We previously developed a tissue-engineered vascular graft (TEVG), created by seeding a biodegradable scaffold with autologous bone marrow–derived mononuclear cells, specifically designed for use in congenital heart surgery. We demonstrated in a clinical trial that this approach is safe and effective for short-term use in children with the added benefit of growth capacity; however, long-term results were complicated by the formation of TEVG stenosis (1). Recently, it was shown that endothelial- to-mesenchymal transition (EndoMT) contributes to post-natal processes, including the development of vein graft remodeling (2). Activation of transforming growth factor-b (TGF-b) signaling is thought to play a central role in this process (3,4). In this study, we explore the contribution of EndoMT to vascular neotissue and stenosis formation, and the potential utility of anti–TGF-b therapy in the prevention of TEVG stenosis.
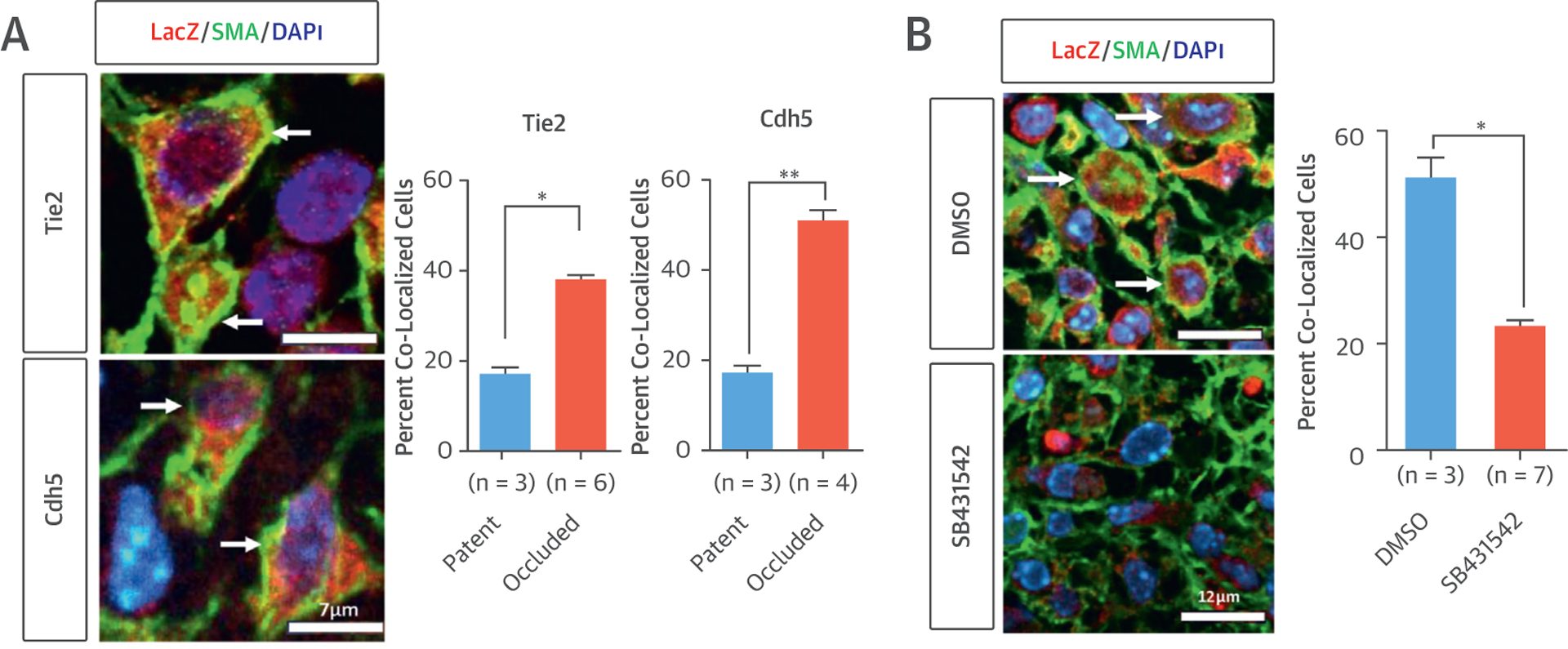
We used endothelial cell lineage-tracing mouse models (Tie2-Cre and Cdh5-CreERT2) to determine whether EndoMT occurs during neovessel formation in a mouse TEVG model. TEVG scaffolds were constructed from a nonwoven polyglycolic acid mesh, with a copolymer sealant solution of poly-L- lactide and -ε-caprolactone, implanted as an inferior vena cava interposition graft and harvested at the 2-week time point. In both models (n 1/4 9 and 7, respectively), we found colocalization of both fate-mapped endothelial cell (LacZ) and mesenchymal cell (smooth muscle actin and Calponin) markers in 38% to 51% of smooth muscle cells in occluded grafts and only 17% in patent grafts (Figure 1A) (p < 0.05, p < 0.01), suggesting that excessive EndoMT significantly contributes to occlusion in our TEVG.
Figure 1.
