Abstract
One of the most critical complications of bariatric surgery (BS), which has been widely discussed recently, is its adverse effects on the health of the bones and skeletal system. Studies show that bone mineral density (BMD) decreases significantly in the early years after BS Nutritional deficiencies are a common complication of BS that can last for months to years after surgery. For example, calcium absorption will significantly reduce after BS The role of gut hormones, endocrine factors, and adipokines in altering bone metabolism should never be overlooked. The available information and guidelines emphasize the periodic evaluation of BMD in patients undergoing BS The method of measuring BMD after BS is essential. DXA and quantitative computed tomography (QCT) are two convenient methods for measuring BMD. Many studies indicate a more detailed study of microarchitecture and cortical and trabecular bone mass with the help of QCT. The overall risk of fractures increases years after BS There are some recommendations for overcoming the adverse effects of BS on bone health. Endurance and resistance exercise after BS can help to mitigate BMD reduction and bone changes. In this review, we will explain each of these points in detail.
Keywords: Bariatric surgery, bone mineral density, quantitative computed tomography, DXA, exercise
INTRODUCTION
Severe obesity and its complications are among the most critical causes of public health and one of the leading preventable causes of death worldwide. Physicians consider this problem to be one of the most severe public health problems of the 21st century.[1,2] This disease is the cause of many diseases such as cardiovascular diseases, Type II diabetes, sleep apnea, some cancers, and osteoarthritis.[3,4,5] Diet and exercise together can control overweight and related disorders, but the most effective obesity treatment is bariatric surgery (BS). This operation is the most effective treatment for weight loss, recovery from dyslipidemia, type II diabetes, high blood pressure, and fatty liver disease after noninvasive therapies for patients with body mass index (BMI) ≥40 kg/m2. Hence, it should be along with training in nutritional and close follow-up.[1] More than 20 years have passed since the 1991 National Institutes of Health Consensus Development Conference statement on gastrointestinal surgery to treat severe obesity. However, BMI is still the essential factor in determining patients who are candidates for these surgeries. In general, accepted criteria for surgical treatment include a BMI higher than 40 kg/m2 or a BMI higher than 35 kg/m2 in combination with high-risk comorbid conditions or lifestyle-limiting obesity-induced physical conditions.
Unfortunately, this treatment is not without side effects. Adverse effects can be gastroesophageal reflux, abdominal pain, malnutrition and deficiency of vitamins and minerals, gallstones, and surgical complications.[6,7,8]
One of the most critical complications of this surgery, which has been widely discussed recently, is its adverse effects on the health of the bones and skeletal system, which have been discussed in many articles.[5,6,7,8,9,10,11] In addition, the side effects of BS can have devastating effects on bone and mineral metabolism.[12] After BS, the bone loss is due to several factors that we will discuss in this article.[10] For example, one of them is the type of operation chosen. Several review articles have noted the negative effect of Roux-en-Y gastric bypass (RYGB) on skeletal system health. However, this type of surgery is still increasingly used to treat obesity.[11,13]
There is not much information about all these interpretations, especially about the pathophysiology of bone loss and diagnostic methods for this complication after BS. Therefore, the primary purpose of this study was to provide a summary of recently published information on the impact of BS on patients’ bone health, including; investigation of pathophysiology and mechanisms and fracture risks of bone loss along with review and comparison of diagnostic methods and evaluation of bone health in patients undergoing BS. Finally, we will discuss the prevention and treatment methods following BS according to the latest studies and published guidelines.
MATERIALS AND METHODS
A literature search was carried out based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.[14] We conducted a systematic search using PubMed, Google Scholar, and Scopus, which was consulted for articles published by May 2021. We used Mesh terms and keywords which included “Sleeve Gastrectomy,” “One Anastomosis Gastric Bypass,” “Roux-en-Y Gastric Bypass,” “OAGB,” “RYGB,” “Bone Loss,” “Bone metabolism,” “Nutrition,” “Bone Mineral Density,” “QCT,” “Quantitative CT,” “DXA,” “Fracture Risk,” “Exercise,” or a combination of them in the titles or abstracts. Studies published before January 2015 were excluded. Two of the authors independently assessed the eligibility of the papers according to the PRISMA guidelines. The references of the articles were manually reviewed for additional relevant documents. The duplicate studies were removed. We updated a previous search on nutrient replacement guidelines following bariatric surgery. Finally, we summarized the results of studies by topic [Figure 1].
Figure 1.
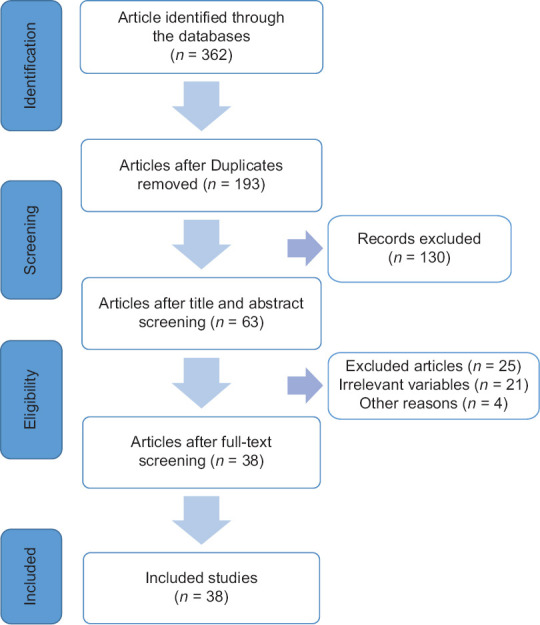
Preferred reporting items for systematic reviews and meta-analyses
AN OVERVIEW OF AVAILABLE METHODS OF BARIATRIC SURGERY
BS is divided into two categories of restrictive and malabsorptive operation. The main effect of restrictive surgeries is to reduce the amount of food consumed by reducing the volume of the stomach. Whereas in malabsorptive procedures, a part of the small intestine is bypassed from the digestive system. Therefore, the success rate of BS depends on the patient's efforts to follow a diet and reduce the amount of food consumed.[15,16,17]
According to these points, BS can be divided into four primary operations; adjustable gastric banding (AGB), sleeve gastrectomy (SG), RYGB, and Biliopancreatic Diversion with Duodenal Switch (BPD-DS) [Figure 2].[18]
Figure 2.
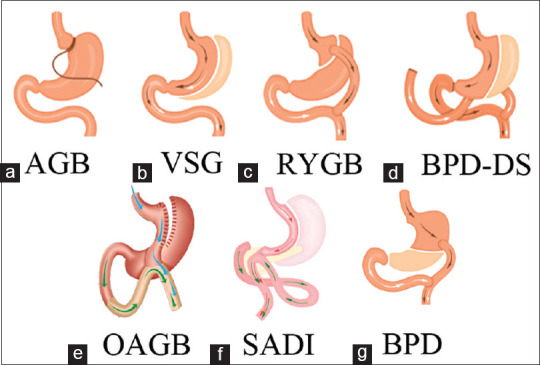
B.S. procedures in order of malabsorption; (a) Adjustable Gastric Band (AGB), (b) Vertical Sleeve Gastrectomy (VSG) or Sleeve Gastrectomy (S.G.), (c) Roux-en-Y Gastric Bypass (RYGB), (d) Biliopancreatic Diversion with a Duodenal Switch (BPD-DS), (e) One Anastomose Gastric Bypass (OAGB), (f) single-anastomosis duodenal-ileal switch (SADI), and (g) Biliopancreatic Diversion (BPD)
ADJUSTABLE GASTRIC BANDING
AGB is a less invasive procedure than gastric bypass (GB) and SG. In AGB, a silicone elastomer ring with an internal inflatable wall is approximated one cm below the gastroesophageal junction.[18] This method has almost no postoperative mortality, and post-operative morbidity is minimal. According to the results, a 6-year follow-up causes 53%–57% weight loss. As mentioned; adjustability and reversibility are significant advantages over most other methods.[19] The mechanism of AGB is produced by reducing the volume of the gastric inlet. However, due to the high recurrence of weight gain and the side effects of banding (erosion, slippage, and prolapse) in long-term use, the tendency to perform this operation has decreased.
SLEEVE GASTRECTOMY
In this type of surgery, removing a large part of the stomach longitudinally (at 5–6 cm from the pyloric valve to the fundus near the cardia) makes the stomach lose more than 85% of its original size. As a result, a sleeve or tube-like structure remains, with an approximate size of about 150 ml.[20] It also causes hormonal changes such as decreased ghrelin secretion. Complications include bleeding and leakage after surgery. The 90-day mortality is 0.06%.[21]
ROUX-EN-Y GASTRIC BYPASS
In this surgery, the stomach is divided into a small gastric pouch (20 mL) at the top and a much larger “remnant” sac at the bottom. The upper part is connected to the small intestine just after the duodenum by the Roux or alimentary limb. Surgeons have developed several different methods for intestinal reconnection, each of which results in a significant reduction in functional gastric volume.[22] This treatment is the gold standard in BS[23] RYGB effects include; decreased stomach volume, moderate absorption, and hormonal changes. In addition, absorption of undigested nutrients in the gastrointestinal tract leads to the secretion of incretins, loss of hunger, and a significant improvement in blood glucose homeostasis from the day of surgery.[24]
According to the results, this type of surgery improves fatty liver disease, osteopathy, and liver fibrosis.[25,26] Acute complications of this operation include peritonitis, anastomotic leakage, and bleeding. Its long-term effects are intestinal obstruction (internal hernia, bezoar) and nutrient deficiencies.[24]
BILIOPANCREATIC DIVERSION WITH A DUODENAL SWITCH
It is a less standard weight loss procedure that involves two essential steps. The first stage is an SG, in which about 85% of the stomach is removed, and a smaller tubular stomach remains. However, pyloric valve remains with the limited part of the small intestine that generally connects to the stomach (first part of duodenum).
The second stage bypasses most of the intestine by connecting the end of the intestine to the first part of the duodenum (duodenoileostomy).[27] In addition to reducing the volume of food, this action also reduces the absorption of nutrients, including proteins and fats. Although this surgery is very effective, it will have more risks, including malnutrition and vitamin deficiencies.[21] These risks make this method rarely performed and is usually recommended for people with a BMI >50.[28]
NEW PROCEDURES
Due to the possibility of weight gain after long-term follow-up of GB patients and the high complications of BPD-DS, surgeons tried to eliminate this problem. A single-anastomosis duodenal-ileal bypass with sleeve (SADIS) was introduced as a life-saving method in BS. To solve the problem, according to studies, promising results were seen in patients losing weight and reducing comorbidity, but the prevalence of macro-nutrient deficiency was also seen following SADIS.[29] SG and transit bipartition (SG + TB) is another new method of BS that leads to early satiety and rapid and continuous weight loss, low complication rate, and lack of absorption. This strategy leads to remission or significant improvement in comorbidities, including diabetes, without removing the duodenum.[30] T. B. is an excellent supplement for SG With all these interpretations, the information obtained about these surgical procedures is limited and requires further study. Omega bypass surgery (one anastomose gastric bypass [OAGB]) is another new method of BS. The gastric pouch is created with a higher height than the gastric pouch in RYGB surgery in this method. Anastomosis is performed by loop gastrojejunostomy at a distance of 150–200 cm from the ligament of Treitz. Weight loss in this procedure is significantly more than RYGB surgery, and due to a fewer anastomosis than RYGB, the duration of surgery is shorter.
