Abstract
Women show a heightened risk for psychosis in midlife that is not observed in men. The menopausal transition (i.e., perimenopause) and accompanying changes in ovarian hormones are theorized to account for this midlife increase in risk. This narrative review aims to empirically examine these theories by reviewing studies of midlife and perimenopausal psychosis risk in women and potential ovarian hormone mechanisms of effects. Clinical and pre-clinical studies examining the effects of midlife age, menopausal stage, and ovarian hormones across adulthood on psychosis risk were identified. Synthesis of this body of work revealed that the peak ages of midlife psychosis risk in women overlap with the age range of key menopausal stages (especially the perimenopausal transition), although studies directly assessing menopausal stage are lacking. Studies examining ovarian hormone effects have almost exclusively focused on earlier developmental stages and events (e.g., pregnancy, the menstrual cycle) and show increases in psychotic symptoms in women and female rats during periods of lower estradiol levels. Estrogen treatment also tends to enhance the effects of neuroleptics in females across species at various reproductive phases. Initial data are promising in suggesting a role for menopausal stage and ovarian hormones in psychosis risk. However, critical gaps in our knowledge base remain, as there is a tendency to rely on indirect and proxy measures of menopausal status and hormones. Opportunities for future research are discussed with the goal of increasing research in this critical area of women’s health.
Schizophrenia is sexually dimorphic1 in its incidence, onset, and course. During the primary reproductive years, women tend to show a lower cumulative incidence, later onset (by ~3-5 years), and milder course than men (Häfner, 2019; Jones, 2013; Riecher-Rössler et al., 2018), yet dramatic shifts in sex-differentiated patterns of risk occur at midlife (~age 40-50 years old). Moreover, while the peak onset age in both men and women is in early adulthood (Häfner et al., 1993), women show a second, midlife peak in the incidence of schizophrenia. Indeed, most midlife onset cases of schizophrenia occur in women (Riecher-Rossler et al., 1997), and first hospital admission rates for psychosis after age 40 have been found to be twice as high in women (21%) than men (10%) (Häfner et al., 1993; Riecher-Rossler et al., 1997). In addition, women with an existing diagnosis experience midlife increases in illness severity, which persist into later stages of adulthood. These midlife changes in incidence and severity are not observed in men of similar ages (Kirkbride et al., 2012; Ochoa et al., 2012; Polachek et al., 2017; Van Der Werf et al., 2014).
Although a handful of studies have failed to find strong age effects on risk for psychosis in women (likely due to methodological limitations, such as truncating data collection at age 45; e.g., Kühl et al., 2016), findings from systematic reviews and the vast majority of empirical studies confirm a female-specific increase in psychosis during midlife (Kirkbride et al., 2012; Ochoa et al., 2012; Van Der Werf et al., 2014). This female predominance of midlife risk is striking and may provide critical clues to etiologic underpinnings of psychosis, particularly sex-specific factors. Indeed, it has been posited that these epidemiological patterns are tied to pathophysiological processes unique to women and the midlife period of life (Castle et al., 1998; Häfner et al., 1993). Naturally, the predominant theory over the past thirty years has focused on the menopausal transition (i.e., perimenopause) and its associated changes in ovarian hormones as potential mechanisms of midlife psychosis risk in women (Häfner, 2019; Häfner et al., 1993; Kulkarni et al., 2012; Riecher-Rössler & Häfner, 1993; Häfner, 1993; Riecher-Rössler et al., 1994).
Nonetheless, menopause and its related hormonal changes are not the only possible explanation for midlife psychosis in women. Other factors relevant to midlife, such as life stressors (e.g., changes in work, health/illness, marital status/residence), could also contribute to increased risk for psychosis (Holtzman et al., 2013; Thomas et al., 2019). Although these factors are not sex-specific, i.e., men endure these same midlife changes/stressors, gender differences in the prevalence of some types of stressors and in responsivity to stress have been described (see Almeida 2005; Bangasser & Wiersielis, 2018; Ter Horst et al., 2009; Thomas et al., 2018). Understanding why the midlife period is a time of heightened risk for psychosis in women may substantially advance etiologic theories, treatment approaches, and women’s health by providing a clearer understanding of key mechanisms of risk and identifying promising targets for more tailored and effective treatment. Such etiological advances may also contribute to discussions of equifinality in schizophrenia— whereby a diversity of pathways may lead to mechanistically disparate but clinically similar syndromes (Green & Glausier, 2016). A better understanding of the etiologic significance of midlife, presumably via its associated hormonal and psychosocial correlates, may help unpack mechanistic heterogeneity in the illness and could have broad implications for understanding differential risk across age groups, genders, and sexes.
In this narrative review, we review clinical and pre-clinical data examining the potential role of menopause and ovarian hormones in heightening midlife psychosis risk in women. A narrative, rather than systematic, review was conducted because of the paucity of data directly examining the midlife/menopause period and our intent to provide a broad topical overview and thematic synthesis of data obtained from an array of methodological approaches (e.g., experimental animal studies; correlational data; pharmaceutical trials). We focus our review on the diagnosis of schizophrenia as well as psychotic symptoms, which are a defining feature of the disorder. Psychosis is broadly characterized by a gross departure from consensual reality and includes delusions (e.g., false beliefs) and hallucinations (e.g., false perceptions), whereas schizophrenia is a heterogeneous disorder that encompasses a number of symptoms, including positive psychotic symptoms (e.g., delusions, hallucinations), negative symptoms (e.g., lack of motivation, flat affect), and disorganized symptoms (American Psychiatric Association, 2013). In addition, we review the influence of ovarian hormones on schizophrenia-like phenotypes in animal studies, which offer a translational perspective on midlife psychosis risk in women. We begin by providing an overview of the menopausal transition, and then we focus on evidence for a role of the menopausal transition and ovarian hormones in psychosis risk. We end by discussing current gaps in the literature and important areas for future research.
Overview of Search Strategy and Criteria
Although this is a narrative review, we believe it is important to outline our broad approach to article identification and selection. Articles were obtained by searching PubMed, PsychInfo, and Google Scholar databases for key terms: psychosis, psychotic disorder, psychotic-like, schizophrenia, midlife, menopause, perimenopause, postmenopause, estrogen, estradiol, progesterone, ovarian hormones, ovarian function. Articles were also identified from references within published articles. Article abstracts were screened for relevance, and studies were included if the study design and findings addressed midlife (ages 40-60), menopausal, and/or ovarian hormone effects on psychotic symptoms in women. Sixty-four manuscripts, including some systematic reviews and meta-analyses, met these criteria and were included in our review.
