ABSTRACT
Background
COVID-19 can cause cardiopulmonary involvement. Physical activity and cardiac complications can worsen prognosis, while pulmonary complications can reduce performance.
Aims
To determine the prevalence and clinical implications of SARS-CoV-2 cardiopulmonary involvement in elite athletes.
Methods
An observational study between 1 July 2020 and 30 June 2021 with the assessment of coronary biomarkers, electrocardiogram, echocardiography, Holter-monitoring, spirometry, and chest X-ray in Danish elite athletes showed that PCR-tested positive for SARS-CoV-2. The cohort consisted of male football players screened weekly (cohort I) and elite athletes on an international level only tested if they had symptoms, were near-contact, or participated in international competitions (cohort II). All athletes were categorized into two groups based on symptoms and duration of COVID-19: Group 1 had no cardiopulmonary symptoms and duration ≤7 days, and; Group 2 had cardiopulmonary symptoms or disease duration >7 days.
Results
In total 121 athletes who tested positive for SARS-CoV-2 were investigated. Cardiac involvement was identified in 2/121 (2%) and pulmonary involvement in 15/121 (12%) participants. In group 1, 87 (72%), no athletes presented with signs of cardiac involvement, and 8 (7%) were diagnosed with radiological COVID-19-related findings or obstructive lung function. In group 2, 34 (28%), two had myocarditis (6%), and 8 (24%) were diagnosed with radiological COVID-19-related findings or obstructive lung function.
Conclusions
These clinically-driven data show no signs of cardiac involvement among athletes who tested positive for SARS-CoV-2 infection without cardiopulmonary symptoms and duration <7 days. Athletes with cardiopulmonary symptoms or prolonged duration of COVID-19 display, exercise-limiting cardiopulmonary involvement.
KEYWORDS: Athletes, coronavirus, return-to-sport, myocarditis, pulmonary involvement
Introduction
Cardiac manifestations of COVID-19 include myocarditis, arrhythmias, rapid-onset heart failure, and thromboembolism [1]. Cardiac involvement can be due to direct damage from the virus, SARS-CoV2, and secondary to a cytokine storm [2]. Hospitalized patients show a prevalence of cardiac injury measured as elevated troponins of 20–40% [3–5], andpatients with known cardiovascular disease (CVD) or CVD risk factors (hypertension, diabetes) have been shown to bemore susceptible to suffering a severe outcome [6,7]. Less is known about cardiac involvement in non-hospitalized patients with COVID-19 without prior cardiac disease or known risk factors. Myocarditis is a concern for athletes that maintain vigorous exercise since it is associated with increased morbidity and mortality in animal models [8], and has been shown to increase the risk of sudden cardiac death (SCD) in athletes [9,10]. Therefore, the diagnosis of myocarditis can have a negative impact on the athletes’ careers since recent guidelines [11] recommend abstinence from moderate to high-intensity exercise for 3–6 months. Furthermore, high-intensity sports participation is not recommended if the myocardial scar or reduced cardiac function persists.
Establishing the diagnosis of myocarditis is based on a combination of symptoms, and clinical findings, including ECG changes, troponin elevation, and, cardiac imaging [12,13], which can be challenging in athletes because of possible exercise-induced cardiac remodeling (athlete’s heart) [14]. COVID-19 has emerged as a multi-organ disease that often affects the lungs, which may reduce exercise capacity. Consequently, examination of both cardiac and pulmonary function to differentiate cardiac from pulmonary involvement is crucial.
Knowledge of when an athlete can safely return to sport (RTS) after COVID-19 infection is fundamental, but is limited because earlier guidelines were proposed before data and experience could be collected [15–17]. Early studies of COVID-19 patients screened with cardiac magnetic resonance imaging (CMR) raised concerns since the incidence of myocarditis ranged from 1to 60% [18–21]. The discrepancy may be attributed to age, comorbidities, the severity of the disease, and different CMR definitions of myocarditis. In contrast in a recent meta-analysis [22] the prevalence of COVID-19-related myocarditis among athletes ranges from 1% to 4%.
The objective of the present study was to determine the prevalence and clinical implications of SARS-CoV-2 cardiopulmonary involvement in elite athletes. The investigation included standard cardiac and lung examinations in elite athletes and further examinations including CMR were only performed if clinically indicated. Thereafter, the athletes were divided into two groups depending on cardiopulmonary symptoms and duration of symptoms and we examined the impact of RTS after COVID-19 in those with cardiopulmonary involvement.
Methods
All athletes supported by the Danish elite sports organization, Team Danmark, the two top-tier Danish male football leagues, and the best Danish women’s national league who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) were offered to participate in the study.
Recruitment, eligibility criteria, and data collection
This study encompasses data from two cohorts of elite athletes examined between 1 July 2020 and 30 June 2021. All athletes were confirmed positive for SARS-CoV-2 by PCR and underwent a voluntary national cardiopulmonary examination for elite athletes.
Cohort I consisted of:
- Male football players from the top two Danish National Leagues who were screened weekly throughout this investigation and described in detail elsewhere [23].
Cohort II consisted of:
- Elite athletes registered under the Danish elite organization, Team Danmark, and female football players in the top Danish National League. These athletes competed on an international level and were not screened routinely but followed the national COVID-19 guidelines throughout the investigation period. All athletes only participated in this study once.
