genetic redundancy: New tricks for old genes. Cell. 2009;136(3):389–392. doi: 10.1016/j.cell.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Social Neuroscience. 2015;10(5):561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Hormones and Behavior. 2011;60(5):498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Social functions of individual vasopressin–oxytocin cell groups in vertebrates: What do we really know? Frontiers in Neuroendocrinology. 2014;35(4):512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kesteren RE, Geraerts WPM. Molecular evolution of ligand-binding specificity in the vasopressin/oxytocin receptor familya. Annals of the New York Academy of Sciences. 1998;839(1 TRENDS IN COM):25–34. doi: 10.1111/j.1749-6632.1998.tb10728.x. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. The Quarterly Review of Biology. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Koganezawa M, Kimura K, Yamamoto D. The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Current Biology. 2016;26(11):1395–1403. doi: 10.1016/j.cub.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences. 1971;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krach, S. (2010). The rewarding nature of social interactions. Frontiers in Behavioral Neuroscience. 10.3389/fnbeh.2010.00022 [DOI] [PMC free article] [PubMed]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton A, Block J, Adler GK. Protocol for an experimental investigation of the roles of oxytocin and social support in neuroendocrine, cardiovascular, and subjective responses to stress across age and gender. BMC Public Health. 2009;9(1):481. doi: 10.1186/1471-2458-9-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanska DJ. The Klüver-Bucy syndrome. In: Bogousslavsky J, editor. Frontiers of Neurology and Neuroscience. S. Karger AG; 2018. pp. 77–89. [DOI] [PubMed] [Google Scholar]
- Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nature Reviews Endocrinology. 2017;13(12):700–709. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Caldwell HK, Macbeth AH, Young WS. Behavioural studies using temporal and spatial inactivation of the oxytocin receptor. Progress in Brain Research. 2008;170:73–77. doi: 10.1016/S0079-6123(08)00407-X. [DOI] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, Treasure J. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neuroscience & Biobehavioral Reviews. 2017;78:125–144. doi: 10.1016/j.neubiorev.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BMC Neuroscience. 2002;3:1. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2001;115(4):910–919. doi: 10.1037/0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: Back to the future. Neuron. 2012;76(1):1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520(7548):499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, II, Glasper ER, Nelson RJ, DeVries AC. Prolonged separation delays wound healing in monogamous California mice, Peromyscus californicus, but not in polygynous white-footed mice, P. leucopus. Physiology & Behavior. 2006;87(5):837–841. doi: 10.1016/j.physbeh.2006.01.035. [DOI] [PubMed] [Google Scholar]
- McCormack SE, Blevins JE, Lawson EA. Metabolic effects of oxytocin. Endocrine Reviews. 2020;41(2):121–145. doi: 10.1210/endrev/bnz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets, D. G., Mehaffey, W. H., Colquitt, B. M., & Brainard, M. S. (2021). Heritable differences in synaptic zinc-transporter levels drive variation in learned birdsong [Preprint]. Neuroscience.10.1101/2021.05.01.442260
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC. A distributed network for social cognition enriched for oxytocin receptors. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2016;36(8):2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Annals of the New York Academy of Sciences. 1999;877(1):242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Newmaster KT, Nolan ZT, Chon U, Vanselow DJ, Weit AR, Tabbaa M, Hidema S, Nishimori K, Hammock EAD, Kim Y. Quantitative cellular-resolution map of the oxytocin receptor in postnatally developing mouse brains. Nature Communications. 2020;11(1):1885. doi: 10.1038/s41467-020-15659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13(1–2):47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother–infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Rutter M. Attachment disorder behavior following early severe deprivation: Extension and longitudinal follow-up. English and Romanian Adoptees Study Team. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(6):703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Opie C, Atkinson QD, Dunbar RIM, Shultz S. Male infanticide leads to social monogamy in primates. Proceedings of the National Academy of Sciences. 2013;110(33):13328–13332. doi: 10.1073/pnas.1307903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré P, Paixão-Côrtes VR, Tovo-Rodrigues L, Vargas-Pinilla P, Viscardi LH, Salzano FM, Henkes LE, Bortolini MC. Oxytocin and arginine vasopressin receptor evolution: Implications for adaptive novelties in placental mammals. Genetics and Molecular Biology. 