Defining Menopausal Stages
Broadly speaking, there are four main reproductive stages during midlife in women: premenopause, perimenopause, menopause, and postmenopause. Women are considered to be in premenopause (typically up to ~age 45) when they are having regular menstrual cycles and are not experiencing the vasomotor symptoms (e.g., hot flashes, flushes, night sweats) that are common during later menopausal stages (see below) (Gold, 2011; Harlow et al., 2012). During premenopause, estradiol and progesterone levels are relatively high and fluctuate in predictable patterns that track with periods of ovulation (e.g., increased estradiol prior to ovulation; increased progesterone after ovulation) (see Figure 1; Harlow et al., 2012; Prior, 2006). Following premenopause, the female hypothalamic-pituitary-gonadal (HPG) axis undergoes dramatic changes, particularly in the production and secretion of ovarian hormones and variability in ovarian follicle development, ovulation, and bleeding patterns. Moreover, as a result of ovarian follicle depletion, the ovary no longer responds to key pituitary hormones (i.e., follicle-stimulating hormone – FSH; luteinizing hormone – LH) that regulate the menstrual cycle, and the production of ovarian estrogen and progesterone eventually ceases (Harlow et al., 2012). These changes associated with reproductive aging occur over the course of the menopause transition (i.e., perimenopause), menopause, and postmenopausal stages.
Figure 1. Changes in Ovarian Hormones across Reproductive Stages.
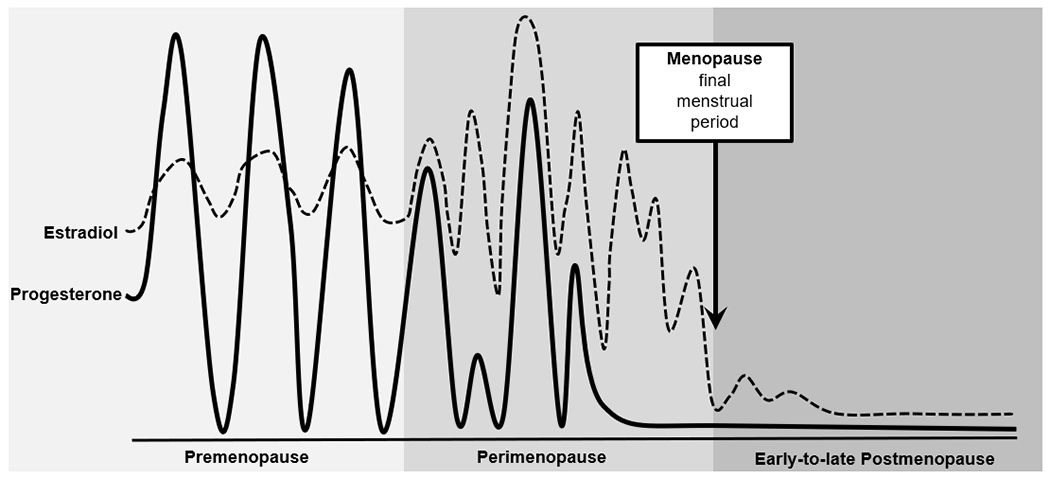
Figure modified from source: Prior (2006).
Most women begin perimenopause in their 40s; the perimenopause transition is marked by changes in menstrual cycle timing and frequency, decreased fertility, and in the vast majority of women (~80%), the onset of vasomotor symptoms and insomnia (Harlow et al., 2012; Williams et al., 2008). Specifically, women shift from premenopausal status to perimenopause once they experience a cycle length change that is ≥7 days and occurs for at least two consecutive cycles and/or have two consecutively skipped cycles (Harlow et al., 2012). The culmination of these reproductive patterns and symptoms largely arises from the increase in FSH (as the number of ovarian follicles drop) and changes in estrogen. Although estrogen declines during perimenopause, levels can dramatically flux – rising and falling unevenly, and in some cases, rising to levels even higher than in premenopause (see Figure 1; Prior, 2006). Progesterone secretion is dependent upon ovulation; in cycling women, progesterone levels rise during the post-ovulatory/luteal phase of the menstrual cycle to prepare the uterus for a fertilized egg and to help maintain early pregnancy. Thus, during the perimenopausal period, progesterone production becomes irregular and will eventually stop, as ovulation ceases (Harlow et al., 2012; Prior, 2006).
Women reach menopause when they have gone 12 consecutive months without menses (Harlow et al., 2012), typically between the ages of 45 and 55 (Boldsen & Jeune, 1990; Chan et al., 2020; Gold, 2011). Following this event, women are considered to be in the last reproductive phase – postmenopause. The postmenopausal period is characterized by low levels of estradiol and progesterone; however, some continued (albeit more intermittent) fluctuations in estradiol (see Figure 1; Harlow et al., 2012; Prior, 2006) and vasomotor symptoms continue to occur during the early postmenopause phase in most women (Williams et al., 2008). Leading guidelines (e.g., STRAW+10) therefore consider this early postmenopause period (e.g., first few years after final menses) to be a part of perimenopause (Harlow et al., 2012).
Psychosis and Menopausal Stage
As noted by others (e.g., Häfner, 2019; Kulkarni et al., 2012; Riecher-Rössler, 2009), the striking overlap between the midlife spike in schizophrenia/psychosis and age of onset of the menopausal transition (both between ages 40-50 years old) strongly suggests that processes involved in menopause may contribute to midlife psychosis risk in women. Remarkably, however, studies generally have not directly examined menopausal stage but have instead have relied on age as an indirect marker of the menopausal transition. Unfortunately, age as a proxy for menopausal changes is problematic since menopausal status cannot be reliably predicted by chronological age (Boldsen & Jeune, 1990; Chan et al., 2020; Gold, 2011; Greer et al., 2003; Stanford et al., 1987). Although most women begin experiencing perimenopausal symptoms in their 40s, there is significant variability in the age at onset and length of perimenopause (mean length = 4 years; range 6 months-8 years) and the age at which postmenopause is reached (median ~51 years; range 30-62 years of age) (Boldsen & Jeune, 1990; Chan et al., 2020; Gold, 2011; Greer et al., 2003; Harlow et al., 2012; Stanford et al., 1987). Consequently, during midlife (i.e., ages 40-60), women of the same age can be in very different menopausal stages (i.e., premenopause, perimenopause, and postmenopause). This variability makes it difficult to conclude that midlife age effects are, de facto, menopausal stage effects.
Only one prior study has explicitly tested whether the menopausal transition is associated with increased psychotic symptoms (e.g., paranoid ideation, psychotism scores; Rössler et al., 2016). This study examined women from the community who were not selected for psychosis risk and found no significant associations between psychotic symptoms and menopausal stage. However, this study also did not find consistent midlife age effects for psychotic symptoms or well-replicated age/menopausal status effects for other psychiatric symptoms (e.g., depression). These null findings lead to concerns that this smaller (N ~ 50 women per menopausal stage) unselected and community sample of participants made it difficult to detect changes in psychosis and psychiatric risk. Furthermore, the broad measure of “psychoticism” employed in this study is better described by two factors (Pedersen et al., 2016): one describing schizotypal signs and one describing core features of clinical psychosis; it is possible that combining all items into a unitary measure may have obscured true relationships between psychotic symptoms and menopausal stage.
