Abstract
Adeno-associated viruses (AAVs) are widespread among vertebrates. AAVs isolated from bats display low capsid protein sequence identities (<60%) to AAV2, AAV5, and other primate AAVs. Here we report the first capsid structure of a non-primate AAV which was isolated from bats. The capsid structure of BtAAV-10HB (10HB) was determined by cryo-electron microscopy and three-dimensional image reconstruction to 3.0 Å resolution. Comparison of empty and genome-containing particles showed that the capsid structures are almost identical except for an ordered nucleotide in a previously described nucleotide-binding pocket, the density in the 5-fold channel, and several amino acids with altered side chain conformations. Compared to other dependoparvoviruses, for example AAV2 and AAV5, 10HB displays unique structural features including insertions and deletions in capsid surface loops. Overall, the 10HB capsid structure superposes with an RMSD of 1.7 Å and 1.8 Å to AAV2 and AAV5, respectively. Currently all approved AAV human gene therapy biologics and vectors in clinical trials are based on primate isolates. However, pre-existing neutralizing antibodies in the human population represents a hurdle to their use. 10HB capsids are capable of packaging AAV2 vector genomes and thus have potential as gene delivery vectors. Significantly, a screen with human sera showed lack of recognition by the 10HB capsid. Thus, the different capsid surface of 10HB vectors likely renders it “invisible” to potential pre-existing neutralizing human antibodies especially because this virus or similar variants do not exist in primate populations.
Keywords: Adeno-associated virus, pre-existing antibodies, cryo-EM, virus capsid
INTRODUCTION
Adeno-associated viruses (AAVs) are members of the Parvoviridae and belong to the Dependoparvovirus genus. Viruses of this genus have been isolated from a wide range of animals including humans (Bantel-Schaal and zur Hausen, 1984), non-human primates (Gao et al., 2004), cattle (Schmidt et al., 2004), goats (Arbetman et al., 2005), pigs (Bello et al., 2014), sea lions (Li et al., 2011), bats (Li et al., 2019), rodents (Lochrie et al., 2006), foxes (Bodewes et al., 2013), birds (Bossis and Chiorini, 2003), and reptiles (Farkas et al., 2004). The AAVs are composed of a small, non-enveloped capsid of ~26 nm in diameter that package a linear single-stranded DNA genome of ~5 kb. The AAVs share a similar genome organization, with two large open reading frames (ORFs) flanked by inverted terminal repeats. The left ORF encodes a series of Rep proteins involved in genome replication, genome packaging, and viral transcription. The right ORF encodes three capsid viral proteins (VPs), VP1, VP2, and VP3, and the assembly-activating protein required for capsid assembly (Sonntag et al., 2010). Recently, another small protein, the membrane-associated accessory protein, was reported to be encoded from this ORF (Ogden et al., 2019). The amino acid sequences of the VPs overlap at the C-terminus, with VP1 being the largest and VP3 the smallest. Sixty VPs are assembled into a capsid in a predicted 1:1:10 (VP1:VP2:VP3) ratio, with T=1 icosahedral symmetry (Chapman and Agbandje-McKenna, 2006). The three-dimensional (3D) capsid structure of many primate AAVs have been determined either by X-ray crystallography or cryo electron microscopy (cryo-EM) (Bennett et al., 2019; Burg et al., 2018; DiMattia et al., 2012; Drouin et al., 2016; Govindasamy et al., 2006; Govindasamy et al., 2013; Guenther et al., 2019; Halder et al., 2015; Kaelber et al., 2020; Lerch et al., 2010; Mietzsch et al., 2020; Mikals et al., 2014; Nam et al., 2007; Ng et al., 2010; Tan et al., 2018; Xie et al., 2002). In all the determined AAV capsid structures, the N-terminus of VP1, VP2, as well as the first ~15 N-terminal residues of VP3 are not observed. The ordered VP3 common region structure contains a jelly roll motif found in most icosahedral viruses, with the βBIDG sheet lining the inside of the capsid (Mietzsch et al., 2019). Large loops inserted between the β-strands assemble the outside surface of the capsid creating characteristic features such as cylindrical channels at the 5-fold axes, protrusions surrounding the 3-fold axes, and depressions at the 2-fold axes and surrounding the 5-fold channels. A raised capsid region between the 2-and 5-fold depressions is termed the 2/5-fold wall (reviewed in (Mietzsch et al., 2019)). Structural comparison of different primate AAVs show that most sequence and structural differences are located in the surface loops whereas the core β-sheets (βBIDG and αCHEF) and α-helical region (αA) are conserved. The clustered differences at the surface loops have been defined as variable regions (VRs) (Govindasamy et al., 2006). The VRs of the AAVs have been associated with receptor attachment (Agbandje-McKenna and Kleinschmidt, 2011; Mietzsch et al., 2019), antigenicity (Bennett et al., 2018; Gurda et al., 2013; Jose et al., 2018; Tseng et al., 2015), transduction (Asokan et al., 2010; Pulicherla et al., 2011; Raupp et al., 2012), and are responsible for the phenotypic differences of the AAVs. Currently no non-primate AAV capsid structure has been determined. Recently, multiple AAV strains were isolated from bats in China (Li et al., 2019). All of the bat AAVs (BtAAVs) display low capsid protein sequence identities (<60%) to AAV2, AAV5 and other primate AAVs.
Vectors based on AAVs are used as gene therapy biologics to treat a large variety of monogenetic diseases. Currently all vectors used in clinical trials are based on primate AAVs. However, pre-existing neutralizing antibodies (NAbs) are a major hurdle to success. As these primate NAbs exist in a large percentage of the human population (up to 70% for AAV2) (Boutin et al., 2010) and target the viral capsids of different AAVs, their presence can lead to vector inactivation and to a loss of treatment efficacy. A strategy to overcome this problem is to utilize AAVs that do not exist in the primate population. Due to the significant sequence differences of their capsids, no or low reactivity to human sera is expected.
This study characterizes the empty and genome-containing capsid structure of the BtAAV-10HB (10HB) isolate of bat AAV and compares it to AAV2 and AAV5. These latter viruses were chosen because they are as diverse from each other as from 10HB and are either actively being developed or already approved as a gene therapy biologic (AAV2) (Keeler and Flotte, 2019; Pasi et al., 2020; Salas et al., 2019). The viral capsid structure of 10HB was determined by cryo-EM and 3D image reconstruction to 3.0 Å resolution. The density map displayed well defined side chain densities. The 10HB capsid maintained the general capsid features previously reported for the other AAVs, including a channel at the 5-fold axes, depressions at the 2-fold and surrounding the 5-fold axes, and protrusions that surround the 3-fold axes. However, compared to AAV2 and AAV5, there are structural differences at the 3-fold protrusions and the 2/5-fold wall due to sequence insertions or deletions as well as sequence differences. Despite these differences, recombinant AAV2 vector genomes could be packaged into the 10HB capsids with AAV2 Rep and thus BtAAV vectors have potential for use in future gene delivery applications.
MATERIALS AND METHODS
Cell culture and BtAAV-10HB production
HEK 293 cells were cultivated as an adherent monolayer at 37°C and 5% CO2 in DMEM supplemented with 1X antibiotic-antimycotic and 10% (v/v) fetal bovine serum (FBS) (Gibco). For AAV2, AAV5, or 10HB production, a standard triple transfection protocol utilizing polyethylenimine was used as previously described (Jose et al., 2018). In addition to pHelper (Stratagene) and an ITR-flanked luciferase reporter gene construct, a Rep-Cap expressing plasmid, containing the AAV2 rep gene with either the AAV2, AAV5 or 10HB cap gene (Li et al., 2019), was transfected. The transfected cells were harvested 72 h post transfection and pelleted by centrifugation at 300 × g for 10 min. The supernatant was subjected to polyethylene glycol 8000 (PEG 8000, 10% w/v) precipitation to recover AAV particles released into the culture medium. Following overnight incubation at 4°C, the PEG-medium solution was spun at 15,000 × g for 90 min and the pellet recovered was resuspended in 1xTD buffer (137 mM NaCl, 2.7 mM KCl, 100 mM Na2HPO4, 2 mM KH2PO4, 2.5 mM KCl, 1 mM MgCl2). In parallel, the pelleted cells were resuspended in 1xTD and subjected to three freeze-thaw cycles. The lysed cells and the resuspended PEG pellet were incubated with 125 units/ml Benzonase (Millipore) for 1 h at 37°C and spun at 10,000 × g for 15 min to pellet cell debris.
