In this study, Cortazar et al. report that exonuclease Xrn2 is recruited to preinitiation complexes and “travels” to 3′ ends of genes, and they propose that processing of all mRNA 3′ ends comprises cleavage and limited 5′–3′ trimming by CPSF73 followed by handoff to Xrn2. Their results suggest that a major fraction of pol II complexes terminates prematurely close to the start site under normal conditions by an Xrn2-mediated torpedo mechanism.
Keywords: 3′ end processing, Xrn2, transcription termination, tRNA processing
Abstract
The exonuclease torpedo Xrn2 loads onto nascent RNA 5′-PO4 ends and chases down pol II to promote termination downstream from polyA sites. We report that Xrn2 is recruited to preinitiation complexes and “travels” to 3′ ends of genes. Mapping of 5′-PO4 ends in nascent RNA identified Xrn2 loading sites stabilized by an active site mutant, Xrn2(D235A). Xrn2 loading sites are approximately two to 20 bases downstream from where CPSF73 cleaves at polyA sites and histone 3′ ends. We propose that processing of all mRNA 3′ ends comprises cleavage and limited 5′–3′ trimming by CPSF73, followed by handoff to Xrn2. A similar handoff occurs at tRNA 3′ ends, where cotranscriptional RNase Z cleavage generates novel Xrn2 substrates. Exonuclease-dead Xrn2 increased transcription in 3′ flanking regions by inhibiting polyA site-dependent termination. Surprisingly, the mutant Xrn2 also rescued transcription in promoter-proximal regions to the same extent as in 3′ flanking regions. eNET-seq revealed Xrn2-mediated degradation of sense and antisense nascent RNA within a few bases of the TSS, where 5′-PO4 ends may be generated by decapping or endonucleolytic cleavage. These results suggest that a major fraction of pol II complexes terminates prematurely close to the start site under normal conditions by an Xrn2-mediated torpedo mechanism.
Termination of transcription by RNA pol II occurs at the ends of genes in a polyA site-dependent process that disassembles the transcription elongation complex (TEC) (Proudfoot 2016). Termination is required to recycle the polymerase, define the boundaries of transcription units, and prevent transcriptional interference between adjacent genes. According to the torpedo model, cleavage of the nascent transcript at the polyA site by CPSF73 provides a 5′-PO4 access point for the nuclear 5′–3′ RNA exonuclease Xrn2/Rat1 (Connelly and Manley 1988; Kim et al. 2004; West et al. 2004), which tracks along the nascent transcript until it encounters the RNA polymerase (Fong et al. 2015) and helps to dismantle the elongation complex. Endonucleolytic cleavage of the nascent transcript alone is not sufficient to trigger termination (Uájvári et al. 2002; Pearson and Moore 2013). Xrn2 is required for most or all termination downstream from genes that make polyA+ transcripts. Xrn2 depletion or inactivation by an active site mutation globally increases RNA synthesis in 3′ flanking regions (Kim et al. 2004; Fong et al. 2015; Eaton et al. 2018, 2020). The Xrn2/Rat1 exonuclease-dependent torpedo is the only known mechanism of inducing termination that is conserved from yeast to humans and it operates on both RNA pol II and pol I, at least in yeast (Kim et al. 2004; West et al. 2004; El Hage et al. 2008; Chalamcharla et al. 2015). A central assumption of the torpedo mechanism is that the 5′-PO4 created at the polyA cleavage site is where Xrn2 engages the nascent transcript and starts degrading it, but this aspect of the model has not been directly tested.
While it is clear that Xrn2 degrades nascent RNA downstream from polyA sites (Fong et al. 2015; Eaton et al. 2018, 2020), it is less well understood whether the exonuclease also cotranscriptionally degrades nascent transcripts elsewhere, including at 3′ ends of histone genes and within gene bodies, where it could instigate premature transcription termination (PTT) (Brannan and Bentley 2012; Wagschal et al. 2012; Kamieniarz-Gdula and Proudfoot 2019). Replication-dependent histone mRNA 3′ end cleavage is catalyzed by the same endonuclease CPSF73 that processes polyA sites. It cleaves downstream from the stem–loop at histone mRNA 3′ ends (Dominski et al. 2005; Kolev et al. 2008; Sun et al. 2020), and in vitro at least its 5′–3′ exonuclease activity trims the 3′ half of molecules generated by endonucleolytic cleavage (Walther et al. 1998; Yang et al. 2009, 2020). Whether the products of histone mRNA 3′ end processing are substrates of Xrn2 in vivo is not known.