Clearly, additional large-scale studies are needed to directly tie menopausal status to midlife psychosis risk in women. In addition to conducting in-depth staging of menopausal status, it will be important for these studies to rule out other factors that could influence or account for increased psychosis risk in women during midlife (e.g., changes in relationship status, employment, stress and distress levels, history of psychopathology; Holtzman et al., 2013; Rössler et al., 2016; Thomas et al., 2019). Arguing against a psychosocial explanation are findings from Castle et al. (1998), who showed that the increased incidence of psychotic symptoms in women during midlife were present even after adjusting for marital status and premorbid work functioning. Nonetheless, other data suggest that psychosocial distress (e.g., discontent in employment, relationships, health) and a history of psychopathology (e.g., psychotic symptoms, depression) prior to age 41 may significantly contribute to individual differences in risk for psychotic-like symptoms in midlife women (Rössler et al., 2016). Given the scant number of studies conducted, more research is needed to examine menopausal risk for psychosis in women and for whom risk may be most amplified. Moreover, men also experience changes/stressors during midlife, yet the midlife shift in psychosis risk is not observed among men. The relative influence of midlife changes/stress on psychosis risk may therefore occur via sex-specific processes (e.g., heightened female responsivity to stress that occurs because of sex-differentiated factors, like ovarian hormones; Bangasser & Wiersielis, 2018; Ter Horst et al., 2009) that would render women more vulnerable to midlife experiences of stress – a possibility that has yet to be examined but warrants further attention.
Ovarian Hormones
Perhaps not surprisingly, theories of menopausal risk have focused quite heavily on the potential role of ovarian hormones (i.e., estrogen, progesterone) in midlife psychosis risk in women. The notion that ovarian hormones might contribute to psychotic symptomatology dates back to the late 1800s and early 1900s when psychiatrists first described chronic hypoestrogenism, “menstrual psychosis,” and increases in psychosis following changes in ovarian functioning (via postpartum or ovarian surgery) (see Bergemann et al., 2007; Riecher-Rössler & Häfner, 1993).
Since that time, there has been increasing acceptance of the “estrogen protection hypothesis” that posits that estradiol’s known neuroprotective effects on cognition, presumably via its effects on neurotransmitter systems (e.g., dopamine, serotonin, glutamate, GABA), decreases female risk for schizophrenia from puberty to the start of perimenopause (Jayashri Kulkarni et al., 2012; Riecher-Rössler et al., 1994, 2018; Seeman, 1996). Estradiol has potent genomic (e.g., regulation of the expression of target genes) and rapid non-genomic (e.g., activation of signaling cascades) neuroprotective properties that have far reaching effects on hormone receptors, regulation of signaling systems, promotion of cell survival and antioxidant processes, enhancement of synaptic plasticity and connectivity, and modulation of inflammatory response (Fuentes & Silveyra, 2019; Villa et al., 2016). Thus, the dramatic fluctuations and/or drops in estradiol during the menopausal transition may leave the brain vulnerable to age-related changes in function, particularly in neurotransmitter systems that are implicated in psychosis (e.g., dopamine, serotonin, glutamate, GABA; Kulkarni et al., 2012; Stahl, 2018). The dopaminergic system may be particularly relevant given evidence of dopamine dysregulation in schizophrenia and the fact that the majority of antipsychotics drugs act via antagonism of dopamine D2 receptor or interact with serotonin receptors (5-HT1A and 5-HT2A) in combination with D2 receptor antagonism (Horacek et al., 2006; Kulkarni et al., 2012; Oyamada et al., 2015).
Surprisingly, to date, no studies have examined whether changes in ovarian hormones across the menopausal transition contribute to psychosis risk in midlife. The paucity of data is striking given past theories and the central role for ovarian hormones in menopause and the regulation of neurobiological systems in women. Nonetheless, animal and human data across other reproductive stages (e.g., menstruation) and studies of treatment effectiveness (e.g., estrogen enhancement of neuroleptics) in younger females show strong associations between lower estradiol levels and psychosis risk.
In terms of reproductive phase effects, animal studies provide strong experimental evidence of a causal link between ovarian hormones and behavioral phenotypes relevant to positive symptoms of schizophrenia. In intact cycling female rats, schizophrenia-like phenotypes (e.g., deficits in sensorimotor gating, latent inhibition) are reduced during proestrous – the estrous cycle phase that immediately precedes estrus and corresponds to the rise and peak in estradiol (Arad & Weiner, 2008; Koch, 1998; Perez et al., 2019). Similarly, ovariectomy of adult female rats, which removes the primary source of estrogen and progesterone, leads to increased expression of schizophrenia-like phenotypes (e.g., attenuated latent inhibition), whereas exogenous estradiol reverses these effects (Arad & Weiner, 2009; Sbisa et al., 2018; Vaillancourt et al., 2002; Van den Buuse & Eikelis, 2001).
Studies in humans provide direct and indirect evidence for a link between ovarian hormones and psychosis risk in women. Women with schizophrenia have high rates of menstrual irregularities and anovulation and are more likely to have circulating levels of estradiol that fall below the normal reference range (Bergemann et al., 2005; Huber et al., 2001; Riecher-Rössler et al., 1994). For example, compared to healthy controls, women with schizophrenia had lower estradiol levels across menstrual cycle days and showed less robust shifts in estradiol concentrations across menstrual cycle phases (Bergemann et al., 2005; Riecher-Rössler et al., 1994). These lower estradiol levels are unlikely to be explained by hyperprolactinemia from antipsychotic drug treatment, as some studies documenting reduced estradiol levels were conducted before the era of antipsychotics (for review, see Gogos et al., 2015); however, whether low estradiol or cycle irregularities/anovulation are driven by other co-occurring factors (e.g., extreme stress, nutritional deficits, low weight from anhedonia; Huhmann, 2020; Toufexis et al., 2014) that are associated with schizophrenia and are known to blunt ovarian hormone production remains to be determined.
