Abstract
To investigate the role of B cells in experimental, superantigen-mediated Staphylococcus aureus arthritis and sepsis, we used gene-targeted B-cell-deficient mice. The mice were inoculated intravenously with a toxic shock syndrome toxin 1 (TSST-1)-producing S. aureus strain. The B-cell-deficient and thus agamma-globulinemic mice showed striking similarities to the wild-type control animals with respect to the development of arthritis, the mortality rate, and the rate of bacterial clearance. Surprisingly, we found that the levels of gamma interferon in serum were significantly lower (P < 0.0001) in B-cell-deficient mice than in the controls, possibly due to impaired superantigen presentation and a diminished expression of costimulatory molecules. In contrast, the levels of interleukin-4 (IL-4), IL-6, and IL-10 in serum were equal in both groups. Our findings demonstrate that neither mature B cells nor their products significantly contribute to the course of S. aureus-induced septic arthritis.
We have previously described a murine model of hematogenously induced Staphylococcus aureus arthritis and sepsis (7, 8). Using this model, approximately 80 to 90% of mice inoculated with S. aureus LS-1 develop clinical arthritis. Immunohistochemical analysis of arthritic joints demonstrated the presence of phagocytes and T cells, predominantly of the CD4 phenotype (4). The infected mice displayed increased levels of inflammatory cytokines, such as tumor necrosis factor and interleukin-6 (IL-6) in serum (7). We have also shown that toxic shock syndrome toxin 1 (TSST-1), a superantigen produced by S. aureus LS-1, contributes to the arthritogenicity of S. aureus (3, 5). A series of studies using this model suggested that S. aureus arthritis is a T-cell-dependent and superantigen mediated disease.
As to the role of B cells in S. aureus arthritis, we have found that a striking feature in this model is the occurrence of polyclonal B-cell activation with highly increased levels of immunoglobulins and autoantibodies in serum (7). Using X-linked immunodeficiency (xid) mice to investigate the contribution of the B1 subset of B cells to the development of septic arthritis, it was found that this defect provided resistance (21). Since the B1 subset is considered to be of importance in the production of autoantibodies, it was hypothesized that the outcome of the experiment might have been due to this fact.
The aim of this study was to investigate if mature B cells, irrespective of their B1 or B2 phenotype, and their products including cytokines, autoantibodies, and antibodies to bacterial constituents would affect the outcome of S. aureus-induced arthritis and sepsis. We report here that a complete absence of mature B cells has no impact on the outcome of these very severe and life-threatening conditions.
MATERIALS AND METHODS
Mice.
Gene-targeted B-cell-deficient μMT mice (C57BL/6 × 129) (11) were backcrossed to B10.Q (H-2q) mice for eight generations and then further intercrossed for two generations to provide homozygous B10.Q mice lacking functional B cells (μMT-BQ) (19). All the offspring were investigated for the presence of serum immunoglobulins (IgM and IgG). The mice were maintained in the animal facility of the Department of Rheumatology, University of Göteborg. Up to 11 mice were kept in each cage, and they were fed standard laboratory chow and water ad libitum. Three independent experiments were performed when the mice were 6, 20, and 24 weeks old.
Bacteria and inoculation.
S. aureus LS-1 used in the experiments has been previously described (8). One of the characteristics of this strain is that it produces large amounts of TSST-1, an exotoxin with superantigenic properties (7). The bacteria were cultured on blood agar for 24 h and then reincubated on blood agar for another 24 h. They were kept frozen at −20°C in phosphate-buffered saline (PBS) (0.13 M sodium chloride, 10 mM sodium phosphate [pH 7.4]) containing 5% bovine serum albumin and 10% dimethyl sulfoxide (C2H6OS) until use. Before the experiments were started, the bacterial solution was thawed, washed in PBS once, and diluted in PBS to achieve the desired concentration of bacteria. Mice were inoculated in one of the tail veins with 0.2 ml of bacterial solution. One group received a low (suboptimal) arthritogenic dose (1 × 107/mouse), the second group received a moderate (optimal) arthritogenic dose (4 × 107 CFU/mouse), and the third group was injected with an high, septic dose (1 × 109 CFU/mouse) of the bacteria. Viable counts in the leftover solution were determined to ascertain the number of bacteria injected.
Clinical evaluation of arthritis.