The athletes who tested positive for SARS-CoV-2 were advised to self-isolate for at least 7 days and were not examined until they were symptom-free for 48 h. In addition, they were recommended to refrain from exercise until they had been cleared for cardiopulmonary involvement. Athletes were advised to gradually resume training if no adverse symptoms appeared 1 week after examination, i.e. after being symptom-free for 2 weeks (Figure 1).
Figure 1.

The recommendation of our study – from tested SARS-CoV-2 positive to retureturn to exercise training/competition if no signs of cardiac or pulmonary affection of the infection.
The project was approved as a health quality investigation by the local and regional Health Research Ethics Committee (nr. 20049575/21012819).
The athletes underwent the following (Figure 2):
Figure 2.

Illustration of the baseline assessment of all participating athletes including clinical investigation, blood samples, echocardiography, Holter monitoring, chest x-ray, and spirometry.
− Personal history and clinical investigation: history of pulmonary or cardiovascular disease, comorbidities, familiar history of SCD or premature cardiac disease, drug therapy, type and duration of symptoms related to SARS-CoV-2 infection, and symptoms indicating cardiac involvement.
− Blood testing, including cardiac biomarkers (high-sensitivity troponin, (TnT), and N-terminal pro-brain-natriuretic peptide (NT-proBNP), D-dimer). All blood measurements were considered abnormal if >99% of the upper limits of normal per assay standards.
− A resting 12-lead electrocardiogram (ECG) was obtained and analyzed according to European Cardiology Society (ECS) guidelines for athletes [24].
− A 24-hour Holter monitoring was performed with a 3-channel Holter device (C3, Cortrium, Copenhagen, Denmark), and data were analyzed by trained staff. More than 500 premature ventricular beats (PVBs)/24 h were defined as abnormal, 200–500 possibly abnormal, and below 200 as normal. Non-sustained ventricular tachycardia (NSVT) was defined as ≥3 ventricular beats at a rate of >100 beats/min with a duration of <30 s.
− Standard transthoracic echocardiography (TTE) including 2D speckle tracking, was obtained using a GE S6 echocardiograph (GE Vingmed Ultrasound AS, Horten, Norway) and stored for offline analysis (EchoPAC version 203; GE Vingmed Ultrasound AS). Analyses were performed according to the standardized criteria [25,26].
- Standard chest X-rays in two projections were obtained and analyzed by an experienced radiologist and a physician who specialized in respiratory diseases. COVID-19-related chest – X-ray findings were defined as ground-glass opacities, pulmonary nodules, and interstitial changes [27].
- Spirometry and diffusion capacity (DLCO) measurements were performed following the American Thoracic Society (ATS) and The European Respiratory Society (ERS) recommendations [28,29]. A reduced lung function was defined as FEV1 < 80% of predicted. An obstructive lung function (FEV1/FVC ratio) and a reduced DLCO were defined as below lower limits of normal [29,30].
Abnormal findings prompted additional clinical investigations according to the principles of good clinical practice and current guidelines to rule out cardiovascular and pulmonary abnormalities, i.e. CMR and chest computed tomography (CT). Cardiopulmonary exercise testing [31] (CPET) was performed (cycle ergometer (Lode Corival, the Netherlands)) in cases of unexplained symptoms of reduced exercise capacity to determine the maximal oxygen uptake (VO2max, (ml/min/kg)).
CMR was performed with a 1.5 T CMR scanner (Magnetom Aera, Siemens Healthcare, Germany). The following sequences were obtained: standard long-axis cine images, T1 and T2 mapping images the short-axis views: basal, mid, and apical slice, and late gadolinium enhancement (LGE).
Key definitions
The study definition of cardiac involvement was adapted from the Updated Lake Louise imaging criteria [12] and involved biomarkers and echocardiography with a high clinical pretest probability of definite cardiac involvement in combination with positive CMR findings as T1 (abnormal T1 values or LGE) or T2 (edema on T2W or increased T2 values on mapping images). SARS-CoV-2 pericardial involvement was defined as small (>5 mm) in end-diastole or greater pericardial effusion or pericardial enhancement on TTE or CMR.
The severity of COVID-19 illness was classified into two groups based on a combination of cardiopulmonary symptoms (as chest pain, palpitations, shortness of breath, and reduced exercise capacity) and duration of disease [16]: Group 1 had no history of cardiopulmonary symptoms and duration of disease ≤7 days, and Group 2 had cardiopulmonary symptoms during acute infection and/or duration of disease above 7 days or persistent reduced exercise performance. The cardiopulmonary symptoms were defined by the athlete’s complaints at the interview on the day of investigations.
Statistical Analysis
The normal distribution of all continuous variables was examined visually and using the Shapiro–Wilk tests. Data are presented as mean ± standard deviation (SD) or median with first and third quartiles depending on the distribution. Categorical variables are expressed as percentages. For continuous data, one-way ANOVA or Kruskal Wallis tests were used to assess the between-group differences, while Fisher’s exact test was used for nominal data in 3 × 2 tables. Posthoc pairwise tests were performed with the Holm correction for multiple comparisons in the case of an overall effect. A p-value < 0.05 was considered statistically significant. Statistics were performed using R (https://www.R-project.org).