Additional indirect evidence of ovarian hormone contributions to psychosis risk come from studies of menarche, pregnancy, the postpartum period, and menstrual cycle phases. Late onset of menarche can be indicative of an underlying disturbance or delay in estrogen availability (Klein et al., 2017). Investigators have therefore explored whether delayed puberty is predictive of schizophrenia and its associated neural abnormalities (e.g., connectivity impairments) that are known to be regulated/mediated by estradiol (Bähner & Meyer-Lindenberg, 2017; Gould, Woolley, Frankfurt, 1990; Woolley, 1998). Consistent with possible estrogen disturbances, later onset of menarche has been associated with increased risk for schizophrenia as well as hippocampal dysconnectivity (Cohen et al., 1999; Damme et al., 2020; Riecher-Rössler et al., 2018). While these effects are promising, the potential mechanisms by which patients with schizophrenia experience late onset of menarche are unclear. Age of onset of menarche is dependent upon a number of genetic and environmental factors, and thus, delayed timing could arise from constitutional growth delays, health/physical abnormalities leading to hypogonadism, and/or life conditions (e.g., stress, inadequate nutrition) that disrupt hormone function (Huhmann, 2020; Klein et al., 2017).
Additionally, pregnancy, the postpartum period, and the menstrual cycle produce clear shifts in ovarian hormone production that map onto shifts in psychosis risk. Women with schizophrenia show improvements in psychotic symptoms and reductions in relapse during pregnancy – a life stage when women experience substantial elevations in estradiol and progesterone (Chang & Renshaw, 1986; Jones et al., 2014; Kendell et al., 1987). Conversely, increased vulnerability to new onset or relapse of psychosis has been observed during the postpartum period when levels of ovarian hormones dramatically drop (Brockington et al., 1988; Jones et al., 2014; Kendell et al., 1987; Mahe & Dumaine, 2001; Mota et al., 2019; Perry et al., 2021; VanderKruik et al., 2017). Similar symptom shifts have been observed across the menstrual cycle in women with schizophrenia, even with the blunted hormone levels described above. An exacerbation of psychotic symptoms and risk for relapse (e.g., rate of hospital admissions) was found during menstrual cycle phases marked by lower estradiol levels (e.g., premenstrual/early follicular), whereas reductions in risk have been observed during phases marked by higher estradiol levels (i.e., mid-luteal) (Bergemann et al., 2007; Brockington et al., 1988; Endo et al., 1978; Gattaz et al., 1994; Hallonquist et al., 1993; Huber et al., 2001; Riecher-Rössler et al., 1994; Rubin et al., 2010; Seeman, 1996).
Phase-based symptom fluctuations have also been tied directly to circulating estradiol levels, providing even stronger evidence of hormone-driven changes in psychosis risk. The severity of patients’ psychotic symptoms are inversely correlated with levels of estradiol at different phases of the menstrual cycle. Specifically, in normally cycling, pre-menopausal women with schizophrenia, higher estradiol levels are associated with a reduction in psychotic symptoms, whereas lower estradiol levels exacerbate symptom severity (Bergemann et al., 2007; Huber et al., 2001; Riecher-Rössler et al., 1994). Because menstrual cycle fluctuations in ovarian hormones occur in a natural and predictive manner among women with normal cycles, it is likely that these hormone-symptom relationships reflect estradiol effects on psychotic symptom expression rather than the reverse.
Finally, studies of shifts in the effectiveness of neuroleptics by estradiol levels have provided additional support for associations between estradiol levels and psychosis. Age-based reductions in antipsychotic medication effectiveness have been observed in women, but not men; before age 40, women with schizophrenia require lower doses of antipsychotic medication yet the dose required for an effective response often increases during midlife (González-Rodríguez & Seeman, 2019; Jayashri Kulkarni, 2009; Jayashri Kulkarni et al., 2012). Key neurobiological targets of antipsychotic medications (e.g., dopaminergic, serotoninergic, and glutamatergic systems) are modulated by estradiol (Becker & Chartoff, 2019; Horacek et al., 2006; Jayashri Kulkarni et al., 2012), leading researchers to explore whether estrogen can enhance the effects of antipsychotic medications. In ovariectomized female rats, administration of estradiol facilitated the efficacy of antipsychotic medications and reversed schizophrenic-like phenotypes (e.g., attenuated latent inhibition; Arad & Weiner, 2009). A similar pattern of effects was observed according to ovarian hormone fluctuations across the estrous cycle – gonadally intact female rats displayed schizophrenic-like symptoms and a decreased response to antipsychotic at low hormonal levels (metestrus-diestrus) whereas antipsychotic medication was effective in disrupting the schizophrenic-like behavior when given in a cycle phase associated with high hormonal levels (i.e., proestrus) (Arad & Weiner, 2008).
Similarly, several clinical trials have provided strong evidence that treatment with estrogens (e.g., estradiol) or selective estrogen receptor modulators (e.g., Raloxifene) in conjunction with antipsychotic medication is beneficial for schizophrenia, in that significant improvements in positive and/or negative psychotic symptoms as well as cognitive performance have been observed in premenopausal and postmenopausal women (Begemann et al., 2012; Brand et al., 2021; Ghafari et al., 2013; Gogos et al., 2015; González-Rodríguez & Seeman, 2019; Kulkarni et al., 2015; Lindamer et al., 2001; Wang et al., 2018; Weiser et al., 2019; Zhu et al., 2021). Moreover, findings from a recent double-blind, randomized, placebo-controlled trial revealed age-based effects. Specifically, compared with placebo, women who received estradiol showed significant improvements in psychotic symptoms, but this effect was limited to those who were in a perimenopausal age range (38 to 46 years old); symptom improvements were not observed in younger women (≤ 37 years old; Weiser et al., 2019). Dose-response effects have also been found – women show greater reductions in psychotic symptoms at higher doses of estrogen (González-Rodríguez & Seeman, 2019; Kulkarni et al., 2001, 2015). Together, these findings advance understanding of the clinical relevance of estrogen deficiency in the pathophysiology of psychosis and the likely importance of estrogen augmentation for neuroleptic drug efficacy, especially for women experiencing low estradiol or estradiol declines.
In summary, animal and human data during earlier reproductive stages or across neuroleptic treatment provide initial support for the effects of lower estradiol levels on increased risk for psychosis in women. These data align with the estrogen protection hypothesis and longstanding theories proposing that the decreasing estradiol levels that characterize perimenopause could contribute to midlife psychosis risk in women. However, to date, studies have been relatively few in number, most studies have not directly examined estradiol levels, and no studies have examined whether estrogen contributes to the observed increased risk for psychosis in midlife or during the critical menopausal transition.