AAV vector purification
For purification of 10HB, the clarified supernatant was initially subjected to sucrose cushion ultracentrifugation. The supernatant was added to an ultracentrifuge tube and 20% sucrose (w/v) in TNET buffer (50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.2% Triton X-100) was carefully pipetted below the supernatant. The 10HB particles were pelleted by ultracentrifugation at 45,000 rpm (Beckman 70-Ti) for 3 h at 4°C. The resulting pellet was resuspended in buffer A (20 mM Tris-HCl, 15 mM NaCl). 10HB was further purified using anion-exchange chromatography as previously described (Zolotukhin et al., 2002). Briefly, the sample was diluted 10-fold in buffer A, applied to a 1 ml HiTrap Q XL column (GE Healthcare) at 1 ml/min, washed with ten column volumes of buffer A and finally eluted with increasing concentration of buffer B (20 mM Tris-HCl, 500 mM NaCl) in 0.5 ml fractions. The individual fractions were analyzed by SDS-PAGE to check for the presence of VP bands and the virus-containing fractions were pooled and concentrated using an Amicon concentrator (Millipore). AAV2 and AAV5 vectors were purified using AVB sepharose columns as previously described (Jose et al., 2018).
Cryo-electron microscopy data collection and image reconstruction
Three microliters of the 10HB sample was applied to glow-discharged Quantifoil copper grids with 2 nm continuous carbon support over holes (Quantifoil R 2/4 400 mesh), blotted, and vitrified using a Vitrobot Mark 4 (FEI) at 95% humidity and 4°C. The particle distribution and ice thickness of the grids were screened in-house using an FEI Tecnai G2 F20-TWIN microscope (FEI Co.) operated under low-dose conditions (200 kV, ~20e-/Å2). Grids optimal for data collection were used for collecting micrograph movie frames using the Leginon semi-automated application (Suloway et al., 2005) on a Titan Krios electron microscope (FEI Co.) operated at 300 kV. The data was collected as part of the NIH “West/Midwest Consortium for High-Resolution Cryo Electron Microscopy” project. Images were recorded on a Gatan K2 Summit direct electron detection camera. The microscope was equipped with a Gatan post-column imaging filter (GIF) utilizing a slit width of 20 eV. A nominal magnification of 130,000x was used for data collection resulting in a pixel size of 1.06 Å. Data collection used counting mode and an accumulated dose of 75 e-/Å2 fractionated into 50 movie frames per micrograph. The movie frames were aligned using MotionCor2 with dose weighting (Zheng et al., 2017). The data collection parameters are provided in Table 1. For three dimensional image reconstruction, the cisTEM software package was utilized (Grant et al., 2018). Briefly, the aligned micrographs were imported into the application and their CTF parameters estimated. This information was used to eliminate micrographs of poor quality. This was followed by automatic particle picking using a particle radius of 130 Å. This set of particles was subjected to 2D classification that eliminated non-AAV particles (ice and debris) from the automatic picking process. While the initial 2D classification, using a box size of ~1.5x the diameter of the 10HB capsids, successfully removed ice particles and other contaminations, it did not separate empty and genome-containing particles. Thus, a second round of 2D classification was performed with the box size reduced to slightly smaller than the diameter of the capsids in order to classify the particles based on their internal content. Following this work around, the capsid structures of empty and genome-containing particles were independently reconstructed using default settings. This included the ab initio 3D model generation, auto refinement, and density map sharpening with a pre-cut off B-factor value of −90 Å2, and variable post-cut off B-factor values such as 0, 20 and 40 Å2. The sharpened density maps were inspected in the Coot and Chimera applications (Emsley and Cowtan, 2004; Pettersen et al., 2004). The −90 Å2 / 0 Å2 sharpened maps were used for assignment of the amino acid main- and side chains. The resolution of the cryo-reconstructed density maps for empty and genome-containing 10HB capsids were estimated to 3.0 Å resolution based on a Fourier Shell Correlation (FSC) of 0.143.
Table 1:
Summary of Data Collection, Image-Processing Parameters, and refinement statistics
| Processing and Refinement Parameters | BtAAV-10HB | |
|---|---|---|
| full | empty | |
| Total number of micrographs | 772 | |
| Defocus range (μm) | 0.99 – 4.03 | |
| Total electron dose (e- / Å2) | 75 | |
| Frames / micrograph | 50 | |
| Pixel size (Å/pixel) | 1.06 | |
| Particles used for final map | 4,661 | 4,088 |
| Resolution of final map (Å) | 3.03 | 3.03 |
| Refinement Statistics | ||
| Map CC | 0.857 | 0.848 |
| RMSD (bonds) | 0.01 | 0.01 |
| RMSD (angles) | 0.92 | 0.86 |
| All-atom clashscore | 7.00 | 7.93 |
| Ramachandran Plot | ||
| Outliers | 0 | 0 |
| Allowed | 2.6 | 2.7 |
| Favored | 97.4 | 97.3 |
| Rotamer outliers | 0 | 0 |
| C-beta deviations | 0 | 0 |
Model building and structure refinement
The cryo-reconstructed density maps were interpreted using a 3D model of the VP3 of 10HB generated with the SWISS Model online server (swissmodel.expasy.org). The VP3 model was used to generate a 60mer capsid model using the online VIPERdb Oligomer Generator by icosahedral matrix multiplication for a T=1 capsid (viperdb.scripps.edu) (Carrillo-Tripp et al., 2009). For model fitting, the cryo-reconstructed density map was converted from the MRC Format to an XPlor format using the “e2proc3D.py” subroutine in EMAN2 (Tang et al., 2007) and subsequently converted to a CCP4 format using the program MAPMAN. The 60mer capsid model was then docked into the cryo-reconstructed density map using the ‘Fit-in-map’ function in the Chimera application by rigid body rotations and translations (Pettersen et al., 2004). During the model fitting, the voxel (pixel) size of the map was surveyed to optimize the correlation coefficient (CC) between the model and the map. The fitted model was saved relative to the map. A VP3 monomer was extracted from the fitted 60mer and the side- and main-chains adjusted into the maps by manual building using the real-space-refinement subroutine in Coot (Emsley and Cowtan, 2004). VP residues 210 to 721 (last C-term residue) was ordered in the cryo-reconstructed density maps of both the empty and genome-packaged capsids. A 60mer capsid was generated from the manually refined VP3 model using the Oligomer Generator in ViperDB (Carrillo-Tripp et al., 2009) and further refined in the Phenix application utilizing the rigid body, real space, and B-factor refinement subroutines (Adams et al., 2010). The CC and refinement statistics, including root mean square deviations (RMSD) from ideal bond lengths and angles were analyzed by Phenix (Table 1).
Native dot immunoblot analysis
AAV capsids were adsorbed onto nitrocellulose membranes (Bio-Rad, Hercules, CA) in a dot blot manifold (Schleicher and Schuell, Dassel, Germany). Excess fluid was drawn through the membrane by vacuum filtration. The membrane was removed from the manifold and blocked with 6% milk in PBS, pH 7.4 for 1 h. Primary antibodies, A1 (anti-AAV VP1u) and A20 (anti-AAV2 capsid) (ARP) diluted 1:1000; hybridoma supernatant for ADK5B (anti-AAV5 capsid) diluted 1:100; or human sera (Valley Biomedical) diluted 1:500, were applied to the membrane in PBS with 6% milk, 0.1% Tween-20, and incubated for 90 min. The membranes were washed with PBS + 0.1% Tween-20 and horse radish peroxidase (HRP)-linked secondary antibodies (GE Healthcare) against mouse or human primary antibodies applied at a dilution of 1:5,000 or 1:20,000, respectively, in PBS with 6% milk, 0.1% Tween-20 and incubated for 1 h. The membranes were washed with PBS with 0.1% Tween-20, Immobilon™ Chemiluminescent Substrate (Millipore, Darmstadt, Germany) applied to the membranes, and signals detected on X-ray film. The A1 antibody was used as a control to confirm the presence of AAVs using denatured capsids.