BARIATRIC SURGERY EFFECTIVENESS AND LIMITATIONS
Deficiencies of vitamins and other nutrients such as protein, iron, Vitamin B12, folate, calcium, and fat-soluble vitamins are common following RYGB, BPD, and BPD-DS surgeries. However, these deficiencies appear to be more significant in malabsorptive procedures (such as BPD) than restrictive procedures.[31] In addition, studies show that bone mineral density (BMD) decreases significantly in the early years after BS. Also, in a study to evaluate long-term bone loss after RYGB and SG, the rate of bone loss during the 1st year was higher than in subsequent years.[32] the types of obesity surgeries based on risks, limitations and effectiveness can be compared and examined in Table 1.[33]
Table 1.
The bariatric procedure is based on risks, limitations, and effectiveness
| Procedure[34] | Percentage TWL | Effectiveness | Risks and limitations |
|---|---|---|---|
| LAGB | 20%-25% | No anatomic alteration Removable Adjustable |
High explant rate Erosion Slip/prolapse |
| S.G. | 25%-30% | Easy to perform No anastomosis Reproducible Few long-term complications Metabolic effects Versatile for challenging patient populations |
Leaks challenging to manage Little data beyond 5 years 20%-30% GERD |
| RYGB | 30%-35% | Strong metabolic effects Standardized techniques<5% significant complication rate Effective for GERD Can be used as the second stage after S.G. |
Few proven revisional options for weight regain Marginal ulcers Internal hernias possible Long-term micronutrient deficiencies |
| BPD/DS | 35%-45% | Powerful metabolic effects Durable weight loss Effective for patients with very high BMI Can be used as the second stage after S.G. |
Malabsorptive 3%-5% protein-calorie malnutrition GERD Potential for internal hernias Duodenal dissection Technically challenging Higher rate of micronutrient deficiencies than RYGB |
| OAGB | 35%-40% | Simpler to perform than RYGB More malabsorptive Strong metabolic effects No mesenteric defects |
Potential for bile reflux Malabsorptive (long BP limb) Little experience in U.S. |
S.G.: Sleeve gastrectomy, RYGB: Roux-en-Y gastric bypass, LAGB: Laparoscopic adjustable gastric band, BPD-DS: Biliopancreatic diversion with a duodenal switch, OAGB: One anastomose gastric bypass, BMI: Body mass index, GERD: Gastroesophageal reflux disease, BP: Biliopancreatic, TWL: Total Weight Loss
BONE HEALTH AND BARIATRIC SURGERY
Bone loss mechanism after bariatric surgery (pathophysiology)
In addition to maintaining the body's integrity, the skeletal system and bones are also part of the human body's endocrine system. As a result, the bones are constantly remodeling and are wholly renewed.[35] According to research, the human body's skeletal system is entirely restored almost every 10 years, but this renewal rate is faster in the spongy parts than in the bone marrow.[36]
Researchers have attributed the higher BMD in obesity to higher bone mechanical load and hormonal activity. Undoubtedly, other genetic and environmental factors such as diet, exercise, and smoking affect BMD, too.[37] According to recent studies, being overweight due to fat may be detrimental to bone health. Factors such as increased adipogenesis in bone marrow (decreased osteoblastogenesis), the activity of inflammatory cytokines (c-reactive protein, interleukin-6 [IL-6], and tumor necrosis factor-alpha [TNF-α]), excessive leptin secretion, and decreased adiponectin are involved in this process.[37,38] With all these interpretations, the relationship between body mass and skeletal status is very complicated. It is not clear to researchers that obesity is a factor in strengthening bone mass or vice versa. According to studies, the rate of BMD reduction with weight loss varies in different populations, but there is a prominent point in this research; weight loss is associated with significant BMD reduction in postmenopausal women and older men.[39,40] Numerous factors and their interactions are involved in changes in the skeletal system during weight loss, but the effects of some of them are not yet fully understood.[41] Undoubtedly, metabolic changes affecting bone loss after surgery occur more extensively and rapidly than standard weight loss.
On the other hand, these rapid changes are accompanied by minimal food intake, micronutrient absorption, and other metabolic changes.[42] Researchers attribute the leading causes of postoperative bone loss mechanisms to mechanical unloading and reduced initial absorption of calcium and vitamin D along with hormonal changes (gut and gonadal hormonal changes). Still, factors such as lean mass loss and increased bone marrow fat are also involved.[43,44]
With all these interpretations, bone loss depends on several different mechanisms, and relatively conflicting information is available on these topics [Figure 3].
Figure 3.
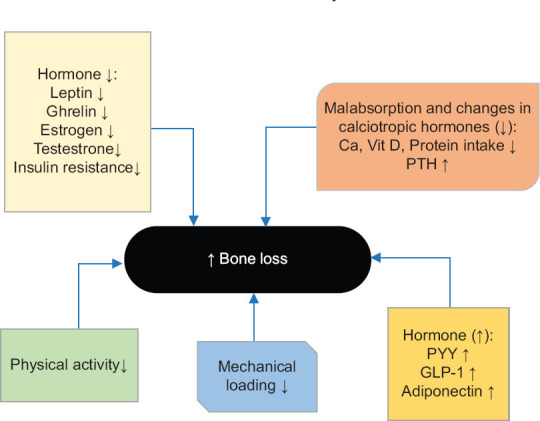
Numerous internal and external factors, including; diet, mechanical loading, hormones, and genetics, play a role in bone health sustainability[149]
MECHANICAL UNLOADING
The size, mass, and biomechanical properties of human bones are affected by mechanical loading. Changes in mechanical loading cause localized bone remodeling by osteocytes through the sclerostin pathway.[45] In this regard, the results of a prospective study of 90 premenopausal women who underwent RYGB and SG showed a direct relationship between increased sclerostin levels and bone loss.[46] It has been seen in situations such as; spinal cord injuries, orthopedic surgeries, bed rest, and astronautics with a reduction in mechanical loading; a significant bone loss has occurred.[47,48,49] Weight loss will be followed by a decrease in bone mechanical loading, which will intensify when not accompanied by physical activity and exercise.[42] Mechanical strain, induced by the weight to the skeletal system during activity and exercise, maintains BMD.[50] In this regard, most studies have reported a strong association between weight loss after BS and bone loss.[51,52,53,54] A study of patients undergoing RYGB surgery found that; Weight loss is strongly associated with bone loss in total hip (TH): (r = 0.65, P = 0.02) and femoral neck (FN): (r = 0.90, P < 0.0001). Another study was performed 2 and 5 years after RYGB surgery; despite constant weight after the 1st year of surgery, a greater decrease in volumetric BMD was reported in the radius bone than in the tibia.[55] Accordingly, later declines may not be a direct result of unloading.[56] In another study using high-resolution peripheral quantitative computed tomography (HR-pQCT), significant changes were observed in the tibia bone, while these changes were not significant in the radius bone 1 year after surgery.[9] These different findings suggest that mechanical unloading affects bone more likely in the 1st year after surgery when maximum weight loss occurs. Long-term changes may be due to an interaction between parathyroid hormone (PTH) and mechanical loading.[56]
NUTRITIONAL DEFICIENCY
Studies have shown that obesity is closely linked to a low micro- and macro-nutrient level. Deficiencies of calcium, protein, and Vitamin D have been observed in these individuals.[57] Despite the success of bariatric surgeries in weight loss, there are many complications in the postoperative period. Nutritional deficiencies are a common complication of BS that can last for months to years after surgery.[58] Deficiencies of vitamins and other nutrients such as protein, iron, Vitamin B12, folate, calcium, and fat-soluble vitamins are common following RYGB, BPD, and BPD-DS surgeries. However, these deficiencies appear to be more significant in malabsorptive methods such as BPD than restrictive procedures.[31] RYGB not only causes vitamin D malabsorption but also reduces calcium absorption by bypassing the duodenum and proximal jejunum, which are the predominant sites of calcium uptake (active, transcellular, 1,25(OH) D-mediated calcium uptake).[59] However, calcium absorption is not only limited to these parts, indeed calcium can be absorbed throughout the gut, and those undergoing RYGB may have adequate calcium uptake. Based on a study that examined the effects of RYGB on intestinal fractional calcium absorption (FCA), FCA decreases dramatically after RYGB even with calcium intake and 25(O. H.) D ≥30 ng/ml level. The authors advised the patients to increase their calcium intake for preventing calcium-induced disorders and maintaining calcium homeostasis. In general, approaches to calcium supplements after BS needs further study.[59] In another study, researchers found that calcium absorption was significantly reduced after SG surgery as well.[60] SG surgery affects bone metabolism through several mechanisms; decreased mechanical unloading following weight loss, decreased ghrelin secretion, decreased calcium absorption due to decreased gastric acid secretion, and increased gastric emptying rate.[61]
Based on a study that assessed the level and absorption of Vitamin D after BS, the results showed that Vitamin D absorption is also reduced, and the mean serum 25(O. H.) D level remains less than 30 ng/ml despite the use of a Vitamin D-containing diet. Vitamin D deficiency was compensated by increasing Vitamin D supplementation to 1500–9100 units/day.[62]
In general, the results of studies show that despite adequate levels of Vitamin D (30 ng/ml) and recommended calcium intake (1200 mg/day) with the help of diets and calcium citrate supplements, calcium absorption in the intestine remains much lower than average in patients undergoing RYGB surgery 6 months after surgery.[59]
To maintain calcium homeostasis in addition to PTH and 1,25-dihydroxy vitamin D, several endocrines, and paracrine factors such as prolactin, estrogen, and insulin-like growth factor directly stimulate intestinal calcium absorption. This cycle is controlled by negative self-regulation. Accordingly, any error in the mentioned processes will alter the serum calcium concentration. A significant point is a decrease in calcium absorption by intestinal epithelial cells at high calcium concentrations in the gastrointestinal tract.[63] This explanation may justify the ineffectiveness of taking oral calcium supplementation in patients that require calcium injections.