TGFβR1 Inhibition Reduces TEVG Stenosis by Blocking EndoMT
(A) Tie2 and Cdh5 models with lineage tracing images in occluded TEVG. Arrows show co-localized cells (left). Quantification demonstrating that EndoMT significantly contributes to TEVG stenosis in both models (right, *p < 0.05, **p < 0.01). (B) SB431542 treatment in the Tie2 lineage tracing model significantly reduces the occurrence of EndoMT in occluded TEVG. Arrows show co-localized cells (*p < 0.05). DMSO = dimethyl sulfoxide; SMA = smooth muscle actin.
Next, we evaluated the expression of TGF-b and TGF-b receptor type 1 (TGFbR1) using our model system with wild-type mice (C57BL/6) to determine their association with the formation of TEVG stenosis. Stenosed grafts expressed higher levels of TGF-b compared with patent grafts and showed increased expression of TGF-b and TGFbR1 by quantitative real-time polymerase chain reaction from day 7 to day 14 as stenosis developed (p < 0.001, n 1/4 5 for each).
We then treated the mice (C57BL/6) with TGFbR1 inhibitor SB431542 at 10 mg/kg twice daily by intraperitoneal administration for 2 weeks following implantation of our TEVG (n 1/4 24). Matched unseeded control and bone marrow–derived mono- nuclear cell–seeded (1 – 106 cells/graft) mice received intraperitoneal injection of sterile dimethyl sulfoxide (n 1/4 25 each). At 8 weeks after implantation, the grafts were evaluated with a high-frequency Doppler ultrasound. The stenosis rate was significantly reduced in the TGFbR1 inhibitor group, similar to that of the seeded control group (p < 0.05, p < 0.01), demonstrating the long-term efficacy of the short-term SB431542 treatment.
To investigate the mechanism by which SB431542 treatment prevents TEVG stenosis, we treated mice from the Tie2 lineage-tracing model (n 1/4 10) with TGFbR1 inhibitor. Drug treatment reduced the occurrence of EndoMT, as demonstrated by significant reduction in the ratio of LacZ-positive smooth muscle cells in occluded grafts in drug-treated mice (Figure 1B) (p < 0.05). We then explored the use of targeted drug delivery in an attempt to maximize efficacy and minimize the risk of any drug-related adverse events, as well as to determine whether local drug delivery could be used to mimic the effects of cell seeding. We developed a poly-lactic glycolic acid microparticle–based delivery system allowing drug release directly from the graft (5). The drug-eluting grafts were implanted in our mouse model (C57BL/6) (n 1/4 10). We then compared stenosis rates to unseeded grafts with empty microparticles (n 1/4 10) and cell-seeded grafts (n 1/4 25) at 2 weeks. These studies showed that the best results were achieved with microparticle drug delivery, which completely eliminated TEVG stenosis, while also permitting vascular neotissue formation.
In this study, we went from the bench to the bedside and back in an attempt to design an improved TEVG. Clinical investigation of our first-generation TEVG demonstrated that the TEVG possessed growth capacity, but its clinical utility was limited by a high incidence of stenosis. These findings prompted us to investigate the cellular and molecular mechanisms underlying vascular neotissue formation, which lead us to discover that excessive EndoMT contributes to neovessel stenosis in the TEVG. We modulated EndoMT by administration of a TGFbR1 inhibitor and showed that TGFbR1 inhibition is a viable strategy for preventing TEVG stenosis without blocking neovessel formation. Finally, we demonstrated that local-controlled release of the TGFbR1 inhibitor recapitulates the functional results achieved using cell seeding. This drug-eluting scaffold will serve as the prototype for our second-generation TEVG.
Sources of funding:
This work was supported by National Institutes of Health Grants (Bethesda, MD) R01-HL 098228 (to C.K.B.), R01-HL 053793 (to M.S.), NSF Career Award (Arlington, VA) 0747577 (to T.M.F), National Institutes of Health (Bethesda, MD) Autoimmunity Center of Excellence Pilot Award (to T.M.F), and Howard Hughes Medical Institute (Chevy Chase, MD) Medical Research Training Fellowship (to D.R.D.).
Disclosures:
C.K.B. receives research funding from Gunze Ltd. (Kyoto, Japan) and Pall Corporation (Port Washington, NY). None of the funding for the work done in this manuscript was provided by Gunze Ltd. or Pall Corporation.
Abbreviations:
- TEVG
tissue engineered vascular graft
- EndoMT
endothelial-to-mesenchymal transition
- TGF-β
transforming growth factor β
- TGFβR1
transforming growth factor β receptor type 1
REFERENCES
- 1.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010;139: 431–6. [DOI] [PubMed] [Google Scholar]
- 2.Cooley BC, Nevado J, Mellad J, et al. TGF-b signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 2014;6(227):227ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res 2002;90:1189–96. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh AK, Nagpal V, Covington JW, Michaels MA, Vaughan DE. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): differential expression of microRNAs during EndMT. Cell Signal 2012;24: 1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahmy TM, Samstein RM, Harness CC, Mark Saltzman W. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials 2005;26:5727–36. [DOI] [PubMed] [Google Scholar]


