Abstract
Our previous study showed that gamma interferon (IFN-γ), a T-helper 1 (Th1)-type cytokine, plays a detrimental role in Staphylococcus aureus infection in mice. In this study, the role of Th2-type cytokines such as interleukin-4 (IL-4) and IL-10 in S. aureus infection was investigated. IL-10 mRNA was induced in parallel with IFN-γ in the spleens and kidneys of mice during S. aureus infection, whereas IL-4 mRNA was induced in the spleens but not in the kidneys of these animals. Spleen cells obtained from S. aureus-infected mice produced lower titers of IFN-γ and higher titers of IL-4 and IL-10 in response to heat-killed S. aureus than did those from uninfected mice. Administration of anti-IL-4 monoclonal antibody (MAb) or anti-IL-10 MAb inhibited the elimination of S. aureus cells from the kidneys of mice. IFN-γ mRNA expression was enhanced in the spleens of anti-IL-4 MAb- or anti-IL-10 MAb-treated mice and also in the kidneys of anti-IL-4 MAb-treated animals. Next, we evaluated the role of IFN-γ in S. aureus infection in IFN-γ−/− mice. An increase in survival rates, a decrease in bacterial numbers in the kidneys, and an amelioration of histologic abnormalities in these organs were observed in IFN-γ−/− mice compared with those in IFN-γ+/+ mice. Administration of MAb against IL-4 or IL-10 failed to affect bacterial growth in the spleens and kidneys of IFN-γ−/− mice irrespective of the expression of Th2 response. These results suggest that S. aureus infection induced a Th2 response and that IL-4 and IL-10 might play a protective role through the regulation of IFN-γ in S. aureus infection.
Staphylococci, including Staphylococcus aureus, which can grow extracellularly and sometimes intracellularly, are a major source of morbidity and mortality in medical facilities. Staphylococci and their products are capable of strongly inducing various cytokines. Endogenous cytokines such as gamma interferon (IFN-γ), tumor necrosis factor alpha, and interleukin-6 (IL-6) are produced in the bloodstreams, spleens, and kidneys of mice during systemic infection with S. aureus (26, 30). Our previous study showed that IFN-γ, but neither tumor necrosis factor alpha nor IL-6, plays a detrimental role in S. aureus infection in mice (26) and that the lethality of this infection could be escaped by the blockade of endogenous IFN-γ by administration of the corresponding monoclonal antibody (MAb). Zhao and Tarkowski (36) also demonstrated that IFN-γ receptor-negative mice developed severe sepsis with higher mortality after S. aureus infection.
Antigen-specific CD4+ helper T (Th)-cell responses can be divided into two types, Th1 and Th2, based on cytokine production and effector function (24, 32). Differentiation of Th1 cells, which produce IL-2, IFN-γ, and lymphotoxin, is driven by IL-12 and IFN-γ, while differentiation of Th2 cells, which produce IL-4, IL-5, IL-10, and IL-13, depends on IL-4. IFN-γ is a representative of the Th1-type cytokines, and it inhibits the outgrowth of Th2 cells (1). IL-10, one of the Th2-type cytokines, shows anti-inflammatory activity and plays a role in protecting the host from endotoxin shock (9, 15), septic shock (2), and staphylococcal enterotoxin shock (8, 12). On the other hand, IL-4 reportedly plays either a protective or detrimental role in S. aureus-induced sepsis, depending on the mouse strains used (17, 18).
We were interested in the Th response and relationship between IFN-γ-mediated pathogenesis and Th2-derived cytokines in S. aureus infection. In this study, we demonstrate that the Th2 response becomes dominant in S. aureus infection and that IL-4 and IL-10 play a protective role. We further show that this protective role might be due to the regulation of IFN-γ.
MATERIALS AND METHODS
Mice.
Outbred ddY mice, IFN-γ deficient mice (IFN-γ−/− mice) on a C57BL/6×Sv129 background (33), and corresponding control mice (IFN-γ+/+ mice), 5 to 8 weeks old, were used. ddY mice were purchased from SLC Japan (Hamamatsu, Shizuoka, Japan). The animals were maintained under specific-pathogen-free conditions at the Institute for Animal Experiment, Hirosaki University School of Medicine.
Bacteria.
S. aureus 834 was prepared as described previously (26). In each experiment, bacteria were cultured on tryptic soy agar (Difco Laboratories, Detroit, Mich.) for 24 h at 37°C, inoculated into tryptic soy broth (Difco), and incubated for another 15 h. The organisms were collected by centrifugation and resuspended in 0.85% saline. The concentration of resuspended cells was adjusted spectrophotometrically at 550 nm. Mice were infected intravenously with 0.2 ml of a solution containing 107 or 108 CFU of viable S. aureus cells in saline. A 50% lethal dose of S. aureus 834 was 4 × 107 CFU in ddY mice and C57BL/6 mice. A heat-killed S. aureus cell suspension at 109 cells per ml in saline, which had been boiled for 10 min (30), was used for stimulation of spleen cells in vitro.