Results
One hundred twenty-one young elite athletes (age 25 ± 4 years, 28% females) who tested positive for SARS-CoV-2 participated in this national study. Athletes were recruited from 10 different sports disciplines, although the majority were football players (74% for both genders). The frequency of SARS-CoV-2 positive test was three times as high in Cohort I compared to Cohort II (74/748 (10%) vs. 48/1507 (3%)). The majority of athletes (81%) exercised ten to twenty hours per week and the remaining athletes exercised more than 20 hours per week. Athletes were examined 12 [9; 17] days (median [1st quartile; 3 rd quartile]) after testing positive. The mean duration of symptoms was 5 ± 4 days; 14% were completely asymptomatic, and none were hospitalized. The five most frequent symptoms were headache (78%), muscle aches (64%), coughing (62%), loss of smell or taste (53%), and fever >37.8 C (49%). Asthma was the most frequent comorbidity, but did not differbetweenthe two groups. Otherwise, there were few reported comorbidities.
The demographic characteristics of the study population are reported in Table 1. Most athletes (72%) were in group 1 with an expected shorter duration of symptoms than group 2 (group 1, 3 ± 2 vs. group 2, 8 ± 4 days, respectively). This was also reflected in the time from positive test to examination (10 [9; 16] vs. 16 [11; 21] ([Median [1st quartile; 3 quartiles]) days, respectively, for groups 1 and 2). The athletes in group 2 had either cardiac symptoms (48%) or duration of symptoms above 1 week (33%) or both (19%). Among the 13 athletes that complained of reduced exercise capacity, we found one (8%) with myocarditis and 6 (46%) with pneumonia or obstructive lung function. Male soccer players were less likely to be symptomatic than other athletes because they were screened weekly. Further examinations, which consisted mainly of CPET and CMR were primarily performed in group 2 and was based either clinically abnormal findings or examination results.
Table 1.
Demography of the athletes divided into total, groups 1 and 2 (depending on cardiopulmonary symptoms and duration of disease, respectively). * denotes different from group 1.
| By athlete grouping |
||||
|---|---|---|---|---|
| Athlete characteristics | Total | Group1 | Group 2 | P-value |
| Number of athletes | 121 | 87 | 34 | |
| Age (years) | 24.7 ± 4.1 | 24.5 ± 4.4 | 25.4 ± 31 | P = 0.20 |
| Females (n (%)) | 34 (28%) | 19 (22%) | 15 (44%)* | P = 0.02 |
| Athletes by sport (n (%)) | ||||
| Male soccer | 63 (52%) | 52 (60%) | 11 (32%)* | P = 0.01 |
| COVID infection (n (%)) | ||||
| Days from positive test to examination ([Median [1st quartile; 3 quartile]) | 12 [9; 17] | 10 [9; 16] | 16 [11; 21]* | P = 0.0001 |
| A known source of infection | 73 (60%) | 50 (57%) | 22 (65%) | P = 0.54 |
| The average duration of symptoms (Days) | 4.6 ± 3.5 | 3.3 ± 2.4 | 8.1 ± 3.6* | P > 0.0001 |
| Asymptomatic patients | 17 (14%) | 17 (20%) | 0* | P = 0.003 |
| Hospitalized | 0 | 0 | 0 | - |
| Comorbidity (n (%)) | ||||
| Known Asthma | 17 (14%) | 10 (11%) | 4 (21%) | P = 0.37 |
| Electrical cardiac disease | 3 (2%) | 3 (3%) | 0 | P = 1 |
| Examinations performed (n (%)) | ||||
| All cardiac examinations | 108 (89%) | 77 (89%) | 31 (91%) | P = 1 |
| All pulmonary examinations | 106 (88%) | 75 (86%) | 31 (91%) | P = 0.55 |
| Further examinations performed | 19 (16%) | 8 (9%) | 11 (32%)* | P = 0.004 |
Pulmonary involvement and clinical findings following SARS-CoV-2 infection
Chest X-ray (CXR) was performed on 113 (93%) athletes. The vast majority (95%) had a normal CXR. Three athletes had typical but very subtle COVID-19-related findings [27]. In the remaining three athletes, CXR raised suspicion of abnormality, but chest CT scans were normal.
Spirometry was performed on 113 (93%) athletes. In Table 2 measurements are presented for the two groups. FVC (%) was significantly higher and FEV1/FVC (%) lower in group 2, and no other between-group differencies existed.
Table 2.
Measurements from spirometry (mean ± SD) are divided into groups 1 and 2, respectively.
| Group 1 | n = 87 | Group 2 | n = 34 | P-value | |
|---|---|---|---|---|---|
| FEV1 (L) | 4.8 ± 0.8 | 81 | 4.6 ± 1.1 | 32 | 0.52 |
| FEV1 (%) | 102 ± 11 | 81 | 105 ± 16 | 32 | 0.39 |
| FVC (L) | 5.7 ± 1.1 | 81 | 5.8 ± 1.4 | 32 | 0.15 |
| FVC (%) | 103 ± 11 | 81 | 109 ± 14* | 32 | 0.02 |
| FEV1/FVC (%) | 84 ± 7 | 81 | 81 ± 7* | 32 | 0.01 |
| DLCO (%) | 101 ± 15 | 78 | 99 ± 13 | 30 | 0.52 |
Abbreviations: FEV1 = Forced expiratory volume in the first second; FVC = Forced vital capacity; DLCO = diffusing capacity of the lungs for carbon monoxide. * denotes different from group 1.