Structure accession numbers
The full and empty cryo-EM reconstructed density maps and models built for their capsids were deposited in the Electron Microscopy Data Bank (EMDB) with accession numbers EMD-21656/PDB ID 6WFT (10HB full) and EMD-21657/PDB ID 6WFU (10HB empty), respectively.
RESULTS and DISCUSSION
BtAAV-10HB vectors generated by triple transfection were suitable for structure determination by cryo-EM and image reconstruction of empty and full particles
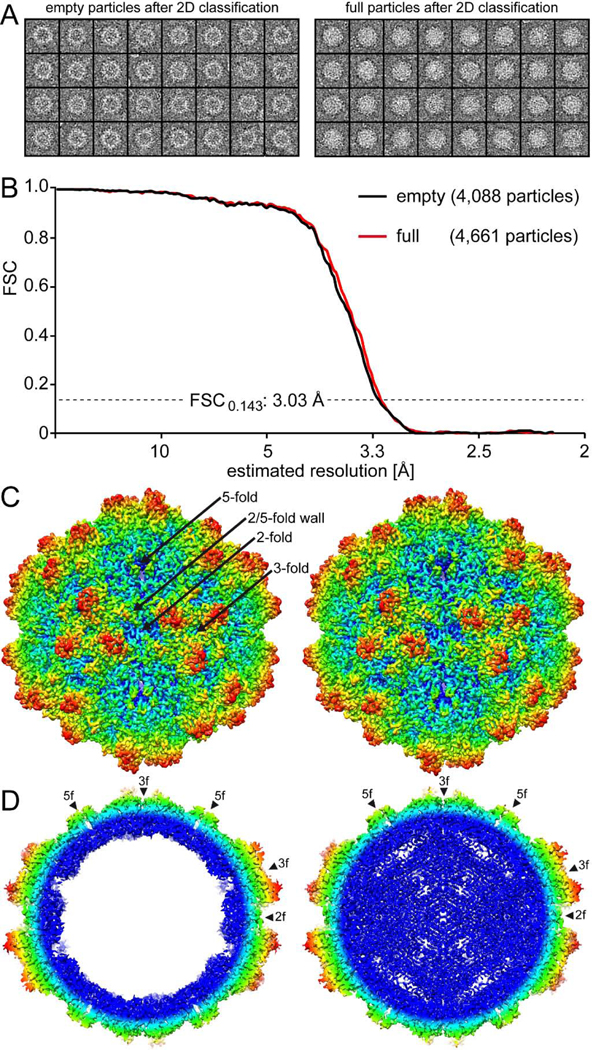
10HB vectors were produced in HEK293 cells and purified for capsid structure determination by cryo-EM. The sample’s purity was verified by SDS-PAGE that showed VP1, VP2, and VP3 at the expected sizes of ~79, 65, and 59 kDa (Fig. 1A). 10HB capsids are capable of packaging recombinant AAV2 vector genomes. Genome packaging appeared to be comparable to AAV2 and AAV5 vectors (Fig. 1B). The sample purification method by sucrose cushion and ion exchange chromatography did not separate empty (no genome) and genome-containing (full) particles. Thus, in the cryo-EM micrographs intact capsids of both types were observed which could be differentiated by their light (empty) and dark (full) interior appearances (Fig. 1C). The empty and full 10HB particles were separated by 2D classification (Fig. 2A) and subsequently reconstructed independently in the cisTEM software package (Grant et al., 2018). A total of 4,088 empty and 4,661 full particles were extracted and used for 3D-reconstructed to 3.03 Å resolution for both particle types (Fig. 2B, Table 1).
FIG. 1.
10HB sample for cryo-EM data collection. A) SDS-PAGE of purified 10HB. The VP1 (~80 kDa), VP2 (~65 kDa) and VP3 (~59 kDa) proteins are present. B) Total yield of AAV2-, AAV5, and 10HB-luciferase vectors after transfection of HEK 293 cells. Benzonase-treated freeze-thaw supernatants were quantified as genomic particles (gp) by qPCR. Yields of three experiments are displayed as mean ± standard deviation. C) Example cryo-electron micrograph of 10HB. Scale bar: 50 nm.
FIG. 2.
The cryo-EM reconstruction of the empty and full 10HB capsid structures. A) Separated empty and full particles following 2D-classification. B) Fourier Shell Correlation (FSC) plotted against resolution for the reconstructed 10HB structures. The resolution of the empty and full maps were estimated to be 3.03 Å, based on a FSC threshold of 0.143. C) The 10HB capsid surface density maps contoured at a sigma (σ) threshold level of 2.0. The maps are radially colored (blue to red) according to distance to the particle center, as indicated by the scale bar below. The icosahedral 2-, 3-, and 5-fold axes and the 2/5-fold wall are indicated on the empty 10HB capsid map. D) Cross-sectional views of the maps from the empty and full particles contoured at 1.0σ. The positions of the icosahedral 2-, 3-, and 5-fold symmetry axes are indicated by arrowheads. This figure was generated using UCSF-Chimera (Pettersen et al., 2004).
The 10HB capsid displayed the characteristic features of other AAVs such as AAV2 and AAV5 which based on the amino acid sequence are as different to each other as to 10HB (VP1 sequence identity: 56–59%). The capsid features include a channel at each 5-fold symmetry axes, trimeric protrusions that surround each 3-fold symmetry axes, and a depression at each 2-fold symmetry axes (Fig. 2C). No major differences on the outer surface of the capsids were visible between empty and full particles (Fig. 2C). Overall, the 10HB capsid looks similar to AAV5 capsids due to the less “spikier” appearance of the 3-fold protrusions compared to the AAV2 capsid (Govindasamy et al., 2013; Xie et al., 2002). In contrast to the capsid surface, the interior of empty and full particles displayed major differences. A cross-sectional view of the capsid showed significant amount of density, likely contributed by the packaged DNA genome, in the interior of the full particles that is absent from the corresponding empty map (Fig. 2D). Similar to previously reported genome-containing AAV capsid structures (Mietzsch et al., 2020) the region just underneath the 5-fold channel in the full structures is “open” compared to the overall interior of the capsid. We hypothesized that the flexible VP1/VP2 common region and VP1u normally occupy the space under the 5-fold channel prior to externalization via the 5-fold channel that is required for its PLA2 enzyme function during the viral life cycle (Venkatakrishnan et al., 2013).
Capsid structures of empty and full BtAAV-10HB particles conserved the AAV features
The high-resolution density maps for both particle types allowed unbiased modeling of the atomic VP structure of BtAAV-10HB. In both density maps the first ordered amino acid at the N-terminus of VP3 common region was aspartic acid 209 which is equivalent to aspartic acid 219 in AAV2 and aspartic acid 209 in AAV5. The lack of ordering of the remainder of the N-terminus is consistent with reports for other AAV VP structures (Burg et al., 2018; DiMattia et al., 2012; Govindasamy et al., 2006; Govindasamy et al., 2013; Guenther et al., 2019; Halder et al., 2015; Lerch et al., 2010; Lerch et al., 2012; Mietzsch et al., 2020; Mikals et al., 2014; Nam et al., 2007; Ng et al., 2010; Xie et al., 2002). However, for some of the AAVs, up to three additional residues are ordered at the N-terminus of VP3 compared to 10HB. Notably, the sequence of the 10HB VP3 N-terminus has five additional amino acids compared to other AAVs due to the start codon being located further upstream within the cap gene. This may introduce flexibility and affect the ordering of this region.