Determination of the numbers of viable S. aureus cells in the organs.
The spleens and kidneys of infected animals were homogenized in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) containing 1% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS; Wako Pure Chemical Co., Osaka, Japan) with a Dounce grinder. The numbers of viable S. aureus cells were established by plating serial 10-fold dilutions of organ homogenates in 0.01 M phosphate-buffered saline (pH 7.4) on tryptic soy agar. Colonies were counted 24 h later.
Spleen cell cultures.
Spleens were removed from uninfected mice and S. aureus-infected mice aseptically, and spleen cells were obtained by squeezing the organs in RPMI 1640 medium containing 2% fetal calf serum. The cell suspension was filtered through stainless-steel mesh (size 100), and erythrocytes were depleted by treatment with 0.83% NH4Cl. After being washed three times with RPMI 1640 medium supplemented with 2% fetal calf serum, the cells were resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 200 U of penicillin G per ml, and 200 μg of streptomycin per ml, and then placed in 24-well tissue culture plates (Greiner, Frickenhausen, Germany) at a cell density of 107 cells per well in a final volume of 1 ml. Heat-killed S. aureus cells were added to the spleen cells at 108 bacteria/well. The culture supernatant was harvested 48 h after incubation and stored at −80°C until the cytokine assays were performed.
Cytokine assays.
IFN-γ, IL-4, and IL-10 assays were carried out by a double-sandwich enzyme-linked immunosorbent assay (ELISA) as described previously (25, 26). Purified rat anti-mouse IFN-γ MAb produced by hybridoma R4-6A2 and rabbit anti-recombinant mouse IFN-γ serum (26) were used for the IFN-γ ELISA. All IFN-γ ELISAs were run with recombinant mouse IFN-γ produced and purified by Genentech, Inc., South San Francisco, Calif. Rat anti-mouse IL-4 MAb (11B11; PharMingen. San Diego, Calif.) or rat anti-mouse IL-10 MAb (JES5-2A5; PharMingen) and biotinylated rat anti-mouse IL-4 MAb (BVD6-24G; PharMingen) or biotinylated rat anti-mouse IL-10 MAb (SXC-1; PharMingen) were used for determination of IL-4 and IL-10. All IL-4 and IL-10 ELISAs were run with recombinant mouse IL-4 (Genzyme Co., Boston, Mass.) or recombinant mouse IL-10 (Genzyme).
In vivo depletion of endogenous cytokines.
Hybridoma cell lines secreting MAbs against mouse IL-4 (11B11; rat immunoglobulin G1), and mouse IL-10 (JES5-2A5; rat immunoglobulin G1) were used. MAbs found in the ascites fluid were partially purified by (NH4)2SO4 precipitation. The mice were given single intravenous injections of 1 mg of anti-IL-4 MAb or anti-IL-10 MAb 1 h before infection. Normal rat globulin (NRG) was injected as a control for the MAbs. NRG was prepared as described previously (30). All in vivo effects of MAbs and NRG described were verified by the use of reagents tested by the Limulus amoebocyte lysate assay to contain <0.1 ng per injected dose.
Reverse transcription-PCR.
Total RNA was isolated from pieces of spleens and kidneys (0.05 g each) using a guanidium thiocyanate-phenol-chloroform single-step method (5). cDNA was prepared by reverse transcription as previously reported (30). PCR amplification was performed with a Program Temp Control System apparatus (PC-700; ASTEC, Inc., Fukuoka, Japan). The reaction mixture consisted of 20 μl of sample cDNA, 8 μl of PCR amplification buffer (Gibco-BRL), 4 μl of 1.25 mM deoxynucleoside triphosphates, 1 μl of 20 mM 5′ and 3′ primers, 0.5 μl (2.5 U) of Taq DNA polymerase (Gibco-BRL), and 47 μl of distilled water to make a final volume of 80.5 μl. Primers for IFN-γ, IL-4, L-10, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were prepared as previously described (25, 30). The predicted sizes of the amplified products for IL-4, IL-10, IFN-γ, and GAPDH were 279, 317, 243, and 277 bp, respectively. After 30 PCR cycles consisting of DNA denaturation at 93°C for 29 s, primer annealing at 55°C for 29 s, and DNA extension at 72°C for 2 min, the reaction was terminated. Then 6 μl of each PCR product was analyzed by electrophoresis and visualized and photographed on a UV transilluminator (Fotodyne Inc., New Berlin, Wis.).
Histological examinations.
