Abstract
Although animals mobilize their innate defenses against gram-negative bacteria when they sense the lipid A moiety of bacterial lipopolysaccharide (LPS), excessive responses to this conserved bacterial molecule can be harmful. Of the known ways for decreasing the stimulatory potency of LPS in blood, the binding and neutralization of LPS by plasma lipoproteins is most prominent. The mechanisms by which host lipoproteins take up the native LPS that is found in bacterial membranes are poorly understood, however, since almost all studies of host-LPS interactions have used purified LPS aggregates. Using native Salmonella enterica serovar Typhimurium outer membrane fragments (blebs) that contained 3H-labeled lipopolysaccharide (LPS) and 35S-labeled protein, we found that two human plasma proteins, LPS-binding protein (LBP) and phospholipid transfer protein (PLTP), can extract [3H]LPS from bacterial membranes and transfer it to human high-density lipoproteins (HDL). Soluble CD14 (sCD14) did not release LPS from blebs yet could facilitate LBP-mediated LPS transfer to HDL. LBP, but not PLTP, also promoted the activation of human monocytes by bleb-derived LPS. Whereas depleting or neutralizing LBP significantly reduced LPS transfer from blebs to lipoproteins in normal human serum, neutralizing serum PLTP had no demonstrable effect. Of the known lipid transfer proteins, LBP is thus most able to transfer LPS from bacterial membranes to the lipoproteins in normal human serum.
Lipopolysaccharide (LPS) recognition by higher animals involves soluble proteins (LPS-binding protein [LBP] and soluble CD14 [sCD14]) (15, 18, 43), membrane receptors (CD14 and Toll-like receptor 4 [TLR4]) (40, 50), and an intracellular signal amplification machinery that rapidly generates and secretes a broad array of response mediators. LBP promotes rapid binding of purified LPS from aggregates to membrane-bound CD14 (mCD14) on cells or to sCD14 in plasma. CD14 is important for confering sensitive cellular responses to LPS (21), and TLR4 appears to be the most important LPS signal transducer (22, 40). Although many details remain to be discovered, much is now known about each of the steps in the LPS-based antibacterial inflammatory response.
Considerably less is understood about host mechanisms for preventing excessive, dangerous acute responses to LPS. Cells can quickly become resistant (tolerant) to LPS stimulation, a desensitization that is at least partly related to overexpression of the p50 subunit of NF-κB (4, 54). Acyloxyacyl hydrolase, an enzyme found in monocytes and neutrophils, can inactivate LPS by removing some of its fatty acyl chains, and intracellular dephosphorylation may also reduce LPS activity (27). In plasma, soluble molecules such as bactericidal permeability-increasing protein, lysozyme, and lactoferrin can bind LPS and neutralize its stimulatory potency (9–11). When gram-negative bacteria or LPS-containing bacterial membrane fragments enter the bloodstream, however, arguably the most important known mechanism for preventing excessive cellular responses is the transfer of LPS to circulating plasma lipoproteins.
When purified LPS (pLPS) aggregates are injected intravenously into animals, approximately half of the LPS is cleared from the plasma within a few minutes, presumably by binding to circulating or marginated leukocytes (14, 16, 31). Most of the remaining LPS binds rapidly to circulating lipoproteins (13) and then (31, 35) can circulate in the plasma for many hours before the lipoproteins are cleared by the liver and other organs (34). Whereas LPS that binds to leukocytes can initiate inflammatory responses, LPS that binds to lipoproteins is essentially inactivated (5, 31, 35, 39, 48). Binding to lipoproteins thus seems to be an important mechanism for LPS detoxification in vivo, and administering lipoproteins to animals can protect them from LPS challenge (12, 38, 41). Although LPS can bind to all of the major plasma lipoproteins (39), most investigative attention has focused on the interactions of LPS with high-density lipoproteins (HDL).
Recent studies have clarified many other aspects of LPS movement in plasma. LBP can transfer pLPS from aggregates to HDL (52). sCD14 accelerates LBP-mediated transfer of purified LPS to HDL when tested using isolated reagents in vitro (51), yet it contributes very little to the movement of purified LPS to HDL in whole plasma (53). Phospholipid transfer protein (PLTP), which shares protein sequence similarity with LBP, can also promote rapid transfer of LPS to HDL in normal plasma (17). In contrast to LBP, PLTP does not promote binding of pLPS to CD14; indeed, in studies using pLPS, PLTP was reported to inhibit the ability of LPS to stimulate CD14-expressing cells (17).
Interpretation of all of these experiments has been limited by the fact that LPS is naturally a constituent of bacterial outer membranes, not an isolated and purified chemical. Studies using pLPS aggregates thus have an uncertain relationship to in vivo phenomena. Although much of the LPS in bacterial membrane fragments (blebs) is known to transfer to HDL when the fragments are injected intravenously into rats (37), nothing is known about the biochemical mechanisms that mediate this transfer. The studies described here were therefore performed to evaluate the ability of the major LPS transfer proteins to transfer LPS from Salmonella enterica serovar Typhimurium blebs to sCD14, lipoproteins, and monocytes.
MATERIALS AND METHODS
Bacteria.
G-30, a galactose epimerase-deficient (galE) strain of Salmonella serovar Typhimurium, was grown in proteose peptone beef extract broth (Difco Laboratories, Detroit, Mich.) that had been dialyzed against distilled water in order to remove methionine and cysteine and then supplemented with M9 salts (42) containing 1 mM MgSO4, 0.11 mM d-galactose, and 0.16% (vol/vol) glycerol or glucose. The broth (100 ml) was then supplemented with 600 μCi of d-[6-3H-galactose] (34.6 Ci/mmol; DuPont NEN Life Sciences, Boston, Mass.), 1.5 mCi of [35S]methionine-cysteine (Tran35S-label; 1,175 Ci/mmol; ICN Pharmaceuticals, Irvine, Calif.), and 1 ml of bacteria that had been incubated overnight in M9 minimal medium. In galE strains, exogenous galactose is incorporated exclusively into LPS; under the growth conditions described here, 35S was found only in bacterial proteins. When the culture reached an optical density at 540 nm of 0.3 to 0.4, 3.75 mM d-galactose was added to the medium and growth was continued to a density of 0.6 to 0.8. The bacteria were pelleted (4,000 × g, 20 min, 4°C), and the supernatant was filtered (0.45-μm-pore-size filter) and saved at 4°C. The pelleted bacteria were washed with 10 mM HEPES buffer (pH 7.4), resuspended in 2 ml of 100 mM HEPES, and stored at −85°C until used to prepare pLPS.