Following glycine 210, the VP3 amino acid side chain densities were ordered (Fig. 3A) and interpretable to the C-terminal residue. There was no significant difference between the empty and full capsid structures in the ordered VP region and superposed with an overall RMSDs of 0.25 Å by pairwise structural alignment. Similar results have been reported for empty and full AAV8, AAVrh.10 and AAVrh.39 particles (Mietzsch et al., 2020), as well as for virus-like particles (VLPs) and full particles of AAV6 (Ng et al., 2010; Xie et al., 2011) and AAV8 (Nam et al., 2011; Nam et al., 2007). The similarity of the empty and full 10HB VP3 structures results in the majority of amino acid side chains adopting the same orientation (Fig. 3A). Even the dual conformation of phenylalanine 690 (F690), located in surface loop VR-IX, was present in both maps (Fig. 3B). F690 is equivalent to tyrosine 704 in AAV2 and 693 in AAV5 and highly conserved among the AAV serotypes except for AAV7 that also possesses a phenylalanine in this position like 10HB. Mutation of tyrosine 704 in AAV2 to alanine (Y704A) resulted in the significant reduction of transduction efficiency and suggested to play a role in transcription (Salganik et al., 2014). In contrast, mutation of Y704 to a phenylalanine in AAV2, to the same residue compared to 10HB, led to an increase of transduction (Zhong et al., 2008b). This phenotype was explained by the removal of a potential phosphorylation residue which acts as a signal for capsid degradation. Thus, 10HB is naturally resistant to phosphorylation at this capsid position. The residue equivalent to this position in AAV8 is pH sensitive, and adopts a different conformation as pH drops (Nam et al., 2011). These observations point to this capsid region as being flexible and functional.
FIG. 3.
Similarity in the density maps of the empty and full 10HB structures. A) Modeled 10HB residues in βB and B) VR-IX are shown inside their density maps (black=empty, red=full). Phenylalanine 690 (F690) displays a dual conformation in both maps. The amino acid residues are shown as stick representation and colored according to atom type: C = yellow, O = red, N = blue. This figure was generated using Chimera (Pettersen et al., 2004).
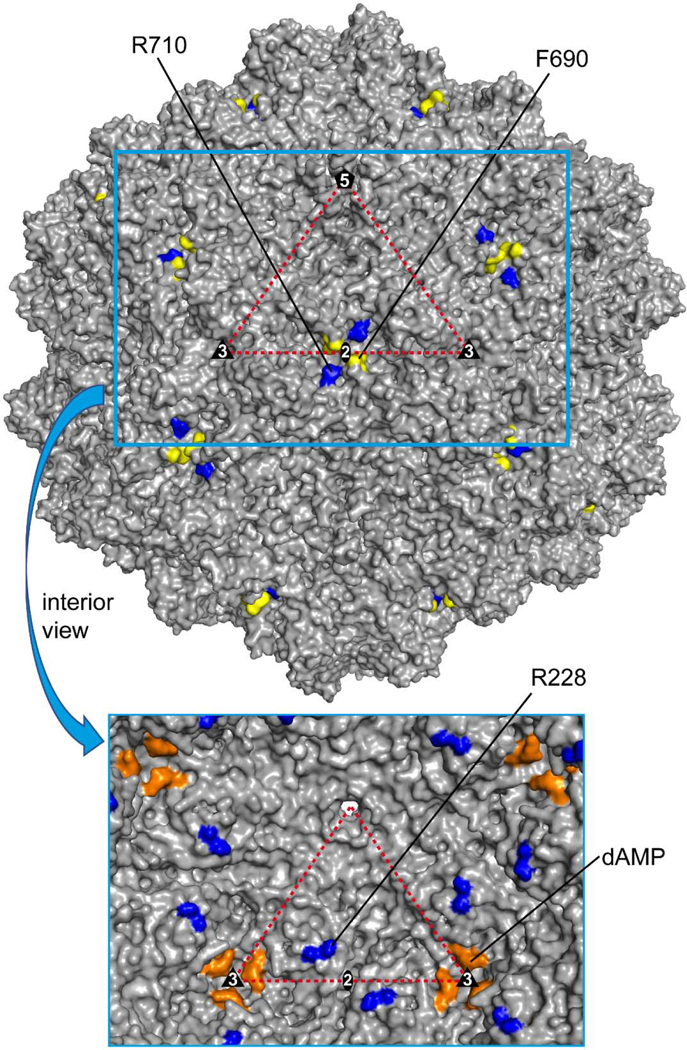
Most amino acid side chains adopted identical conformations in the empty and full density maps of 10HB with two exceptions. Several arginines displayed dual conformations in the full density map while only a single conformation in the empty map (Fig. 4A and B). Arginine 710 (R710) is located in close proximity to F690 (distance: ~9 to 11 Å) and present on the capsid surface near the 2-fold symmetry axis (Fig. 5). It is unclear how genome packaging affects side chain orientations on the exterior of the capsid. Notably, this arginine is completely conserved in all AAV serotypes and equivalent to position 724 in AAV2 or 713 in AAV5. The other arginine displaying a dual conformation in the full but not empty density map is R228 (Fig. 4A). This residue is also located near the 2-fold symmetry axis but on the inside of the capsid (Fig. 5). At a low sigma threshold level of <1σ, the genome density inside the capsid makes contact with the alternative side chain orientation in the full map (not shown) which explains the presence of the dual configuration of the arginine exclusively in the genome-filled capsid. R228 is also conserved among the AAV serotypes except for AAV4 and AAV11 that have a histidine or lysine, respectively, in that position. The equivalent residue position in AAV2 is R238 that was previously identified as a potential DNA binding residue in the AAV2 capsid (Levy et al., 2009). Despite the conservation, no dual conformation of this arginine was reported for the full AAV8, AAVrh.10, and AAVrh.39 (Mietzsch et al., 2020). Mutation of R238 to alanine led to an assembly defect in AAV2 (Wu et al., 2000). However, this mutation may have also affected AAP (unknown at the time of original analysis) which is encoded in another ORF within the cap ORF and could prevent capsid assembly if altered (Sonntag et al., 2010; Wu et al., 2000). Another region on the inside of the capsid known to interact with the DNA genome for members of genus Dependoparvovirus is the nucleotide binding pocket in the interior near the 3-fold symmetry axis (Govindasamy et al., 2006; Halder et al., 2015; Lerch et al., 2010; Mietzsch et al., 2020; Mikals et al., 2014; Nam et al., 2007; Ng et al., 2010). The amino acids located in this region are conserved in the 10HB capsid. Consequently, an ordered nucleotide, interpreted as a dAMP, was observed in the full map between P412 and H615/P616 at ~2σ which was absent in the empty map (Fig. 4C and Fig. 5). Recently, it was suggested that DNA interacts with this region by entering the pocket, and binds to two symmetry-related VPs, P412 and H615/P616, before exiting the 3-fold pocket (Fig. 4D) (Mietzsch et al., 2020). Binding to only two VPs at the 3-fold symmetry axis disrupts symmetry imposed during 3D image reconstruction and results in a less ordered state of the DNA relative to the VPs. While the above-mentioned dAMP between P412 and H615/P616 is ordered at ~2σ the neighboring nucleotides are disordered. At a lower σ-level of 1, density could be seen for less ordered nucleotides (Fig. 4D). This suggests that the dAMP exists in each pocket while the other nucleotides could be a mixture.
FIG. 4.
Differences between the empty and full 10HB capsid structures. A) Modeled 10HB residues aa227 to 229 and B) aa709 to 711 shown inside their density maps (black=empty, red=full). Arginine 228 and 710 display a dual conformation exclusively in the full map. C) Modeled residues at the nucleotide binding pocket shown inside their density maps. Extra density for an ordered nucleotide (dAMP) is exclusively in the full map. D) A pentanucleotide built based on density at the 3-fold pocket is shown for the full 10HB capsid structure inside a red mesh density map at a 2 and 1 σ. The location of the 3-fold symmetry axis is indicated. E) Density in the channel at the 5-fold axis for the empty and full 10HB maps is contoured at a 1σ. Residues 188–199 are modeled inside the channel. This figure was generated using UCSF-Chimera (Pettersen et al., 2004).
FIG. 5.
Capsid surface representation of 10HB. The location of amino acids with dual side chain orientations and the ordered dAMP on the full 10HB capsid structure are shown. F690 (yellow) and R710 (blue) are highlighted on the outside surface. In the cutout image below R228 (blue) and dAMP (orange) are highlighted on a surface representation viewed from the interior of the 10HB capsid. The viral asymmetric unit, with the 5-fold (pentagon), the 2-fold (ellipse), and the two 3-folds (triangles), is shown. This image was generated using PyMOL (DeLano, 2002).