Mice which were infected with 107 CFU of S. aureus were sacrificed, and their kidneys were fixed in 10% neutral-buffered formalin. Tissues were processed and embedded in paraffin routinely. Sections were stained with hematoxylin and eosin.
Statistical evaluation of the data.
Data were expressed as means ± standard deviations, and the Wilcoxon rank sum test was used to determine the significance of the differences of bacterial counts in the organs and the cytokine titers between the control and experimental groups. The generalized Wilcoxon test was used to determine the significance of differences in the survival rate. Each experiment was repeated at least three times and accepted as valid only when the trials showed similar results.
RESULTS
Induction of IFN-γ, IL-4, and IL-10 mRNA expression during S. aureus infection.
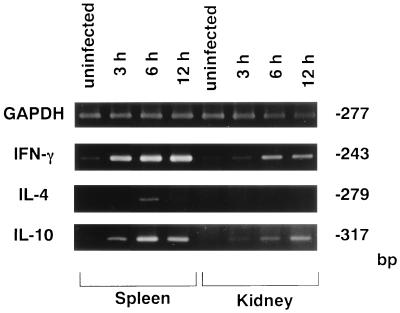
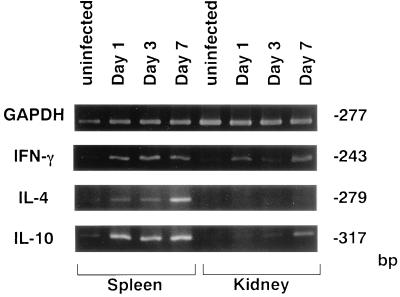
Mice were infected with 108 CFU of S. aureus cells, and IFN-γ, IL-4, and IL-10 mRNA expression in the spleens and kidneys was investigated by reverse transcription-PCR at 3, 6, and 12 h of infection (Fig. 1). There was no expression of IFN-γ, IL-4, or IL-10 mRNA in any of the organs from uninfected mice. IFN-γ and IL-10 mRNA was detected in the spleens and kidneys of S. aureus-infected mice at 3, 6, and 12 h, whereas IL-4 mRNA was expressed only in the spleens at 6 h. When mice were infected with 107 CFU of S. aureus cells (Fig. 2), IFN-γ mRNA was detected in the spleens and kidneys on days 1, 3, and 7 of infection; IL-4 mRNA was induced in the spleens of S. aureus-infected mice on day 7 after infection; and IL-10 mRNA was detected in the spleens from days 1 to 7 and in the kidneys on day 7 after infection.
FIG. 1.
PCR-assisted mRNA amplification analysis of IFN-γ, IL-4, and IL-10 in the spleens and kidneys of S. aureus-infected or uninfected mice within 12 h of infection with 108 CFU of S. aureus. The results were reproduced in three repeated experiments.
FIG. 2.
PCR-assisted mRNA amplification analysis of IFN-γ, IL-4, and IL-10 in the spleens and kidneys of S. aureus-infected or uninfected mice on days 1, 3, and 7 after infection with 107 CFU of S. aureus. The results were reproduced in three repeated experiments.
IFN-γ, IL-4, and IL-10 production in spleen cell cultures obtained from S. aureus-infected mice.
Next, we investigated the in vitro production of IL-4 and IL-10 in mouse spleen cell cultures. Spleen cells obtained from uninfected mice and S. aureus-infected mice on days 7 and 21 of infection were stimulated with heat-killed S. aureus, and the titers of IFN-γ, IL-4, and IL-10 in the culture supernatants were estimated (Table 1). On day 7 of infection, IFN-γ production was decreased in the infected mice compared with the uninfected mice (P < 0.01) whereas IL-10 production in the infected mice was higher than that in the controls (P < 0.01). There was no difference in IL-4 production between the infected mice and uninfected mice (P > 0.05). On day 21 of infection, IFN-γ production in the infected mice was lower than that in uninfected mice (P < 0.01). In contrast, both IL-4 and IL-10 production in the infected mice was augmented compared with that in the uninfected mice (P < 0.01).
TABLE 1.
Production of IFN-γ, IL-4, and IL-10 in the spleen cell cultures obtained from S. aureus-infected micea
| Time postinfection and infection status | Cytokine titerb
|
||
|---|---|---|---|
| IFN-γ (ng/ml) | IL-4 (pg/ml) | IL-10 (ng/ml) | |
| 7 days | |||
| Uninfected | 59.8 ± 3.2 | 43 ± 0.4 | 14.5 ± 3.0 |
| Infected | 7.7 ± 0.2c | 41 ± 2.5 | 32.5 ± 2.3c |
| 21 days | |||
| Uninfected | 34.6 ± 2.2 | 91 ± 10.3 | 28.2 ± 1.3 |
| Infected | 17.9 ± 1.9c | 143 ± 13.5c | 42.7 ± 6.2c |
Mice were infected with 107 CFU of S. aureus or left uninfected.