Importantly, there has also been a lack of consideration of progesterone in psychosis risk (Bergemann et al., 2007; González-Rodríguez & Seeman, 2019; Sun et al., 2016), including the potential interplay between estrogen and progesterone. Progesterone exerts neuroprotective effects within the CNS (e.g., promotion of cell survival, synaptic plasticity and connectivity; enhanced cognitive function; Singh & Su, 2013; Sun et al., 2016) via its involvement in the modulation of neurotrophic expression (e.g., BDNF) and several neurotransmitter systems (e.g., dopamine, serotonin, glutamate, GABA; Singh & Su, 2013) implicated in psychotic disorders. Progesterone also has strong, antagonistic effects on estrogen that alters estradiol action (Asarian & Geary, 2006; Miner et al., 2011) and its associated effects on behavior (e.g., binge eating, anxiety; Graham & Daher, 2016; Klump et al., 2013). Because women with schizophrenia have high rates of anovulation and the progesterone surge is dependent on ovulation, at least a subgroup of women with schizophrenia experience persistently low levels of progesterone. Menstrual cycle phase differences in psychotic symptom expression, as well as symptom changes associated with pregnancy and the postpartum period, also indirectly support the possibility that low progesterone could also be risky. Indeed, progesterone is elevated (in addition to estradiol) in reproductive phases (i.e., pregnancy; midluteal cycle phase) associated with reductions in psychotic symptoms, and both progesterone and estradiol are low in phases (e.g., postpartum period; premenstrual/early follicular cycle phases) associated with increased risk. Given progesterone’s effects within the CNS and the dramatic decreases in progesterone levels across the menopausal transition, future studies should aim to elucidate the potential joint effects of estrogen and progesterone on psychosis risk.
Discussion
Women show striking midlife increases in psychosis incidence and severity, which are not observed in men. These sex-differentiated patterns provide initial support for a role for the menopausal transition and ovarian hormones in psychosis risk in women. However, the paucity of data directly examining these mechanisms is surprising given extensive theories that have proposed these factors as causal. Thus, there are many gaps in the literature that are critical areas for future research.
The inability of age to reliably predict menopausal status in women highlights the strong need for studies to directly assess menopausal status. There are straightforward criteria available for staging women (e.g., STRAW+10; Harlow et al., 2012)) that do not require biological samples. These criteria rely on readily identifiable, objective physical signs of menopause (e.g., skipped periods, vasomotor symptoms). There are also easy to administer self-report questionnaires that can be used to identify menopausal stage and evaluate the severity of menopausal symptoms, which may offer additional information on the impact of the menopausal transition on psychosis risk. Future studies are strongly encouraged to directly assess menopausal status rather than rely on age as a proxy measure. Age is correlated with other factors (e.g., midlife stressors and role transitions; Thomas et al., 2019) that could enhance risk for psychosis, especially in vulnerable women (Myin-Germeys et al., 2005; Reininghaus et al., 2016). Further, although the midlife shift in risk (~ages 40-50) aligns with the general timing of perimenopausal period, there could be continuing risk into postmenopause given its hormonal milieu of chronically low estradiol (and progesterone) levels. Directly examining menopausal status and other factors associated with midlife (e.g., stressors; role transitions) would substantially increase understanding of psychosis risk in middle-aged women.
Studies directly assessing ovarian hormone mechanisms are desperately needed as well. To date, no studies have examined links between natural physiological changes in estrogen in midlife or the critical menopausal transition and psychotic symptoms. Although animal and human data during earlier reproductive stages suggest that decreasing estradiol levels contribute to psychosis risk in women, it is unclear whether effects at earlier life stages (e.g., menstrual cycle, pregnancy, and postpartum) directly translate to midlife and the menopausal transition. Additionally, not all women experience perimenopausal/postmenopausal hormonal changes in the same way, so assessing menopausal stages, in the absence of capturing individual differences in ovarian hormone effects, could obscure or hinder the detection of potential menopause-psychosis relationships. Moving forward, it will be important for future studies to use comprehensive and multiple/longitudinal measures of hormones to accurately capture the dramatic hormonal fluctuations and drops (see Figure 1) that characterize this stage of development. Ovarian hormones can now reliably be assessed in saliva multiple times per day and/or across several days, making the characterization of menopausal hormone profiles much more feasible (and accurate). These saliva sampling techniques have been used to examine ovarian hormone effects across the menstrual cycle for other phenotypes (e.g., worry, binge eating; Gloe et al., 2021; Klump et al., 2013) and would be an incredibly useful addition to the literature examining hormone influences on psychosis, particularly during menopause. In addition, there are well-documented sex differences in responsivity to stress, including heightened stress sensitivity in women, that are at least partially modulated by ovarian hormones and may help explain observed midlife increases in risk among women but not men (Bangasser & Wiersielis, 2018; Ter Horst et al., 2009; Toufexis et al., 2014). The hormonal/biological changes and symptoms (e.g., hot flashes/night sweats, sleep disturbances) that accompany the menopausal transition are also stressful and decrease wellbeing, which could further contribute to psychosis risk in women. Thus, concurrent measurement of ovarian hormones, menopausal status, and stress responsivity will likely be important for fully elucidating female-specific vulnerability to psychosis in midlife.
Finally, it would also be helpful for future studies to characterize population-level risk for psychosis during menopause and hormonal transitions. There is a surprising lack of research on psychosis risk during midlife in non-treatment seeking, community-based samples. We therefore lack critical data on midlife, perimenopausal and population-level risk. Data indicate that it is possible to examine psychotic symptoms in population-based and community samples – even symptoms that fall short of diagnostic thresholds and affect a much larger proportion of women (McGrath et al., 2015; Nuevo et al., 2012). Studies of population-based samples during the menopausal transition would provide critical data on psychosis risk that can be used in mental health and physician screenings to increase identification of midlife psychosis in women. These public health benefits, along with the potential treatment implications of estrogen modulation of psychosis risk during midlife (see section on neuroleptic treatment above), could substantially decrease the burden of mental illness in this critical area of women’s health.
Funding Statement:
This research was supported by the National Institute of Mental Health (R01 MH128196) grant awarded to KLK, KMC, and KNT.
Footnotes
Conflicts of Interest: None.
We acknowledge that sex and gender are not synonymous terms, and the extent to which these epidemiological patterns are due to biological sex differences, and/or social differences related to gender identity, is currently unclear. Herein we tend to use the term sex, rather than gender, because most studies have reported male-female comparisons according to sex assigned at birth. That said, research exploring gender identity is needed, and it is possible that examination of effects by sex versus gender identity could lead to different patterns/results (Ristori et al., 2020).