Five of 11 athletes with obstructive lung function accepted follow-up. Four athletes initiated inhalation treatment for asthma.
Pulmonary involvement defined as obstructive lung function (11), reduced DLCO (1), or radiological signs of COVID-19 by chest x-ray (3) was found in 15 (12%) of all participants.
Cardiac involvement following SARS-CoV-2 infection
Resting ECGs were obtained in 110 (91%) athletes. Eight recordings were obtained at local hospitals and described as normal, but not available to the investigators of this study. For the remaining four athletes, a resting ECG was not performed.
Five athletes displayed T-wave inversion (TWI) in anterior precordial leads extending beyond V2: (1) one athlete with concomitant ST-depression and CMR determined findings of myocarditis CMR. (2) One athlete who had been examined before the infection due to TWI V1-4 including CMR with normal findings and asymptomatic COVID-19 disease. (3) One athlete with African ethnicity with minor diphasic TWI in anterior leads extending to V5, but asymptomatic COVID-19 disease and otherwise unremarkable examinations. The changes were considered unrelated to the COVID-19 disease. (4) One asymptomatic athlete with diphasic TWI V1-3, and otherwise unremarkable examinations. Although the findings were not considered to be related to the infection, the athlete was offered further investigation with CMR but declined to proceed. (5) One athlete with TWI in V1-2 had concomitant signs of right ventricle hypertrophy and right axis; all other examinations were normal.
Holter monitor data was obtained in 118 (98%) athletes. Five athletes presented an increased number of ventricular ectopy (between 200 and 500/24 hours), and one of these athletes had myocarditis. The remaining four athletes were examined with CPET, which was normal. Two athletes had single runs of NSVT but had otherwise a normal cardiac workup, including CPET. One athlete had intermittent widening of the QRS complex and displayed exercise-induced right bundle branch block (RBBB) during CPET.
TTE was performed in 98% of athletes (Table 3). The systolic function of the left and right ventricles did not differ between groups. Eight athletes had LVEF <50% but none <45% and or GLS < −15. None of the measurements was related to more symptoms or longer duration of COVID-19.
Table 3.
Echocardiographic measurements (mean ± SD) divided into group 1 and 2, respectively.
| Group 1 | Group 2 | P-value | |
|---|---|---|---|
| Number | 87 (72%) | 34 (28%) | |
| LV size | |||
| LVEDD (mm) | 51 ± 4 | 51 ± 5 | 0.96 |
| LVEDD/BSA (mm/m2) | 26.3 ± 2.3 | 26.9 ± 2.8 | 0.33 |
| RWT | 0.35 ± 0.05 | 0.34 ± 0.04 | 0.39 |
| LV systolic function | |||
| LVEF (%) | 57 ± 5 | 56 ± 4 | 0.25 |
| GLS (%) | −19 ± 2 | −19 ± 2 | 0.89 |
| LV diastolic function | |||
| E/e | 5 ± 1 | 4 ± 1 | 0.41 |
| RV size | |||
| RVOTplax (mm) | 30 ± 5 | 30 ± 5 | 0.55 |
| RVOTplax/BSA (mm/m2) | 15.6 ± 2.4 | 15.8 ± 2.4 | 0.74 |
| RV/LV-ratio | 0.60 ± 0.1 | 0.59 ± 0.1 | 0.58 |
| RV function | |||
| TAPSE (mm) | 28 ± 6 | 27 ± 4 | 0.51 |
| RV FAC (%) | 38 ± 6 | 40 ± 6 | 0.17 |
| RVFWLS (%) | −25 ± 3 | −24 ± 4 | 0.42 |
Abbreviations: LV = left ventricle; RV = right ventricle; LVEDD = left ventricle end-diastolic diameter; BSA = body surface area; RWT = relative wall thickness; LVEF = left ventricle ejection fraction; GLS = global longitudinal strain; E/e* = Transmitral inflow velocity early rapid passive atrial emptying (E-wave)/Mitral annular velocity tissue Doppler (e*); RVOT = right ventricle outflow tract; TAPSE = Tricuspid annular plane systolic excursion, FAC = Fractional area change; FWLS = Free wall longitudinal strain. * denotes different from group 1.
Troponins were obtained in 99% and was elevated in three athletes; one had myocarditis and two had been exercising before examination and were without any other indications of cardiac involvement. Troponin measurements were repeated the following day in the two without other signs of cardiac involvement and were normal. Dividing the blood measurements into different groups (Table 4) did not show any association with more symptoms or longer duration of COVID-19.
Table 4.