HORMONAL CHANGES
Gut hormones
BS dramatically increases bone turnover, decreases BMD, and increases the prevalence of fractures in these patients.[10,37,64,65] The role of gut hormones in altering bone metabolism should never be overlooked.[66,67] Gut hormones play a significant role in patients benefiting from BS, especially RYGB, and studies in this field support the “gut-bone” theory.[68,69] Among the Gut hormones, peptide YY (PYY) and ghrelin may be involved in changes in the body's skeletal system after BS.[70]
Peptide YY
PYY is a peptide with 36 amino acids that is secreted by intestinal L-cells in the terminal ileum and colon. The most crucial factor for this peptide secretion is the stimulation of these cells by food. This hormone induces satiety and is very important in regulating energy homeostasis.[71] This hormone secretion is reduced in obese people.[72] According to animal studies, an inverse relationship was found between PYY overproduction and bone mass formation. Deficiency of this hormone was associated with the expansion and strengthening of bone mass in mice.[73] PYY affects the Y1 receptors of osteoblasts, and it has been shown that deletion of this receptor increases bone mass.[74]
In a study that examined changes in fasting serum PYY with bone turnover marker levels (Tartrate-resistant acid phosphatase, C-terminal telopeptide of type 1 collagen (CTx), and N-terminal telopeptide of type I collagen) and BMD loss after RYGB, the 6-month changes of these markers and BMD (using DXA and QCT) were analyzed. The results of this study showed that the increase in PYY after surgery was associated with a weak rise in procollagen 1 N-terminal propeptide (a marker of bone formation) and a further decline in spinal BMD (measured by QCT). These results clearly show the role of PYY in bone loss after RYGB and the relationship between intestinal and skeletal metabolism.[70]
Ghrelin
Ghrelin is a peptide with 28 amino acids.[75] Despite the adverse effects of PYY on bone mass, ghrelin may stimulate bone formation. Ghrelin is secreted by X-A cells of the oxyntic glands in the gastric fundus. The secretion of this hormone increases with prolonged fasting and strengthens hunger. Therefore, this hormone also plays an essential role in regulating energy homeostasis.[75,76]
Ghrelin can bind to the growth hormone secretagogue receptor 1a,[77] so it may cause the bone formation and increase bone mass by affecting growth hormone and the insulin-like growth factor-1 (IGF-1) secretion.[78] Ghrelin also directly stimulates osteoblast proliferation and differentiation in vitro. Intraperitoneal injection of this hormone increased BMD in rats.[79] However, human studies have not yet found a clear association between serum ghrelin levels and BMD.[80] Based on current studies, plasma levels of ghrelin are reduced in most cases after BS, especially RYGB, but surprisingly, there is no correlation between Ghrelin levels and bone parameters.[70,81,82]
Others Gut Hormone
Levels of some other gut Hormones, such as glucagon-like peptide-1 (GLP-1), show extensive changes after RYGB, SG, and BPD-DS surgeries. Although these hormonal changes have many metabolic benefits, bone mass loss is also reported.[83,84,85] Furthermore, glucose-dependent insulin tropic polypeptide is a peptide with 42 amino acids secreted by K-cells in the proximal small intestine. According to laboratory results, this peptide can inhibit osteoblast apoptosis.[86] Limited studies have shown a decrease in the synthesis of this peptide after RYGB surgery.[87]
ENDOCRINE FACTOR
Estrogen
Estrogen is one of the sex hormones responsible for creating the female reproductive system and secondary sexual characteristics.[88] Estrogens play an essential role in growth spurts during puberty, accelerating linear growth and closing the epiphysis. Therefore, these hormones have stimulating effects on the skeletal system of the body. Published studies confirm the protective effects of these hormones on the maintenance of bone mass. They are one of the most important reasons for maintaining BMD throughout life, and hypoestrogenism is one reason for the increased risk of osteoporosis during menopause.[89,90,91] A study evaluated the effects of BS (VBG) and estradiol levels on bone metabolism simultaneously. The results showed that the estradiol level in patients who underwent BS showed decreased estradiol (sex steroids) compared to patients who underwent medical treatment. According to studies, adipose tissue converts testosterone to estradiol by the aromatase enzyme.[92] However, the estrogen therapy study results in postmenopausal women did not show beneficial effects on callus BMD (evaluated by QCT). Extensive studies are needed in this field.[93]
Testosterone
Testosterone is an essential steroid hormone in humans with wide androgenic (gender) and anabolic (constructive, growth) effects. Age-related testosterone deficiency is one of the most important causes of bone loss in older men.[94] According to published studies, treatments using testosterone reduce fat mass (abdominal adipose tissue) and increase hip BMD.[95] Androgens can be converted to estrogen by binding directly to androgen receptors or estrogen receptors (through aromatization) and affect bone health. Cellular studies show that androgen causes the proliferation of preosteoblasts and the differentiation of osteoblasts.
On the other hand, by converting to estrogen in adipose tissue or at the surface of receptors, it suppresses osteoclast formation and absorption activity. Thus, estrogen is needed to inhibit bone resorption, but androgen and estrogen are essential for both to build bone mass.[96] Other activities of androgens in regulating bone metabolism include their effects on cytokines, such as the regulation of growth factor-beta, IGF-1, and interleukin-6.[97] These cytokines, which stimulate osteoclast activation, have higher levels in obese individuals than normal BMIs.[98]
According to studies, male hypogonadism is associated with obesity. For example, a study looked at testosterone levels in patients with severe obesity who underwent BS. The results showed an increase in blood testosterone levels after BS.[99] In another study, testosterone levels returned to normal after RYGB surgery.[100]
Insulin
A peptide hormone produced by the pancreatic islet beta cells is the primary anabolic hormone in the body. According to studies, BMD is higher in menopausal women and men who have been resistant to insulin than others. Furthermore, these studies did not report evidence in favor of the association between insulin resistance and increased fracture risk after BMI and BMD adjustment.[101,102,103] Another study was conducted to investigate the relationship between BMD and insulin resistance in women with polycystic ovary syndrome. Insulin resistance was introduced as a protective factor against bone mineral loss in these women.[104] With all these interpretations, the link between BMD and obesity and insulin is still unclear.
In one study, lowering blood insulin levels and improving insulin sensitivity after RYGB surgery were identified as detrimental factors for bone tissue.[105] Other studies have shown an improvement in insulin sensitivity from a few days to several months after RYGB, SG, and BPD-DS surgeries with increased 5’ AMP-activated protein kinase activity. BPD cures insulin resistance faster than other surgeries.[106] According to studies, bariatric surgeries can have adverse effects on bone mass by improving and treating insulin resistance. However, to achieve this certainty, more extensive studies are needed.
Adipokines
Adipokines, or adipocytokines, are secreted from adipose tissue and first discovered in 1994.[107] So far, over 100 Adipokines have been found. Essential adipokines are leptin, adiponectin, IL-6, TNF-α, visfatin, and vaspin.[108,109] On the other hand, bone cells secrete various proteins called osteokines to maintain bone homeostasis, energy, and glucose. Thus, the body's relation between adipose and bone tissue forms a hemostatic feedback system associated with each other by adipokines and osteokine.[11] According to studies, adipokines, especially leptin and adiponectin secreted by adipose tissue, substantially affect bone mass.[110]
Leptin
Leptin is a cytokine-like hormone secreted by fat cells.[111] For this reason, the more adipose tissue a person has, the more leptin he will release.[112] Leptin plays a vital role in feeling full and hungry, so this hormone is also essential in energy consumption and energy balance.[113] In addition to establishing energy homeostasis, this hormone plays a critical role in regulating nerves, endocrine glands, and bone metabolism.[114] Various studies have reported different effects of this hormone on BMD and bone metabolism.[115] According to studies, leptin regulates bone mass by directly affecting bone cells and indirectly affecting the hypothalamus.[116] Studies to investigate the direct effects of leptin on bone tissue in mice showed that leptin increases bone mass by inhibiting osteoclast differentiation and not stimulating osteoblast differentiation.[103,117,118] However, in another study, leptin was introduced as an anti-osteogenic substance that only affects trabecular bone mass.[119] In some other independent studies, leptin deficiency was associated with a decrease in BMD.[120,121,122] The results of a recent meta-analysis of studies by Liu et al. leptin intake in postmenopausal women is positively associated with increased BMD and bone mineral content (BMC).[123]
Changes in this hormone are pretty evident after RYGB surgery. The results of studies show that leptin decreases rapidly after BS. Assuming that leptin levels decrease after BS, the direct stimulatory effects of this hormone on bone cells will be reduced, followed by a decrease in bone mass in BMD and BMC.[124]
Adiponectin
Adiponectin is a peptide with 244 amino acids produced and secreted by fat cells.[125] The secretion of this hormone is inversely related to obesity and central fat.[126,127] In addition to increasing insulin sensitivity and reducing the incidence of diabetes, this hormone-like also has anti-inflammatory and cardioprotective effects.[11,128] This hormone stimulates osteoblastogenesis and inhibits osteoclastogenesis in vitro, but its impact on the body appears different. In most studies, an inverse relationship between adiponectin levels and BMD, independent of BMI, has been reported.[129]
Studies have examined adiponectin levels after BS, such as RYGB, confirm an increase in postoperative hormone levels[105] and present this hormone as an independent factor in reducing BMD[130] However, in another study, researchers did not find a link between adiponectin levels and bone turnover marker after RYGB surgery.[131] Based on the discrepancies in the study results, it seems that this issue still needs more comprehensive investigation.
Others adipokine
Inflammatory cytokines such as TNFα and IL-6 are among the most well-known activators of osteoclasts and BMD degraders, with higher levels in obese individuals.[132,133] Studies have shown that these inflammatory cytokines decrease after BS, especially SG and RYGB, and may positively affect BMD.[134]
Cysteine-rich Visfatin and Resistin, like other adipokines, affect the skeletal system. In one study, Visfatin was introduced as an independent predictor of BMD.[135] Another study between Visfatin and BMD found an inverse relationship between Visfatin and BMD.[136] Overall, reports of adipokines such as Visfatin and Resistin indicate different effects on BMD and are generally expected to inhibit bone mass.[11] Furthermore, in the study that examined the level of Resistin before and after GB surgery, no significant changes were observed.[137]
EXERCISE AND BONE HEALTH
Various treatments, including nutritional, pharmacological, psychological therapies, supplements, and surgery, are performed to combat obesity.[138,139,140] No nonsurgical intervention is as effective as BS.[15,140,141] However, surgery never eliminates unhealthy habits.[142] Therefore, changing lifestyle and having a healthy eating pattern and good physical activity will undoubtedly improve surgeries outcome.[143]
Physical activity after BS plays a vital role in eliminating lean body mass.[141] Exercise in patients undergoing BS can cause weight loss of more than 4% of BMI.[144] According to studies, exercise and physical activity are associated with higher BMD, and the least practical effect on BMD was reported 4 h/week.[145] Studies suggest exercise as a treatment for bone loss due to BS.[146]
Physical activity includes endurance and stretching-resistance exercises. Many studies have shown the positive effects of endurance (aerobic) exercise after BS limited information is available on stretching-resistance exercises. Gozde et al. studied aerobic exercise (AE group) with aerobic plus progressive-resistance exercise (AEPR group) in a 12-week program after BS Weight loss, muscle mass, function capacity, and upper-body strength were significantly increased at the end of the 12th week in the AEPR group compared to the AE group.[147]
A clinical trial also showed that appropriate physical activity could be considered an effective strategy to improve bone health in patients undergoing BS.[146] Therefore, researchers suggest that physical activity be added to the treatment process after BS, as these activities improve body composition, BMD, increased muscle strength, and physical fitness.[148] Researchers state that exercise should focus on fat mass, muscle mass, and endurance exercise capacity.[144]
In general, in patients undergoing BS, exercise interventions under the supervision of a physician have many benefits. Some benefits include a sense of self-efficacy, motivation, increased functional muscle strength and walking capacity, insulin sensitivity, and most importantly, maintaining BMD.[141,146,148] Table 2 lists the endurance and resistance exercises before and after surgery in detail.
Table 2.
Exercise before and after bariatric surgery
| Endurance exercise | Resistance exercise |
|---|---|
| Before BS | |
| 20%-45% min/session | <60% of 1-repetetion maximum (1 RM) |
| 45%-54% peak HR | 12-15 repetitions ≥6 muscle group |
| 2%-3% days/week | 3 series/large muscle 2-3 days/week |
| After BS | |
| Weekly exercise volume up to 250-400 min | Moderate to high-intense resistance exercise (≥70% of 1 RM) |
| Moderate effort (50%-70% peak HR) Involving large muscle (walking, rowing,.) 3 up to 5 days/week |
12-15 repetitions for 3 series/session Targeting large muscle groups (femoris, hamstring, calfs, abdominal and back muscle, …) |
BS: Bariatric surgery, 1 RM: Repetitions maximum, HR: Heart rate
DIAGNOSTIC METHODS
The risk of fracture in people who have undergone various BS procedures is 2.3 times higher than normal population. Therefore, accurate measurement of bone mass and BMD is significant to assess and manage bone health accurately.[150]
The available information and guidelines emphasize the periodic evaluation of BMD using DXA in patients undergoing BS.[151,152] Any imaging technique has its limitation and benefits.[10] Most clinical studies have used the DXA to assess BMD, although this method may not report BMD correctly due to changes in the soft tissue around the bone.[153,154]
DXA is used to determine BMD for the diagnosis of osteopenia and osteoporosis.[155] BMD is compared with two norms-healthy young adults (T-score) and age-matched adults (Z-score). A T-score of 1–2.5 standard deviation (SD) below the young adult mean (−1 to −2.5 SD) indicates osteopenia.[156] Whereas, A T-score of 2.5 SD or more below the young adult mean (more than-2.5 SD) indicates the presence of osteoporosis.[157]
Studies using DXA have shown BMD reduction after BS Studies have discussed densitometry changes based on site (spine vs. hip), type of surgery (RYGB, SG, and OAGB), follow-up duration (short term vs. long term), and age of patients.