References
- Almeida DM (2005). Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science, 14, 64–68. 10.1111/j.0963-7214.2005.00336.x [DOI] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5). American Psychiatric Association. [Google Scholar]
- Arad M, & Weiner I (2008). Fluctuation of latent inhibition along the estrous cycle in the rat : Modeling the cyclicity of symptoms in schizophrenic women? Psychoneuroendocrinology, 33, 1401–1410. 10.1016/j.psyneuen.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Arad M, & Weiner I (2009). Disruption of latent inhibition induced by ovariectomy can be reversed by estradiol and clozapine as well as by co-administration of haloperidol with estradiol but not by haloperidol alone. Psychopharmacology, 206, 731–740. 10.1007/s00213-009-1464-0 [DOI] [PubMed] [Google Scholar]
- Asarian L, & Geary N (2006). Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1471), 1251–1263. 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähner F, & Meyer-Lindenberg A (2017). Hippocampal–prefrontal connectivity as a translational phenotype for schizophrenia. European Neuropsychopharmacology, 27(2), 93–106. 10.1016/j.euroneuro.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, & Wiersielis KR (2018). Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones, 17(1), 5–13. 10.1007/s42000-018-0002-z [DOI] [PubMed] [Google Scholar]
- Becker JB, & Chartoff E (2019). Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology, 44(1), 166–183. 10.1038/s41386-018-0125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann MJH, Dekker CF, Lunenburg M. Van, & Sommer IE (2012). Estrogen augmentation in schizophrenia: A quantitative review of current evidence. Schizophrenia Research, 141, 179–184. 10.1016/j.schres.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Bergemann N, Mundt C, Parzer P, Jannakos I, Nagl I, Salbach B, …Resch F (2005). Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophrenia Research, 73, 357–366. 10.1016/j.schres.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Bergemann N, Parzer P, Runnebaum B, Resch F, & Mundt C (2007). Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychological Medicine, 37(10), 1427–1436. 10.1017/S0033291707000578 [DOI] [PubMed] [Google Scholar]
- Boldsen JL, & Jeune B (1990). Distribution of age at menopause in two Danish samples. Human Biology, 62(2), 291–300. [PubMed] [Google Scholar]
- Brand BA, de Boer JN, & Sommer IEC (2021). Estrogens in schizophrenia: progress, current challenges and opportunities. Current Opinion in Psychiatry, 34(3), 228–237. 10.1097/YCO.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington IF, Kelly A, Hall P, & Deakin W (1988). Premenstrual relapse of puerperal psychosis. Journal of Affective Disorders, 14(3), 287–292. 10.1016/0165-0327(88)90046-8 [DOI] [PubMed] [Google Scholar]
- Castle D, Sham P, & Murray R (1998). Differences in distribution of ages of onset in males and females with schizophrenia. Schizophrenia Research, 33(3), 179–183. 10.1016/S0920-9964(98)00070-X [DOI] [PubMed] [Google Scholar]
- Chan S, Gomes A, & Singh RS (2020). Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women’s health. BMC Women’s Health, 20(1), 1–15. 10.1186/s12905-020-00932-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, & Renshaw D (1986). Psychosis and pregnancy. Comprehensive Therapy, 12(10), 36–41. [PubMed] [Google Scholar]
- Cohen RZ, Seeman MV, Gotowiec A, & Kopala L (1999). Earlier puberty as a predictor of later onset of schizophrenia in women. American Journal of Psychiatry, 156(7), 1059–1064. 10.1176/ajp.156.7.1059 [DOI] [PubMed] [Google Scholar]
- Damme KSF, Ristanovic I, Vargas T, & Mittal VA (2020). Timing of menarche and abnormal hippocampal connectivity in youth at clinical-high risk for psychosis. Psychoneuroendocrinology, 117, 104672. 10.1016/j.psyneuen.2020.104672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS (1990). Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. The Journal of Neuroscience, 10, 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Daiguji M, Asano Y, Yamashita I, & Takahashi S (1978). Periodic psychosis recurring in association with menstrual cycle. The Journal of Clinical Psychiatry, 39, 456–466. [PubMed] [Google Scholar]
- Fuentes N, & Silveyra P (2019). Estrogen receptor signaling mechanisms. Advances in Protein Chemistry and Structural Biology, 116, 135–170. 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattaz WF, Vogel P, Riecher-Rössler A, & Soddu G (1994). Influence of the menstrual cycle phase on the therapeutic response in schizophrenia. Biological Psychiatry, 36(2), 137–139. 10.1016/0006-3223(94)91195-9 [DOI] [PubMed] [Google Scholar]
- Ghafari E, Fararouie M, Shirazi HG, Farhangfar A, Ghaderi F, & Mohammadi A (2013). Combination of estrogen and antipsychotics in the treatment of women with chronic schizophrenia: A double-blind, randomized, placebo-controlled clinical trial. Clinical Schizophrenia and Related Psychoses, 6(4), 172–176. 10.3371/CSRP.GHFA.01062013 [DOI] [PubMed] [Google Scholar]
- Gloe LM, Kashy DA, Jacobs EG, Klump KL, & Moser JS (2021). Examining the role of ovarian hormones in the association between worry and working memory across the menstrual cycle. Psychoneuroendocrinology, 131, 105285. 10.1016/j.psyneuen.2021.105285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, & Dean B (2015). A Role for Estrogen in schizophrenia: clinical and preclinical findings. International Journal of Endocrinology, 2015. 10.1155/2015/615356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB (2011). The timing of the age at which natural menopause occurs. Obstetrics and Gynecology Clinics of North America, 38(3), 425–440. 10.1016/j.ogc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rodríguez A, & Seeman MV (2019). The association between hormones and antipsychotic use: a focus on postpartum and menopausal women. Therapeutic Advances in Psychopharmacology, 9, 1–20. 10.1177/2045125319859973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, & Daher M (2016). Estradiol and progesterone have opposing roles in the regulation of fear extinction in female rats. Neuropsychopharmacology, 41(3), 774–780. 10.1038/npp.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green IW, & Glausier JR (2016). Different paths to core pathology: The equifinal model of the schizophrenia syndrome. Schizophrenia Bulletin, 42(3), 542–549. 10.1093/schbul/sbv136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer W, Sandridge AL, & Chehabeddine RS (2003). The frequency distribution of age at natural menopause among Saudi Arabian women. Maturitas, 46(4), 263–272. 10.1016/S0378-5122(03)00215-9 [DOI] [PubMed] [Google Scholar]
- Häfner H (2019). From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: how sex , age , and other risk factors influence incidence and course of illness. Psychiatry Journal, 2019, 1–15. 10.1155/2019/9804836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H, Maurer K, Loffler W, & Riecher-Rossler A (1993). The influence of age and sex on the onset of early course of schizophrenia. British Journal of Psychiatry, 162, 80–86. 10.1192/bjp.162.1.80 [DOI] [PubMed] [Google Scholar]