2016;39(4):646–657. doi: 10.1590/1678-4685-gmb-2015-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Fung LK, Garner JP, Hardan AY. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Science Translational Medicine. 2019;11(491):eaau7356. doi: 10.1126/scitranslmed.aau7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science (New York, N.Y.) 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Perry DC, Datta S, Sturm VE, Wood KA, Zakrzewski J, Seeley WW, Miller BL, Kramer JH, Rosen HJ. Reward deficits in behavioural variant frontotemporal dementia include insensitivity to negative stimuli. Brain. 2017;140(12):3346–3356. doi: 10.1093/brain/awx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RM, Lerner U, Pontelli H. The effect of progesterone on oxytocin-induced contraction of the three separate layers of human gestational myometrium in the uterine body and lower segment. American Journal of Obstetrics and Gynecology. 1967;98(4):547–554. doi: 10.1016/0002-9378(67)90109-3. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121(1):355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Reichard UH, Boesch C, editors. Monogamy: Mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press; 2003. [Google Scholar]
- Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Reviews in the Neurosciences. 2013;24(1):51–60. doi: 10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson LN, Porter-Stransky KA, Nevárez N, McLean JW, Kuhnmuench MA, Murphy AZ, Mathews TA, Aragona BJ. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. ELife. 2016;5:e15325. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SRM, Lubin S, Waltma R, Delson B, Tisdall L. Status of membranes and uterine contraction characteristics as criteria for clinical success of failure in the use of pitocin by continuous intravenous drip for induction of labor. American Journal of Obstetrics and Gynecology. 1950;59(5):1062–1068. doi: 10.1016/s0002-9378(16)39170-0. [DOI] [PubMed] [Google Scholar]
- Rich ME, deCárdenas EJ, Lee H-J, Caldwell HK. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. Plos One. 2014;9(6):e98839. doi: 10.1371/journal.pone.0098839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychological Bulletin. 2014;140(1):140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(5):1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, Stainier DYR. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015;524(7564):230–233. doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia Research. 2010;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Andersen-Wood L, Beckett C, Bredenkamp D, Castle J, Groothues C, Kreppner J, Keaveney L, Lord C, O’Connor TG. Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40(4):537–549. doi: 10.1111/1469-7610.00472. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Annals of the New York Academy of Sciences. 1992;652:448–451. doi: 10.1111/j.1749-6632.1992.tb34380.x. [DOI] [PubMed] [Google Scholar]
- Sikich L, Kolevzon A, King BH, McDougle CJ, Sanders KB, Kim S-J, Spanos M, Chandrasekhar T, Trelles MDP, Rockhill CM, Palumbo ML, Witters Cundiff A, Montgomery A, Siper P, Minjarez M, Nowinski LA, Marler S, Shuffrey LC, Alderman C, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. New England Journal of Medicine. 2021;385(16):1462–1473. doi: 10.1056/NEJMoa2103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Riley PR. Thymosin β4 in vascular development response to research commentary. Circulation Research. 2013;112(3):e29–e30. doi: 10.1161/CIRCRESAHA.112.300555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: Oxytocin, dopamine and attachment. Journal of Neuroendocrinology. 2011;23(11):1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streelman JT, Peichel CL, Parichy DM. Developmental genetics of adaptation in fishes: The case for novelty. Annual Review of Ecology, Evolution, and Systematics. 2007;38(1):655–681. doi: 10.1146/annurev.ecolsys.38.091206.095537. [DOI] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborski, S., Mintz, E. M., & Caldwell, H. K. (2016). Sex differences in the embryonic development of the central oxytocin system in mice. Journal of Neuroendocrinology, 28(4). 10.1111/jne.12364 [DOI] [PubMed]
- Tautz D. Problems and paradigms: Redundancies, development and the flow of information. BioEssays. 1992;14(4):263–266. doi: 10.1002/bies.950140410. [DOI] [PubMed] [Google Scholar]