Similar to previous reports for other AAVs (Halder et al., 2015; Mietzsch et al., 2020), a rod-shaped density was observed in the 5-fold channels of genome-containing 10HB particles which was absent in the empty particle map (Fig. 4E). Due to its position at the 5-fold symmetry axis, residues in the center of the channel are icosahedrally averaged making the interpretation of the amino acids difficult. A stretch of VP3 N-terminus residues with small amino acid side chains were suggested to be located in the channel (Halder et al., 2015; Mietzsch et al., 2020). For 10HB aa 188 to 199 (MASAEVAAGGGG) was built into the density (Fig. 4E). The majority of the Parvovirinae subfamily possess a glycine-rich stretch of residues near the N-terminus of their major VP (Ilyas et al., 2018; Mietzsch et al., 2019). While some parvoviruses have up to sixteen consecutive glycine residues in a row, most AAVs have only three consecutive glycines (Mietzsch et al., 2019). 10HB and AAV5, both have four consecutive glycines whereas AAV2 only has spaced single glycines in this region (Fig. 6A). The presence of several glycines was suggested to confer flexibility to the VP N-terminus to facilitate externalization of the enzymatic VP1u region through the 5-fold channel (Venkatakrishnan et al., 2013).
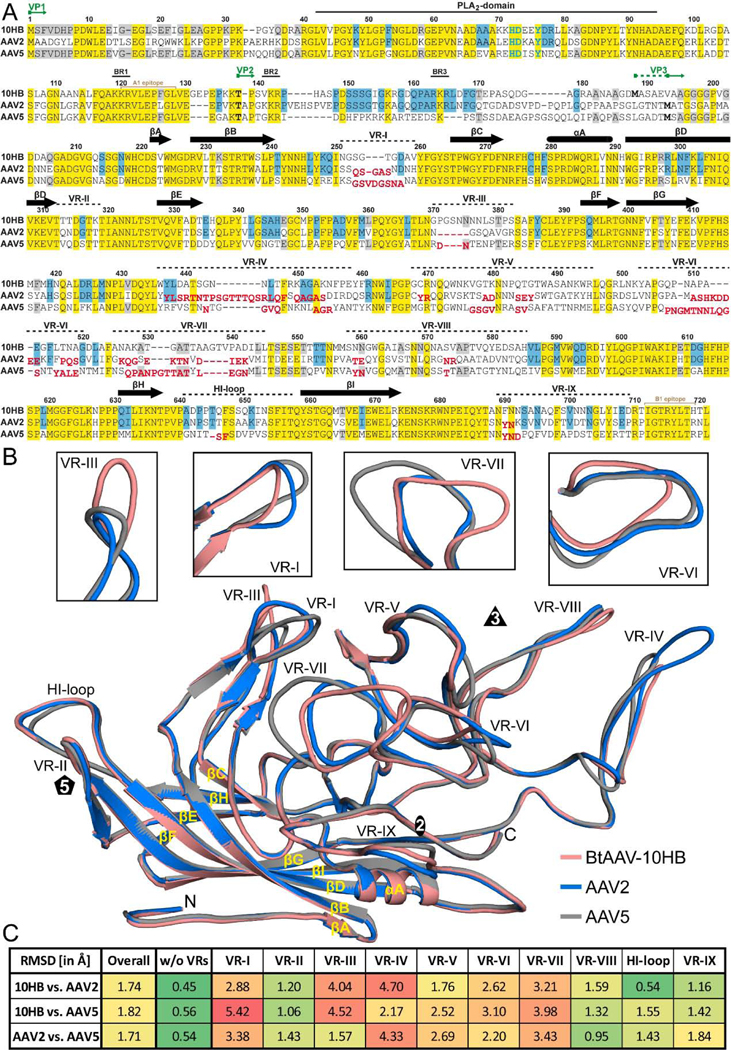
FIG. 6.
Capsid structure comparison of 10HB, AAV2, and AAV5. A) Structure-based sequence alignment, except for aa 1 to 209 (10HB numbering) which is based exclusively on the amino acid sequence. The VP1, VP2, and VP3 N-termini residue numbers based on 10HB VP1, the phospholipase (PLA2) domain, the basic regions (BR), and the location of the variable regions (VRs) are indicated above the amino acid sequence. Secondary structure elements such as β-strands and α-helices, are shown as black arrows and black cylinders, respectively. Amino acids highlighted in yellow indicate sequence identity among all three viruses whereas amino acids in blue or gray indicate an identical residue in 10HB compared to AAV2 or AAV5, respectively. Amino acids in AAV2 or AAV5 whose Cα atoms are further than 2 Å apart when superposed onto 10HB are shown offset and in red below the aligned residues. The A1 and B1 epitope are indicated in light brown. B) Structural superposition of 10HB (salmon), AAV2 (blue), and AAV5 (gray) shown as ribbon diagrams with the position of VR-I to VR-IX, the HI-loop, β-strand A-I, α-helixA, the N- and C-terminus, and the icosahedral 2-, 3-, and 5-fold axis labeled. Close-ups of VR-I, VR-III, VR-VI, and VR-VII are shown above separately for clarity. This image was generated using PyMOL (DeLano, 2002). C) Calculated root mean square deviations (RMSDs) of the VPs of 10HB, AAV2, and AAV5 to each other for the overall structure and for specific VRs.
BtAAV-10HB VP3 structure exhibits diversity in surface loop conformation
The 10HB VP3 topology has a conserved core β-strand A, eight-stranded antiparallel β -barrel (βB-βI), and α-helix A (Fig. 6 A and B), as described previously for all other AAVs (Bennett et al., 2019; Burg et al., 2018; DiMattia et al., 2012; Govindasamy et al., 2006; Guenther et al., 2019; Halder et al., 2015; Kaelber et al., 2020; Lerch et al., 2010; Mietzsch et al., 2020; Mikals et al., 2014; Nam et al., 2007; Ng et al., 2010; Tan et al., 2018; Xie et al., 2002). This conserved core of the VP3 structure is superposable between 10HB, AAV2, and AAV5 with RMSD of ~0.5Å (Fig. 6B). Between the conserved core, inserted loops form the surface topology of the capsid. Structural superposition of 10HB, AAV2 and AAV5 showed that the majority of the differences are located within the previously defined variable regions (VRs) (Govindasamy et al., 2006) (Fig. 6A and B). Among those surface loops, some regions showed minor structural differences, e.g., VR-II, VR-VIII, VR-IX, and the Hl-loop (local RMSD <2Å) while others were larger, e.g., VR-I, VR-III, VR-IV, VR-V, VR-VI, and VR-VII (local RMSD >2Å) (Fig. 6C). The absence of significant differences in the 5-fold region, which includes VR-II and the HI-loop, are likely due to the function these loops have to fulfill such as DNA packaging and VP1u externalization (Bleker et al., 2005; Venkatakrishnan et al., 2013). The only variation is observed in the AAV5 HI-loop, which is slightly shorter due to a single amino acid deletion compared to 10HB and AAV2 (Fig. 6A and B). Notably, the Rep proteins of AAV2 are capable of packaging vector genomes into all three capsids likely via binding to this structurally conserved capsid region during the DNA packaging process. Despite the structural conservation, sequence differences exist that prevent purification of 10HB vectors by AVB affinity chromatography (data not shown). This nanobody is proposed to bind around the 5-fold symmetry axis (Wang et al., 2015). Compared to the 5-fold region, the 3-fold protrusions and the 2/5-fold wall display high structural differences among the three viruses and lead morphological differences in appearances of the AAV capsids. The less pointed characteristics of the 10HB capsid compared to AAV2 is due to a 7 amino acid truncation in VR-IV (Fig. 6A and B). Similarly, AAV5 VR-IV has a deletion of 6 amino acids compared to AAV2 resulting in the 10HB and AAV5 VPs appearing similar (Fig. 6A and B). Unique to 10HB is the 5 and 3 amino acid insertion in VR-III compared to AAV2 and AAV5, respectively. At the same time 10HB’s VR-I is truncated by 2 and 3 amino acids compared to AAV2 and AAV5, respectively (Fig. 6A and B). Both of these loops form the 2/5-fold wall that displays significant differences between 10HB, AAV2, and AAV5 (not shown). VR-VI and VR-VII located at the side of the 3-fold protrusions also show clear structural variations. While VR-VI in 10HB is 4 amino acids shorter compared to both AAV2 and AAV5, VR-VII possesses a 4 amino acid insertion compared to AAV2 and a single amino acid insertion compared to AAV5 (Fig. 6A and B). Overall, it appears that insertions and deletions in the surface loops for different AAVs are balanced so that no capsid has exclusively localized deletions or insertions and thus the capsids have a similar general morphology (as previously discussed (Govindasamy et al., 2006)). Structural superposition of the Cα coordinates for the entire VP resulted in an overall RMSD of 1.7 Å and 1.8 Å for 10HB vs AAV2 and 10HB vs AAV5, respectively (Fig. 6C). An overall RMSD of 1.7 Å between the AAV2 and AAV5 capsid is consistent with the three capsid VP structures being equally different to each other.