Preparation of bacterial membrane fragments (blebs).
The blebs were precipitated by adding 2.5 volumes of saturated ammonium sulfate to the filtered culture supernatant and allowing the mixture to stand at 4°C overnight (37). The fine precipitate was pelleted by centrifugation (4,000 × g, 2 h, 4°C) and dialyzed against phosphate-buffered saline (PBS) that contained 0.02% NaN3. The blebs were separated from labeled soluble proteins by centrifugation through a 12% sucrose solution onto a 60% sucrose cushion (150,000 × g, 4.5 h, 4°C). The 60% sucrose cushion and blebs were then dialyzed against PBS that contained 0.02% NaN3. The final concentration of bleb LPS was estimated by its [3H]LPS content as described below.
Electron microscopy of blebs.
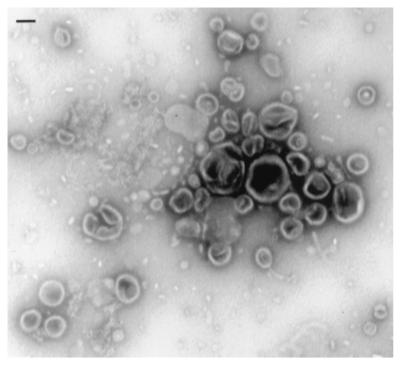
The blebs in PBS with 0.02% NaN3 were concentrated by ultracentrifugation (150,000 × g, 4.5 h, 4°C); 100-mesh nickel grids (Electron Microscopy Sciences, Fort Washington, Pa.) coated with Formvar and carbon by standard methods were floated over droplets of the concentrated bleb suspension for 5 min. The grids were then washed with distilled water and negatively stained by floating the grids over 1% aqueous uranyl acetate grids (Electron Microscopy Sciences) for 1 to 2 min. Excess uranyl acetate was removed by wicking onto filter paper, and the grids were dried in a vacuum desiccator. The specimens were viewed by electron microscopy in a Jeol JEM 100SX microscope, photographed at a magnification of ×30,000 on the negative film plate, and printed on photographic paper. Negative control grids, floated over distilled water and negatively stained, did not reveal any artifact structures.
Preparation of pLPS.
[3H]LPS was extracted from the whole bacteria by the hot phenol-water method (49). The pLPS was suspended in 10 mM Tris HCl with 0.01% triethylamine and quantitated by comparing the intensity of silver staining (46) on a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel to that of known amounts of wild-type Salmonella serovar Typhimurium pLPS. The specific activity (3H disintegrations per minute per microgram) of the pLPS was then calculated, and the amount of LPS in the blebs was estimated based on 3H content. The specific activities of the preparations ranged from 51,000 to 171,000 dpm/μg of LPS. Estimations of LPS molar concentrations assumed an average molecular mass of 5,500 Da.
Preparation and quantification of LBP, PLTP, and sCD14.
CHO cells stably transfected with recombinant human LBP or empty vector (pRc/RSV) were provided by P. Tobias (Scripps Research Institute, La Jolla, Calif.). The cells (CHO-rLBP or CHO-RSV) were incubated in roller bottles containing 200 ml of serum-free medium (CHO-S-SFM-II; Life Technologies, Grand Island, N.Y.) with 1% (vol/vol) SP-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) for 48 h. The beads were sedimented by gravity, washed in PBS containing 0.5 mM phenylmethylsulfonyl fluoride and 0.02% NaN3, packed in a pyrogen-free column, and equilibrated with 0.4 M NaCl in 20 mM sodium acetate (pH 4.0) (45). LBP was eluted by increasing the NaCl concentration to 1.4 M. Protein elution was monitored spectrophotometrically (280 nm). The peak fractions were pooled and placed in a dialysis bag (12,000- to 14,000-molecular-weight cutoff), dialyzed against PBS containing 0.02% NaN3, and then concentrated by coating the outside of the dialysis bag with polyethelene glycol (molecular weight, 20,000). The LBP concentration was measured by enzyme-linked immunosorbent assay (ELISA) using reagents generously provided by P. Tobias. Recombinant human His-tagged PLTP was prepared as described elsewhere (2, 17). Recombinant human sCD14 was a gift from Rolf Thieringer and Samuel Wright (Merck, Rahway, N.J.).
Lipoproteins.
Native HDL (nHDL) was isolated from human plasma by sequential ultracentrifugation in increasing concentrations of potassium bromide (KBr) (8, 20) and then thoroughly dialyzed against cold 0.9% NaCl containing 0.25 mM EDTA (pH 8). Lyophilized reconstituted HDL (R-HDL) (30) was a gift from P. Lerch (Swiss Red Cross Blood Transfusion Service, Bern, Switzerland). It was resuspended in distilled water and dialyzed against cold 0.9% NaCl containing 0.25 mM EDTA (pH 8) to remove free cholate. R-HDL contained apolipoprotein A-I (apoA-I) and phosphatidylcholine in a 1:160 molar ratio. The concentrations of nHDL and R-HDL were determined by apoA-I assay (Sigma, St. Louis, Mo.). For some experiments, plasma was obtained from blood that was anticoagulated with streptokinase (Calbiochem).
In vitro assay of LPS release from blebs.
Sucrose density gradients (4 to 24% sucrose in 5 ml) were made as previously described (35). Samples (0.15 ml) containing radiolabeled blebs were added to the tops of the gradients, and the tubes were centrifuged (150,000 × g, 4.5 h, room temperature). Fractions (approximately 0.35 ml) were collected from the bottom of the each tube with an 18-gauge needle. 3H and 35S radioactivity was measured by adding the fractions to 4 ml of Budget-Solve scintillation cocktail (Research Products International Corp., Mount Prospect, Ill.) that contained 0.2 ml of 1% SDS in 10 mM EDTA and counting in a Packard MINAXI TRI-CARB 4000 series scintillation counter (Packard Instrument Company, Downer's Grove, Ill.) with external standardization and automatic quench correction.
Immunoprecipitation of [3H]LPS-sCD14 complexes.