Different BMD changes have been reported after various types of BS. According to the results, the rate of BMD reduction after RYGB is also different in multiple sites. For example, a decrease in BMD in the 1st year after surgery in the lumbar spine (LS) has been reported to be 3%-7% and 8%-11% in hips. Areal BMD (aBMD) changes in LS are less affected by RYGB than in TH. In a meta-analysis, V Jaruvongvanich et al. discussed BMD changes after LSG surgery. Significant reductions were observed in aBMD at FN and TH, while no difference in LS was observed [Table 3].[164]
Table 3.
Studies were evaluating bone mineral density after bariatric surgery
| Time of Study | Author | Year | Surgery | Follow up (months) | BMD loss (%) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| F.N. | T.H. | L.S. | |||||
| Short term follow up | Chen et al.[158] | 2020 | LSG | 12 | 6* | 10.5 | 0.9 |
| Gerber P et al.[159] | 2021 | LSG/RYGB | 12 | LSG <RYGB | - | LSG <RYGB | |
| Luger et al.[160] | 2018 | OAGB | 12 | - | 13 | 7 | |
| Ieong et al.[161] | 2021 | RYGB | 24 | 9 | 8 | 3 | |
| LSG | 7 | 10 | 1 | ||||
| LSG/RYGB | NS | NS | NS | ||||
| Long term follow up | Brzozowska et al.[162] | 2021 | LSG | 36 | - | 9 | 0.1 |
| RYGB | - | 14 | 7.2 | ||||
| LSG/RYGB | - | LSG <RYGB | LSG <RYGB | ||||
| Cadart et al.[32] | 2020 | LSG | 48 | 8.1±5.5 | 7.4±6.4 | 2.0±7.2 | |
| RYGB | 8.6±5 | 10.9±6.3 | 2.8±5.8 | ||||
| LSG/RYGB | NS | LSG <RYGB | NS | ||||
| Lindeman et al.[163] | 2020 | RYGB | 60 | 14.1±8.0 | 15.3±6.3 | 7.8±7.6 | |
*Highlight; significant. NS: Nonsignificant, BMD: Bone mineral density, F.N.: Femoral neck, T.H.: Total hip, L.S.: Lumbar spine, RYGB: Roux-en-Y gastric bypass, LAGB: Laparoscopic adjustable gastric band, LSG: Laparoscopic sleeve gastrectomy
We will discuss studies that compared the effect of RYGB and SG on aBMD. Carrasco et al. concluded that aBMD at FN decreased more after RYGB in 2 years follow up.[60] Kelly Ieong et al. reported no significant changes in FN, TH, and LS aBMD 2 years after RYGB versus LSG, and surgery type was not a significant risk factor in bone loss.[161] Studies in 36 and 48-month follow-up have shown that RYGB causes a more significant aBMD reduction in TH than LSG surgery. The results of LS aBMD reduction were different. The inconformity of this result might be due to the other follow-up times. The changes in aBMD after LSG and RYGB have been investigated through a meta-analysis by Tian et al. they concluded that BMI and BMD changes were comparable in each group. Furthermore, RYGB and SG had similar effects on postoperative BMD.[165]
A meta-analysis performed by Byung-Joon et al. compared aBMD changes between the surgical and non-surgical control groups. They concluded that regardless of the type of surgery, FN aBMD was lower in the surgical, but between the two groups, at LS aBMD no difference was found. In cases where the BMI of the surgical group was higher than or equal to the control group, LS and FN aBMD in the surgical group did not differ from the nonsurgical group regardless of the type of surgery. Whereas in studies where the BMI of the surgical group was lower than the control group at the time of the BMD, only the FNA aBMD was lower in the surgical group, both for all types of surgery and RYGB.[166] Therefore, weight loss due to BS mainly affects aBMD in FN rather than the spine. It should be noted that the wide range of follow-up in the studies of this meta-analysis is one of the limitations of this study.
QCT is a convenient method for measuring volumetric BMD in the axial and appendicular regions. HR-pQCT is sometimes used, which has a high ability to defined cortical and trabecular bone microarchitecture and measures strength.[167] The results of the present studies in the evaluation of BMD using QCT and DXA after BS are contradictory.[168] For example, in a survey that measured aBMD and vBMD (TH and FN) by DXA and QCT in a group of patients 1 year after SG or RYGB, incompatible QCT and DXA results were reported.[169] In one study, Yu and Elaine W examined the LS and proximal femur density using DXA and QCT 1 year after RYGB surgery versus the non-surgical control group, and inconsistent and contradictory results were reported between the two methods. LS BMD was significantly decreased in DXA and QCT assessments. However, despite the decrease in FN and TH densitometry measured by DXA, QCT did not change.[168]
In a 2017 study, Bredella et al. measured BMD using QCT and DXA methods 1 year after SG and RYGB. DXA results in TH and FN in RYGB decreased more than the SG group, while QCT results in FN vBMD did not show significant changes.[83]
Another study examined aBMD and vBMD using central DXA and QCT 12 months after SG The results of this study indicated the incompatibility of BMD measurement between QCT and DXA methods. They showed that technical or physiological factors might play a role in the difference between QCT and DXA results during short-term follow-up.[169]
Overall, the measured BMD by DXA depends on excess fat and tissue changes seen during rapid weight loss. Most studies have been performed using DXA, except for a few limited studies, while the results indicate a more detailed survey of trabecular and cortical bone changes using QCT.[170,171,172] According to published studies, HR-QCT is more accurate than DXA in measurement and diagnosing microarchitectural changes. Also, this method is less prone to magnification errors and extraosseous tissue changes.[10,168] The most important limitations of QCT against DXA are high radiation exposure and high cost.[173] Obesity and weight loss may affect QCT assessment, but this method avoids DXA bias due to 2-dimensional, single-projection data acquisition.[174] As mentioned, studies have shown inconsistencies and differences in the measurement of BMD by QCT and DXA methods. Most studies have shown a reduction in BMD at various sites, either by DXA or QCT. However, limited studies have also denied these changes. All studies indicate a more detailed study of microarchitecture and cortical and trabecular bone mass with the QCT. In general, the most appropriate imaging method for measuring BMD is still unclear, and meta-analysis studies seem very helpful for surgeons and physicians.
FRACTURE RISK AFTER BARIATRIC SURGERY
BS is associated with reduced bone density. Although the bone resorption process begins 10 days after surgery and continues for years after surgery, it is not clear that a decrease in bone density is associated with an increased risk of fracture. Studies in this field show different and even contradictory results. According to studies, reducing BMD of the skeletal system can increase the risk of fracture.[175] This section will review the studies conducted in this field and compare their results with each other.
In a Swedish obesity surgery study, 2007 patients underwent BS (13.3% RYGB, 18.7% AGB, 68% VGB) with 2040 obese patients in the control group over 15.1–17.9 years (depending on the type of surgery) were followed. This study showed that fracture risk was higher in the RYGB group than in the nonsurgical control group and the group undergoing AGB and VGB.[176] A noteworthy point in this study is that SG was not enrolled.
Another case study in Canada was conducted by the Québec Integrated Chronic Disease Surveillance System. In this study, 12676 patients who underwent BS were compared with 38028 obese patients and 12676 nonobese patients who did not undergo surgery in the control group during a follow-up of 4.4 years. In this study, the prevalence and site of fractures between groups were investigated. Fracture risk before and after surgery was also studied based on the type of surgery. During follow-up, the rate of fractures in the operated group (514: 4.1%) was higher than the obese control group (1013: 2.7%) and nonobese (3008: 2.4%). The risk of fracture was higher in the BS group than in the obese (r: 1.38, 95% confidence interval [CI]; 1.23–1.55) and non-obese (r: 1.44, 95% CI; 1.29–1.59) control group. In the study of the fracture site, before surgery, the risk of fracture in the distal lower extremities was higher and in the upper extremities was lower. However, after surgery, the risk of distal fractures of the lower extremities decreased (0.66, 95% CI; 0.56–0.78) and increased (1.64, 95% CI; 1.40–1.93) in the upper extremities. The risk of clinical fractures in the spine, pelvis, hip, and femur was also increased (Shift fracture pattern to osteoporotic pattern). The results on fracture risk after RYGB and SG were inconclusive, but fracture risk after BPD increased.[65]
Zhang et al., in a meta-analysis, showed that obesity surgeries increase the risk of complete fractures, especially in the upper extremities. This article showed that the risk of any fracture was higher in the operated group than in the nonsurgical group. In this study, the risk of postoperative fractures in the nonvertebral site, especially in the upper limb, was significantly increased. Therefore, surgeons and physicians should consider bone health and the risk of postoperative fractures.[175] Another study found that the risk of fractures after BS increased 2.3 times over the next 7.7 years.[150,177]
Many factors such as age, fat distribution, fracture site characteristics, and most importantly, comorbidity seem to affect the relationship between obesity and fracture risk.[178] The risk of fracture in patients who underwent RYGB surgery was not significantly different from patients who did not undergo surgery (with the same condition). Several other studies showed no significant association between BS and fracture risk.[179,180,181]
Another study using the U. K. general practice research database was performed on 2079 patients who underwent BS Patients were matched with a control group (10442 patients) without a history of BS The average follow-up was 2.2 years. In this study, the risk of fracture did not increase significantly in patients who underwent surgery. Although it showed that an increase in fracture risk was observed within three to 5 years after surgery, it was not significant. In this study, 60% of patients underwent AGB, and 11% underwent other surgeries (SG and BPD).[180] Short follow-up time (2.2 years) and a significant percentage of AGB patients (60%) are the reasons for the different results of this study from others.
A recent retrospective cohort study showed a reduction in the risk of nontraffic accidents related to obese patients undergoing BS. Obese patients were divided into two groups: surgical (1322) and nonsurgical (1322). The third group was the general population. The risk of overall fracture in the operated group was similar to that in the nonoperated group but was higher than in the general population. The risk of nontraffic accident-related fracture was lower in the surgical group than in the nonsurgical group and higher than in the general population.[182]
Each type of BS may have a specific level of risk. In one study, AGB (a completely restrictive method) was introduced as a method without increasing the risk of fracture.[179] In another study, the association of AGB (excess weight loss [EWL] percentage: low) with malabsorption of nutrients affecting bone metabolism was utterly ruled out.[180] In contrast, SG, RYGB, and BPD-DS methods, which cause weight loss through food absorption (EWL percentage: high), increase the risk of fractures.[150,183,184]
In one study, BS patients, aged 40–65 years, with BMI ≥40 kg/m2, were matched to one control (1:1) by age, sex, comorbidity index, and obesity class (≤50 kg/m2 vs. ≥50 kg/m2) in which 46% of participants underwent SG, and 35% experienced RYGB. The results showed that patients undergoing RYGB had a 70% increased risk of major osteoporotic fractures (hip, proximal humorous, wrist, and distal forearm, and clinical spine) than controls (r: 1.70, 95% CI 1.46–1.98). In contrast, the risk of fracture in patients who underwent SG (r: 0.95, 95% CI 0.79–1.14) and AGB (HR 0.95, 95% CI 0.72–1.25) was not significantly different from the control group.[184]
The study by Syed et al. tried to evaluate the risk of fracture in those who do not undergo BS and those who undergo RYGB or SG. In this study, patients were divided into three groups - (1) patients are eligible for BS who did not undergo surgery (16371 patients), (2) Patients who underwent RYGB (16371 patients), (3) Patients who underwent SG (16371 patients). After 3 years of patient follow-up, the risk of overall fracture and even fracture site was similar between the RYGB and patients who did not have surgery. Still, in patients who underwent SG, fracture risk was lower than in the nonsurgical group. Compared between the RYGB and SG groups, the risk of overall fracture and humerus fracture was significantly higher in the RYGB group. There was no difference in fracture risk in ulnar, radius, pelvis, hip, and spine.[178] Studies to evaluate the increased risk of fracture after BPD-DS is limited, but available results indicate an increased risk of fracture after this surgery.[65]
We should note that BMI may be a confounding factor in the studies mentioned above. For example, in one study, the association between BS and the risk of subsequent fractures while controlling BMI was ruled out.[180] However, another study maintaining BMI showed a 22% increase in major osteoporotic fracture after BS.[21] Random clinical trial (RCT) can also help clarify this issue.