All the mice were followed up individually, and arthritis was evaluated. The limbs were evaluated by a blinded observer on days 0, 2 to 4, 7, and 10 to 11 after bacterial inoculation. The joints inspected included finger/toe and ankle/wrist joints. Arthritis was defined as visible erythema and/or swelling of a joint. To evaluate the intensity of arthritis, a clinical scoring (arthritis index) was carried out using a system where macroscopic inspection yielded a score of 0 to 3 points for each limb (0, neither swelling nor erythema; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema) (2). The total score was calculated by adding all the scores for each animal tested. The overall condition was evaluated by assessment of weight and general appearance.
Histopathologic examination.
Histopathologic examination of the joints was performed after routine fixation, decalcification, and paraffin embedding. Tissue sections from fore- and hindpaws from the experiment with the lowest (1 × 107) bacterial dose were cut and stained with hematoxylin-eosin. All the slides were coded and evaluated by two blinded observers. The specimens were evaluated with regard to synovial hypertrophy, pannus formation, and cartilage/subchondral-bone destruction. The degree of synovitis and destruction yielded each a score from 0 to 3 in every joint, i.e., finger/toes, wrists/ankles, elbows, and knees.
Bacteriological examination.
Bacterial growth in blood was examined after 18 h and 3 days in the “septic-dose” experiment. The bacterial content in both kidneys and the liver was examined at the time of sacrifice. The different organs were aseptically removed, ground, and diluted with 10 ml of PBS. Appropriate dilutions were made, and 0.1-ml samples of tissue suspension or blood were plated on agar dishes containing 5% horse blood. Samples for bacteriological examination of joints were obtained using sterilized cotton sticks, after dissection of talocrural and radiocarpal joints, and transferred to 5% horse blood agar. After incubation for 48 h the colonies were counted and the results were expressed as the number of CFU per milliliter blood or per whole organ.
Serological analyses. (i) Immunoglobulins.
Levels of IgG and IgM in serum were measured by radial immunodiffusion (12). Antiserum and Ig were purchased from Sigma Chemical Co. (St. Louis, Mo.).
(ii) IL-6 assay.
Cell line B13.29, subclone B9, which is dependent on IL-6 for its growth, was used for IL-6 determinations (1, 10). B9 cells were harvested from tissue culture flasks, seeded into microtiter plates (Nunc, Roskilde, Denmark) at 5,000 cells/well, and cultured in Iscove's medium supplemented with 5 × 10−5 M 2-mercaptoethanol, 5% fetal calf serum (Integro B.V., Leuvenheim, The Netherlands), and 50 μg of gentamicin per ml and serum samples were added. [3H]thymidine was added after 68 h of culturing, and the cells were harvested 4 h later. The samples were tested in twofold dilutions and compared with a recombinant mouse IL-6 standard (Genzyme, Cambridge, Mass.) (6). B9 cells were previously shown not to react with several recombinant cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor, and gamma interferon (IFN-γ). There was only a weak reactivity with IL-4 (10).
(iii) IFN-γ assay.
Levels of IFN-γ were measured by an enzyme-linked immunosorbent assay using 2 μg of purified rat anti-mouse IFN-γ monoclonal antibody (PharMingen, San Diego, Calif.) per ml in sodium bicarbonate (pH 9.6) for coating. All sera were serially diluted in Tris-NaCl and incubated in wells. Biotinylated rat anti-mouse IFN-γ monoclonal antibody (2 μg/ml) (PharMingen) was added to measure the level of IFN-γ bound to the solid phase. This procedure was followed by stepwise addition of streptavidin alkaline phosphatase (Dako, Glostrup, Denmark). The enzyme substrate was then added, and the absorbance was measured in a SpectraMax PLUS photometer (Molecular Devices) at 405 nm. The samples were tested in twofold dilutions and compared with a recombinant mouse IFN-γ standard (Genzyme).
(iv) IL-4 and IL-10 assay.
Kits for detection of these cytokines were purchased from R&D Systems. Detection limits in our assays were 47 pg/ml and 2 pg/ml, respectively.
Statistical analysis.
The mortality rate and the frequency of arthritis were analyzed using the χ2 test with Yates' correction. All the remaining parameters were analyzed by the Mann-Whitney U test. All data are expressed as means ± standard errors of the means unless otherwise indicated.
RESULTS
B lymphocytes do not affect the course of S. aureus arthritis or sepsis.