Samples (0.15 ml) containing radiolabeled blebs (80 ng of LPS or 0.54 μg/ml), LBP (0.32 μg/ml), and sCD14 (13.4 μg/ml) were incubated for 30 min at 37°C with occasional mixing, and the mixtures were centrifuged onto sucrose density gradients as described above. The top (released LPS) and bottom (blebs) halves of the density gradient were separated, and levels of 3H and 35S radioactivity were measured in an aliquot of each by scintillation counting; 1 ml of each half was then supplemented with 25 μl of 10% bovine serum albumin and either 5 μg of mouse anti-human 2G9 antibody (anti-sCD14 antibody supplied by Richard Darveau, University of Washington, Seattle) or 5 μg of control antibody MOPC 141 (Sigma, St. Louis, Mo.), and the mixtures were allowed to stand for 1 h at 4°C. Fifty microliters of a 1:1 suspension of Pansorbin (Calbiochem-Novabiochem Corporation, La Jolla, Calif.) that had been washed in immunoprecipitation buffer (20 mM Tris [pH 7.5], 250 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, 1 μg of pepstatin A per ml) was then added to each sample and rocked on a platform for 1 h at 4°C. The Pansorbin was pelleted by centrifugation (4,000 rpm, 2 min, 4°C), washed three times with immunoprecipitation buffer, suspended in 200 μl of 1% SDS in 10 mM EDTA, and placed in a 50°C water bath for 5 min. Levels of 3H and 35S radioactivity were measured in the supernatants and Pansorbin pellets by scintillation counting.
Assay of [3H]LPS transfer from blebs to lipoproteins.
After incubating tritium-labeled pLPS or blebs with plasma or purified lipoproteins and LPS transfer proteins, the [3H]LPS-lipoprotein complexes were isolated from the mixtures by sequential ultracentrifugation (244,000 × g, 45 h, 4°C) in KBr at densities of 1.019 (very low density lipoproteins [VLDL]), 1.063 (low-density lipoproteins [LDL]), and 1.21 (HDL) g/ml. The tubes were sliced two-thirds of the distance from the bottom. Top and bottom fractions were brought to a total volume of 10 ml with distilled water, and the radioactivity in 1-ml aliquots was measured as described above. Radioactivity located in the top fraction was assumed to be associated with lipoprotein particles since pLPS or bleb LPS, in the absence of lipoproteins, was recovered from the bottom fraction of the tube.
Depletion of LBP and PLTP activity from serum.
To neutralize PLTP, 100 μl of normal serum was incubated for 30 min on ice with 30 μl of Rb72 rabbit anti-human PLTP (purified immunoglobulin G [IgG]; 17 mg of IgG/ml); the remaining PLTP activity was 2% of that in the untreated serum. To neutralize LBP, we added 10 μg of anti-human LBP monoclonal antibody (MAb) 1E8 (a gift from P. Tobias) to 100 μl of serum and incubated the mixture for 30 min on ice. This method resulted in nearly complete inhibition of LBP-mediated LPS binding to mCD14-expressing cells, indicating effective LBP neutralization (not shown). To neutralize sCD14, we added 10 μg of anti-CD14 MAb 60bca to 100 μl of serum and incubated the mixture for 30 min on ice.
To deplete LBP from serum, anti-human LBP MAb 18G4 (a gift from P. Tobias) or nonimmune mouse IgG was covalently bound to Avidchrom hydrazide F gel beads (Unisyn Technologies, Hopkinton, Mass.) according to the manufacturer's instructions. The antibody-coated beads (1-ml packed volume) were suspended in fresh human serum (4 to 5 ml) obtained from healthy volunteers, mixed overnight on a rocking platform at 4°C, and then centrifuged (850 × g, 2 min, 4°C). The LBP concentration was measured in the control and LBP-depleted sera by ELISA. PLTP activity was measured as described elsewhere (6). Over 99% of the LBP was removed from the serum. Since the control antibody also removed more than 50% of LBP from serum, the unadsorbed serum was used as the control. Approximately 30% of the serum PLTP activity was also removed during LBP depletion.
IL-8 release assay.
THP-1 cells (47) were obtained from D. Altieri (Scripps Research Institute) and cultured as previously described (28). To induce CD14 expression, the cells were cultured in 0.05 μM 1,25-dihydroxyvitamin D3 for 48 to 72 h. The differentiated THP-1 cells were washed with cold RPMI 1640 and resuspended in Cellgro complete serum-free medium (Mediatech, Herndon, Va.) containing 20 mM HEPES buffer (pH 7.4) at 6 × 106 to 8 × 106 cells/ml. Radiolabeled blebs or pLPS from the same G-30 Salmonella serovar Typhimurium culture were incubated with LBP and sCD14 before centrifugation on 4 to 24% sucrose density gradients as previously described. Aliquots of the top half of the density gradient (sCD14-[3H]LPS complexes) were then added to differentiated THP-1 cells in serum-free medium and incubated for 2 h at 37°C. Blebs that were not incubated with LBP and sCD14 or centrifuged on sucrose density gradients were tested in parallel. The cells were removed by centrifugation (750 × g, 2 min, 4°C), and the culture supernatants were assayed for interleukin-8 (IL-8) (DuoSet ELISA development system; Genzyme, Cambridge, Mass.). In further experiments, blebs were incubated with or without LBP, PLTP, and sCD14 (30 min, 37°C) before direct addition to the differentiated THP-1 cells and incubation for 2 h at 37°C. The cells were again removed by centrifugation, and the culture supernatants were assayed for IL-8.
RESULTS
Electron microscopy of the blebs revealed small vesicular structures that ranged in size from 25 to 300 nm (Fig. 1). This size range is similar to that found in previous ultrastructural analyses of bacterial membrane blebs (33). Very few flagella and no intact bacteria were found in the preparations.
FIG. 1.
Ultrastructure of bacterial membrane fragments. Outer membrane blebs were isolated from Salmonella serovar Typhimurium culture supernatants as described in Materials and Methods. The blebs were concentrated by ultracentrifugation, resuspended in PBS, allowed to adhere to Formvar-coated grids, negatively stained with 1% uranyl acetate, and viewed by transmission electron microscopy. The bar represents 100 nm.
LBP transfers LPS from bacterial membranes to sCD14; PLTP does not.