Blood measurements reported as median [1st quartile; 3 quartiles] and divided into groups 1 and 2, respectively. * denotes significantly different from group 1.
| Group 1 | n = 87 | Group 2 | n = 34 | P-value | |
|---|---|---|---|---|---|
| Haemoglobin (mmol/L) | 9.2 [8.8; 9.7] | 83 | 9.0 [8.7; 9.1] | 31 | 0.41 |
| Leukocytes (E9/L) | 5.7 [5.0; 6.9] | 83 | 5.9 [5.0; 6.7] | 31 | 0.88 |
| Ferritin (µg/L) | 130 [81; 178] | 85 | 126 [74; 211] | 32 | 0.75 |
| D-dimer (FEU/L) | 0.1 [0.1; 0.3] | 86 | 0.1 [0.1; 0.2] | 33 | 0.37 |
| Creatinine (µmol/L) | 89 [81; 95] | 82 | 84 [68; 92] | 31 | 0.08 |
| Alkaline phosphatase (U/L) | 72 [61; 86] | 82 | 66 [55; 76] | 31 | 0.11 |
| LDH (U/L) | 190 [169; 214] | 64 | 181 [170; 191] | 24 | 0.28 |
| CK (U/L) | 131 [94; 236] | 82 | 119 [85; 189] | 33 | 0.31 |
| CK-MB (µg/L) | 2.0 [1.0; 3.0] | 84 | 2.0 [1.0; 2.0] | 32 | 0.27 |
| TnT (ng/L) | 7.0 [5.0; 9.0] | 86 | 6.0 [4.0; 7.0]* | 33 | 0.03 |
| CRP (mg/L) | 1.0 [1.0; 1.0] | 82 | 1.0 [1.0; 1.0] | 31 | 0.82 |
| Pro-BNP (pmol/L) | 4.0 [2.2; 6.1] | 82 | 4.9 [2.6; 5.7] | 32 | 0.53 |
Abbreviations: N = number of participants for each measurement; CRP = C-Reactive protein; LDH = Lactate Dehydrogenase; CK = Creatine kinase; CK-MB = Creatine kinase myocardial bond; TnT = Troponin T, Pro-BNP = Pro-Brain natriuretic peptide.
We identified two definitive cases of myocarditis as shown in Figure 3. The two athletes were reexamined with CMR within 6 months after testing PCR positive, and both athletes were recommended to abstain from competitive sports during that period. One athlete resolved completely and continued her sport at her usual level. The other athlete had a persistent myocardial scar but decided to stop his career for other reasons. Three other athletes were examined with CMR and displayed normal findings.
Figure 3.
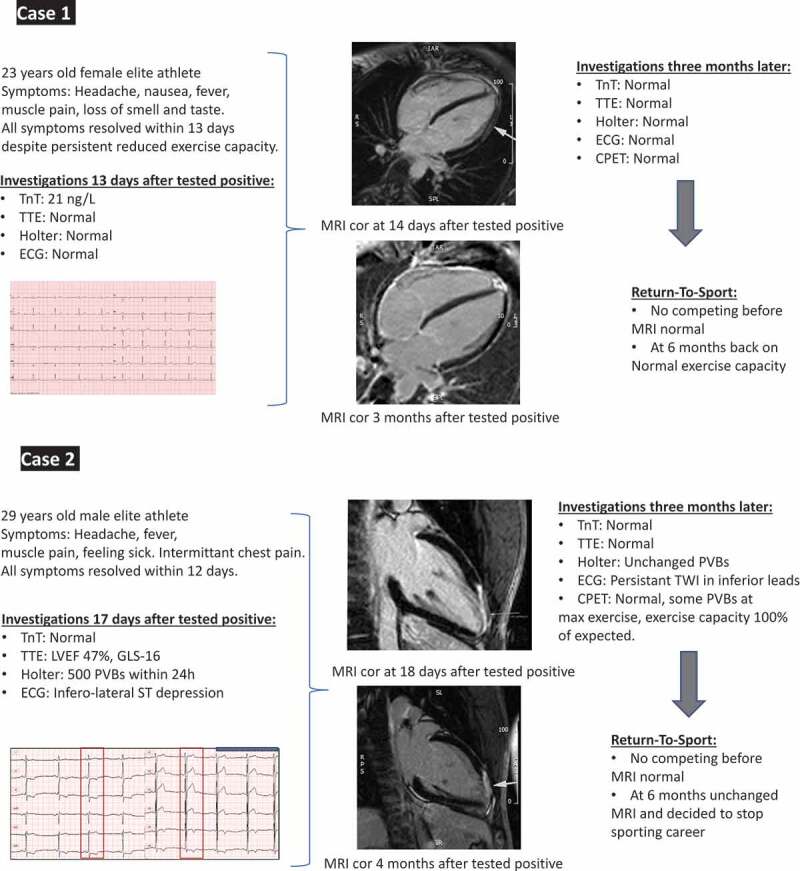
Characteristics of athletes (cases 1 and 2) with myocarditis from diagnosis to follow-up.
As shown in Figure 4, none in group 1 had signs of cardiac involvement, but seven (8%) had an obstructive lung function, one had slightly reduced DLCO, and one had radiological signs of COVID-19. In group 2, two athletes’ symptoms could be explained by myocarditis (6%), and eight others displayed obstructive lung function (24%). The remaining 20 athletes were without signs of cardiac or pulmonary involvement, and their symptoms were subsequently resolved, and all continued their sports at the pre-COVID-19 level. Of the 13 athletes that complained of reduced exercise capacity, 46% could be explained by pulmonary involvement due to obstructive lung function (four athletes) or radiological signs of COVID-19 by chest x-ray (two athletes). One athlete had myocarditis, while it was not possible to identify a cause in the remaining six athletes. These athletes reported that they were unable to exercise as usual until two to 4 months after testing positive. During the follow-up (from 3 to 12 months), no adverse clinical events were reported.
Figure 4.

Clinical findings of participants who tested positive for SARS-CoV-2 divided into groups 1 and 2 based on cardiopulmonary symptoms and duration of disease.
No cases of pericarditis were detected.