Spleen cells were stimulated with heat-killed S. aureus for 48 h, and the cytokine titer in each culture supernatant was determined. Each result represents the mean ± standard deviation for a group of four mice. The results were reproduced in three repeated experiments.
Significantly different from the value for the uninfected group (P < 0.01).
Effect of in vivo administration of MAbs against IL-4 and IL-10 on host resistance to S. aureus infection.
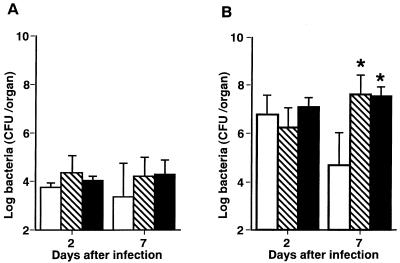
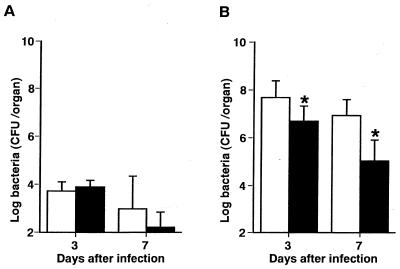
To determine the role of endogenous IL-4 and IL-10 during S. aureus infection, mice were given single intravenous injections of anti-IL-4 MAb, anti-IL-10 MAb, or NRG 1 h before being infected with 107 CFU of S. aureus, and the numbers of bacteria in the spleens and kidneys were determined on days 2 and 7 of infection (Fig. 3). No significant differences in bacterial numbers were shown in the spleens of NRG-injected and MAb-injected animals (P < 0.01) (Fig. 3A). The bacterial numbers in the kidneys were significantly higher in anti-IL-4 MAb- or anti-IL-10 MAb-pretreated mice than in NRG-pretreated mice on day 7 (P < 0.01) but not on day 2 (P > 0.05) of infection (Fig. 3B).
FIG. 3.
Effect of in vivo administration of anti-IL-4 MAb or anti-IL-10 MAb on the growth of bacterial cells in the organs of mice which were infected with 107 CFU of S. aureus. Mice were injected with normal rat globulin (open bars), anti-IL-4 MAb (hatched bars), or anti-IL-10 MAb (filled bars) 1 h before infection. The numbers of bacterial cells in the spleens (A) and kidneys (B) were determined on days 2 and 7 of infection. Each result represents the mean and standard deviation for a group of five mice. A single asterisk indicates a significant difference from the control group at P < 0.01. The results were reproduced in three repeated experiments.
Effect of in vivo administration of MAbs against IL-4 and IL-10 on IFN-γ mRNA expression during S. aureus infection.
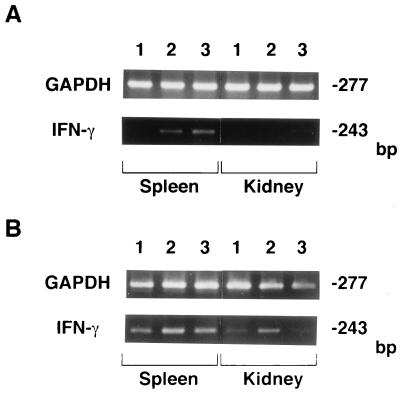
To investigate whether IFN-γ mRNA expression would be regulated by endogenous IL-4 and IL-10 during S. aureus infection, mice were given single intravenous injections of anti-IL-4 MAb, anti-IL-10 MAb, or NRG 1 h before being infected with 107 CFU of S. aureus, and IFN-γ mRNA expression in the spleens and kidneys was then detected by reverse transcription-PCR on days 2 and 7 of infection (Fig. 4). Induction of IFN-γ mRNA was observed in the spleens on day 2 in anti-IL-4 MAb- and anti-IL-10 MAb-injected mice but not in NRG-treated animals, while IFN-γ mRNA was detected in the spleens of both groups of mice on day 7. In the kidneys, IFN-γ mRNA was expressed on day 7 only when mice were injected with anti-IL-4 MAb.
FIG. 4.
Effect of administration of MAbs against IL-4 and IL-10 on IFN-γ mRNA expression in the spleens and kidneys of S. aureus-infected mice. Mice were injected with NRG (lane 1), anti-IL-4 MAb (lane 2), or anti-IL-10 MAb (lane 3) and infected with 107 CFU of S. aureus 1 h later. The spleens and kidneys were obtained on day 2 (A) or day 7 (B) of infection for PCR-assisted mRNA amplification of IFN-γ and GAPDH. The results were reproduced in three repeated experiments.
Fate of S. aureus infection in IFN-γ-deficient mice.