Differences in the 10HB VRs alter antigenic reactivity compared to AAV2 and AAV5
The VRs of the AAVs are described as important determinants of tissue tropism and antigenicity (Agbandje-McKenna and Kleinschmidt, 2011). Thus, based on the high variability of the VRs, a differential tissue tropism and altered antigenicity would be expected of 10HB compared to AAV2 and AAV5. Unlike AAV2, 10HB vectors carrying a luciferase transgene transduced permanent cells lines such as HEK 293, U87, Neuro2A, A549, HT29, Huh7 and CHO cells poorly (data not shown). However, in vivo mouse transduction assays have shown that 10HB vectors are capable of transducing muscle tissue (Li et al., 2019). For human gene delivery applications, pre-existing NAbs, against a wide range of primate AAV capsids represents a major hurdle to achieving treatment efficacy. Due to the difference in 10HB VRs compared to AAV2 and AAV5, the prediction is that NAbs that bind the latter viruses are unlikely to bind to the 10HB capsid. The only region that might display cross-reactivity is the structurally conserved 5-fold region. However, despite this structural conservation, the high amino acid variation in the DE- and HI-loops assembling the 5-fold region of these viruses is likely to prevent NAb binding. This possibility is supported by the lack of binding to AVB to 10HB while AAV2 and AAV5 are recognized by this nanobody.
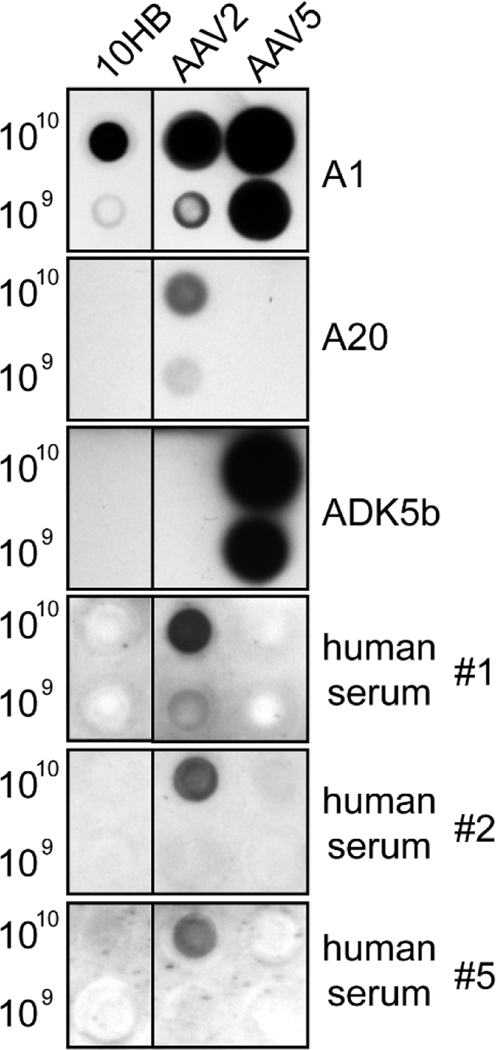
10HB capsids probed with example monoclonal antibodies against AAV2 and AAV5 and human serum samples, by a native dot immunoblot assay, showed no cross-reactivity. As expected neither the A20 antibody, which binds at the 2/5-fold wall of AAV2 capsids (McCraw et al., 2012), nor ADK5B antibody that binds around the 5-fold region of AAV5 capsids (Tseng et al., 2015) detected 10HB capsids (Fig. 7). These antibodies bound their respective capsids (Fig. 7). All three human sera samples tested contained antibodies reactive against AAV2 capsids while none bound to 10HB or AAV5 capsids (Fig. 7). Antibodies against AAV2 capsids are common (>70%) in the human population whereas anti-AAV5 capsid antibodies are less prevalent (~40%) (Boutin et al., 2010). While this small sampling indicates low seropositive reactivity against 10HB, further testing is required before conclusions are made. The A1 antibody used as positive control confirmed the presence of all three capsid types (Fig. 7). This antibody recognizes a conserved epitope within the VP1u region in denatured capsids (Fig. 6A) (Wobus et al., 2000). In contrast to A1, the B1 antibody, also commonly used as a positive control, failed to recognize 10HB (not shown). This is due to an amino acid difference, histidine instead of arginine, within the B1 epitope at the VP C-terminus (Fig. 6A). A similar difference in AAV4 also prevents binding to B1.
FIG. 7.
Native dot immunoblot of 10HB, AAV2, or AAV5 capsids. The amount of genome-containing particles loaded onto membrane is indicated. The membranes were probed with the monoclonal antibodies A1, A20, ADK5b or different commercially available human donor sera. For the membrane probed with A1 antibody heat-denatured AAVs were used. The black dots indicate a positive response.
Bat AAVs exhibit sequence differences in VRs likely linked to host diversity
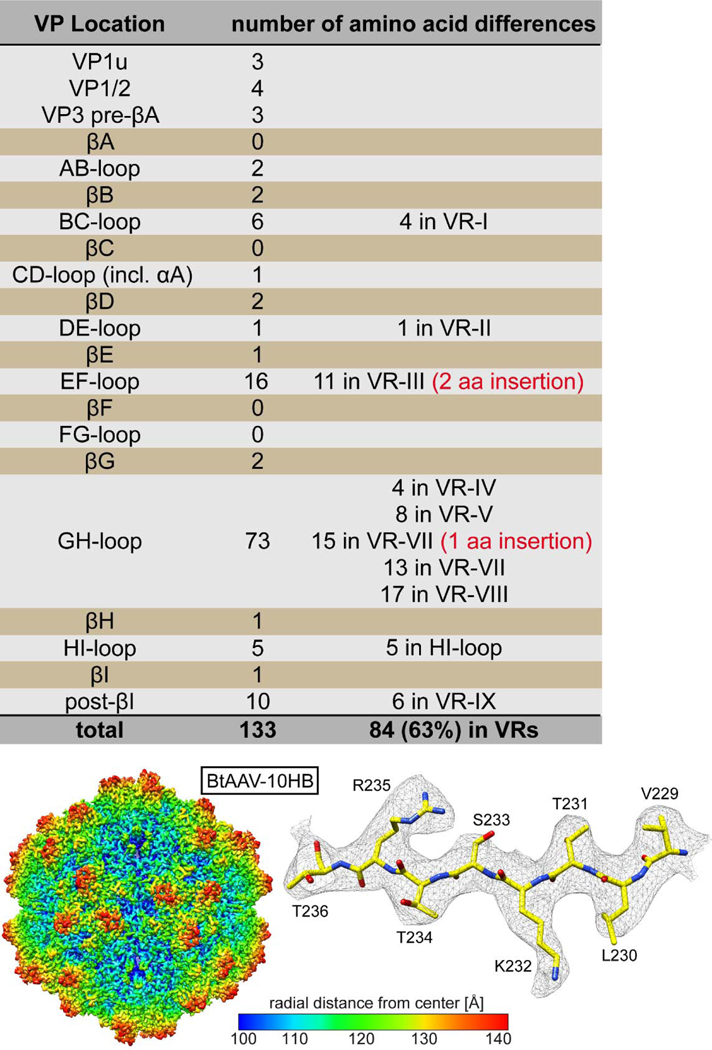
Several strains of AAV viruses have been isolated from bats in addition to 10HB. One of these is called BtAAV-YNM (YNM) (accession number: YP_003858572) (Li et al., 2010). The VP1 of YNM has a sequence identity of 81.6% (133 amino acid differences) compared to 10HB. Thus, the two BtAAV strains likely represent two separate serotypes, although serology tests would be required to confirm this. Interestingly, the VP1u and the VP1/2 common regions are highly conserved with only 7 amino acid differences (Fig. 8). The majority (95%) of the amino acid differences between 10HB and YNM are located within VP3 but less than 7% (9 amino acids) in the core β-barrel. Most differences are localized to the VRs (63%) or in close proximity to these (Fig. 8). Interestingly, the already large VR-III of 10HB (compared to AAV2 and AAV5) has an additional 2 amino acids insertion in YNM. Another 30% of the amino acid differences were located in VP regions mostly conserved among primate AAVs. This observation might be due to the high diversity of bats and adaptations of the capsids to specific bat species (Calisher et al., 2006).