None of the athletes participating reported adverse cardiac events through the available follow-up period (maximum 12 months).
Discussion
Our study is the first national single-center study of cardiac and pulmonary involvement in elite athletes that tested PCR positive for SARS-CoV-2. The main findings were as follows: (1) no athletes in group 1 had signs of cardiac involvement. (2) Fifteen (12%) had signs of pulmonary involvement with obstructive lung function or radiological signs of COVID-19, but only two (2%) athletes had cardiac involvement with CMR determined myocarditis . (3) 13 (46%) complaining of reduced exercise capacity after SARS-CoV-2 infection displayed diminished lung function due to asthma, post-viral airway hyperreactivity or radiological signs of COVID-19.
In the beginning of the SARS-CoV-2 epidemic, 8–12% of hospitalized patients with COVID-19 were reported to have cardiac involvement [32,33], while less was known about non-hospitalized and previously healthy patients with COVID-19. In the last year, screening studies of cardiac involvement [34,35] in athletes positive for SARS-CoV-2 have been published examining cardiopulmonary involvement [36], but none including pulmonary findings. In one study [34], 789 professional (98% male) asymptomatic or mildly symptomatic athletes that tested positive for SARS-Cov-2 were examined with troponins, ECG and TTE. Three athletes were diagnosed with myocarditis and two with pericarditis. In a seperate study , 3018 college athletes who tested positive for SARS-Cov-2 were also examined with troponins, ECG, and TTE [35], and CMR was performed irrespective of clinical indication in 198 athletes and when clinically indicated in the remaining athletes. Cardiac involvement was identified in 0.5% of athletes based on clinically indicated CMR and in 3% of those with primary CMR screening. In yet another study[36], 90 elite athletes screened with the same triad together with spirometry, 24 h Holter monitoring, CPET, 3.3% had cardiac involvement (one with peri-myocarditis and two with pericarditis) but there were no reported cases of pulmonary involvement.
Our data on cardiac involvement in athletes with COVID-19 are in accordance with findings from these studies [34–36]. In addition, we extend on previous findings by demonstrating that a high number of athletes display pulmonary involvement, which is an important differential diagnosis in those with reduced exercise capacity. A recent follow-up study found [37] a low prevalence of athletes with persistent (>3 weeks of symptoms ~1%) or exertional cardiopulmonary symptoms (~4%) on return to exercise, and only 9% could be explained by cardiopulmonary causes. In comparison, we found ~10% with exertional symptoms and ~50% could be explained by cardiopulmonary causes with asthma/bronchial hyperreactivity as the main cause.
Fifteen athletes reported a reduced exercise capacity. These athletes were contacted after 3 months and all reported prolonged time to recover in accordance with earlier reported [38,39], but all of our athletes were back to normal training capacity within one to 2 months except for the one with cardiac involvement who was recommended to abstain from sports. The high number of athletes with pulmonary involvement in this group highlights the importance of adding primarily spirometry when athletes complain of reduced exercise capacity post-COVID. The prevalence of asthma and airway hyperresponsiveness is known to be high in elite athletes [40], in our study, 14% (17/121) had self-reported asthma. Of these 17 athletes, nine had cardiopulmonary symptoms or duration of symptoms >7 days. However, only one had obstructive lung function at the time of examination, and the remaining 10 athletes with obstructive lung function after COVID-19 were not known to have asthma. The diagnosis of asthma can be difficult to establish, and studies have shown a higher prevalence of airway hyperresponsiveness and asthma (‘lower airway dysfunction’) when using objective testing methodology [40,41]. We have no information on how the diagnosis of asthma had been determined before entering our study, but one possible explanation for the high number of athletes with obstructive lung function could be that they had undiagnosed asthma before the COVID infection. However, viral infection can also cause temporary post-viral hyperreactivity that mimics asthma [41].
Based on our findings, we suggest that: All elite athletes are initially evaluated with a questionnaire and that those with cardiopulmonary symptoms, undergo a cardiac examination, including the triad of troponin, TTE, and ECG in addition spirometry and exercise testing. The athletes with reduced exercise capacity before restarting exercise training are difficult to identify, and therefore, we recommend re-evaluation including exercise testing and spirometry if the improvement in exercise capacity is poor within the first week of restarting exercise.
Limitations and strengths
Our study is a nationwide study but has inherent limitations, including small sample size, and therefore interpretation of our findings should be made with that caution. None of the examined athletes in this cohort had been vaccinated at the time of the investigation, and our findings might have been different in a cohort with a high vaccination rate. Whether the number of athletes with cardiopulmonary involvement differ from an age-matched, non-hospitalized, non-athlete cohort is unknown. One athlete with a myocardial scar was identified as having the Delta variant, but we do not know the subtype of the others. Finally, we cannot exclude that some of the athletes with normal troponins, ECG and TTE might have had signs of myocarditis on CMR, although the clinical significance of such finding is unknown.
The strength of our study is that it is the first study with a comprehensive number of both cardiac and pulmonary investigations in this population with very few missing data. Furthermore, it pinpoints the diagnostic importance of both cardiac and pulmonary investigations.
Conclusion
COVID-19-induced myocarditis is rare and pulmonary complications more frequent among elite athletes. We suggest that athletes with cardiopulmonary symptoms or prolonged duration of COVID-19 symptoms should be offered a cardiopulmonary examination. Furthermore, it seems like follow-up examination of athletes including spirometry and exercise testing, would be a reasonable approach if cardiopulmonary symptoms occur or persist after resuming exercise.