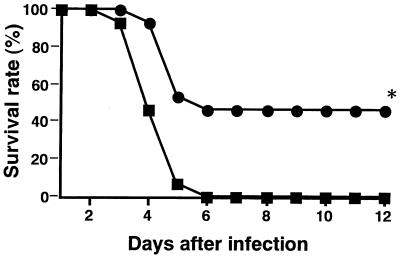
Our previous study showed that administration of anti-IFN-γ MAb protected mice from lethal S. aureus infection (26). In the present study, we investigated the fate of S. aureus infection in IFN-γ−/− mice. When IFN-γ−/− and IFN-γ+/+ mice were infected with 108 CFU of S. aureus, the IFN-γ+/+ mice died 3 to 6 days after infection; however, 46% of the IFN-γ−/− mice survived until 12 days after infection (P < 0.01) (Fig. 5). Next, we measured the numbers of S. aureus cells in the spleens and kidneys of IFN-γ−/− mice. IFN-γ−/− and IFN-γ+/+ mice were infected with 107 CFU of S. aureus, and the bacterial numbers in the organs were determined 3 and 7 days later (Fig. 6). The bacterial numbers in the kidneys of IFN-γ−/− mice were significantly lower than those in IFN-γ+/+ mice (P < 0.01), while the bacterial numbers in the spleens of IFN-γ−/− mice were comparable to those in IFN-γ+/+ mice (P > 0.05).
FIG. 5.
Survival rate of IFN-γ-deficient mice infected with a lethal dose of S. aureus. IFN-γ+/+ mice (solid squares) and IFN-γ−/− mice (solid circles) were infected with 108 CFU of S. aureus. The asterisk indicates a significant difference from IFN-γ+/+ mice at P < 0.01. The results were reproduced in three repeated experiments.
FIG. 6.
Growth of S. aureus cells in the spleens and kidneys of IFN-γ-deficient mice. IFN-γ+/+ mice (open bars) and IFN-γ−/− mice (filled bars) were infected with 107 CFU of S. aureus, and the numbers of bacterial cells in the spleens (A) and kidneys (B) were determined on days 3 and 7 of infection. Each result is the mean and standard deviation for a group of five mice. The asterisks indicate a significant difference from IFN-γ+/+ mice at P < 0.01. The results were reproduced in three repeated experiments.
Histologic examination of specimens from IFN-γ-deficient mice.
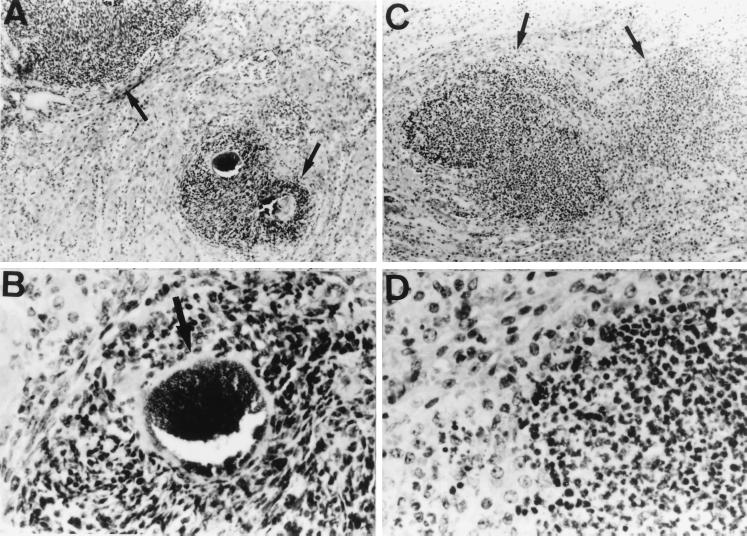
Microscopic examination of the kidneys obtained from IFN-γ−/− and IFN-γ+/+ mice was performed on day 3 after infection with 107 CFU of S. aureus. The common histopathological findings in both groups included multiple abscesses of various sizes and vasculitis with bacterial thrombosis, with or without infarction, so-called septic infarction. In IFN-γ+/+ mice, the abscesses were composed mainly of neutrophils and monocytes, and in the center of the abscesses were some microcolonies surrounded by “clubs” of reactive protein material (Fig. 7A and B). In contrast, IFN-γ−/− mice had fewer and smaller abscesses in the kidneys. The abscesses consisted mainly of neutrophils, and their borders were unclear. The club-shaped colony of the microorganisms was also obscure (Fig. 7C and D). Histologic observations of kidneys specimens obtained from both groups of mice on day 7 of infection were similar to those of specimens obtained on day 3 (data not shown).
FIG. 7.
Histology of kidneys from S. aureus-infected IFN-γ-deficient mice. The kidney sections from IFN-γ+/+ mice (A and B) and IFN-γ−/− mice (C and D) were obtained after infection with 107 CFU of S. aureus. The sections were stained with hematoxylin and eosin. Magnification, ×100 (A and C) and ×400 (B and D). The results were reproduced in three repeated experiments.