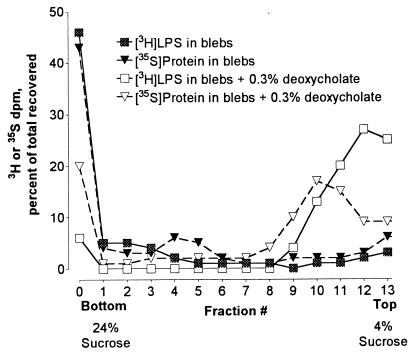
Sucrose gradient centrifugation was used to evaluate LPS release from blebs (35). Blebs containing [3H]LPS and 35S-labeled protein sedimented to the bottom of the 4 to 24% sucrose gradient. In contrast, after bacterial membranes were disrupted with deoxycholate, a large percentage of both radiolabels remained in the top fractions of the gradient (Fig. 2). In subsequent experiments, release of LPS from the blebs was assumed when [3H]LPS was found at the top of the gradient while 35S-protein pelleted to the bottom.
FIG. 2.
Sucrose gradient analysis of LPS release from blebs. Blebs containing 3,300 dpm of [3H]LPS and 2,800 dpm of 35S-protein were incubated with 0.3% deoxycholate for 30 min at 37°C and then centrifuged onto 4 to 24% sucrose gradients. Untreated blebs were used as controls. After centrifugation, fractions were collected, and the level of 3H and 35S radioactivity in each fraction was determined. The experiment was repeated with similar results.
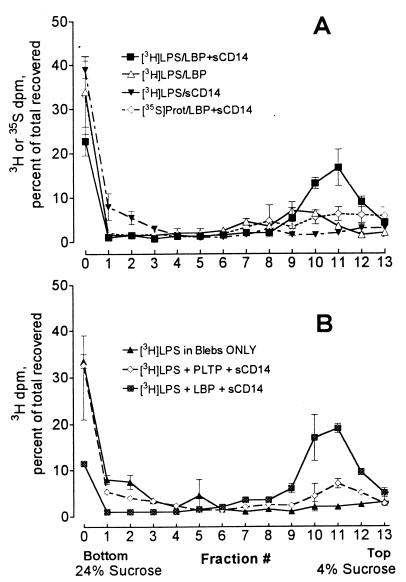
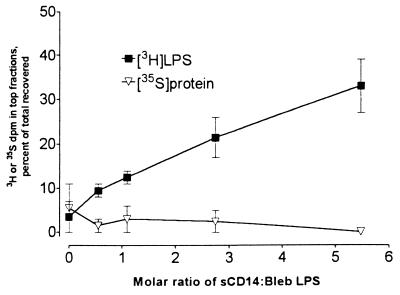
To investigate the roles of plasma LPS transfer proteins in releasing LPS from bacterial membranes, radiolabeled blebs containing 80 ng of LPS (0.54 μg/ml) were exposed to LBP (0.32 μg/ml) with or without sCD14 (27 μg/ml) at 37°C for 30 min. The mixtures were then analyzed by centrifugation onto sucrose gradients. Incubation of blebs with LBP alone or sCD14 alone did not release LPS from the blebs, while incubation with both LBP and sCD14 released 49% ± 7.4% (mean ± standard deviation, n = 4) of the LPS (Fig. 3A). In another experiment, very little release of LPS occurred when blebs were incubated with as much as 3.2 μg of LBP per ml (data not shown). When blebs were incubated with PLTP (10 μg/ml) and sCD14, there was no significant release of [3H]LPS (Fig. 3B). Increasing the sCD14 concentration in the presence of LBP increased LPS release from the blebs, while only trivial amounts of 35S-labeled protein were released (Fig. 4).
FIG. 3.
When CD14 is present, LBP releases LPS from blebs. (A) Blebs containing 80 ng of [3H]LPS (0.54 μg/ml) were incubated for 30 min at 37°C with a 0.06:1 molar ratio of LBP (0.324 μg/ml) to LPS, with a 5.5:1 molar excess of sCD14 (26.7 μg/ml), or with LBP plus sCD14. The mixtures were then centrifuged onto 4 to 24% sucrose gradients. Symbols show means ± standard errors of four independent experiments. (B) Blebs containing 80 ng of [3H]LPS (0.54 μg/ml) were incubated for 30 min at 37°C with a 2.8:1 molar excess of sCD14 (13.4 μg/ml) and either a 0.06:1 molar ratio of LBP (0.324 μg/ml) or a 1.4:1 molar excess of PLTP (10.7 μg/ml). The mixtures were then centrifuged onto 4 to 24% sucrose gradients. Symbols indicate the means and ranges from two independent experiments.
FIG. 4.
sCD14 enhances LBP-mediated LPS release from blebs. Blebs containing 80 ng of [3H]LPS (0.54 μg/ml) were incubated with a 0.06:1 molar ratio of LBP (0.324 μg/ml) and increasing amounts of sCD14 (0 to 26.7 μg/ml) for 30 min at 37°C and then centrifuged onto 4 to 24% sucrose gradients. Symbols indicate the means and ranges from two independent experiments.
Previous studies have suggested that LPS can bind to sCD14, which then functions as an LPS carrier or transfer protein (15, 19, 51), and we have previously shown that pLPS-sCD14 complexes remain in the top fractions of sucrose gradients (26). To determine whether LBP can transfer membrane [3H]LPS to sCD14, we used anti-CD14 MAbs to immunoprecipitate CD14 from the incubation mixtures. We found that 32% of the LPS in the top gradient fractions and only 3% of the bleb LPS in the bottom gradient fractions could be coimmunoprecipitated. Very little 35S-labeled protein was recovered from the top of the gradient, and only 6% of the 35S-labeled protein in this fraction was coimmunoprecipitated by the anti-CD14 MAb. Likewise, only 3% of the 35S-labeled protein was found in immunoprecipitates from the bottom gradient fractions.
LBP and PLTP can transfer LPS from blebs to HDL.