The clinical outcome of arthritis varied among the three experiments due to inoculation of different numbers of bacteria. However, no statistically significant differences with respect to arthritis between the μMT and the wild-type mice were observed in any of the experiments. In the experiment where the mice were inoculated with an optimal arthritogenic dose of bacteria (4 × 107/mouse), 70% of the μMT mice (n = 10) developed arthritis by day 7 whereas the corresponding rate for their littermate controls (n = 11) was 81%. The mice in the low-dose experiment (1 × 107/mouse) had a lower frequency of arthritis: on day 7, 36% of the μMT mice (n = 11) had developed arthritis and 27% of the wild-type controls had done so. In the sepsis experiment, the mice were inoculated with a high dose of bacteria (1 × 109 CFU/mouse); 86% of the μMT mice (n = 11) and 60% of the controls (n = 7) developed arthritis by day 4. Also, the severity of arthritis was similar between μMT and wild-type controls in all three experiments (data not shown). The clinical observations were confirmed by histopathological analysis of joints (Table 1).
TABLE 1.
Absence of B cells does not affect histopathological progression of S. aureus arthritis in wild-type or μMT micea
| Mice | Frequency of synovitis (%) | Severity of synovitisb | Severity of bone or cartilageb destruction | Frequency of pannus (%) |
|---|---|---|---|---|
| μMT (n = 11) | 91 | 4.5 ± 1.6 | 4.4 ± 2.3 | 36 |
| Wild type (n = 11) | 91 | 3.4 ± 0.7 | 2.7 ± 1.1 | 27 |
In all cases, the differences between μMT and wild-type mice are not statistically significant.
For a definition of the numbers used to indicate severity, see the text.
No deaths were recorded as a result of inoculation with the arthritogenic and subarthritogenic doses of bacteria (1 × 107 and 4 × 107, respectively). Inoculation of B-cell-deficient mice with a septic dose (1 × 109) of S. aureus resulted in a somewhat increased mortality compared to that of wild-type controls (45% [5 of 11] and 13% [1 of 7], respectively). However, these data did not reach statistical significance. Furthermore, the general condition of the mice as measured by weight gain or loss showed no differences between the groups (data not shown).
B cells do not influence the elimination of S. aureus in vivo.
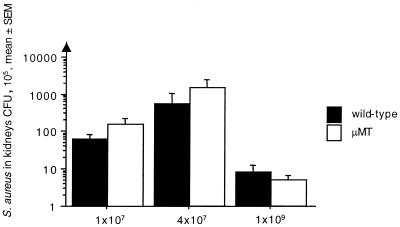
To assess the elimination of S. aureus during infection, bacterial counts in blood were measured after 18 h and 3 days. In addition, bacterial counts in the kidneys, liver, and joints were measured at the time of sacrifice. There were no significant differences between the mice, irrespective of their B-cell status, in any of the experiments (Fig. 1). The shorter life span of the mice in the septic-dose experiment explains the lower bacterial burden.
FIG. 1.
B-cell deficiency does not influence bacterial clearance in the kidneys. In the low-dose (1 × 107 bacteria/mouse) experiment, μMT (n = 11) and wild-type (n = 11) mice were sacrificed on day 14; in the moderate-dose (4 × 107 bacteria/mouse) experiment, μMT (n = 11) and wild-type (n = 10) mice were sacrificed on day 11; and, finally, in the septic-dose experiment (1 × 109 bacteria/mouse), the μMT (n = 6) and wild-type (n = 6) mice were sacrificed on day 7.
Decreased production of IFN-γ in response to S. aureus infection in B-cell-deficient mice.
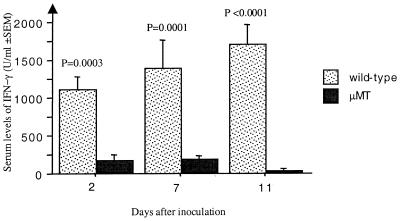
To further study the cellular basis of responses to S. aureus, levels of cytokines in serum were determined. As shown in Fig. 2, there was a striking reduction of IFN-γ levels in the μMT mice following infection with S. aureus. No differences in the levels of IL-4, IL-6, and IL-10 were found (Table 2).
FIG. 2.
Levels of IFN-γ in serum are significantly reduced in the B10.Q (H-2q) μMT mice compared to the wild-type controls. The levels were measured in the moderate-dose (4 × 107 bacteria/mouse) experiment on days 2, 7, and 11.
TABLE 2.