Acknowledgments
Special thanks to our collaborators, Team Danmark and the Danish National Football League. Study nurse Liselotte Klint Christiansen is thanked for her excellent contribution to the Holter analyses. Medical students, Tino Severinsen and Kristine Lund Michaelsen for fast and efficient data collection. Secretaries Christina Tegner Björnberg Kobelecki, Trine Ridorf-Hansen, and Karen Marie O´Neill for excellent service to ensure all logistics made the study possible.
Biographies
Hanne Kruuse Rasmusen is a specialist in Sports Cardiology at the Department of Cardiology, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. Her research focuses on cardiac remodeling in athletes and imaging.
Mikkel Aarøe is a Ph.D. student at the Department of Cardiology, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on cardiac remodeling in athletes.
Christoffer Valdorff Madsen is a Ph.D. student at the Department of Cardiology, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on arrhythmogenesis and thrombogenesis related to atrial cardiomyopathy.
Helga Lillian Gudmundsdottir is a Ph.D. student at the Department of Cardiology, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. Her research focuses on hypertrophic cardiomyopathy.
Kenneth Hudlebusch Mertz is a postdoc at the Institute of Sports medicine, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on musculoskeletal injuries in athletes.
Astrid Duus Mikkelsen is a Ph.D. student at the Heart Centre, Rigshospitalet, Copenhagen. Her research focuses on lung remodeling and hemodynamics in heart disease.
Christian Have Dall is a senior researcher and physiotherapist at the Department of Cardiology, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on exercise in health and cardiovascular disease.
Christoffer Brushøj is a medical consultant for Team DK and a specialist in orthopedic surgery at the Institute of Sports medicine, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on overuse-related sports injuries.
Jesper Løvind Andersen is a Senior Scientist and Head of the Laboratory at the Institute of Sports medicine, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on focuses on resistance training, muscle hypertrophy, and aging.
Maria Helena Dominguez Vall-Lamora is a specialist in Cardiology at the Department of Cardiology, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. Her research focuses on arrhythmogenesis and thrombogenesis related to atrial cardiomyopathy.
Ann Bovin is a specialist in Cardiology at the Department of Cardiology, Vejle Hospital, part of Lillebaelt Hospital, Denmark. Her research focuses on cardiovascular risk assessment and secondary prevention.
S. Peter Magnusson is a professor at the Institute of Sports Medicine Copenhagen & Dept of Physical Therapy, University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on musculoskeletal rehabilitation.
Jens Jakob Thune is a specialist in Cardiology at Copenhagen University Hospital – Bispebjerg and Frederiksberg, Denmark. His research focuses on cardiomyopathy and imaging.
Redi Pecini is a specialist in Cardiology at the Department of Cardiology, Copenhagen University Hospital - Rigshospitalet, Denmark. His research focuses on cardiac imaging.
Lars Pedersen is a specialist in Respiratory Medicine at Copenhagen University Hospital - Bispebjerg and Frederiksberg, Denmark. His research focuses on respiratory illness in athletes.
Funding Statement
This work was funded by Team Denmark, Novo Nordisk, and the Danish National Football League. Novo Nordisk Foundation.
Highlights
What is already known on this topic?
COVID-19 can cause cardiac and respiratory manifestations, such as myocarditis and respiratory illness (RI).
Recent guidelines recommend that athletes abstain from vigorous exercise for months if myocarditis is diagnosed since exercise is supposed to worsen outcomes.
Acute RI symptoms are associated with a prolonged return to sport in athletes with COVID-19 compared to acute RI due to other infections.
What does this study add?
More than 70% of athletes do not have clinical signs of cardiac or pulmonary involvement after being tested positive for SARS-CoV-2.
A pulmonary involvement is frequently found after SARS-CoV-2, but both cardiac and pulmonary involvement after SARS-CoV-2 can cause the same symptoms.
How this study might affect clinical practice in the future?
The majority of athletes without cardiopulmonary symptoms and a duration of less than 1 week may not need further examination if resumption of exercise is also without cardiopulmonary symptoms.
Athletes with cardiopulmonary symptoms, including reduced exercise capacity or symptoms of more than 7 days should be referred for further cardiopulmonary examinations.
To separate cardiac from pulmonary causes in those athletes with dyspnea or reduced exercise capacity, the examination should include exercise testing and spirometry since pulmonary causes are more prevalent.
Authors´contribution
Hanne Kruuse Rasmusen (HKR), Mikkel Aarøe (MAA), Christoffer Valdorff Madsen (CVM), Helga Lillian Gudmundsdottir (HLG), Kenneth Hudlebisch Mertz (KHM), Astrid Duus Mikkelsen (ADM), Christian Have Dall (CHD), Christoffer Brushøj (CB), Jesper Løvind Andersen (JLA), Maria Helena Dominguez Vall-Lamora (MHVD), Ann Bovin (AB), S. Peter Magnusson (SPM), Jens Jakob Thune (JJT), Redi Pecini (RP), Lars Pedersen (LP)
HKR, JLA, and LP conceptualized and obtained funding for the study. HKR and LP designed and initiated the trial. HKR, MAA, CVM, HLG, ADM, CHD, MHVD, JLA, SPM, AB, JJT, RP, and LP contributed to the data collection. KHM and HKR did the statistical analyses. HKR drafted the manuscript. All authors contributed to the revision of the manuscript for crucial intellectual content.