IL-4 and IL-10 production in spleen cell cultures obtained from S. aureus-infected IFN-γ-deficient mice.
Spleen cells were obtained on days 7 and 21 postinfection from IFN-γ−/− mice and IFN-γ+/+ mice infected with 107 CFU of S. aureus. Control cells were obtained from uninfected IFN-γ−/− mice and IFN-γ+/+ mice. The spleen cells were stimulated with heat-killed S. aureus, and the production of IL-4 and IL-10 in the culture supernatants was assayed (Table 2). IL-10 production was augmented in spleen cell cultures obtained from either IFN-γ−/− mice or IFN-γ+/+ mice after infection on both days 7 and 21 of infection (P < 0.01).
TABLE 2.
IL-10 production in spleen cell cultures obtained from IFN-γ-deficient mice
| Infection status and time postinfectiona | IL-10 titer (ng/ml)b in:
|
|
|---|---|---|
| IFN-γ+/+ mice | IFN-γ−/− mice | |
| Uninfected | 4.5 ± 3.0 | 4.3 ± 2.5 |
| Infected | ||
| 7 days | 18.5 ± 6.8c | 16.7 ± 5.4c |
| 21 days | 21.0 ± 10.5c | 13.4 ± 5.0c |
Mice were infected with 107 CFU of S. aureus.
Spleen cells were stimulated with heat-killed S. aureus for 48 h, and the IL-10, titer in each culture supernatant was determined. Each result represents the mean ± standard deviation for a group of four mice. The results were reproduced in three repeated experiments.
Significantly different from the value for the uninfected group (P < 0.01).
Effect of administration of MAbs against IL-4 and IL-10 in IFN-γ-deficient mice.
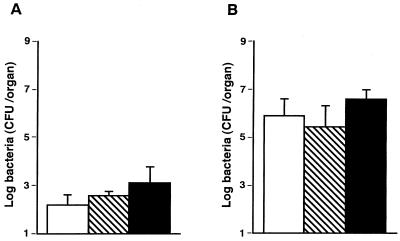
To determine whether IL-4 and IL-10 are involved in host resistance to S. aureus infection in IFN-γ−/− mice, IFN-γ−/− mice were given single intravenous injections of anti-IL-4 MAb, anti-IL-10 MAb, or NRG 1 h before being infected with 107 CFU of S. aureus, and the numbers of bacterial cells in the spleens and kidneys were determined on day 7 of infection (Fig. 8). There were no differences in the numbers of bacteria in the spleens and kidneys among the three groups (P < 0.01).
FIG. 8.
Effect of in vivo administration of anti-IL-4 MAb or anti-IL-10 MAb on the growth of bacterial cells in the organs of IFN-γ-deficient mice which were infected with 107 CFU of S. aureus. Mice were injected with NRG (open bars), anti-IL-4 MAb (hatched bars), or anti-IL-10 MAb (filled bars) 1 h before infection. The numbers of bacterial cells in the spleens (A) and kidneys (B) were determined on day 7 of infection. Each result represents the mean and standard deviation for a group of five mice. The results were reproduced in three repeated experiments.
DISCUSSION
In this study, we demonstrated that S. aureus infection induced a Th2 response and that IL-4 and IL-10 might play a protective role through the regulation of IFN-γ in S. aureus infection.
Endogenous IL-4 and IL-10 in the spleens and kidneys of S. aureus-infected mice were assayed by ELISAs. However, the levels of these cytokines were below the limits of detection in any specimen. Therefore, we investigated endogenous cytokines in the organs by reverse transcriptase-PCR. IL-10 mRNA was induced in parallel with IFN-γ in the spleens and kidneys of mice during S. aureus infection, whereas IL-4 mRNA was induced in the spleens but not in the kidneys of these animals (Fig. 1 and 2). The different expressions of IL-4 and IL-10 in the kidneys might be due to differences in the spectra of cell types found in these organs, because various types of cells including macrophages can produce IL-10 (23) but IL-4 production is reportedly limited to cell populations such as T cells, NK cells, and mast cells (27).
Spleen cells obtained from S. aureus-infected mice produced lower titers of IFN-γ and higher titers of IL-4 and IL-10 in response to heat-killed S. aureus than did those obtained from uninfected mice (Table 1), suggesting that S. aureus infection polarizes Th0 toward Th2. Polarization of Th1 and Th2 reportedly depends on the genetic background of mice in some microbial infections such as Leishmania major (13, 31) and Borrelia burgdorferi (21, 34) infections. In the case of S. aureus infection, a Th2 response occurred in both C57BL/6-background mice (Table 2), which make a predominantly Th1 response (13, 32, 34), and BALB/c mice (data not shown), which make a predominantly Th2 response (13, 21, 31), in addition to outbred ddY mice (Table 1), showing that S. aureus infection induces a Th2 response irrespective of the mouse strain. It has been reported that S. aureus infection also induced a Th2 response in human peripheral blood CD4+ T cells (22).