Levels of cytokines in serum following S. aureus infection in B-cell-deficient (μMT) and wild-type congeneic mice
| Cytokine | Level of cytokinea (mean ± SEM) at days after inoculation:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2
|
3
|
7
|
11
|
|||||
| μMT | Wild type | μMT | Wild type | μMT | Wild type | μMT | Wild type | |
| IL-4 | 62 ± 15 | 47 ± 0b | 49 ± 2 | 47 ± 0b | ND | ND | 119 ± 50 | 55 ± 8b |
| IL-6 | 2,962 ± 908 | 1,544 ± 437b | NDf | ND | 4,631 ± 1,859 | 6,967 ± 349b | 1,761 ± 406 | 1,449 ± 448b |
| IL-10 | 5 ± 1 | 5 ± 1b | 4.0 ± 0 | 58 ± 52b | ND | ND | 5 ± 1 | 5 ± 1b |
| IFN-γ | 166 ± 79 | 1,107 ± 172c | ND | ND | 185 ± 51 | 1,396 ± 364d | 34 ± 34 | 1,704 ± 267e |
Levels of IL-4, IL-6, and IL-10 are expressed in picograms per milliliter. Levels of IFN-γ are expressed in units per milliliter.
Not statistically significant.
P = 0.0003.
P = 0.0001.
P < 0.0001.
ND, not done.
DISCUSSION
This study demonstrates that a complete absence of functional B cells in the μMT mice affects neither susceptibility to nor outcome of S. aureus-induced septic arthritis and sepsis-related mortality. In addition, B cells do not influence the rate of in vivo elimination of staphylococci.
We have previously shown that mice with X-linked immunodeficiency (xid mice) are less susceptible to septic arthritis than are their congeneic controls, probably because of a changed cytokine profile, characterized by decreased production of IL-1β and IL-6 and increased synthesis of IFN-γ combined with poor antibody responses (21). The xid mice have relatively few functional B cells of the B2 subset and have no B1 cells. Since the B1 subset of B cells is an important source of autoantibody production, it may be hypothesized that deletion of this population might have had a beneficial impact on the outcome of S. aureus arthritis, a disease characterized by the production of high levels of rheumatoid factors, collagen II antibodies, and anti-DNA antibodies (7). In contrast, the B2 subset of B cells has the capacity to produce antibodies to extrinsic molecules, e.g., bacterial antigens. These antibodies might, at least under certain circumstances, be important in the defense against S. aureus sepsis and arthritis. Indeed, we have recently shown that antibodies specific to staphylococcal collagen adhesin (15) and enterotoxins (16) have a protective capacity. Thus, we hypothesize that the opposing properties of B1 versus B2 cell products might have neutralized each other's effect.
It has recently been shown that B cells are crucial to the progression of collagen type II-induced arthritis (19). It was suggested that B cells were important antigen-presenting cells and that some of them (i.e., those specific for collagen type II) were important for antigen uptake and thereby enhanced macrophage activity. The type of challenge used in different studies of B-cell-deficient mice is vital to the experimental outcome. In the present study, rather than a single antigen, live bacteria encompassing a multitude of antigens, mitogens, and superantigen were employed.
Is the ability of the B cells to present antigens important to the acquisition of an efficient immune response to the infectious agent? In this study, this does not seem to be the case. Previously it has been shown that B cells are not mandatory as antigen-presenting cells for T-cell priming, at least with respect to soluble protein antigens (9, 17, 20). Interestingly, a recent study indicated that intraperitoneal inoculation of μMT mice with Listeria monocytogenes did not affect the clinical outcome of the disease but led to significantly lower production of IFN-γ (13). This outcome is similar to our findings with S. aureus, an extracellularly growing bacterium. The mechanism responsible for the decrease of IFN γ production is unclear but may be dependent on the expression of costimulatory molecules, such as B7 and CD40, on antigen-presenting cells. There are several studies which show the importance of CD40/CD40L costimulation for regulation of Th1 responses. Thus CD40L-deficient (−/−) mice fail to produce IFN-γ during the induction phase of 2,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis (18). In addition, stimulation with a CD40L agonist enhanced IFN-γ production by human peripheral blood mononuclear cells (14).
