Abstract
Faster and better wound healing is a critical medical issue. Because the repair process of wounds is closely related to revascularization, accurate early assessment and postoperative monitoring are very important for establishing an optimal treatment plan. Herein, we present an extended depth-of-field photoacoustic microscopy system (E-DOF-PAM) that can achieve a constant spatial resolution and relatively uniform excitation efficiency over a long axial range. The superior performance of the system was verified by phantom and in vivo experiments. Furthermore, the system was applied to the imaging of normal and trauma sites of volunteers, and the experimental results accurately revealed the morphological differences between the normal and traumatized skin of the epidermis and dermis. These results demonstrated that the E-DOF-PAM is a powerful tool for observing and understanding the pathophysiology of cutaneous wound healing.
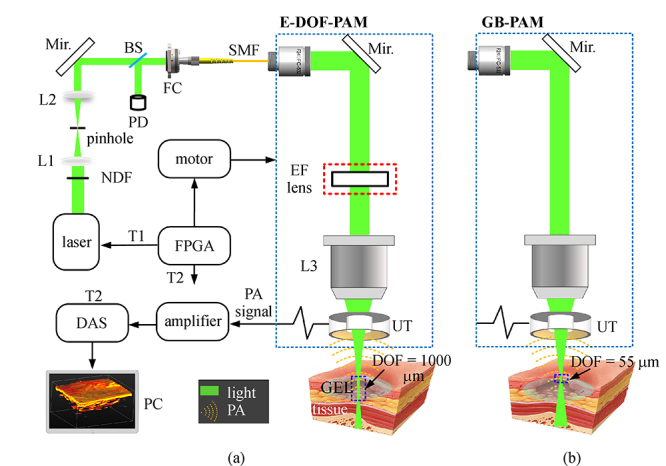
Keywords: photoacoustic microscopy (PAM), extended depth-of-field, traumatized skin
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 61822505, 11774101, 61627827, and 81630046), the Science and Technology Planning Project of Guangdong Province, China (No. 2015B020233016), and the Science and Technology Program of Guangzhou (No. 2019050001).
Footnotes
Zhongwen Cheng is a Ph.D. candidate student from College of Biophotonics, South China Normal University, China. His research interests includes photoacoustic microscopy and photoacoustic tomography.
Haigang Ma is a Ph.D. candidate student from College of Biophotonics, South China Normal University, China. His research interests includes utilizing photoacoustic imaging for detection of skin diseases and clinical applications.
Zhiyang Wang is a Ph.D. candidate student from College of Biophotonics, South China Normal University, China. His research interests focus on photoacoustic microscopy and its clinical applications.
Sihua Yang is a professor at MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, South China Normal University, China. His research focuses on photoacoustic imaging, photoacoustic endoscopic technology, instrumentation and their biomedical applications.