Disclosure statement
None of the authors has any conflict of interest.
Data availability
Data from this study can be shared upon request to the first author.
References
- [1].Atri D, Siddiqi HK, Lang JP, et al. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5(5):518–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chilazi M, Duffy EY, Thakkar A, et al. COVID and cardiovascular disease: what we know in 2021. Curr Atheroscler Rep. 2021;23(7):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chapman AR, Bularga A, Mills NL.. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141(22):1733–1735. [DOI] [PubMed] [Google Scholar]
- [5].Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of Myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tomasoni D, Inciardi RM, Lombardi CM, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the cardio-COVID-Italy multicentre study. Eur J Heart Fail. 2020;22(12):2238–2247. [DOI] [PubMed] [Google Scholar]
- [7].Xie J, Tong Z, Guan X, et al. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Network Open. 2020;3(4):e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karjalainen J, Heikkilä J, Nieminen MS, et al. Etiology of mild acute infectious myocarditis. Relation to clinical features. Acta Med Scand. 1983;213(1):65–73. [DOI] [PubMed] [Google Scholar]
- [9].Eckart RE, Scoville, SL, Campbell, CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann. Intern Med. 2004;141(11):829–834. [DOI] [PubMed] [Google Scholar]
- [10].Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the USA, 1980-2006. Circulation. 2009;119(8):1085–1092. [DOI] [PubMed] [Google Scholar]
- [11].Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42(1):17–96. [DOI] [PubMed] [Google Scholar]
- [12].Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in Nonischemic Myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. [DOI] [PubMed] [Google Scholar]
- [13].Tschope C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prior DL, La GA. The athlete’s heart. Heart. 2012;98(12):947–955. [DOI] [PubMed] [Google Scholar]
- [15].Wilson MG, Hull, JH, Rogers, J, et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br J Sports Med. 2020;54(19):1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bhatia RT, Marwaha S, Malhotra A, et al. Exercise in the severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of sports cardiology & exercise of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2020;27(12):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) Infection. JAMA Cardiol. 2020;5(10):1085. [DOI] [PubMed] [Google Scholar]
- [18].Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brito D, Meester S, Yanamala N, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2020;14(3):541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Malek LA, Marczak, M, Milosz-Wieczorek, B, et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53(6):1723-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Modica G, Bianco M, Sollazzo F, et al. Myocarditis in Athletes recovering from COVID-19: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(7):4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pedersen L, Lindberg J, Lind RR, et al. Reopening elite sport during the COVID-19 pandemic: experiences from a controlled return to elite football in Denmark. Scand J Med Sci Sports. 2021;31(4):936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39(16):1466–1480. [DOI] [PubMed] [Google Scholar]
- [25].Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28(1):1–39. [DOI] [PubMed] [Google Scholar]
- [26].Pelliccia A, Caselli S, Sharma S, et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur Heart J. 2018;39(21):1949–1969. [DOI] [PubMed] [Google Scholar]
- [27].Benameur N, Mahmoudi, R, Zaid, S, et al. SARS-CoV-2 diagnosis using medical imaging techniques and artificial intelligence: a review. Clin Imaging. 2021;76:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Graham BL, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. [DOI] [PubMed] [Google Scholar]
- [30].Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2018;39(14):1144–1161. [DOI] [PubMed] [Google Scholar]
- [32].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guan WJ, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martinez MW, Tucker AM, Bloom OJ, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6(7):745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moulson N, Petek BJ, Drezner JA, et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cavigli L, Frascaro F, Turchini F, et al. A prospective study on the consequences of SARS-CoV-2 infection on the heart of young adult competitive athletes: implications for a safe return-to-play. Int J Cardiol. 2021;336:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Petek BJ, Moulson, N, Baggish, AL, et al. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: a study from the outcomes registry for cardiac conditions in Athletes (ORCCA). Br J Sports Med. 2022;56(16):913-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hull JH, Wootten M, Moghal M, et al. Clinical patterns, recovery time and prolonged impact of COVID-19 illness in international athletes: the UK experience. Br J Sports Med. 2022;56(1):4–11. [DOI] [PubMed] [Google Scholar]
- [39].Schwellnus M, Sewry N, Snyders C, et al. Symptom cluster is associated with prolonged return-to-play in symptomatic athletes with acute respiratory illness (including COVID-19): a cross-sectional study-AWARE study I. Br J Sports Med. 2021;55(20):1144–1152. [DOI] [PubMed] [Google Scholar]
- [40].Price OJS, Schwellnus N, Backer M, et al. Prevalence of lower airway dysfunction in athletes: a systematic review and meta-analysis by a sub-group of the IOC consensus group on “acute respiratory illness in the athlete. Br J Sports Med. 2022;56(4):213-222. [DOI] [PubMed] [Google Scholar]
- [41].Schwellnus M, Adami, PE, Bougault, V, et al. International Olympic Committee (IOC) consensus statement on acute respiratory illness in athletes part 2: non-infective acute respiratory illness. Br J Sports Med. 2022. May 27;bjsports-2022-105567. doi: 10.1136/bjsports-2022-105567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study can be shared upon request to the first author.