FIG. 8.
Comparison of 10HB to BtAAV-YNM. Overview of the locations and number of amino acid differences with the VP protein between both viruses are provided.
Summary
Prior to this study, the only AAV capsid structures known were for isolates from primates. Due to advances in DNA sequencing technology new AAVs are continuously being discovered in a wide range of animals and not only primates. These AAVs hold great potential as gene delivery vectors compared to primate AAVs. While their core VP features such as the β-barrel and nucleotide binding site are maintained, their different capsid surfaces properties offer new tissue tropisms and the potential to evade pre-existing neutralizing antibodies. The 3.0 Å capsid structure of the divergent 10HB, with potentially low human sera recognition, can serve as a platform for further capsid engineering. This can be in the form of identifying and modifying surface-exposed tyrosine, serine, threonine, and lysine amino acids, to prevent phosphorylation or ubiquitination of the capsid following cellular entry (Zhong et al., 2008a; Zhong et al., 2008b). This can also be in the form of grafting receptor attachment sites or targeting peptides into surface loops. The goal of these modifications is to improve transduction characteristics in human cells and therapeutic efficacy.
the first reported capsid structure of a non-primate AAV isolated from bats (10HB)
10HB displays unique structural features compared to AAV2 and AAV5
alternative side chain orientations in empty and full particles
10HB vectors likely “invisible” to pre-existing neutralizing human antibodies
ACKNOWLEDGEMENTS
We thank the University of Florida (UF) Interdisciplinary Center for Biotechnology Research (ICBR) electron microscopy lab for providing negative-stain EM and cryo-EM screening services. We thank Dr. Hong Zhou (University of California Los Angeles) and the NIH “West/Midwest Consortium for High-Resolution Cryo Electron Microscopy” project for access to the Electron Imaging Center for Nanomachines’s Titan Krios and K2 DED utilized for high-resolution data collection (MPI, HZ, MAM, and others). The University of Florida COM and NIH GM082946 (to MAM and RM) provided funds for the research efforts at the University of Florida.
Footnotes
Conflict of Interest
MAM is a SAB member for Voyager Therapeutics, Inc., and AGTC, has a sponsored research agreement with AGTC, Voyager Therapeutics, and Intima Biosciences, Inc., and is a consultant for Intima Biosciences, Inc. MAM is a co-founder of StrideBio, Inc. This is a biopharmaceutical company with interest in developing AAV vectors for gene delivery application.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH, 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica. Section D, Biological crystallography 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbandje-McKenna M, Kleinschmidt J, 2011. AAV capsid structure and cell interactions. Methods Mol Biol S07, 47–92. [DOI] [PubMed] [Google Scholar]
- Arbetman AE, Lochrie M, Zhou S, Wellman J, Scallan C, Doroudchi MM, Randlev B, Patarroyo-White S, Liu T, Smith P, Lehmkuhl H, Hobbs LA, Pierce GF, Colosi P, 2005. Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties. J Virol 79, 1523S–15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, Yadav S, DiPrimio N, Nam HJ, Agbandje-McKenna M, McPhee S, Wolff J, Samulski RJ, 2010. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol 28, 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel-Schaal U, zur Hausen H, 1984. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology 134, 52–63. [DOI] [PubMed] [Google Scholar]
- Bello A, Chand A, Aviles J, Soule G, Auricchio A, Kobinger GP, 2014. Novel adeno-associated viruses derived from pig tissues transduce most major organs in mice. Scientific reports 4, 6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A, Keravala A, Makal V, Kurian J, Belbellaa B, Aeran R, Tseng YS, Sousa D, Spear J, Gasmi M, Agbandje-McKenna M, 2019. Structure comparison of the chimeric AAV2.7mS vector with parental AAV2. J Struct Biol, 107433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AD, Wong K, Lewis J, Tseng YS, Smith JK, Chipman P, McKenna R, Samulski RJ, Kleinschmidt J, Agbandje-McKenna M, 2018. AAV6 K531 serves a dual function in selective receptor and antibody ADK6 recognition. Virology 518, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleker S, Sonntag F, Kleinschmidt JA, 2005. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J Virol 79, 2528–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R, van der Giessen J, Haagmans BL, Osterhaus AD, Smits SL, 2013. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J Virol 87, 7758–7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossis I, Chiorini JA, 2003. Cloning of an avian adeno-associated virus (AAAV) and generation of recombinant AAAV particles. J Virol 77, 6799–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C, 2010. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 21, 704–712. [DOI] [PubMed] [Google Scholar]
- Burg M, Rosebrough C, Drouin LM, Bennett A, Mietzsch M, Chipman P, McKenna R, Sousa D, Potter M, Byrne B, Jude Samulski R, Agbandje-McKenna M, 2018. Atomic structure of a rationally engineered gene delivery vector, AAV2.5. J Struct Biol 203, 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T, 2006. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Tripp M, Shepherd CM, Borelli IA, Venkataraman S, Lander G, Natarajan P, Johnson JE, Brooks CL 3rd, Reddy VS, 2009. VIPERdb2: an enhanced and web API enabled relational database for structural virology. Nucleic Acids Res 37, D436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MS, Agbandje-McKenna M, 2006. Atomic structure of viral particles, p. 109–123, in: Bloom SFCME, Linden RM, Parrish CR, and Kerr JR, (Ed.), Parvoviruses, Edward Arnold, Ltd., London. [Google Scholar]
- DeLano WL 2002. The PyMOL Molecular Graphics Syste. DeLano Scientific, San Carlos, CA, USA. [Google Scholar]
- DiMattia MA, Nam HJ, Van Vliet K, Mitchell M, Bennett A, Gurda BL, McKenna R, Olson NH, Sinkovits RS, Potter M, Byrne BJ, Aslanidi G, Zolotukhin S, Muzyczka N, Baker TS, Agbandje-McKenna M, 2012. Structural insight into the unique properties of adeno-associated virus serotype 9. J Virol 86, 6947–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin LM, Lins B, Janssen M, Bennett A, Chipman P, McKenna R, Chen W, Muzyczka N, Cardone G, Baker TS, Agbandje-McKenna M, 2016. Cryo-electron Microscopy Reconstruction and Stability Studies of the Wild Type and the R432A Variant of Adeno-associated Virus Type 2 Reveal that Capsid Structural Stability Is a Major Factor in Genome Packaging. J Virol 90, 8542–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K, 2004. Coot: model-building tools for molecular graphics. Acta crystallographica. Section D, Biological crystallography 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Farkas SL, Zadori Z, Benko M, Essbauer S, Harrach B, Tijssen P, 2004. A parvovirus isolated from royal python (Python regius) is a member of the genus Dependovirus. J Gen Virol 85, 555–561. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM, 2004. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 78, 6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy L, Padron E, McKenna R, Muzyczka N, Kaludov N, Chiorini JA, Agbandje-McKenna M, 2006. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J Virol 80, 11556–11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy L, Dimattia MA, Gurda BL, Halder S, McKenna R, Chiorini JA, Muzyczka N, Zolotukhin S, Agbandje-McKenna M, 2013. Structural insights into adeno-associated virus serotype 5. J Virol 87, 11187–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant T, Rohou A, Grigorieff N, 2018. cisTEM, user-friendly software for single-particle image processing. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther CM, Brun MJ, Bennett AD, Ho ML, Chen W, Zhu B, Lam M, Yamagami M, Kwon S, Bhattacharya N, Sousa D, Evans AC, Voss J, Sevick-Muraca EM, Agbandje-McKenna M, Suh J, 2019. Protease-Activatable Adeno-Associated Virus Vector for Gene Delivery to Damaged Heart Tissue. Molecular Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurda BL, DiMattia MA, Miller EB, Bennett A, McKenna R, Weichert WS, Nelson CD, Chen WJ, Muzyczka N, Olson NH, Sinkovits RS, Chiorini JA, Zolotutkhin S, Kozyreva OG, Samulski RJ, Baker TS, Parrish CR, Agbandje-McKenna M, 2013. Capsid antibodies to different adeno-associated virus serotypes bind common regions. J Virol 87, 9111–9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S, Van Vliet K, Smith JK, Duong TT, McKenna R, Wilson JM, Agbandje-McKenna M, 2015. Structure of neurotropic adeno-associated virus AAVrh.8. J Struct Biol 192, 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas M, Mietzsch M, Kailasan S, Vaisanen E, Luo M, Chipman P, Smith JK, Kurian J, Sousa D, McKenna R, Soderlund-Venermo M, Agbandje-McKenna M, 2018. Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy. Viruses 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A, Mietzsch M, Smith K, Kurian J, Chipman P, McKenna R, Chiorini J, Agbandje-McKenna M, 2018. High resolution structural characterization of a new AAV5 antibody epitope toward engineering antibody resistant recombinant gene delivery vectors. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelber JT, Yost SA, Webber KA, Firlar E, Liu Y, Danos O, Mercer AC, 2020. Structure of the AAVhu.37 capsid by cryoelectron microscopy. Acta crystallographica. Section F, Structural biology communications 76, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler AM, Flotte TR, 2019. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu Rev Virol 6, 601–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch TF, Xie Q, Chapman MS, 2010. The structure of adeno-associated virus serotype 3B (AAV-3B): insights into receptor binding and immune evasion. Virology 403, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch TF, O’Donnell JK, Meyer NL, Xie Q, Taylor KA, Stagg SM, Chapman MS, 2012. Structure of AAV-DJ, a retargeted gene therapy vector: cryo-electron microscopy at 4.5 A resolution. Structure (London, England : 1993) 20, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy HC, Bowman VD, Govindasamy L, McKenna R, Nash K, Warrington K, Chen W, Muzyczka N, Yan X, Baker TS, Agbandje-McKenna M, 2009. Heparin binding induces conformational changes in Adeno-associated virus serotype 2. J Struct Biol 165, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shan T, Wang C, Cote C, Kolman J, Onions D, Gulland FM, Delwart E, 2011. The fecal viral flora of California sea lions. J Virol 85, 9909–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Liu Y, Shi Z, Liu H, Wei Y, Yang L, 2019. Bat adeno-associated viruses as gene therapy vectors with the potential to evade human neutralizing antibodies. Gene Ther 26, 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ge X, Hon CC, Zhang H, Zhou P, Zhang Y, Wu Y, Wang LF, Shi Z, 2010. Prevalence and genetic diversity of adeno-associated viruses in bats from China. J Gen Virol 91, 2601–2609. [DOI] [PubMed] [Google Scholar]
- Lochrie MA, Tatsuno GP, Arbetman AE, Jones K, Pater C, Smith PH, McDonnell JW, Zhou SZ, Kachi S, Kachi M, Campochiaro PA, Pierce GF, Colosi P, 2006. Adeno-associated virus (AAV) capsid genes isolated from rat and mouse liver genomic DNA define two new AAV species distantly related to AAV-5. Virology 353, 68–82. [DOI] [PubMed] [Google Scholar]
- McCraw DM, O’Donnell JK, Taylor KA, Stagg SM, Chapman MS, 2012. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology 431, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzsch M, Penzes JJ, Agbandje-McKenna M, 2019. Twenty-Five Years of Structural Parvovirology. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzsch M, Barnes C, Hull JA, Chipman P, Xie J, Bhattacharya N, Sousa D, McKenna R, Gao G, Agbandje-McKenna M, 2020. Comparative Analysis of the Capsid Structures of AAVrh.10, AAVrh.39, and AAV8. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikals K, Nam HJ, Van Vliet K, Vandenberghe LH, Mays LE, McKenna R, Wilson JM, Agbandje-McKenna M, 2014. The structure of AAVrh32.33, a novel gene delivery vector. J Struct Biol 186, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Gurda BL, McKenna R, Potter M, Byrne B, Salganik M, Muzyczka N, Agbandje- McKenna M, 2011. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J Virol 85, 11791–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Lane MD, Padron E, Gurda B, McKenna R, Kohlbrenner E, Aslanidi G, Byrne B, Muzyczka N, Zolotukhin S, Agbandje-McKenna M, 2007. Structure of adeno-associated virus serotype 8, a gene therapy vector. J Virol 81, 12260–12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Govindasamy L, Gurda BL, McKenna R, Kozyreva OG, Samulski RJ, Parent KN, Baker TS, Agbandje-McKenna M, 2010. Structural characterization of the dual glycan binding adeno-associated virus serotype 6. J Virol 84, 12945–12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden PJ, Kelsic ED, Sinai S, Church GM, 2019. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 366, 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasi KJ, Rangarajan S, Mitchell N, Lester W, Symington E, Madan B, Laffan M, Russell CB, Li M, Pierce GF, Wong WY, 2020. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N Engl J Med 382, 29–40. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE, 2004. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M, Asokan A, 2011. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther 19, 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupp C, Naumer M, Muller OJ, Gurda BL, Agbandje-McKenna M, Kleinschmidt JA, 2012. The threefold protrusions of adeno-associated virus type 8 are involved in cell surface targeting as well as postattachment processing. J Virol 86, 9396–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas D, Kwikkers KL, Zabaleta N, Bazo A, Petry H, van Deventer SJ, Aseguinolaza GG, Ferreira V, 2019. Immunoadsorption enables successful rAAV5-mediated repeated hepatic gene delivery in nonhuman primates. Blood advances 3, 2632–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salganik M, Aydemir F, Nam HJ, McKenna R, Agbandje-McKenna M, Muzyczka N, 2014. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J Virol 88, 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Katano H, Bossis I, Chiorini JA, 2004. Cloning and characterization of a bovine adeno-associated virus. J Virol 78, 6509–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag F, Schmidt K, Kleinschmidt JA, 2010. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci U S A 107, 10220–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B, 2005. Automated molecular microscopy: the new Leginon system. J Struct Biol 151, 41–60. [DOI] [PubMed] [Google Scholar]
- Tan YZ, Aiyer S, Mietzsch M, Hull JA, McKenna R, Grieger J, Samulski RJ, Baker TS, Agbandje-McKenna M, Lyumkis D, 2018. Sub-2 A Ewald curvature corrected structure of an AAV2 capsid variant. Nature communications 9, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ, 2007. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157, 38–46. [DOI] [PubMed] [Google Scholar]
- Tseng YS, Gurda BL, Chipman P, McKenna R, Afione S, Chiorini JA, Muzyczka N, Olson NH, Baker TS, Kleinschmidt J, Agbandje-McKenna M, 2015. Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J Virol 89, 1794–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan B, Yarbrough J, Domsic J, Bennett A, Bothner B, Kozyreva OG, Samulski RJ, Muzyczka N, McKenna R, Agbandje-McKenna M, 2013. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J Virol 87, 4974–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lock M, Prongay AJ, Alvira MR, Petkov B, Wilson JM, 2015. Identification of an adeno-associated virus binding epitope for AVB sepharose affinity resin. Mol Ther Methods Clin Dev 2, 15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Hugle-Dorr B, Girod A, Petersen G, Hallek M, Kleinschmidt JA, 2000. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J Virol 74, 9281–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, Flotte T, Muzyczka N, 2000. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol 74, 8635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Lerch TF, Meyer NL, Chapman MS, 2011. Structure-function analysis of receptor-binding in adeno-associated virus serotype 6 (AAV-6). Virology 420, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS, 2002. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A 99, 10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, Agard DA, 2017. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nature methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A, 2008a. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 381, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH Jr., Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A, 2008b. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A 105, 7827–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr., Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO, 2002. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28, 158–167. [DOI] [PubMed] [Google Scholar]