References
- 1.Valvis S M, Waithman J, Wood F M, Fear M W, Fear V S. The immune response to skin trauma is dependent on the etiology of injury in a mouse model of burn and excision. Journal of Investigative Dermatology. 2015;135(8):2119–2128. doi: 10.1038/jid.2015.123. [DOI] [PubMed] [Google Scholar]
- 2.Qin W, Li Y, Wang J, Qi X, Wang R K. In vivo monitoring of microcirculation in burn healing process with optical microangiography. Advances in Wound Care. 2016;5(8):332–337. doi: 10.1089/wound.2015.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Shi L, Qin J, Yousefi S, Li Y, Wang R K. Multimodal optical imaging can reveal changes in microcirculation and tissue oxygenation during skin wound healing. Lasers in Surgery and Medicine. 2014;46(6):470–478. doi: 10.1002/lsm.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeksema H, Van de Sijpe K, Tondu T, Hamdi M, Van Landuyt K, Blondeel P, Monstrey S. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns. 2009;35(1):36–45. doi: 10.1016/j.burns.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Deegan A J, Wang W, Men S, Li Y, Song S, Xu J, Wang R K. Optical coherence tomography angiography monitors human cutaneous wound healing over time. Quantitative Imaging in Medicine and Surgery. 2018;8(2):135–150. doi: 10.21037/qims.2018.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar I, Staton C A, Cross S S, Reed M W, Brown N J. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. British Journal of Surgery. 2009;96(12):1484–1491. doi: 10.1002/bjs.6778. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Wei H, Jin Y, Chen L, Zhang Q, Deng X. Monitoring skin trauma healing in mice using second-harmonic generation combined with optical coherence tomography. IEEE Photonics Journal. 2017;9(4):1–12. [Google Scholar]
- 8.Atiyeh B S, Gunn S W, Hayek S N. State of the art in burn treatment. World Journal of Surgery. 2005;29(2):131–148. doi: 10.1007/s00268-004-1082-2. [DOI] [PubMed] [Google Scholar]
- 9.Still J M, Law E J, Klavuhn K G, Island T C, Holtz J Z. Diagnosis of burn depth using laser-induced indocyanine green fluorescence: a preliminary clinical trial. Burns. 2001;27(4):364–371. doi: 10.1016/S0305-4179(00)00140-6. [DOI] [PubMed] [Google Scholar]
- 10.McUmber H, Dabek R J, Bojovic B, Driscoll D N. Burn depth analysis using indocyanine green fluorescence: a review. Journal of Burn Care & Research. 2019;40(4):513–516. doi: 10.1093/jbcr/irz054. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Jiang J, An L, Gareau D, Wang R K. In vivo volumetric imaging of microcirculation within human skin under psoriatic conditions using optical microangiography. Lasers in Surgery and Medicine. 2011;43(2):122–129. doi: 10.1002/lsm.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke-Smith A, Collier J, Jones I. A comparison of non-invasive imaging modalities: infrared thermography, spectrophotometric intracutaneous analysis and laser Doppler imaging for the assessment of adult burns. Burns. 2015;41(8):1695–1707. doi: 10.1016/j.burns.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Pape S A, Skouras C A, Byrne P O. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns. 2001;27(3):233–239. doi: 10.1016/S0305-4179(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 14.Pierce M C, Sheridan R L, Hyle Park B, Cense B, de Boer J F. Collagen denaturation can be quantified in burned human skin using polarization-sensitive optical coherence tomography. Burns. 2004;30(6):511–517. doi: 10.1016/j.burns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Jiao S, Yu W, Stoica G, Wang L V. Contrast mechanisms in polarization-sensitive Mueller-matrix optical coherence tomography and application in burn imaging. Applied Optics. 2003;42(25):5191–5197. doi: 10.1364/AO.42.005191. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz M, Soliman D, Omar M, Buehler A, Ovsepian S V, Aguirre J, Ntziachristos V. Optoacoustic dermoscopy of the human skin: tuning excitation energy for optimal detection bandwidth with fast and deep imaging in vivo. IEEE Transactions on Medical Imaging. 