PREVENTION AND TREATMENT
Obesity surgeries, in varying degrees, will affect the nutrition of these patients and potentially cause significant micronutrient deficiencies.[152] In patients with severe obesity, micronutrient deficiencies such as iron, ferritin, folate, calcium, Vitamin D, Fat-soluble vitamins deficiencies are common, and obesity surgeries exacerbate this problem.[42,185,186,187,188]
A combination of rapid weight loss, reduced food intake, and decreased nutrient uptake put BS patients at relatively high risk of long-term bone loss through various mechanisms.[9] According to published studies, obesity surgeries that affect food absorption (such as RYGB and BPD) put patients at greater risk of bone health deterioration than restrictive surgeries (such as AGB and SG). Studies show that exercise and dietary interventions with calcium, vitamin D, and protein consumption, are the most critical strategies for managing this complication. Also, examining some of the factors that we will discuss below will help physicians and patients before and after surgery, and in case of a deficiency of vitamins and micronutrients, they could take the necessary precautions.
According to the latest published guidelines, all candidates for BS should have a comprehensive nutritional assessment before surgery, and a nutritionist should make an accurate assessment of the patient's diet.[189,190] As mentioned before, vitamin D deficiency is common in people with severe and complex obesity.[191] Table 4 summarizes the pre- and post-operative measures which will help preventing BMD reduction after surgery.
Table 4.
Clinical evaluation and preventive strategies for bone health in bariatric surgery
| Before surgery | Prevention | Follow up |
|---|---|---|
| Calcium | ||
| Serum PTH | 1200-1500 mg/day (after AGB, RYGB, and S.G.) | Every 6-12 month (S.G., RYGB, BPD/BPD-DS) |
| Serum calcium | 1800-2400 mg/day (after BPD/BPD-DS) (food and supplements)** | Every 12 month (AGB) then annually |
| Serum 25(OH) D | ||
| DXA at spine and hip (RYGB, BPD, BPD-DS; in higher-risk patients)* | DXA at spine and hip 2 years postoperatively (all patients) | |
| Vitamin D | ||
| Serum 25(OH) D | 3000 IU D3/day (normal range 25(OH) D>30 ng/mL)*** | Every 6-12 month (S.G., RYGB, BPD/BPD-DS) |
| Serum PTH | Every 12 month (AGB) | |
| Protein | ||
| Serum albumin | 46 g/day - women | 6-12 month |
| 56 g/day - men | Serum albumin (S.G., RYGB, BPD/BPD-DS) | |
| Protein needs | 12 month (AGB), then annually for all patients | |
| Should constitute: | ||
| 10%-35% of daily caloric intake | ||
| Weight maintenance: 0.8-1.2 g/kg body weight/day | ||
| Active weight loss: 1.2 g/kg body weight (BPD/DS may require 1.5-2.0 g/kg body weight/day) |
*Women aged ≥65 years, men aged ≥70 years, men above age 50-69; based on the risk factor profile, and men aged 50 and older who have had an adult age fracture. **Calcium citrate is preferable over calcium carbonate because it is independent of stomach acidity absorption. Calcium should be given in divided doses (single doses should not exceed 600 mg), separated by ≥2-h intervals from iron-containing supplements. Calcium carbonate should be taken with meals, whereas calcium citrate can be taken with or without meals. ***D3 is recommended as more potent than D2, but both forms can be effective and dose-dependent. It is recommended that both D2 and D3 be taken with a meal containing fat to ensure maximum absorption. PTH: Parathyroid hormone, 25(OH) D: 25-hydroxyvitamin D, RYGB: Roux-en-Y gastric bypass, LAGB: Laparoscopic adjustable gastric band, BPD-DS: Biliopancreatic diversion with a duodenal switch, AGB: Adjustable gastric banding, DXA: Dual-energy X-ray absorptiometry, S.G.: Sleeve gastrectomy
Bone loss markers are monitored by urinary or serum CTx. CTx is recommended in people with significant risk factors for osteoporosis and pre- and post-menopausal women with low estrogen levels.[33]
After surgery, the need for Vitamin D3 supplementation is to maintain optimal 25(O. H.) D serum levels at >75 nmol/L.[192] Therefore, it is recommended to start 2000–4000 international Vitamin D3 per day to keep the serum 25(O. H.) D level in normal limits. The dose adjustment depends on laboratory results.[193]
Several guidelines also recommend taking calcium supplements after BS.[194] However, the optimal intake of calcium is not precise. Parrott et al. recommend a daily intake of 1,200–1,500 mg of calcium in AGB, SG, and RYGB surgeries and 1,800–2,400 mg/day following BPD-DS surgery.[190] In regards to preventing bone loss after BS, few trials are assessing the effect of bisphosphonates on bone health following different types of BS.[195,196]
According to studies, strength training can minimize the adverse effects on bone.[157] The type of physical activity may be an essential factor in maintaining bone mass.[197] However, the impact of AE has been proven in some studies.[198] Clinical evaluation and preventive measures for bone health before and after obesity surgery are given in Table 4.[33,189]
CONCLUSION
Studies show that BMD decreases significantly in the early years after BS. The rate of bone loss during the 1st year is higher than in subsequent years. Mechanical unloading, Gut-hormone alternations (decreased Leptin and Ghrelin, increased PYY and GLP-1), and reduced insulin levels due to improved insulin resistance all play a significant role in affecting bone changes after BS.
The available information and guidelines emphasize the periodic evaluation of BMD in patients undergoing BS.[151,152] Most clinical studies have used the DXA to assess BMD. Studies assessing bone loss with DXA method show that BS affects TH site more than LS at both short and long-term follow-ups regardless of surgery type (RYGB or SG). In the comparison between RYGB and SG, BMD reduction in the LS showed discordant results at long-term follow-up. However, RYGB had more destructive effects than SG in TH density. The results were conflicted in the short-term follow-up. The meta-analysis performed by Tian et al. yielded unreliable results due to the many limitations (Few RCT studies, small sample size, incomplete data) mentioned by the authors. Therefore, it is recommended to carry out a meta-analysis with appropriate follow-up with sufficient studies.
Although DXA is a more common method of measuring aBMD, it is possible to measure vBMD using QCT, which is a three-dimensional indicator of bone density. Therefore, in the studies, we reviewed the densitometry results differed between the QCT and DXA methods. All studies indicate a more detailed survey of microarchitecture and cortical and trabecular bone mass with the help of QCT. In general, the most appropriate imaging method for measuring BMD is still unclear, and meta-analysis studies seem very helpful for surgeons and physicians.
Studies have compared the fracture risk after BS in a different type of surgery and also with the normal population. Based on our assessment, compared with obese and non-obese people, fracture risk increases after BS Fracture risk after malabsorptive surgery (RYGB and BPD-DS) was higher than restrictive operation (SG). A meta-analysis carried out in 2018 shows that obesity surgeries increase fracture risk, especially in the upper extremities. The pattern of fracture also shifts from lower limbs (obesity fracture type) to upper extremities (osteoporotic fracture type).
Numerous strategies have been proposed to prevent bone destruction after obesity surgery. In this regard, preoperative laboratory assessments are of great importance. The evaluation of Vitamin D, calcium, and PTH levels is necessary to diagnose secondary hyperparathyroidism due to vitamin D deficiency. Preventive measures after surgery include medication intervention and exercise. The recommended prophylactic dose of calcium and Vitamin D to overcome adverse bone effects of BS is 1200–1500 mg/day and 3000 IU/day, respectively. The suggested dose prevents bone loss-induced hyperparathyroidism. Exercise after B. S mitigates the adverse effects of BS on bone. It improves body composition, maintains BMD, increases muscle strength, helps physical fitness and improves insulin sensitivity. Therefore, it is recommended in the recently updated guidelines for the prevention of BMD loss. For all patients, a target of moderate aerobic physical activity that includes a minimum of 150 min/week and a goal of 300 min/week, including strength training 2–3 times/week, is recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodhouse R. Obesity in art: A brief overview. Front Horm Res. 2008;36:271–86. doi: 10.1159/000115370. [DOI] [PubMed] [Google Scholar]
- 3.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Jehan S, Myers AK, Zizi F, Pandi-Perumal SR, Jean-Louis G, McFarlane SI. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: Epidemiology and pathophysiologic insights. Sleep Med Disord. 2018;2:52–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biobaku F, Ghanim H, Monte SV, Caruana JA, Dandona P. Bariatric surgery: Remission of inflammation, cardiometabolic benefits, and common adverse effects. J Endocr Soc. 2020;4:bvaa049. doi: 10.1210/jendso/bvaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston EH. Complications of bariatric surgery. Surg Clin North Am. 2005;85:853–68. doi: 10.1016/j.suc.2005.04.007. vii. [DOI] [PubMed] [Google Scholar]
- 8.Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331:219–25. doi: 10.1097/00000441-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Stein EM, Carrelli A, Young P, Bucovsky M, Zhang C, Schrope B, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98:541–9. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein EM, Silverberg SJ. Bone loss after bariatric surgery: Causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2:165–74. doi: 10.1016/S2213-8587(13)70183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013;14:52–67. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 12.Scibora LM, Ikramuddin S, Buchwald H, Petit MA. Examining the link between bariatric surgery, bone loss, and osteoporosis: A review of bone density studies. Obes Surg. 2012;22:654–67. doi: 10.1007/s11695-012-0596-1. [DOI] [PubMed] [Google Scholar]
- 13.Canales BK, Schafer AL, Shoback DM, Carpenter TO. Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY. Surg Obes Relat Dis. 2014;10:878–84. doi: 10.1016/j.soard.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutton B, Catalá-López F, Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc) 2016;147:262–6. doi: 10.1016/j.medcli.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25:1822–32. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–11. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 18.Franco JV, Ruiz PA, Palermo M, Gagner M. A review of studies comparing three laparoscopic procedures in bariatric surgery: Sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21:1458–68. doi: 10.1007/s11695-011-0390-5. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien PE, Dixon JB, Brown W, Schachter LM, Chapman L, Burn AJ, et al. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002;12:652–60. doi: 10.1381/096089202321019639. [DOI] [PubMed] [Google Scholar]
- 20.Karmali S, Schauer P, Birch D, Sharma AM, Sherman V. Laparoscopic sleeve gastrectomy: An innovative new tool in the battle against the obesity epidemic in Canada. Can J Surg. 2010;53:126–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Paccou J, Caiazzo R, Lespessailles E, Cortet B. Bariatric surgery and osteoporosis. Calcif Tissue Int. 2021 doi: 10.1007/s00223-020-00798-w. https://doi.org/10.1007/s00223-020-00798-w. [DOI] [PubMed] [Google Scholar]