IL-4, a representative of Th2-type cytokines, plays a detrimental role in host resistance to microbial infections including Listeria monocytogenes (11, 25), Candida albicans (28), and Leishmania major (13, 31). Hultgren et al. (17, 18) reported that IL-4 plays either a protective or detrimental role in S. aureus-induced sepsis, depending on the mouse strain used. IL-10, another Th2-type cytokine, plays a detrimental role in host resistance to microbial infections including L. monocytogenes (6), Mycobacterium avium (3), Klebsiella pneumoniae (10), and Candida albicans (29). In contrast, IL-10 plays a beneficial role in protecting the host from endotoxin shock (9, 15), septic shock (2), and staphylococcal enterotoxin shock (8, 12). In this study, administration of anti-IL-4 MAb or anti-IL-10 MAb to mice inhibited the elimination of S. aureus from the kidneys, in which infectious foci were easily formed (Fig. 3), suggesting that both IL-4 and IL-10 might be involved in host resistance to S. aureus infection. Although IL-4 mRNA was expressed in the kidneys only in the early phase of infection (Fig. 1), it is possible that the IL-4 may have been supplied by other sites such as the spleen via the circulation. Alternatively, bacterial numbers in the spleens may not have been affected by the administration of anti-IL-4 MAb or anti-IL-10 MAb (Fig. 3 and 6). Our previous study indicated that S. aureus preferentially colonizes and proliferates, with abscess formation, in kidneys but not in spleens (26). We presumed that S. aureus cells detected in the spleens might have been derived from the circulation and that elimination of bacteria might not be controlled by cytokines including IL-4 and IL-10. However, a precise mechanism for the different effects of these cytokines in the spleen and kidneys is unsolved.
We demonstrated that administration of anti-IFN-γ MAb resulted in the suppression of bacterial growth in the kidneys and protected mice from the lethal effects of S. aureus infection (26). Zhao and Tarkowski (36) also demonstrated that IFN-γ receptor-negative mice developed severe sepsis with high mortality after S. aureus infection. Recently, however, Zhao et al. reported that anti-IFN-γ MAb treatment had no significant influence on survival rates and was accompanied by an increase in the bacterial numbers in the kidneys, and they also reported that administration of recombinant IFN-γ ameliorated S. aureus sepsis (35). Therefore, we evaluated the role of IFN-γ in S. aureus infection in IFN-γ−/− mice. Our present results showed that IFN-γ plays an important role in the pathogenesis of S. aureus infection, because there was an increase in the survival rates (Fig. 5), a decrease in bacterial numbers in the kidneys (Fig. 6), and an amelioration of histologic changes observed in the kidneys (Fig. 7) in IFN-γ−/− mice compared with those in IFN-γ+/+ mice.
Our present study indicated that IL-4 and IL-10 might play a beneficial role in host resistance to S. aureus infection. These cytokines are known to have anti-inflammatory actions in various inflammatory diseases (2, 8, 9, 15, 17–19). It is possible that these cytokines may regulate excess inflammatory responses in S. aureus infection. On the other hand, IL-4 and IL-10 are known to suppress Th1 development by inhibiting the production of IFN-γ and IL-12 (16). In our hands, IFN-γ mRNA expression in the spleens of anti-IL-4 MAb- or anti-IL-10 MAb-treated mice and also in the kidneys of the anti-IL-4 MAb-treated animals was enhanced (Fig. 4). Interestingly, administration of MAb against IL-4 or IL-10 failed to affect the bacterial growth in the spleens and kidneys of IFN-γ−/− mice (Fig. 8) irrespective of the expression of the Th2 response (Table 2). These results suggested that IL-4 and IL-10, rather than affecting host resistance by acting directly against S. aureus infection, may show their protective effect through the regulation of IFN-γ production.
Staphylococcal enterotoxin C reportedly induces Th2-type cytokines (7). The S. aureus strain used herein produces staphylococcal enterotoxin C and toxic shock syndrome toxin 1 (26, 30). We are planning to elucidate the role of staphylococcal enterotoxin C in Th2 polarization and host resistance to S. aureus infection. Whereas IFN-γ is well known to be a key mediator in various immune responses, it is also reportedly involved in the pathogenesis of infectious diseases such as endotoxin shock (4, 14) and in the severity of gram-negative bacterial infections (20). More studies are necessary to clarify the precise mechanism of IFN-γ action in the pathogenesis of S. aureus infection and its protective effects as a potential immunotherapeutic agent against S. aureus sepsis.