2017;36(6):1287–1296. doi: 10.1109/TMI.2017.2664142. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Yao J. Photoacoustic microscopy: principles and biomedical applications. Biomedical Engineering Letters. 2018;8(2):203–213. doi: 10.1007/s13534-018-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao J, Wang L, Yang J M, Maslov K I, Wong T T, Li L, Huang C H, Zou J, Wang L V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nature Methods. 2015;12(5):407–410. doi: 10.1038/nmeth.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zhang G, Wu M, Shang Q, Huang L, Jiang H. Photoacoustic assessment of hemodynamic changes in foot vessels. Journal of Biophotonics. 2019;12(6):e201900004. doi: 10.1002/jbio.201900004. [DOI] [PubMed] [Google Scholar]
- 20.Ma H, Cheng Z, Wang Z, Xiong K, Yang S. Fast controllable confocal focus photoacoustic microscopy using a synchronous zoom opto-sono objective. Optics Letters. 2019;44(7):1880–1883. doi: 10.1364/OL.44.001880. [DOI] [PubMed] [Google Scholar]
- 21.Xu D, Yang S, Wang Y, Gu Y, Xing D. Noninvasive and high-resolving photoacoustic dermoscopy of human skin. Biomedical Optics Express. 2016;7(6):2095–2102. doi: 10.1364/BOE.7.002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, Hu Y, Chen Z, Xing D. All-optical photoacoustic and reflectance confocal microscopy for melanoma characterization. Applied Physics Letters. 2019;114(16):163704. doi: 10.1063/1.5087906. [DOI] [Google Scholar]
- 23.Ma H, Cheng Z, Wang Z, Gu Y, Zhang T, Qiu H, Yang S. Fast linear confocal scanning photoacoustic dermoscopy for non-invasive assessment of chromatodermatosis. Applied Physics Letters. 2018;113(8):083704. doi: 10.1063/1.5041769. [DOI] [Google Scholar]
- 24.Li X, Dinish U S, Aguirre J, Bi R, Dev K, Attia A B E, Nitkunanantharajah S, Lim Q H, Schwarz M, Yew Y W, Thng S T G, Ntziachristos V, Olivo M. Optoacoustic mesoscopy analysis and quantitative estimation of specific imaging metrics in Fitzpatrick skin phototypes II to V. Journal of Biophotonics. 2019;12(9):e201800442. doi: 10.1002/jbio.201800442. [DOI] [PubMed] [Google Scholar]
- 25.Aguirre J, Schwarz M, Garzorz N, Omar M, Buehler A, Kilian Eyerich K, Ntziachristos V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nature Biomedical Engineering. 2017;1(5):0068. doi: 10.1038/s41551-017-0068. [DOI] [Google Scholar]
- 26.Zhang W, Li Y, Nguyen V P, Huang Z, Liu Z, Wang X, Paulus Y M. High-resolution, in vivo multimodal photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy imaging of rabbit retinal neovascularization. Light, Science & Applications. 2018;7(1):103. doi: 10.1038/s41377-018-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian C, Zhang W, Mordovanakis A, Wang X, Paulus Y M. Noninvasive chorioretinal imaging in living rabbits using integrated photoacoustic microscopy and optical coherence tomography. Optics Express. 2017;25(14):15947–15955. doi: 10.1364/OE.25.015947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H, Xiong K, Wu J, Ji X, Yang S. Noncontact photoacoustic angiography with an air-coupled ultrasonic transducer for evaluation of burn injury. Applied Physics Letters. 2019;114(13):133701. doi: 10.1063/1.5088857. [DOI] [Google Scholar]
- 29.Yang J, Gong L, Xu X, Hai P, Shen Y, Suzuki Y, Wang L V. Motionless volumetric photoacoustic microscopy with spatially invariant resolution. Nature Communications. 2017;8(1):780. doi: 10.1038/s41467-017-00856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajireza P, Forbrich A, Zemp R J. Multifocus optical-resolution photoacoustic microscopy using stimulated Raman scattering and chromatic aberration. Optics Letters. 2013;38(15):2711–2713. doi: 10.1364/OL.38.002711. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Qin H, Yang S, Xing D. In vivo fast variable focus photoacoustic microscopy using an electrically tunable lens. Optics Express. 2014;22(17):20130–20137. doi: 10.1364/OE.22.020130. [DOI] [PubMed] [Google Scholar]