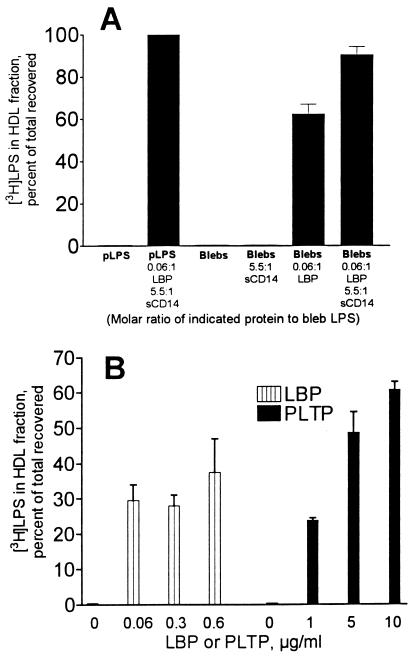
Since both LBP and sCD14 were needed to release LPS from bacterial membranes, we next asked whether both proteins were necessary to transfer LPS from blebs to HDL. For this analysis we used R-HDL (a defined reagent that contains only apoA-I and phosphatidylcholine) and separated blebs from R-HDL-bound LPS by ultracentrifugal flotation in KBr (ρ = 1.21 g/ml). Purified [3H]LPS aggregates were used as a control. In preliminary experiments, we found aggregates of pLPS and pLPS-sCD14 complexes at the bottom of the centrifuge tube (data not shown), whereas R-HDL-bound pLPS (produced by incubating pLPS with R-HDL and LBP with or without sCD14) was recovered from the top (Fig. 5A).
FIG. 5.
LBP and PLTP transfer LPS from blebs to HDL. (A) Blebs containing 80 ng of [3H]LPS (0.8 μg/ml) were incubated with R-HDL (1 mg of apoA-I/ml) for 30 min at 37°C with or without LBP (0.05 μg/ml) or sCD14 (4.0 μg/ml). The HDL was then separated by ultracentrifugal flotation, and the fraction of the [3H]LPS that bound to the HDL was measured. sCD14 enhanced the ability of LBP to promote LPS transfer to HDL. (B) Blebs containing 80 ng of [3H]LPS (0.8 μg/ml) were incubated for 30 min at 37°C with nHDL (0.5 mg of apoA-I/ml) and the indicated amounts of LBP or PLTP. Bars indicate the means and ranges of two independent experiments.
sCD14 alone did not transfer LPS from membrane blebs to R-HDL, while LBP transferred over 62% (standard deviation = 8%, n = 3 experiments) of the membrane LPS to R-HDL. LPS transfer activity was maximal at substoichiometric concentrations of LBP (Fig. 5B). When sCD14 was incubated with blebs, LBP, and R-HDL, over 90% of the LPS transferred from blebs to R-HDL (Fig. 5A). sCD14 thus enhances the ability of LBP to transfer LPS to R-HDL.
LBP also transferred LPS from blebs to nHDL. However, the amount of nHDL required to accept LPS from membranes in a 30-min incubation was greater than the amount of R-HDL needed for the same reaction (data not shown). PLTP also transferred LPS from bacterial membranes to nHDL (Fig. 5B).
LBP transfers LPS to lipoproteins in plasma.
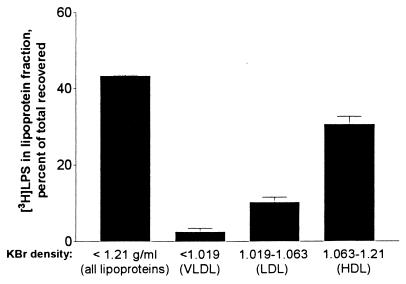
When blebs were incubated in undiluted normal human plasma for 30 min at 37°C, 43% of the [3H]LPS floated in the ρ ≤ 1.21-g/ml KBr fractions, and the majority of the lipoprotein-bound [3H]LPS was found in HDL (Fig. 6).
FIG. 6.
Bleb LPS transfers to lipoproteins in normal human plasma. Blebs containing 80 ng of [3H]LPS (0.8 μg/ml) were incubated for 2 h at 37°C with human platelet-free plasma (prepared from whole blood that contained streptokinase [150 U/ml] to prevent clotting), and the VLDL, LDL, and HDL were separated sequentially by ultracentrifugal flotation according to the indicated densities. Bars indicate the means and ranges of two experiments performed in duplicate.
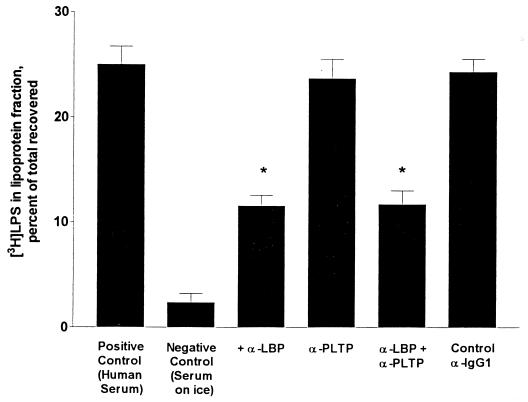
To determine whether endogenous LBP and PLTP were responsible for the transfer of bleb LPS to lipoproteins, we used antibodies to neutralize the two proteins in normal human serum. When an anti-LBP MAb was added to normal serum, LPS transfer from blebs to serum lipoproteins decreased by nearly 50% (Fig. 7). In contrast, inhibiting PLTP activity in normal serum did not reduce LPS transfer from blebs to lipoproteins. Normal levels of LBP are thus able to compensate for the absence of PLTP. When both anti-LBP and anti-PLTP antibodies were added to the serum, the reduction in LPS transfer was identical to that seen with anti-LBP alone. Adding anti-CD14 antibodies did not further reduce LPS transfer (not shown). In other experiments we depleted over 99% of the serum LBP by immunoadsorption (see Materials and Methods); this reduced LPS transfer from blebs to serum lipoproteins by nearly 50% (not shown). When the LBP-depleted serum was supplemented with recombinant LBP, LPS transfer from blebs to HDL was restored to the levels seen in normal human serum (not shown). These data thus suggest that (i) LBP contributes substantially to the transfer of LPS from membranes to lipoproteins in human serum in vitro and (ii) serum contains factor(s) other than LBP, PLTP, and sCD14 that can also promote LPS transfer to lipoproteins.
FIG. 7.
LBP neutralization inhibits LPS transfer from blebs to lipoproteins. A 0.1-ml aliquot of normal human serum was preincubated for 30 min on ice with or without 5 μl of anti-LBP (10 μg), 30 μl of anti-PLTP (0.53 mg), or 10 μl of control mouse anti-IgG1 (10 μg). The mixtures were then diluted to 1 ml with PBS, blebs containing 80 ng of [3H]LPS were added, incubation was continued for 30 min at 37°C, and the lipoproteins were isolated by flotation at ρ = 1.21 g/ml. The bars show the means and ranges of three experiments performed in duplicate. ∗, significantly different from positive control (P < 0.05, analysis of variance).