ACKNOWLEDGMENTS
This work was supported in part by grants-in-aid for general scientific research (grants 10670247 and 40261440) provided by the Japanese Ministry of Education, Science, Sports and Culture.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Berg D J, Kühn R, Rajewsky K, Müller W, Menon S, Davidson N, Grünig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxin shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Champsi J. Infection with Mycobacterium avium induced production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Car B D, Eng V M, Schnyder B, Ozmen L, Huang S, Galley P, Heumann D, Aguet M, Ryffel B. Interferon γ receptor deficient mice are resistant to endotoxin shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single step method for RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Dai W, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 7.Ferens W A, Goff W L, Davis W C, Fox L K, Deobald C, Hamilton M J, Bohach G A. Induction of type 2 cytokines by a staphylococcal enterotoxin superantigen. J Nat Toxins. 1998;7:193–213. [PubMed] [Google Scholar]
- 8.Florquin S, Amraoui Z, Abramowicz D, Goldman M. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J Immunol. 1994;153:2618–2623. [PubMed] [Google Scholar]
- 9.Gérard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberger M J, Strieter R M, Kunkel S T, Danforth J M, Goodman R E, Standiford T J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 11.Haak-Frendsho M, Brown J F, Iizawa Y, Wagner R D, Czuprynski C J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992;148:3978–3985. [PubMed] [Google Scholar]
- 12.Hasko G, Virag L, Egnaczyk G, Salzman A L, Szabo C. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur J Immunol. 1998;28:1417–1425. doi: 10.1002/(SICI)1521-4141(199804)28:04<1417::AID-IMMU1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heremans H, van Damme J, Dillen C, Dijkmans R, Billiau A. Interferon γ, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990;171:1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin-10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh C-S, Heimberger A B, Gold J S, O'Garra A, Murphy K M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultgren O, Kopf M, Tarkowski A. Staphylococcus aureus-induced septic arthritis and septic death is decreased in IL-4-deficient mice: role of IL-4 as promoter for bacterial growth. J Immunol. 1998;160:5082–5087. [PubMed] [Google Scholar]
- 18.Hultgren O, Kopf M, Tarkowski A. Outcome of Staphylococcus aureus-triggered sepsis and arthritis in IL-4-deficient mice depends on the genetic background of the host. Eur J Immunol. 1999;29:2400–2405. doi: 10.1002/(SICI)1521-4141(199908)29:08<2400::AID-IMMU2400>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Joosten L A B, Lubberts E, Durez P, Helsen M M A, Jacobs M J M, Goldmam M, Van den Berg W B. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 20.Kohler, J., D. Heumann, G. Garotta, D. LeRoy, S. Bailat, C. Barras, J.-D. Baumgartner, and M. P. Glauser. IFN-γ involvement in the severity of gram-negative infections in mice. J. Immunol. 151:916–921. [PubMed]
- 21.Matynaik J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme diseases. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayet W J, Marker-Hermann E, Schlaak J, Meyer zum Buschenfelde K H. Irregular cytokine pattern of CD4+ T lymphocytes in response to Staphylococcus aureus in patients with Wegener's granulomatosis. Scand J Immunol. 1999;49:585–594. doi: 10.1046/j.1365-3083.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 23.Moore K W, O'Garra A, De Waal Malefy R, Vieira P, Mossman T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T R, Cherwinski H, Bond M, Giedlin M A, Coffman R L. Two types of murine helper T-cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;36:2348–2357. [PubMed] [Google Scholar]
- 25.Nakane A, Nishikawa S, Sasaki S, Miura T, Asano M, Kohanawa M, Ishiwata K, Minagawa T. Endogenous interleukin-4, but not interleukin-10, is involved in suppression of host resistance against Listeria monocytogenes infection in gamma interferon-depleted mice. Infect Immun. 1996;64:1252–1258. doi: 10.1128/iai.64.4.1252-1258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakane A, Okamoto M, Asano M, Kohanawa M, Minagawa T. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun. 1995;63:1165–1172. doi: 10.1128/iai.63.4.1165-1172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelms K, Keegan A D, Zamorano J, Ryan J J, Paul W E. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 28.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induced systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 30.Sasaki S, Miura T, Nishikawa S, Yamada K, Hirasue M, Nakane A. Protective role of nitric oxide in Staphylococcus aureus in mice. Infect Immun. 1998;66:1017–1022. doi: 10.1128/iai.66.3.1017-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 32.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 33.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 34.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y-X, Nilsson I M, Tarkowski A. The dual role of interferon-γ in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology. 1998;93:80–85. doi: 10.1046/j.1365-2567.1998.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y-X, Tarkowski A. Impact of interferon-γ receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J Immunol. 1995;155:5736–5742. [PubMed] [Google Scholar]