- 22.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 23.Misra S, Nandhini BD, Christinajoice S, Kumar SS, Prabhakaran S, Palanivelu C, et al. Is laparoscopic Roux-en-Y gastric bypass still the gold standard procedure for indians? Mid- to long-term outcomes from a tertiary care center. Obes Surg. 2020;30:4482–93. doi: 10.1007/s11695-020-04849-x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt JB, Pedersen SD, Gregersen NT, Vestergaard L, Nielsen MS, Ritz C, et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: A randomized controlled clinical trial. Int J Obes (Lond) 2016;40:281–90. doi: 10.1038/ijo.2015.162. [DOI] [PubMed] [Google Scholar]
- 25.Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159:1290–301.e5. doi: 10.1053/j.gastro.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Thereaux J, Lesuffleur T, Païta M, Czernichow S, Basdevant A, Msika S, et al. Long-term follow-up after bariatric surgery in a national cohort. Br J Surg. 2017;104:1362–71. doi: 10.1002/bjs.10557. [DOI] [PubMed] [Google Scholar]
- 27.Kim WW, Gagner M, Kini S, Inabnet WB, Quinn T, Herron D, et al. Laparoscopic vs. open biliopancreatic diversion with duodenal switch: A comparative study. J Gastrointest Surg. 2003;7:552–7. doi: 10.1016/S1091-255X(02)00149-X. [DOI] [PubMed] [Google Scholar]
- 28.Strain GW, Torghabeh MH, Gagner M, Ebel F, Dakin GF, Abelson JS, et al. The impact of biliopancreatic diversion with duodenal switch (BPD/DS) over 9 years. Obes Surg. 2017;27:787–94. doi: 10.1007/s11695-016-2371-1. [DOI] [PubMed] [Google Scholar]
- 29.Shoar S, Poliakin L, Rubenstein R, Saber AA. Single anastomosis duodeno-ileal switch (SADIS): A systematic review of efficacy and safety. Obes Surg. 2018;28:104–13. doi: 10.1007/s11695-017-2838-8. [DOI] [PubMed] [Google Scholar]
- 30.Santoro S, Klajner S, Sampaio R. Sleeve gastrectomy and transit bipartition. In: Obesity and Diabetes. Obesity and Diabetes:Springer. 2015:89–110. [Google Scholar]
- 31.Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: What have we learned? Obes Surg. 2005;15:145–54. doi: 10.1381/0960892053268264. [DOI] [PubMed] [Google Scholar]
- 32.Cadart O, Degrandi O, Barnetche T, Mehsen-Cetre N, Monsaingeon-Henry M, Pupier E, et al. Long-term effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density: A 4-year longitudinal study. Obes Surg. 2020;30:3317–25. doi: 10.1007/s11695-020-04568-3. [DOI] [PubMed] [Google Scholar]
- 33.Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures–2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Endocr Pract. 2019;25:1–75. doi: 10.4158/GL-2019-0406. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, et al. Long-term outcomes after bariatric surgery: A systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29:3–14. doi: 10.1007/s11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blom-Høgestøl IK, Mala T, Kristinsson JA, et al. Change in bone marrow Adipose tissue one year after Roux-en-Y gastric Bypass: aprospective cohort study. JBMR. 2101;34(10):1815–23. doi: 10.1002/jbmr.3814. [DOI] [PubMed] [Google Scholar]
- 36.Modi AC, Zeller MH, Xanthakos SA, Jenkins TM, Inge TH. Adherence to vitamin supplementation following adolescent bariatric surgery. Obesity (Silver Spring) 2013;21:E190–5. doi: 10.1002/oby.20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, et al. Voluntary weight reduction in older men increases hip bone loss: The osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 40.Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA. Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause. 2014;21:501–8. doi: 10.1097/GME.0b013e3182a76fd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: Focus on metabolic bone disease. Cleve Clin J Med. 2008;75:333–4. doi: 10.3949/ccjm.75.5.333. 336, 338. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Porat T, Elazary R, Sherf-Dagan S, Goldenshluger A, Brodie R, Mintz Y, et al. Bone health following bariatric surgery: Implications for management strategies to attenuate bone loss. Adv Nutr. 2018;9:114–27. doi: 10.1093/advances/nmx024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory NS. The effects of bariatric surgery on bone metabolism. Endocrinol Metab Clin North Am. 2017;46:105–16. doi: 10.1016/j.ecl.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Saad R, Habli D, El Sabbagh R, Chakhtoura M. Bone health following bariatric surgery: An update. J Clin Densitom. 2020;23:165–81. doi: 10.1016/j.jocd.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Lin D, Chen J, Zhang Z, Liao Z, Swain M, et al. Role of mechanical stimuli in oral implantation. J Biosci Med. 2014;2:63–68. [Google Scholar]
- 46.Muschitz C, Kocijan R, Haschka J, Zendeli A, Pirker T, Geiger C, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: The BABS study. J Bone Miner Res. 2016;31:672–82. doi: 10.1002/jbmr.2707. [DOI] [PubMed] [Google Scholar]
- 47.Kazakia GJ, Tjong W, Nirody JA, Burghardt AJ, Carballido-Gamio J, Patsch JM, et al. The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT. Bone. 2014;63:132–40. doi: 10.1016/j.bone.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maïmoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: From physiopathology to therapy. Spinal Cord. 2006;44:203–10. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- 49.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 50.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: A randomized controlled trial. Arch Intern Med. 2006;166:2502–10. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 51.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burckhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: Potential role of serum C telopeptides for follow-up. Int J Obes (Lond) 2005;29:1429–35. doi: 10.1038/sj.ijo.0803040. [DOI] [PubMed] [Google Scholar]
- 53.Katznelson L, Laws ER, Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933–51. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 54.Pluskiewicz W, Bužga M, Holéczy P, Bortlík L, Šmajstrla V, Adamczyk P. Bone mineral changes in spine and proximal femur in individual obese women after laparoscopic sleeve gastrectomy: A short-term study. Obes Surg. 2012;22:1068–76. doi: 10.1007/s11695-012-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindeman KG, Greenblatt LB, Rourke C, Bouxsein ML, Finkelstein JS, Yu EW. Longitudinal 5-year evaluation of bone density and microarchitecture after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2018;103:4104–12. doi: 10.1210/jc.2018-01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krez AN, Stein EM. The skeletal consequences of bariatric surgery. Curr Osteoporos Rep. 2020;18:262–72. doi: 10.1007/s11914-020-00579-2. [DOI] [PubMed] [Google Scholar]
- 57.Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2:121–33. doi: 10.1002/jbm4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chamberlain C, Terry R, Shtayyeh T, Martinez C. Recognizing postoperative nutritional complications of bariatric surgery in the primary care patient: A narrative review. J Osteopath Med. 2021;121:105–12. doi: 10.7556/jaoa.2020.135. [DOI] [PubMed] [Google Scholar]
- 59.Schafer AL, Weaver CM, Black DM, Wheeler AL, Chang H, Szefc GV, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30:1377–85. doi: 10.1002/jbmr.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrasco F, Basfi-Fer K, Rojas P, Csendes A, Papapietro K, Codoceo J, et al. Calcium absorption may be affected after either sleeve gastrectomy or Roux-en-Y gastric bypass in premenopausal women: A 2-y prospective study. Am J Clin Nutr. 2018;108:24–32. doi: 10.1093/ajcn/nqy071. [DOI] [PubMed] [Google Scholar]
- 61.Vilarrasa N, San José P, García I, Gómez-Vaquero C, Miras PM, de Gordejuela AG, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21:465–72. doi: 10.1007/s11695-010-0338-1. [DOI] [PubMed] [Google Scholar]
- 62.Chakhtoura MT, Nakhoul NN, Shawwa K, Mantzoros C, El Hajj Fuleihan GA. Hypovitaminosis D in bariatric surgery: A systematic review of observational studies. Metabolism. 2016;65:574–85. doi: 10.1016/j.metabol.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wongdee K, Rodrat M, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Factors inhibiting intestinal calcium absorption: Hormones and luminal factors that prevent excessive calcium uptake. J Physiol Sci. 2019;69:683–96. doi: 10.1007/s12576-019-00688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muschitz C, Kocijan R, Marterer C, Nia AR, Muschitz GK, Resch H, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100:891–901. doi: 10.1210/jc.2014-3367. [DOI] [PubMed] [Google Scholar]
- 65.Rousseau C, Jean S, Gamache P, Lebel S, Mac-Way F, Biertho L, et al. Change in fracture risk and fracture pattern after bariatric surgery: Nested case-control study. BMJ. 2016;354:i3794. doi: 10.1136/bmj.i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25:423–39. doi: 10.1007/s00198-013-2480-9. [DOI] [PubMed] [Google Scholar]
- 67.Slater GH, Ren CJ, Siegel N, Williams T, Barr D, Wolfe B, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Quercia I, Dutia R, Kotler DP, Belsley S, Laferrère B. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40:87–94. doi: 10.1016/j.diabet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong IP, Baldock PA, Herzog H. Gastrointestinal peptides and bone health. Curr Opin Endocrinol Diabetes Obes. 2010;17:44–50. doi: 10.1097/MED.0b013e3283344a05. [DOI] [PubMed] [Google Scholar]
- 70.Kim TY, Shoback DM, Black DM, Rogers SJ, Stewart L, Carter JT, et al. Increases in PYY and uncoupling of bone turnover are associated with loss of bone mass after gastric bypass surgery. Bone. 2020;131:115115. doi: 10.1016/j.bone.2019.115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–8. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 72.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 73.Wong IP, Driessler F, Khor EC, Shi YC, Hörmer B, Nguyen AD, et al. Peptide YY regulates bone remodeling in mice: A link between gut and skeletal biology. PLoS One. 2012;7:e40038. doi: 10.1371/journal.pone.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee NJ, Nguyen AD, Enriquez RF, Doyle KL, Sainsbury A, Baldock PA, et al. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011;48:461–7. doi: 10.1016/j.bone.2010.10.174. [DOI] [PubMed] [Google Scholar]
- 75.Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab. 2015;4:437–60. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 77.Camiña JP, Carreira MC, El Messari S, Llorens-Cortes C, Smith RG, Casanueva FF. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology. 2004;145:930–40. doi: 10.1210/en.2003-0974. [DOI] [PubMed] [Google Scholar]
- 78.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–84. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, et al. Ghrelin directly regulates bone formation. J Bone Miner Res. 2005;20:790–8. doi: 10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- 80.van der Velde M, van der Eerden BC, Sun Y, Almering JM, van der Lely AJ, Delhanty PJ, et al. An age-dependent interaction with leptin unmasks ghrelin's bone-protective effects. Endocrinology. 2012;153:3593–602. doi: 10.1210/en.2012-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carrasco F, Basfi-Fer K, Rojas P, Valencia A, Csendes A, Codoceo J, et al. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: Relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes Surg. 2014;24:877–84. doi: 10.1007/s11695-014-1179-0. [DOI] [PubMed] [Google Scholar]
- 82.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 83.Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90. doi: 10.1016/j.bone.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ivaska KK, Huovinen V, Soinio M, Hannukainen JC, Saunavaara V, Salminen P, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54. doi: 10.1016/j.bone.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Mônaco-Ferreira DV, Leandro-Merhi VA, Aranha NC, Brandalise A, Brandalise NA. Vitamin D deficiency and paratohommonium increase in late postoperative gastric bypass in Roux-En-Y. Arq Bras Cir Dig. 2018;31:e1407. doi: 10.1590/0102-672020180001e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhong Q, Itokawa T, Sridhar S, Ding KH, Xie D, Kang B, et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab. 2007;292:E543–8. doi: 10.1152/ajpendo.00364.2006. [DOI] [PubMed] [Google Scholar]