- Hallonquist JD, Seeman MV, Lang M, & Rector NA (1993). Variation in symptom severity over the menstrual cycle of Schizophrenics. Biological Psychiatry, 33(3), 207–209. 10.1016/0006-3223(93)90141-Y [DOI] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, …De Villiers TJ (2012). Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause, 19(4), 387–395. 10.1097/gme.0b013e31824d8f40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman CW, Trotman HD, Goulding SM, Ryan AT, MacDonald AN, Shapiro DI, Brasfield JL, & Walker EF (2013). Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience, 249, 172–191. 10.1016/j.neuroscience.2012.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, & Cyril H (2006). Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs, 20(5), 389–409. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Emrich HM, & Schneider U (2001). Estradiol levels in psychotic disorders. Psychoneuroendocrinology, 26, 27–35. [DOI] [PubMed] [Google Scholar]
- Huhmann K (2020). Menses requires energy: a review of how disordered eating, excessive exercise, and high stress lead to menstrual irregularities. Clinical Therapeutics, 42(3), 401–407. 10.1016/j.clinthera.2020.01.016 [DOI] [PubMed] [Google Scholar]
- Jones I, Chandra PS, Dazzan P, & Howard LM (2014). Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. The Lancet, 384(9956), 1789–1799. 10.1016/S0140-6736(14)61278-2 [DOI] [PubMed] [Google Scholar]
- Jones PB (2013). Adult mental health disorders and their age at onset. British Journal of Psychiatry, 202, 5–10. 10.1192/bjp.bp.112.119164 [DOI] [PubMed] [Google Scholar]
- Kendell RE, Chalmers JC, & Platz C (1987). Epidemiology of puerperal psychoses. British Journal of Psychiatry, 150, 662–673. 10.1192/bjp.150.5.662 [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, Boydell J, …Jones PB (2012). Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PLoS ONE, 7(3), e31660. 10.1371/journal.pone.0031660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DA, Emerick JE, Sylvester JE, & Vogt KS (2017). Disorders of puberty: an approach to diagnosis and management. American family physician, 96(9), 590–599. [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, …Hu JY (2013). The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology, 122(1), 131–137. 10.1037/a0029524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M (1998). Sensorimotor gating changes across the estrous cycle in female rats. Physiology and Behavior, 64(5), 625–628. 10.1016/S0031-9384(98)00098-5 [DOI] [PubMed] [Google Scholar]
- Kühl JOG, Laursen TM, Thorup A, & Nordentoft M (2016). The incidence of schizophrenia and schizophrenia spectrum disorders in Denmark in the period 2000–2012. A register-based study. Schizophrenia Research, 176(2–3), 533–539. 10.1016/j.schres.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, Gurvich C, …Burger H (2015). Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Molecular Psychiatry, 20(6), 695–702. 10.1038/mp.2014.33 [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Riedel A, De Castella AR, Fitzgerald PB, Rolfe TJ, Taffe J, & Burger H (2001). Estrogen - a potential treatment for schizophrenia. Schizophrenia Research, 48(1), 137–144. 10.1016/S0920-9964(00)00088-8 [DOI] [PubMed] [Google Scholar]
- Kulkarni Jayashri. (2009). Oestrogen — a new treatment approach for schizophrenia? The Medical Journal of Australia, 190(S4), S37–38. 10.5694/j.1326-5377.2009.tb02373.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni Jayashri, Hayes E, & Gavrilidis E (2012). Hormones and schizophrenia. Current Opinion in Psychiatry, 25(2), 89–95. 10.1097/YCO.0b013e328350360e [DOI] [PubMed] [Google Scholar]
- Lindamer LA, Buse DC, Lohr JB, & Jeste DV (2001). Hormone replacement therapy in postmenopausal women with schizophrenia: positive effect on negative symptoms? Biological Psychiatry, 49, 47–51. [DOI] [PubMed] [Google Scholar]
- Mahe V, & Dumaine A (2001). Oestrogen withdrawal associated psychoses. Acta Psychiatrica Scandinavica, 104, 323–331. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Saha S, Al-Hamzawi A, Alonso J, Bromet EJ, Bruffaerts R, … Kessler RC (2015). Psychotic experiences in the general population: a cross-national analysis based on 31,261 respondents from 18 countries. JAMA Psychiatry, 72(7), 697–705. 10.1001/jamapsychiatry.2015.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, & Minson CT (2011). Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. American Journal of Physiology - Heart and Circulatory Physiology, 301(4), 1716–1722. 10.1152/ajpheart.00405.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota NP, Chartier M, Ekuma O, Nie Y, Hensel JM, MacWilliam L, McDougall C, Vigod S, & Bolton JM (2019). Mental disorders and suicide attempts in the pregnancy and postpartum periods compared with non-pregnancy: a population-based study. Canadian Journal of Psychiatry, 64(7), 482–491. 10.1177/0706743719838784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul P, & Van Os J (2005). Behavioral sensitization to daily life stress in psychosis. Psychological Medicine, 35(5), 733–741. 10.1017/S0033291704004179 [DOI] [PubMed] [Google Scholar]
- Nuevo R, Chatterji S, Verdes E, Naidoo N, Arango C, & Ayuso-Mateos JL (2012). The continuum of psychotic symptoms in the general population: A cross-national study. Schizophrenia Bulletin, 38(3), 475–485. 10.1093/schbul/sbq099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S, Usall J, Cobo J, Labad X, & Kulkarni J (2012). Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophrenia Research and Treatment, 2012, 1–9. 10.1155/2012/916198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada Y, Horiguchi M, & Rajagopal L (2015). Receptor antagonism reproduces atypical antipsychotic drug effects on phencyclidine-impaired novel object recognition in rats. Behavioural Brain Research, 285, 165–175. [DOI] [PubMed] [Google Scholar]
- Pedersen G, Urnes O, Kvarstein EH, & Karterud S (2016). The three factors of the psychoticism scale of SCL-90-R. Personality and Mental Health, 10, 244–255. 10.1002/pmh.1278 [DOI] [PubMed] [Google Scholar]
- Perez SM, Donegan JJ, & Lodge DJ (2019). Effect of estrous cycle on schizophrenia-like behaviors in MAM exposed rats. Behavioural Brain Research, 362, 258–265. 10.1016/j.bbr.2019.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Gordon-Smith K, Jones L, & Jones I (2021). Phenomenology, epidemiology and aetiology of postpartum psychosis: A review. Brain Sciences, 11(1), 1–14. 10.3390/brainsci11010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polachek IS, Manor A, Baumfeld Y, & Bagadia A (2017). Sex differences in psychiatric hospitalizations of individuals with psychotic disorders. The Journal of Nervous and Mental Disease, 205(4), 313–317. 10.1097/NMD.0000000000000645 [DOI] [PubMed] [Google Scholar]