Our findings showing deficient production of IFN-γ are confirmed by the results of Matsazuki et al. (13) and may be explained by a diminished number of major histocompatibility complex class II molecules, CD40, B7-1, and B7-2 molecules, and thereby an impaired T-cell priming. In addition, the decreased number of class II-expressing cells due to lack of the B-cell population might have affected the superantigenic responses, since the IFN-γ is a cytokine whose secretion is readily triggered by TSST-1 (22). Finally, it has recently been shown that clonal expansion of superantigen-reactive T cells is diminished in μMT mice compared to intact controls (20).
This study demonstrates that the clinical and histopathological outcome of septic arthritis and sepsis-related mortality in response to intravenously inoculated S. aureus is identical in B-cell-deficient mice and their congeneic controls. Interestingly, significantly decreased IFN-γ levels and absence of Ig production in the μMT mice did not affect the in vivo clearance of bacteria.
ACKNOWLEDGMENTS
We thank Lena Svensson and Margareta Verdrengh for excellent technical assistance.
This work was supported by the Göteborg Medical Society, the Swedish Association against Rheumatism, the King Gustaf V. Foundation, the Swedish Medical Research Council, Inflammation Network, Infection and Vaccinology Network, the Nanna Swartz Foundation, the AME Wolff Foundation, and the University of Göteborg.
REFERENCES
- 1.Aarden L A, De Groot E R, Schaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Abdelnour A, Arvidson S, Bremell T, Ryden C, Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelnour A, Bremell T, Holmdahl R, Tarkowski A. Clonal expansion of T lymphocytes causes arthritis and mortality in mice infected with toxic shock syndrome toxin-1-producing staphylococci. Eur J Immunol. 1994;24:1161–1166. doi: 10.1002/eji.1830240523. [DOI] [PubMed] [Google Scholar]
- 4.Abdelnour A, Bremell T, Holmdahl R, Tarkowski A. Role of T lymphocytes in experimental Staphylococcus aureus arthritis. Scand J Immunol. 1994;39:403–408. doi: 10.1111/j.1365-3083.1994.tb03392.x. [DOI] [PubMed] [Google Scholar]
- 5.Abdelnour A, Bremell T, Tarkowski A. Toxic shock syndrome toxin 1 contributes to the arthritogenicity of Staphylococcus aureus. J Infect Dis. 1994;170:94–99. doi: 10.1093/infdis/170.1.94. [DOI] [PubMed] [Google Scholar]
- 6.Brakenhoff J P, de Groot E R, Evers R F, Pannekoek H, Aarden L A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987;139:4116–4121. . (Erratum, 140:4413, 1988.) [PubMed] [Google Scholar]
- 7.Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremell T, Lange S, Yacoub A, Ryden C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein M M, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helle M, Boeije L, Aarden L A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 12.Mancini G, Carbonara A O, Heremans J F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki G, Vordermeier H M, Hashimoto A, Nomoto K, Ivanyi J. The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell Immunol. 1999;194:178–185. doi: 10.1006/cimm.1999.1503. [DOI] [PubMed] [Google Scholar]
- 14.McDyer J F, Goletz T J, Thomas E, June C H, Seder R A. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J Immunol. 1998;160:1701–1707. [PubMed] [Google Scholar]
- 15.Nilsson I M, Patti J M, Bremell T, Hook M, Tarkowski A. Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. J Clin Investig. 1998;101:2640–2649. doi: 10.1172/JCI1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson I M, Verdrengh M, Ulrich R G, Bavari S, Tarkowski A. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J Infect Dis. 1999;180:1370–1373. doi: 10.1086/315023. [DOI] [PubMed] [Google Scholar]
- 17.Phillips J A, Romball C G, Hobbs M V, Ernst D N, Shultz L, Weigle W O. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vella A T, Scherer M T, Schultz L, Kappler J W, Marrack P. B cells are not essential for peripheral T-cell tolerance. Proc Natl Acad Sci USA. 1996;93:951–955. doi: 10.1073/pnas.93.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y X, Abdelnour A, Holmdahl R, Tarkowski A. Mice with the xid B cell defect are less susceptible to developing Staphylococcus aureus-induced arthritis. J Immunol. 1995;155:2067–2076. [PubMed] [Google Scholar]
- 22.Zhao Y X, Abdelnour A, Ljungdahl A, Olsson T, Tarkowski A. Patterns of interferon-gamma mRNA expression in toxic shock syndrome toxin-1 expanded V beta 11+ T lymphocytes. Cell Immunol. 1995;161:28–33. doi: 10.1006/cimm.1995.1005. [DOI] [PubMed] [Google Scholar]