- 87.Rao RS, Kini S. GIP and bariatric surgery. Obes Surg. 2011;21:244–52. doi: 10.1007/s11695-010-0305-x. [DOI] [PubMed] [Google Scholar]
- 88.Brakta S, Chorich LP, Kim HG, Coons LA, Katzenellenbogen JA, Hall JE, et al. Long-term follow-up and treatment of a female with complete estrogen insensitivity. J Clin Endocrinol Metab. 2020;105:1478–88. doi: 10.1210/clinem/dgaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cutler GB., Jr The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61:141–4. [PubMed] [Google Scholar]
- 90.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–91. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 91.Sumino H, Ichikawa S, Abe M, Endo Y, Nakajima Y, Minegishi T, et al. Effects of aging and postmenopausal hypoestrogenism on skin elasticity and bone mineral density in Japanese women. Endocr J. 2004;51:159–64. doi: 10.1507/endocrj.51.159. [DOI] [PubMed] [Google Scholar]
- 92.Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T. Effect of weight loss on bone metabolism: Comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13:383–8. doi: 10.1381/096089203765887705. [DOI] [PubMed] [Google Scholar]
- 93.Jäckle K, Kolb JP, Schilling AF, Schlickewei C, Amling M, Rueger JM, et al. Analysis of low-dose estrogen on callus BMD as measured by pQCT in postmenopausal women. BMC Musculoskelet Disord. 2020;21:693. doi: 10.1186/s12891-020-03713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rinonapoli G, Ruggiero C, Meccariello L, Bisaccia M, Ceccarini P, Caraffa A. Osteoporosis in men: A review of an underestimated bone condition. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20:378–87. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 96.Mohamad NV, Soelaiman IN, Chin KY. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317–24. doi: 10.2147/CIA.S115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: A critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 98.Vettor R, Milan G, Rossato M, Federspil G. Review article: Adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;22(Suppl 2):3–10. doi: 10.1111/j.1365-2036.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 99.Botella-Carretero JI, Balsa JA, Gómez-Martin JM, Peromingo R, Huerta L, Carrasco M, et al. Circulating free testosterone in obese men after bariatric surgery increases in parallel with insulin sensitivity. J Endocrinol Invest. 2013;36:227–32. doi: 10.3275/8469. [DOI] [PubMed] [Google Scholar]
- 100.Woodard G, Ahmed S, Podelski V, Hernandez-Boussard T, Presti J, Jr, Morton JM. Effect of Roux-en-Y gastric bypass on testosterone and prostate-specific antigen. Br J Surg. 2012;99:693–8. doi: 10.1002/bjs.8693. [DOI] [PubMed] [Google Scholar]
- 101.Cherif R, Mahjoub F, Sahli H, Cheour E, Vico L, Sakly M, et al. Positive association of obesity and insulin resistance with bone mineral density in tunisian postmenopausal women. J Clin Densitom. 2018;21:163–71. doi: 10.1016/j.jocd.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 102.Napoli N, Conte C, Pedone C, Strotmeyer ES, Barbour KE, Black DM, et al. Effect of insulin resistance on BMD and fracture risk in older adults. J Clin Endocrinol Metab. 2019;104:3303–10. doi: 10.1210/jc.2018-02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas T, Burguera B, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29:114–20. doi: 10.1016/s8756-3282(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 104.Yüksel O, Dökmetaş HS, Topcu S, Erselcan T, Sencan M. Relationship between bone mineral density and insulin resistance in polycystic ovary syndrome. J Bone Miner Metab. 2001;19:257–62. doi: 10.1007/s007740170029. [DOI] [PubMed] [Google Scholar]
- 105.Dirksen C, Jørgensen NB, Bojsen-Møller KN, Jacobsen SH, Hansen DL, Worm D, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55:1890–901. doi: 10.1007/s00125-012-2556-7. [DOI] [PubMed] [Google Scholar]
- 106.Xu XJ, Apovian C, Hess D, Carmine B, Saha A, Ruderman N. Improved insulin sensitivity 3 months after RYGB surgery is associated with increased subcutaneous adipose tissue AMPK activity and decreased oxidative stress. Diabetes. 2015;64:3155–9. doi: 10.2337/db14-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conde J, Scotece M, Gómez R, López V, Gómez-Reino JJ, Lago F, et al. Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors. 2011;37:413–20. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 108.Adali E, Yildizhan R, Kolusari A, Kurdoglu M, Bugdayci G, Sahin HG, et al. Increased visfatin and leptin in pregnancies complicated by pre-eclampsia. J Matern Fetal Neonatal Med. 2009;22:873–9. doi: 10.1080/14767050902994622. [DOI] [PubMed] [Google Scholar]
- 109.Chwalba A, Machura E, Ziora K, Ziora D. The role of adipokines in the pathogenesis and course of selected respiratory diseases. Endokrynol Pol. 2019;70:504–10. doi: 10.5603/EP.a2019.0051. [DOI] [PubMed] [Google Scholar]
- 110.Legroux-Gérot I, Vignau J, Viltart O, Hardouin P, Chauveau C, Cortet B. Adipokines and bone status in a cohort of anorexic patients. Joint Bone Spine. 2019;86:95–101. doi: 10.1016/j.jbspin.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 111.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 112.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 113.Williams KW, Scott MM, Elmquist JK. From observation to experimentation: Leptin action in the mediobasal hypothalamus. Am J Clin Nutr. 2009;89:985S–90S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015;64:105–13. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greco EA, Lenzi A, Migliaccio S. The obesity of bone. Ther Adv Endocrinol Metab. 2015;6:273–86. doi: 10.1177/2042018815611004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 117.Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002;17:1563–9. doi: 10.1359/jbmr.2002.17.9.1563. [DOI] [PubMed] [Google Scholar]
- 118.Whipple T, Sharkey N, Demers L, Williams N. Leptin and the skeleton. Clin Endocrinol (Oxf) 2002;57:701–11. doi: 10.1046/j.1365-2265.2002.01630.x. [DOI] [PubMed] [Google Scholar]
- 119.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004;19:1607–11. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 120.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 121.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–8. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 122.Takeshita N, Mutoh S, Yamaguchi I. Osteopenia in genetically diabetic DB/DB mice and effects of 1alpha-hydroxyvitamin D3 on the osteopenia. Basic Research Group. Life Sci. 1995;56:1095–101. doi: 10.1016/0024-3205(95)00046-9. [DOI] [PubMed] [Google Scholar]
- 123.Liu K, Liu P, Liu R, Wu X, Cai M. Relationship between serum leptin levels and bone mineral density: A systematic review and meta-analysis. Clin Chim Acta. 2015;444:260–3. doi: 10.1016/j.cca.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 124.Münzberg H, Björnholm M, Bates SH, Myers MG., Jr Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–52. doi: 10.1007/s00018-004-4432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–6. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 126.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 127.Yatagai T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T, Kuroe A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–8. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 128.Carsote M, Petrescu R, Nica AE, Ghemigian A, Paduraru DN, Valea A. Bariatric surgery and osteoporosis. Romanian Med J. 2016;63:297–299. [Google Scholar]
- 129.Biver E, Salliot C, Combescure C, Gossec L, Hardouin P, Legroux-Gerot I, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2703–13. doi: 10.1210/jc.2011-0047. [DOI] [PubMed] [Google Scholar]
- 130.Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19:41–6. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 131.Bruno C, Fulford AD, Potts JR, McClintock R, Jones R, Cacucci BM, et al. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: Correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010;95:159–66. doi: 10.1210/jc.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang T, He C. TNF-α and IL-6: The Link between Immune and Bone System. Curr Drug Targets. 2020;21:213–27. doi: 10.2174/1389450120666190821161259. [DOI] [PubMed] [Google Scholar]
- 133.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viana EC, Araujo-Dasilio KL, Miguel GP, Bressan J, Lemos EM, Moyses MR, et al. Gastric bypass and sleeve gastrectomy: The same impact on IL-6 and TNF-α. Prospective clinical trial. Obes Surg. 2013;23:1252–61. doi: 10.1007/s11695-013-0894-2. [DOI] [PubMed] [Google Scholar]
- 135.Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta. 2008;387:31–5. doi: 10.1016/j.cca.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 136.Sucunza N, Barahona MJ, Resmini E, Fernández-Real JM, Ricart W, Farrerons J, et al. A link between bone mineral density and serum adiponectin and visfatin levels in acromegaly. J Clin Endocrinol Metab. 2009;94:3889–96. doi: 10.1210/jc.2009-0474. [DOI] [PubMed] [Google Scholar]
- 137.Parreño Caparrós E, Illán Gómez F, Gonzálvez Ortega M, Orea Soler I, Pérez Paredes M, Lozano Almela ML, et al. Resistin in morbidly obese patients before and after gastric bypass surgery. Nutr Hosp. 2017;34:1333–7. doi: 10.20960/nh.1028. [DOI] [PubMed] [Google Scholar]
- 138.Barnes AS. Obesity and sedentary lifestyles: Risk for cardiovascular disease in women. Tex Heart Inst J. 2012;39:224–7. [PMC free article] [PubMed] [Google Scholar]
- 139.Bertisch SM, Wee CC, McCarthy EP. Use of complementary and alternative therapies by overweight and obese adults. Obesity (Silver Spring) 2008;16:1610–5. doi: 10.1038/oby.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neff KJ, Ferrannini E, Le Roux CW. Treatment of obesity: Bariatric surgery. International Textbook of Diabetes Mellitus. Wiley Online Library. 2015:505–18. [Google Scholar]
- 141.Hassannejad A, Khalaj A, Mansournia MA, Rajabian Tabesh M, Alizadeh Z. The effect of aerobic or aerobic-strength exercise on body composition and functional capacity in patients with BMI≥35 after bariatric surgery: A randomized control trial. Obes Surg. 2017;27:2792–801. doi: 10.1007/s11695-017-2717-3. [DOI] [PubMed] [Google Scholar]
- 142.Welch G, Wesolowski C, Piepul B, Kuhn J, Romanelli J, Garb J. Physical activity predicts weight loss following gastric bypass surgery: Findings from a support group survey. Obes Surg. 2008;18:517–24. doi: 10.1007/s11695-007-9269-x. [DOI] [PubMed] [Google Scholar]
- 143.Bond DS, Phelan S, Wolfe LG, Evans RK, Meador JG, Kellum JM, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity (Silver Spring) 2009;17:78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]
- 144.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Exercise following bariatric surgery: Systematic review. Obes Surg. 2010;20:657–65. doi: 10.1007/s11695-010-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lorentzon M, Mellström D, Ohlsson C. Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: The GOOD study. J Bone Miner Res. 2005;20:1936–43. doi: 10.1359/JBMR.050709. [DOI] [PubMed] [Google Scholar]
- 146.Diniz-Sousa F, Veras L, Boppre G, Sa-Couto P, Devezas V, Santos-Sousa H, et al. The effect of an exercise intervention program on bone health after bariatric surgery: A randomized controlled trial. J Bone Miner Res. 2021;36:489–99. doi: 10.1002/jbmr.4213. [DOI] [PubMed] [Google Scholar]
- 147.In G, Taskin HE, Al M, Alptekin HK, Zengin K, Yumuk V, et al. Comparison of 12-week fitness protocols following bariatric surgery: Aerobic exercise versus aerobic exercise and progressive resistance. Obes Surg. 2021;31:1475–84. doi: 10.1007/s11695-020-05144-5. [DOI] [PubMed] [Google Scholar]