LBP transfers LPS from blebs to host cell membranes.
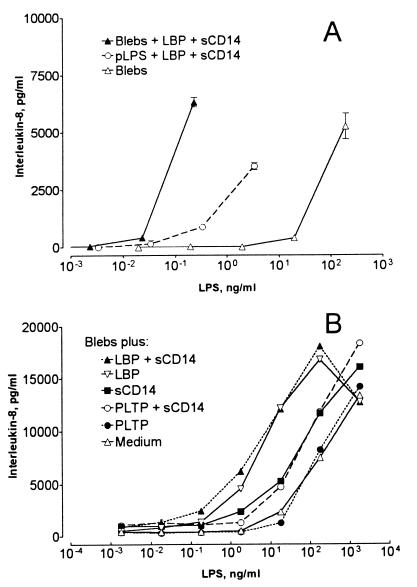
We next compared sCD14-[3H]LPS complexes derived from blebs and pLPS for the ability to stimulate IL-8 production by differentiated THP-1 cells. Bleb-derived sCD14-[3H]LPS complexes were approximately 30-fold more potent than complexes derived from purified LPS and sCD14 (Fig. 8A). In further experiments, we compared the potency of blebs that had been incubated for 30 min with or without LBP, PLTP, or sCD14. Incubation with LBP and sCD14 increased bleb-induced IL-8 secretion approximately 100-fold (Fig. 8B). PLTP (1:3 [mol/mol] to bleb LPS and 16:1 to LBP, sufficient to transfer most of the bleb LPS to lipoproteins in a 30-min incubation) neither enhanced nor inhibited the ability of the blebs to stimulate IL-8 secretion (Fig. 8B). In further experiments, a molar excess of PLTP (3:1 to bleb LPS and 160:1 to LBP) only partially inhibited LBP's ability to increase bleb-induced IL-8 secretion (data not shown).
FIG. 8.
LBP and sCD14 enhance monocyte stimulation by bacterial membranes; PLTP does not. (A) To prepare complexes of sCD14 with bleb or pLPS, 100 ng of pLPS or blebs containing 100 ng of LPS were incubated at 37°C for 30 min with 0.06 μg of LBP and 0.5 μg of sCD14. The mixtures were centrifuged onto 4 to 24% sucrose gradients, and aliquots of the top halves of the gradients were counted to measure the sCD14-associated [3H]LPS. As a control, blebs were added to a sucrose gradient without LBP and sCD14. Equal amounts of [3H]LPS from the three preparations were then diluted in serum-free medium and added to vitamin D3-differentiated THP-1 cells (7 × 105), followed by incubation for 2 h at 37°C. The cells were pelleted by centrifugation, and IL-8 was measured in the culture medium. (B) Blebs containing 100 ng of LPS were incubated at 37°C for 30 min with or without 0.03 μg of LBP, 0.5 μg of sCD14, or 0.5 μg of PLTP, and the mixtures were incubated with THP-1 cells as described above. IL-8 was measured in the culture supernatants. Symbols show the means of duplicate determinations. Each of these experiments was repeated with similar results.
DISCUSSION
As they grow in vivo, gram-negative bacteria release membrane blebs (3, 36). When they were isolated from bacterial cultures and injected intravenously into rats, blebs surrendered much more of their LPS to plasma lipoproteins than did bacterial outer membranes or intact bacteria (37). It thus seems likely that blebs contain a significant fraction of the bacterial membrane LPS that interacts with lipoproteins in vivo. Although the composition of the blebs released by bacteria in vivo is not known, when Salmonella serovar Typhimurium blebs were compared to bacterial outer membranes isolated from the same in vitro cultures, the blebs were enriched in LPS and relatively depleted in protein and phospholipid (37); these differences may account for the greater susceptibility of bleb LPS to extraction by plasma transfer proteins.
LPS may also be transferred directly from intact bacterial membranes in sufficient quantities to stimulate host cells (25, 37). Although other constituents of the gram-negative bacterial membrane can also stimulate cells, LPS seems to play a dominant role. In keeping with this conclusion, Somerville et al. showed that an Escherichia coli mutant (msbB) expressing an altered LPS was 100-fold less stimulatory to endothelial cells and monocytes than was wild-type E. coli (44). Similarly, we found that blebs were approximately 100- to 1,000-fold more stimulatory when they were incubated with LBP, which released the LPS from the blebs and transferred it to mCD14 on the THP-1 cells.
We found that low concentrations of LBP can remove LPS from blebs when an appropriate acceptor (lipoprotein or sCD14) is present. Incubating blebs with sCD14 alone did not release LPS. This finding suggests that LBP and CD14 cooperate to extract LPS or that sCD14 functions as an acceptor for LPS that is released from the blebs by LBP. The latter interpretation is favored by the following observations: (i) LBP alone, but not sCD14 alone, can transfer LPS from blebs to HDL, another LPS acceptor, and (ii) when both LBP and sCD14 were present in the incubation mixtures, much of the released LPS was found in complexes with sCD14 (see Results). Previous studies found that sCD14 can slowly bind the LPS in purified aggregates (18), and we cannot rule out the possibility that it also slowly removes LPS from blebs. However, this activity is unlikely to be physiologically significant because it occurs very slowly and because LBP is always present in plasma to promote the transfer of LPS to sCD14. PLTP, which does not transfer LPS from purified aggregates to CD14 (17), also did not transfer LPS from blebs to sCD14. Under the conditions of our experiments, PLTP removed LPS from blebs only when HDL was present to accept it.
In the model proposed by Wurfel et al. for LPS transfer from purified aggregates to lipoproteins, LBP mediates LPS transfer (51). sCD14 did not contribute greatly to this process in whole plasma (53), a finding that we confirmed in these experiments. The LPS transfer activity of sCD14 may become more important when serum LBP levels rise and PLTP levels fall during acute infection and inflammation (1, 24).