- Teng X, Dayhoff-Brannigan M, Cheng W-C, Gilbert CE, Sing CN, Diny NL, Wheelan SJ, Dunham MJ, Boeke JD, Pineda FJ, Hardwick JM. Genome-wide consequences of deleting any single gene. Molecular Cell. 2013;52(4):485–494. doi: 10.1016/j.molcel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofanopoulou C, Gedman G, Cahill JA, Boeckx C, Jarvis ED. Universal nomenclature for oxytocin–vasotocin ligand and receptor families. Nature. 2021;592(7856):747–755. doi: 10.1038/s41586-020-03040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LM, Young AR, Römpler H, Schöneberg T, Phelps SM, Hoekstra HE. Monogamy evolves through multiple mechanisms: Evidence from V1aR in deer mice. Molecular Biology and Evolution. 2010;27(6):1269–1278. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- Tye KM. Neural circuit motifs in valence processing. Neuron. 2018;100(2):436–452. doi: 10.1016/j.neuron.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Hammock EAD. Oxytocin receptor dynamics in the brain across development and species: OXT receptor dynamics in the brain. Developmental Neurobiology. 2017;77(2):143–157. doi: 10.1002/dneu.22403. [DOI] [PubMed] [Google Scholar]
- Villella, A., & Hall, J. C. (2008). Chapter 3 Neurogenetics of courtship and mating in Drosophila. In Advances in Genetics (Vol. 62, pp. 67–184). Elsevier. 10.1016/S0065-2660(08)00603-2 [DOI] [PubMed]
- Vitaterna MH, King DP, Chang A-M, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P, Bondolfi G, Sander D, Vuilleumier P. The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience. 2012;7(5):473–493. doi: 10.1080/17470919.2011.647410. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150(3811):563–565. doi: 10.1038/150563a0. [DOI] [Google Scholar]
- Waddington CH. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183(4676):1654–1655. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wagner A. Circuit topology and the evolution of robustness in two-gene circadian oscillators. Proceedings of the National Academy of Sciences. 2005;102(33):11775–11780. doi: 10.1073/pnas.0501094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP, Booth G, Bagheri-Chaichian H. A population genetic theory of canalization. Evolution. 1997;51(2):329–347. doi: 10.1111/j.1558-5646.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Dyball RE, Lincoln DW. Milk ejection in the rat: The result of a selective release of oxytocin. The Journal of Endocrinology. 1973;57(3):557–558. doi: 10.1677/joe.0.0570557. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ. Ontogeny of oxytocin and vasopressin receptor binding in the lateral septum in prairie and montane voles. Brain Research. Developmental Brain Research. 1997;104(1–2):191–195. doi: 10.1016/S0165-3806(97)00138-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ, De Vries GJ, Insel TR. Voles and vasopressin: A review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Progress in Brain Research. 1998;119:483–499. doi: 10.1016/S0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- Weber JN, Peterson BK, Hoekstra HE. Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature. 2013;493(7432):402–405. doi: 10.1038/nature11816. [DOI] [PubMed] [Google Scholar]
- Weissbourd, B., Momose, T., Nair, A., Kennedy, A., Hunt, B., & Anderson, D. J. (2021). Functional modules within a distributed neural network control feeding in a model medusa [Preprint]. Neuroscience.10.1101/2021.02.22.432372
- White JK, Gerdin A-K, Karp NA, Ryder E, Buljan M, Bussell JN, Salisbury J, Clare S, Ingham NJ, Podrini C, Houghton R, Estabel J, Bottomley JR, Melvin DG, Sunter D, Adams NC, Tannahill D, Logan DW, MacArthur DG, et al. Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell. 2013;154(2):452–464. doi: 10.1016/j.cell.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Hormones and Behavior. 1992;26(3):339–349. doi: 10.1016/0018-506X(92)90004-F. [DOI] [PubMed] [Google Scholar]
- Wiltschko AB, Tsukahara T, Zeine A, Anyoha R, Gillis WF, Markowitz JE, Peterson RE, Katon J, Johnson MJ, Datta SR. Revealing the structure of pharmacobehavioral space through motion sequencing. Nature Neuroscience. 2020;23(11):1433–1443. doi: 10.1038/s41593-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow, J. T., & Insel, T. R. (2002). The social deficits of the oxytocin knockout mouse. Neuropeptides, 36(2–3), 221–229. 10.1054/npep.2002.0909 [DOI] [PubMed]
- Winslow, J. T., Hastings, N., Carter, C. S., Harbaugh, C. R., & Insel, T. R. (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature, 365(6446), 545–548. 10.1038/365545a0 [DOI] [PubMed]
- Winslow, J. T., Shapiro, L., Carter, C. S., & Insel, T. R. (1993). Oxytocin and complex social behavior: Species comparisons. Psychopharmacology Bulletin, 29(3), 409–414. [PubMed]
- Woolley SC, Rajan R, Joshua M, Doupe AJ. Emergence of context-dependent variability across a basal ganglia network. Neuron. 2014;82(1):208–223. doi: 10.1016/j.neuron.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148(3):596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125(4):947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Yang CF, Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82(2):261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Shepard E, Amico J, Hennighausen L, Wagner KU, LaMarca ME, McKinney C, Ginns EI. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. Journal of Neuroendocrinology. 1996;8(11):847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends in Neurosciences. 1998;21(2):71–75. doi: 10.1016/S0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.