- Prior JC (2006). Perimenopause lost - Reframing the end of menstruation. Journal of Reproductive and Infant Psychology, 24(4), 323–335. 10.1080/02646830600974071 [DOI] [Google Scholar]
- Reininghaus U, Gayer-Anderson C, Valmaggia L, Kempton MJ, Calem M, Onyejiaka A, … Morgan C (2016). Psychological processes underlying the association between childhood trauma and psychosis in daily life: an experience sampling study. Psychological Medicine, 46(13), 2799–2813. 10.1017/S003329171600146X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rössler A (2009). Psychotic Disorders and Menopause: The Untold Story. In Soares CN, Warren M (eds): The Menopausal Transition. Interface between Gynecology and Psychiatry. Key Issues in Mental Health. Basel, Karger, vol 175, pp 115–126. doi: 10.1159/000209606 [DOI] [Google Scholar]
- Riecher-Rössler A, Butler S, & Kulkarni J (2018). Sex and gender differences in schizophrenic psychoses—a critical review. Archives of Women’s Mental Health, 21(6), 627–648. 10.1007/s00737-018-0847-9 [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A, & Häfner H (1993). Schizophrenia and oestrogens - is there an association? European Archives of Psychiatry and Clinical Neuroscience, 242, 323–328. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A, Häfner H, Stumbaum M, Maurer K, & Schmidt R (1994). Can estradiol modulate schizophrenic symptomatology? Schizophrenia Bulletin, 20(1), 203–214. 10.1093/schbul/20.1.203 [DOI] [PubMed] [Google Scholar]
- Riecher-Rossler A, Loffler W, & Munk-Jorgnsen P (1997). What do we really know about late-onset schizophrenia? European Archives of Psychiatry and Clinical Neuroscience, 247, 195–208. [DOI] [PubMed] [Google Scholar]
- Ristori J, Cocchetti C, Romani A, Mazzoli F, Vignozzi L, Maggi M, & Fisher AD (2020). Brain sex differences related to gender identity development: Genes or hormones? International Journal of Molecular Sciences, 21(6). 10.3390/ijms21062123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler W, Ajdacic-Gross V, Riecher-Rössler A, Angst J, & Hengartner MP (2016). Does menopausal transition really influence mental health? Findings from the prospective long-term Zurich study. World Psychiatry, 15(2), 146–154. 10.1002/wps.20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajfi-Nazarloo H, Sweeney JA, & Maki PM (2010). Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia Research, 124, 13–21. 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbisa A, Van Den Buuse M, & Gogos A (2018). The effect of estrogenic compounds on psychosis-like behaviour in female rats. PLoS ONE, 13(3), 1–14. 10.1371/journal.pone.0193853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV (1996). The role of estrogen in schizophrenia. Journal of Psychiatry and Neuroscience, 21(2), 123–127. [PMC free article] [PubMed] [Google Scholar]
- Singh M, & Su C (2013). Progesterone and neuroprotection. Hormones and Behavior, 63(2), 284–290. 10.1016/j.yhbeh.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM (2018). Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis : dopamine, serotonin, and glutamate. CNS Spectrums, 23, 187–191. 10.1017/S1092852918001013 [DOI] [PubMed] [Google Scholar]
- Stanford J, Hartge P, Brinton LA, Hoover RN, & Brookmeyer R (1987). Factors influencing age at natural menopause. Journal of Chronic Diseases, 40(11), 995–1002. 10.1111/jog.14614 [DOI] [PubMed] [Google Scholar]
- Sun J, Walker AJ, Dean B, van den Buuse M, & Gogos A (2016). Progesterone: The neglected hormone in schizophrenia? A focus on progesterone-dopamine interactions. Psychoneuroendocrinology, 74, 126–140. 10.1016/j.psyneuen.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, & Lin Y (2009). Sex differences in stress responses: focus on ovarian hormones. Physiology & behavior, 97(2), 239–249. 10.1016/j.physbeh.2009.02.036 [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Mitchell ES, & Woods NF (2018). The challenges of midlife women: themes from the Seattle midlife Women’s health study. Womens Midlife Health, 4(8), 1–10. 10.1186/s40695-018-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Mitchell ES, & Woods NF (2019). Undesirable stressful life events, impact, and correlates during midlife: observations from the Seattle midlife women’s health study. Women’s Midlife Health, 5(1), 1–13. 10.1186/s40695-018-0045-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis D, Rivarola MA, Lara H, & Viau V (2014). Stress and the reproductive axis. Journal of neuroendocrinology, 26(9), 573–586. 10.1111/jne.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt C, Cyr M, Rochford J, Boksa P, & Di Paolo T (2002). Effects of ovariectomy and estradiol on acoustic startle responses in rats. Pharmacology Biochemistry and Behavior, 74(1), 103–109. 10.1016/S0091-3057(02)00967-X [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, & Eikelis N (2001). Estrogen increases prepulse inhibition of acoustic startle in rats. European Journal of Pharmacology, 425, 33–41. [DOI] [PubMed] [Google Scholar]
- Van Der Werf M, Hanssen M, Köhler S, Verkaaik M, Verhey FR, Van Winkel R, …Allardyce J (2014). Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychological Medicine, 44(1), 9–16. 10.1017/S0033291712002796 [DOI] [PubMed] [Google Scholar]
- VanderKruik R, Barreix M, Chou D, Allen T, Say L, Cohen LS, … von Dadelszen P (2017). The global prevalence of postpartum psychosis: a systematic review. BMC Psychiatry, 17(1), 1–9. 10.1186/s12888-017-1427-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Vegeto E, Poletti A, & Maggi A (2016). Estrogens, neuroinflammation, and neurodegeneration. 37, 372–402. 10.1210/er.2016-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Dong X, Wang Y, & Li X (2018). Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia : a meta-analysis of randomized controlled trials. Archives of Women’s Mental Health, 21, 31–41. 10.1007/s00737-017-0773-2 [DOI] [PubMed] [Google Scholar]
- Weiser M, Levi L, Zamora D, Biegon A, SanGiovanni JP, Davidson M, ... & Davis JM (2019). Effect of adjunctive estradiol on schizophrenia among women of childbearing age: a randomized clinical trial. JAMA psychiatry, 76(10), 1009–1017. doi: 10.1001/jamapsychiatry.2019.1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RE, Kalilani L, Dibenedetti DB, Zhou X, Granger AL, & Fehnel SE (2008). Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric, 11, 32–43. 10.1080/13697130701744696 [DOI] [PubMed] [Google Scholar]
- Woolley CS (1998). Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and Behavior, 34(2), 140–148. https://pdf.sciencedirectassets.com/272297/1-s2.0-S0018506X00X00256/1-s2.0- [DOI] [PubMed] [Google Scholar]
- Zhu X, Zheng W, Li X, Cai D, Yang X, Ungvari GS, …Xiang Y (2021). Adjunctive raloxifene for postmenopausal women with schizophrenia: a meta-analysis of randomized , double-blind , placebo-controlled trials. Schizophrenia Research, 197, 288–293. 10.1016/j.schres.2018.01.017 [DOI] [PubMed] [Google Scholar]