- 32.Yeh C, Soetikno B, Hu S, Maslov K I, Wang L V. Microvascular quantification based on contour-scanning photoacoustic microscopy. Journal of Biomedical Optics. 2014;19(9):096011. doi: 10.1117/1.JBO.19.9.096011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Jiang B, Song X, Wei J, Luo Q. Fast axial-scanning photoacoustic microscopy using tunable acoustic gradient lens. Optics Express. 2017;25(7):7349–7357. doi: 10.1364/OE.25.007349. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Song X, Jiang B, Luo Q. Multifocus optical-resolution photoacoustic microscope using ultrafast axial scanning of single laser pulse. Optics Express. 2017;25(23):28192–28200. doi: 10.1364/OE.25.028192. [DOI] [Google Scholar]
- 35.Jiang B, Yang X, Luo Q. Reflection-mode Bessel-beam photoacoustic microscopy for in vivo imaging of cerebral capillaries. Optics Express. 2016;24(18):20167–20176. doi: 10.1364/OE.24.020167. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Huang X, Gou D, Zeng J, Chen G, Pang M, Hu Y, Zhao Z, Zhang Y, Zhou Z, Wu H, Cheng H, Zhang Z, Xu C, Li Y, Chen L, Wang A. Rapid volumetric imaging with Bessel-beam three-photon microscopy. Biomedical Optics Express. 2018;9(4):1992–2000. doi: 10.1364/BOE.9.001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Wang L, Noordam C, Wang L V. Bessel-beam Grueneisen relaxation photoacoustic microscopy with extended depth of field. Journal of Biomedical Optics. 2015;20(11):116002. doi: 10.1117/1.JBO.20.11.116002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Fulop G F, Flores A, Wang M R, Yang J J. Infrared imaging lens with extended depth of focus; Bellingham: SPIE; 2005. pp. 841–848. [Google Scholar]
- 39.Durnin J, Miceli J, Jr, Eberly J H. Diffraction-free beams. Physical Review Letters. 1987;58(15):1499–1501. doi: 10.1103/PhysRevLett.58.1499. [DOI] [PubMed] [Google Scholar]
- 40.Song W, Wu Y, Gao Y, Chen T, Zheng W, Fang H, Song L, Yuan X. Flexibly adjustable depth-of-focus photoacoustic microscopy with spatial light modulation. Applied Physics Letters. 2018;113(16):163502. doi: 10.1063/1.5042805. [DOI] [Google Scholar]
- 41.Flores A, Wang M R, Yang J J. Achromatic hybrid refractive-diffractive lens with extended depth of focus. Applied Optics. 2004;43(30):5618–5630. doi: 10.1364/AO.43.005618. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Xiong K, Yang S. Large-depth-of-field optical-resolution colorectal photoacoustic endoscope. Applied Physics Letters. 2019;114(16):163703. doi: 10.1063/1.5093789. [DOI] [Google Scholar]
- 43.Cheng Z, Ma H, Wang Z, Yang S. 3D depth-coded photoacoustic microscopy with a large field of view for human skin imaging. Chinese Optics Letters. 2018;16(8):081701. doi: 10.3788/COL201816.081701. [DOI] [Google Scholar]
- 44.Zhang W, Ma H, Cheng Z, Wang Z, Zhang L, Yang S. Miniaturized photoacoustic probe for in vivo imaging of subcutaneous microvessels within human skin. Quantitative Imaging in Medicine and Surgery. 2019;9(5):807–814. doi: 10.21037/qims.2019.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Guo H, Jin T, Qi W, Xie H, Xi L. Ultracompact highresolution photoacoustic microscopy. Optics Letters. 2018;43(7):1615–1618. doi: 10.1364/OL.43.001615. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Yang X, Liu Y, Jiang B, Luo Q. Reflection-mode optical-resolution photoacoustic microscopy based on a reflective objective. Optics Express. 2013;21(20):24210–24218. doi: 10.1364/OE.21.024210. [DOI] [PubMed] [Google Scholar]
- 47.Matts P J, Dykes P J, Marks R. The distribution of melanin in skin determined in vivo. British Journal of Dermatology. 2007;156(4):620–628. doi: 10.1111/j.1365-2133.2006.07706.x. [DOI] [PubMed] [Google Scholar]
- 48.Hu Y, Chen Z, Xiang L, Xing D. Extended depth-of-field all-optical photoacoustic microscopy with a dual non-diffracting Bessel beam. Optics Letters. 2019;44(7):1634–1637. doi: 10.1364/OL.44.001634. [DOI] [PubMed] [Google Scholar]