Removing LBP from serum (by immunoadsorption) and neutralizing it (with a MAb) both resulted in a 50% decrease in the transfer of LPS from blebs to lipoproteins. In contrast, polyclonal anti-PLTP immunoglobulins (which decreased serum phospholipid transfer activity by 98%) had no effect when added either to normal serum or to serum that contained the MAb to LBP. Although purified PLTP could clearly promote LPS transfer to isolated HDL, we were thus unable to show that native PLTP transfers LPS from blebs to lipoproteins in normal serum. In this more physiological setting, LBP accounted for approximately half of the transfer activity, while the remaining activity presumably reflects the contribution of unknown transfer molecules. It is noteworthy that the anti-PLTP antibodies inhibited most of the LPS transfer activity that remained in LBP-depleted serum (data not shown); this observation indicates that the antibodies can inhibit the LPS transfer activity of PLTP while suggesting that, like LBP, the unknown transfer molecules can bind nonspecifically to the beads used for immunoadsorption, leaving PLTP as the sole LPS transfer protein in the serum.
Our finding that LBP contributes significantly to LPS-lipoprotein transfer in serum may seem to conflict with the observation of Jack et al. that LPS clearance was not altered in mice that cannot synthesize LBP (23). In both LBP-producing and homozygous LBP knockout mice, they found that >90% of a bolus of radiolabeled LPS was cleared from the circulation within 5 min. Since LPS that binds to lipoproteins remains in the circulation for hours (7, 32, 34), it is possible that the LPS used in the experiments of Jack et al. did not bind to plasma lipoproteins even in the control (wild-type) mice. Alternatively, a 50% reduction in LPS-lipoprotein binding might not be detectable in vivo, or other LPS transfer proteins might be able to compensate for the absence of LBP in the genetically altered mice. Further experiments are needed to determine the precise contribution that LBP makes to LPS-lipoprotein transfer in vivo.
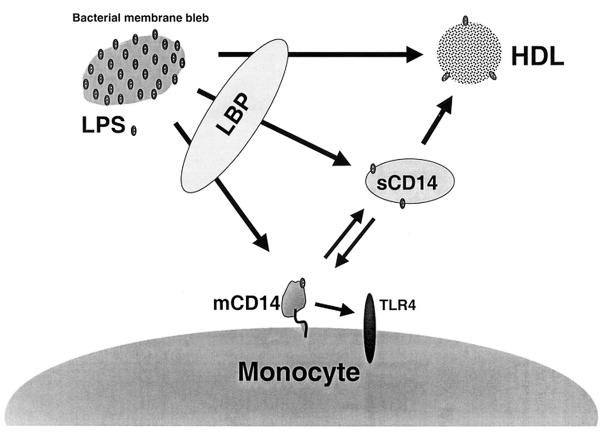
Our data are most consistent with the model shown in Fig. 9. When blebs are released into plasma, LPS is rapidly extracted from them, principally by LBP. The LPS from blebs may be neutralized either after LBP-mediated transfer to HDL or indirectly by transfer to sCD14 with subsequent binding to HDL. The LPS from blebs may also be stimulatory when transferred by LBP directly to mCD14-expressing cells, or to sCD14 with subsequent transfer to cells. Also shown in this model is the ability of sCD14, but not LBP or PLTP, to transfer LPS from monocyte cell membranes to plasma lipoproteins (29). Whether bleb LPS is neutralized by lipoproteins is probably determined by the relative concentrations of LBP, sCD14, other LPS transfer proteins, lipoproteins, and target cells in the blood compartment, as well as by the rate at which LPS is cleared from the blood into tissues.
FIG. 9.
Model for the interactions between bacterial membranes, lipid transfer proteins, and mCD14-expressing cells in human plasma. LBP can transfer LPS from blebs to HDL, mCD14-expressing cells, or sCD14. sCD14 may transfer LPS from blebs to mCD14-expressing cells or HDL, and it can also transfer LPS from mCD14-expressing cells to lipoproteins. Although PLTP can transfer LPS from blebs to isolated HDL particles, a role in LPS transfer to lipoproteins in complete serum could not be shown.
ACKNOWLEDGMENTS
This research was supported by grants AI18188 from the National Institute for Allergy and Infectious Diseases and HL30086 from the National Heart, Lung, and Blood Institute. C. J. Vesy was supported by USPH training grant T32-DK07745.
We thank David Spady, Richard Darveau, Peter Tobias, Rolf Thieringer, and Samuel Wright for providing essential reagents and John Dietschy for critical manuscript review.
REFERENCES
- 1.Albers J J, Tu A Y, Wolfbauer G, Cheung M C, Marcovina S M. Molecular biology of phospholipid transfer protein. Curr Opin Lipidol. 1996;7:88–93. doi: 10.1097/00041433-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Albers J J, Wolfbauer G, Cheung M C, Day J R, Ching A F, Lok S, Tu A Y. Functional expression of human and mouse plasma phospholipid transfer protein: effect of recombinant and plasma PLTP on HDL subspecies. Biochim Biophys Acta. 1995;1258:27–34. doi: 10.1016/0005-2760(95)00091-p. [DOI] [PubMed] [Google Scholar]
- 3.Arditi M, Ables L, Yogev R. Cerebrospinal fluid endotoxin levels in children with H. influenzae meningitis before and after administration of intravenous ceftriaxone. J Infect Dis. 1989;160:1005–1011. doi: 10.1093/infdis/160.6.1005. [DOI] [PubMed] [Google Scholar]
- 4.Bohuslav J, Kravchenko V V, Parry G C, Erlich J H, Gerondakis S, Mackman N, Ulevitch R J. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Investig. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavaillon J-M, Fitting C, Haeffner-Cavaillon N, Kirsch S J, Warren H S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990;58:2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung M C, Wolfbauer G, Albers J J. Plasma phospholipid mass transfer rate: relationship to plasma phospholipid and cholesteryl ester transfer activities and lipid parameters. Biochim Biophys Acta. 1996;1303:102–110. doi: 10.1016/0005-2760(96)00082-3. [DOI] [PubMed] [Google Scholar]
- 7.Coulthard M G, Swindle J, Munford R S, Gerard R D, Meidell R S. Adenovirus-mediated transfer of a gene encoding acyloxyacyl hydrolase (AOAH) into mice increases tissue and plasma AOAH activity. Infect Immun. 1996;64:1510–1515. doi: 10.1128/iai.64.5.1510-1515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein C, Scanu A M. Precautionary measures for collecting blood destined for lipoprotein isolation. Methods Enzymol. 1986;128:151–170. doi: 10.1016/0076-6879(86)28065-9. [DOI] [PubMed] [Google Scholar]
- 9.Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias P S, Mazurier J, Spik G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun. 1998;66:486–491. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison R T, III, Giehl T J. Killing of Gram-negative bacteria by lactoferrin and lysozyme. J Clin Investig. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsbach P, Weiss J. Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol. 1998;10:45–49. doi: 10.1016/s0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 12.Feingold K R, Funk J L, Moser A H, Shigenaga J K, Rapp J H, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun. 1995;63:2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freudenberg M A, Bog-Hansen T C, Back U, Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980;28:373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freudenberg N, Freudenberg M A, Guzman J, Mittermayer C, Bandara K, Galanos C. Identification of endotoxin-positive cells in the rat lung during shock. Virchows Arch. 1984;404:197–211. doi: 10.1007/BF00704064. [DOI] [PubMed] [Google Scholar]