- 148.Hansen D, Decroix L, Devos Y, Nocca D, Cornelissen V, Dillemans B, et al. Towards optimized care after bariatric surgery by physical activity and exercise intervention: A review. Obes Surg. 2020;30:1118–25. doi: 10.1007/s11695-020-04390-x. [DOI] [PubMed] [Google Scholar]
- 149.Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37:135–87. doi: 10.1210/er.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakamura KM, Haglind EG, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ, 3rd, et al. Fracture risk following bariatric surgery: A population-based study. Osteoporos Int. 2014;25:151–8. doi: 10.1007/s00198-013-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C, et al. Endocrine and nutritional management of the post-bariatric surgery patient: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4823–43. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 152.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: Cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract. 2013;19:337–72. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tothill P, Hannan WJ, Cowen S, Freeman CP. Anomalies in the measurement of changes in total-body bone mineral by dual-energy X-ray absorptiometry during weight change. J Bone Miner Res. 1997;12:1908–21. doi: 10.1359/jbmr.1997.12.11.1908. [DOI] [PubMed] [Google Scholar]
- 154.Van Loan MD. Is Dual-Energy X-ray Absorptiometry Ready for Prime Time in the Clinical Evaluation of Body Composition? The American Journal Of Clinical Nutrition. 1998;68:1155–56. doi: 10.1093/ajcn/68.6.1155. [DOI] [PubMed] [Google Scholar]
- 155.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: Review of physical concepts. Am J Physiol. 1996;271:E941–51. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- 156.Zou D, Sun Z, Zhou S, Zhong W, Li W. Hounsfield units value is a better predictor of pedicle screw loosening than the T-score of DXA in patients with lumbar degenerative diseases. Eur Spine J. 2020;29:1105–11. doi: 10.1007/s00586-020-06386-8. [DOI] [PubMed] [Google Scholar]
- 157.Wosje KS, Binkley TL, Specker BL. Comparison of bone parameters by dual-energy X-ray absorptiometry and peripheral quantitative computed tomography in Hutterite vs. non-Hutterite women aged 35-60 years. Bone. 2001;29:192–7. doi: 10.1016/s8756-3282(01)00495-1. [DOI] [PubMed] [Google Scholar]
- 158.Chen X, Zhang C, Li J, Liu W, Zhang J, Zhou Z. Effects of Laparoscopic Sleeve Gastrectomy on Bone Mineral Density and Bone Metabolism in Chinese Patients with Obesity. Diabetes Metab Syndr Obes. 2020;13:4095–103. doi: 10.2147/DMSO.S274614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gerber P. Bariatric Surgery: Predictors of Outcome: Results from a National Database (SOREG) with Particular Emphasis on Patients’ Age; Karolina Institute/Dept of Clinical Science. 2021 [Google Scholar]
- 160.Luger M, Kruschitz R, Winzer E, Schindler K, Grabovac I, Kainberger F, et al. Changes in bone mineral density following weight loss induced by one-anastomosis gastric bypass in patients with vitamin D supplementation. Obes Surg. 2018;28:3454–65. doi: 10.1007/s11695-018-3353-2. [DOI] [PubMed] [Google Scholar]
- 161.Ieong K, Ardila-Gatas J, Yang J, Zhang X, Tsui ST, Spaniolas K, et al. Bone mineral density changes after bariatric surgery. Surg Endosc. 2021;35:4763–70. doi: 10.1007/s00464-020-07953-2. [DOI] [PubMed] [Google Scholar]
- 162.Brzozowska MM, Tran T, Bliuc D, Jorgensen J, Talbot M, Fenton-Lee D, et al. Roux-en-Y gastric bypass and gastric sleeve surgery result in long term bone loss. Int J Obes (Lond) 2021;45:235–46. doi: 10.1038/s41366-020-00660-x. [DOI] [PubMed] [Google Scholar]
- 163.Lindeman KG, Rushin CC, Cheney MC, Bouxsein ML, Hutter MM, Yu EW. Bone density and trabecular morphology at least 10 years after gastric bypass and gastric banding. J Bone Miner Res. 2020;35:2132–42. doi: 10.1002/jbmr.4112. [DOI] [PubMed] [Google Scholar]
- 164.Jaruvongvanich V, Vantanasiri K, Upala S, Ungprasert P. Changes in bone mineral density and bone metabolism after sleeve gastrectomy: A systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:1252–60. doi: 10.1016/j.soard.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 165.Tian Z, Fan XT, Li SZ, Zhai T, Dong J. Changes in bone metabolism after sleeve gastrectomy versus gastric bypass: A meta-analysis. Obes Surg. 2020;30:77–86. doi: 10.1007/s11695-019-04119-5. [DOI] [PubMed] [Google Scholar]
- 166.Ko BJ, Myung SK, Cho KH, Park YG, Kim SG, Kim do H, et al. Relationship between bariatric surgery and bone mineral density: A meta-analysis. Obes Surg. 2016;26:1414–21. doi: 10.1007/s11695-015-1928-8. [DOI] [PubMed] [Google Scholar]
- 167.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–24. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM, et al. Bone loss after bariatric surgery: Discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29:542–50. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tan HC, Tan MZ, Tham KW, Pasupathy S, Eng AK, Ganguly S, et al. One year changes in QCT and DXA bone densities following bariatric surgery in a multiethnic Asian cohort. Osteoporos Sarcopenia. 2015;1:115–20. [Google Scholar]
- 170.Bolotin HH. DXA in vivo BMD methodology: An erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41:138–54. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 171.Javed F, Yu W, Thornton J, Colt E. Effect of fat on measurement of bone mineral density. Int J Body Compos Res. 2009;7:37–40. [PMC free article] [PubMed] [Google Scholar]
- 172.Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8:31–8. doi: 10.1385/jcd:8:1:031. [DOI] [PubMed] [Google Scholar]
- 173.Kim J, Nimeri A, Khorgami Z, El Chaar M, Lima AG, Vosburg RW, et al. Metabolic bone changes after bariatric surgery: 2020 update, American Society for Metabolic and Bariatric Surgery Clinical Issues Committee position statement. Surg Obes Relat Dis. 2021;17:1–8. doi: 10.1016/j.soard.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 174.Schafer AL, Kazakia GJ, Vittinghoff E, Stewart L, Rogers SJ, Kim TY, et al. Effects of gastric bypass surgery on bone mass and microarchitecture occur early and particularly impact postmenopausal women. J Bone Miner Res. 2018;33:975–86. doi: 10.1002/jbmr.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Q, Chen Y, Li J, Chen D, Cheng Z, Xu S, et al. A meta-analysis of the effects of bariatric surgery on fracture risk. Obes Rev. 2018;19:728–36. doi: 10.1111/obr.12665. [DOI] [PubMed] [Google Scholar]
- 176.Axelsson KF, Werling M, Eliasson B, Szabo E, Näslund I, Wedel H, et al. Fracture risk after gastric bypass surgery: A retrospective cohort study. J Bone Miner Res. 2018;33:2122–31. doi: 10.1002/jbmr.3553. [DOI] [PubMed] [Google Scholar]
- 177.Berarducci A, Haines K, Murr MM. Incidence of bone loss, falls, and fractures after Roux-en-Y gastric bypass for morbid obesity. Appl Nurs Res. 2009;22:35–41. doi: 10.1016/j.apnr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 178.Khalid SI, Omotosho PA, Spagnoli A, Torquati A. Association of bariatric surgery with risk of fracture in patients with severe obesity. JAMA Netw Open. 2020;3:e207419. doi: 10.1001/jamanetworkopen.2020.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the United Kingdom: A cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015;12:e1001925. doi: 10.1371/journal.pmed.1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lalmohamed A, de Vries F, Bazelier MT, Cooper A, van Staa TP, Cooper C, et al. Risk of fracture after bariatric surgery in the United Kingdom: Population based, retrospective cohort study. BMJ. 2012;345:e5085. doi: 10.1136/bmj.e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maghrabi AH, Wolski K, Abood B, Licata A, Pothier C, Bhatt DL, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity. 2015;23:2344–8. doi: 10.1002/oby.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chin WL, Chi PJ, Hung WC, Lin CW, Chen CY, Chen JH. Bariatric surgery decreases the risk of non-traffic accident-related fractures in patients with obesity: Real-world data from Taiwan. Obes Surg. 2021;31:2231–40. doi: 10.1007/s11695-021-05262-8. [DOI] [PubMed] [Google Scholar]
- 183.Yu EW, Kim SC, Sturgeon DJ, Lindeman KG, Weissman JS. Fracture risk after Roux-en-Y gastric bypass vs adjustable gastric banding among medicare beneficiaries. JAMA Surg. 2019;154:746–53. doi: 10.1001/jamasurg.2019.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paccou J, Martignène N, Lespessailles E, Babykina E, Pattou F, Cortet B, et al. Gastric bypass but not sleeve gastrectomy increases risk of major osteoporotic fracture: French population-based cohort study. J Bone Miner Res. 2020;35:1415–23. doi: 10.1002/jbmr.4012. [DOI] [PubMed] [Google Scholar]
- 185.Aasheim ET, Hofsø D, Hjelmesaeth J, Birkeland KI, Bøhmer T. Vitamin status in morbidly obese patients: A cross-sectional study. Am J Clin Nutr. 2008;87:362–9. doi: 10.1093/ajcn/87.2.362. [DOI] [PubMed] [Google Scholar]
- 186.Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB) – A prospective study. Obes Surg. 2010;20:447–53. doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 187.Krzizek EC, Brix JM, Herz CT, Kopp HP, Schernthaner GH, Schernthaner G, et al. Prevalence of micronutrient deficiency in patients with morbid obesity before bariatric surgery. Obes Surg. 2018;28:643–8. doi: 10.1007/s11695-017-2902-4. [DOI] [PubMed] [Google Scholar]
- 188.O’Kane M, Parretti HM, Pinkney J, Welbourn R, Hughes CA, Mok J, et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes Rev. 2020;21:e13087. doi: 10.1111/obr.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e77–137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 190.Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the surgical weight loss patient 2016 update: Micronutrients. Surg Obes Relat Dis. 2017;13:727–41. doi: 10.1016/j.soard.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 191.Elhag W, El Ansari W, Abdulrazzaq S, Abdullah A, Elsherif M, Elgenaied I. Evolution of 29 anthropometric, nutritional, and cardiometabolic parameters among morbidly obese adolescents 2 years post sleeve gastrectomy. Obes Surg. 2018;28:474–82. doi: 10.1007/s11695-017-2868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bassatne A, Chakhtoura M, Saad R, Fuleihan GE. Vitamin D supplementation in obesity and during weight loss: A review of randomized controlled trials. Metabolism. 2019;92:193–205. doi: 10.1016/j.metabol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 193.Flores L, Moizé V, Ortega E, Rodríguez L, Andreu A, Filella X, et al. Prospective study of individualized or high fixed doses of vitamin D supplementation after bariatric surgery. Obes Surg. 2015;25:470–6. doi: 10.1007/s11695-014-1393-9. [DOI] [PubMed] [Google Scholar]
- 194.Aills L, Blankenship J, Buffington C, Furtado M, Parrott J Allied Health Sciences Section Ad Hoc Nutrition Committee. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4:S73–108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y, Côté MM, Cheney MC, Lindeman KG, Rushin CC, Hutter MM, et al. Zoledronic acid for prevention of bone loss in patients receiving bariatric surgery. Bone Rep. 2021;14:100760. doi: 10.1016/j.bonr.2021.100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rino Y, Aoyama T, Atsumi Y, Yamada T, Yukawa N. Metabolic bone disorders after gastrectomy: Inevitable or preventable. Surg Today. 2021 doi: 10.1007/s00595-021-02253-1. https://doi.org/10.1007/s00595 021-02253-1. [DOI] [PubMed] [Google Scholar]
- 197.Karthik L, Kumar G, Keswani T, Bhattacharyya A, Chandar SS, Bhaskara Rao KV. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS One. 2014;9:e90972. doi: 10.1371/journal.pone.0090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mahawar KK, Parmar C, Graham Y. One anastomosis gastric bypass: Key technical features, and prevention and management of procedure-specific complications. Minerva Chir. 2019;74:126–36. doi: 10.23736/S0026-4733.18.07844-6. [DOI] [PubMed] [Google Scholar]