- 15.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge Y, Ezzell R M, Tompkins R G, Warren H S. Cellular distribution of endotoxin after injection of chemically purified lipopolysaccharide differs from that after injection of live bacteria. J Infect Dis. 1994;169:95–104. doi: 10.1093/infdis/169.1.95. [DOI] [PubMed] [Google Scholar]
- 17.Hailman E, Albers J J, Wolfbauer G, Tu A Y, Wright S D. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J Biol Chem. 1996;271:12172–12178. doi: 10.1074/jbc.271.21.12172. [DOI] [PubMed] [Google Scholar]
- 18.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C T, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 20.Havel R J, Eder H A, Bragdon J H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Investig. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1997;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 23.Jack R S, Fan X, Bernhelden M, Rune C, Ehlers M, Weber A, Kirsch G, Mentel R, Fürll B, Freudenberg M, Schmitz G, Stelter F, Schütt C. Lipopolysaccharide-binding protein is required to combat a murine Gram-negative bacterial infection. Nature. 1997;389:742–744. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X-C, Bruce C. Regulation of murine plasma phospholipid transfer protein activity and mRNA levels by lipopolysaccharide and high cholesterol diet. J Biol Chem. 1995;270:17133–17138. doi: 10.1074/jbc.270.29.17133. [DOI] [PubMed] [Google Scholar]
- 25.Katz S S, Chen K, Chen S, Doerfler M E, Elsbach P, Weiss J. Potent CD14-mediated signalling of human leukocytes by Escherichia coli can be mediated by interaction of whole bacteria and host cells without extensive prior release of endotoxin. Infect Immun. 1996;64:3592–3600. doi: 10.1128/iai.64.9.3592-3600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitchens R L, Munford R S. CD14-dependent internalization of lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J Immunol. 1998;160:1920–1928. [PubMed] [Google Scholar]
- 27.Kitchens R L, Munford R S. Internalization of LPS by phagocytes. In: Brade H, Opal S, Vogel S, Morrison D C, editors. Endotoxin in health and disease. New York, N.Y: Marcel Dekker; 1999. pp. 521–536. [Google Scholar]
- 28.Kitchens R L, Ulevitch R J, Munford R S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;1760:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitchens R L, Wolfbauer G, Albers J J, Munford R S. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J Biol Chem. 1999;274:34116–34122. doi: 10.1074/jbc.274.48.34116. [DOI] [PubMed] [Google Scholar]
- 30.Lerch P G, Förtsch V, Hodler G, Bolli R. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 1996;71:155–164. doi: 10.1046/j.1423-0410.1996.7130155.x. [DOI] [PubMed] [Google Scholar]
- 31.Mathison J C, Ulevitch R J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979;123:2133–2143. [PubMed] [Google Scholar]
- 32.Mathison J C, Ulevitch R J. In vivo interaction of bacterial lipopolysaccharide (LPS) with rabbit platelets: modulation by C3 and high density lipoproteins. J Immunol. 1981;126:1575–1580. [PubMed] [Google Scholar]
- 33.Mayrand D, Grenier D. Biological activities of outer membrane vesicles. Can J Microbiol. 1989;35:607–613. doi: 10.1139/m89-097. [DOI] [PubMed] [Google Scholar]
- 34.Munford R S, Andersen J M, Dietschy J M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Investig. 1981;68:1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munford R S, Hall C L, Dietschy J M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981;34:835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munford R S, Hall C L, Grimm L. Detection of free endotoxin in cerebrospinal fluid by the Limulus lysate test. Infect Immun. 1984;45:531–533. doi: 10.1128/iai.45.2.531-533.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munford R S, Hall C L, Lipton J M, Dietschy J M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Investig. 1982;70:877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pajkrt D, Doran J E, Koster F, Lerch P G, Arnet B, van der Poll T, ten Cate J W, van Deventer S J H. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker T S, Levine D M, Chang J C C, Laxer J, Coffin C C, Rubin A L. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect Immun. 1995;63:253–258. doi: 10.1128/iai.63.1.253-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poltorak A, He X, Smirnova I, Liu M-Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 41.Read T E, Grunfeld C, Kumwenda Z L, Calhoun M C, Kane J P, Feingold K R, Rapp J H. Triglyceride-rich lipoproteins prevent septic death in rats. J Exp Med. 1995;182:267–272. doi: 10.1084/jem.182.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide-binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 44.Somerville J E, Jr, Cassiano L, Bainbridge B, Cunningham M D, Darveau R P. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Investig. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theofan G, Horwitz A H, Williams R E, Liu P-S, Chan I, Birr C, Carroll S F, Mészáros K, Parent J B, Kasler H, Aberle S, Trown P W, Gazzano-Santoro H. An amino-terminal fragment of human lipopolysaccharide-binding protein retains lipid A binding but not CD14-stimulatory activity. J Immunol. 1994;152:3623–3629. [PubMed] [Google Scholar]
- 46.Tsai C-M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 48.Warren H S, Novitsky T J, Ketchum P A, Roslansky P F, Kania S, Siber G R. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985;22:590–595. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 50.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 51.Wurfel M M, Hailman E, Wright S D. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wurfel M M, Kunitake S T, Lichenstein H, Kane J P, Wright S D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wurfel M M, Wright S D. Lipopolysaccharide-binding protein and soluble CD14 transfer lipopolysaccharide to phospholipid bilayers—preferential interaction with particular classes of lipid. J Immunol. 1997;158:3925–3934. [PubMed] [Google Scholar]
- 54.Ziegler-Heitbrock H W L, Wedel A, Schraut W, Ströbel M, Wendelgass P, Sternsdorf T, Bäuerle P A, Haas J G, Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappaB with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]