Several lines of evidence suggest that PTT can limit pol II transcription within gene bodies, especially under stress conditions. PTT results in the poor processivity of transcription within genes upon depletion of the CTD-bound SCAF proteins (Gregersen et al. 2019), heat shock (Cugusi et al. 2022), and exposure to splicing inhibitors (Davidson et al. 2012; Sousa-Luís et al. 2021). In these cases, PTT is associated with elevated intragenic polyadenylation, which is coupled to termination almost certainly by the torpedo mechanism (Di et al. 2019).
Whether PTT occurs at a significant level under normal conditions and whether it involves the Xrn2 torpedo are important unresolved questions. Xrn2 has been implicated in PTT, but the importance of this phenomenon is unclear, as the effects reported have been modest (Brannan et al. 2012; Davidson et al. 2012; Wagschal et al. 2012; Nojima et al. 2015). A recent study demonstrated a role for Xrn2 in PTT downstream from intronic polyA sites activated by splicing inhibition, but under normal conditions it only affected termination downstream from genes (Sousa-Luís et al. 2021). Hence, whether PTT occurs at a significant level independent of premature polyadenylation and the potential role of the Xrn2 torpedo in this process remain unclear.
There is evidence for distinct pathways of PTT in metazoans involving the exosome (Wagschal et al. 2012), the pol II-associated proteins ZC3H4/WDR82 (Austenaa et al. 2015, 2021; Brewer-Jensen et al. 2015; Estell et al. 2021), and the integrator whose endonuclease subunit IntS11 a member of the same β-CASP family of endonucleases as CPSF73 (Elrod et al. 2019; Tatomer et al. 2019; Beckedorff et al. 2020; Lykke-Andersen et al. 2021). Some or all of these pathways have been implicated in termination of the relatively short, poorly adenylated, divergent transcripts made at many promoters and enhancers (Flynn et al. 2011; Hah et al. 2013; Ntini et al. 2013). Importantly, none of these putative PTT factors has identified biochemical activities sufficient to disrupt TECs and cause termination. Therefore, it is important to determine whether Xrn2, arguably the best-validated pol II termination factor, functions in PTT, especially under normal conditions.
Consistent with a possible role in PTT, Xrn2 localizes not only at 3′ ends but also at 5′ ends of human and yeast genes (Kim et al. 2004; Jimeno-González et al. 2010; Brannan et al. 2012; Aoi et al. 2020). Furthermore, in yeasts, Rat1 is implicated in premature termination of elongation complexes with uncapped transcripts (Jimeno-González et al. 2010) and at sites of heterochromatin establishment (Chalamcharla et al. 2015). However, it is unknown whether localization of Xrn2 at 5′ ends results from direct interaction or as an indirect result of gene looping that brings 5′ and 3′ ends into proximity (Grzechnik et al. 2014). Here we report that Xrn2 is recruited to pol II transcription start sites (TSSs) before RNA synthesis begins and “travels” with pol II to the 3′ ends of genes. Therefore, Xrn2 is localized within genes, where it could function in PTT.
We screened for points of Xrn2 entry onto RNA substrates by identifying 5′-PO4 ends in nascent RNA that are stabilized by an active site mutant of the enzyme. This analysis identified 5′ ends two to 20 bases downstream from polyA cleavage sites and histone mRNA 3′ ends that are candidate entry points for Xrn2 to initiate degradation. In addition, we identified 5′ ends of debranched introns and RNase Z cleavage sites in primary tRNA transcripts as Xrn2 substrates. Exonuclease-dead Xrn2 not only inhibits termination downstream from genes but also has a substantial effect on nascent transcription at 5′ ends, as measured by metabolic labeling and eNET-seq. These results demonstrate a high level of PTT operating under normal physiological conditions and implicate the Xrn2-dependent torpedo in this process for divergent transcripts at promoters and enhancers.
Results
Xrn2 is recruited at transcription start sites
To investigate the mechanism of Xrn2 recruitment to promoter regions, we asked whether it was dependent on transcription initiation. Transcription initiation is inhibited by triptolide, which causes accumulation of preinitiation complexes (PICs) at TSSs. As expected, triptolide treatment for 10 min led to pol II depletion within genes (Fig. 1A,D,E) and accumulation in PICs located slightly upstream of the peak of initiated and paused pol II present under control conditions (Supplemental Fig. S1A). Importantly, when initiation was blocked by triptolide, Xrn2 localization was maintained at 5′ ends and, like pol II, was shifted slightly upstream of the promoter-proximal pause region toward the TSS (Fig 1A; Supplemental Fig. S1C). The triptolide effect on Xrn2 localization was similar for the endogenous Xrn2 (Supplemental Fig. S1C) and overexpressed epitope-tagged Xrn2 (Fig. 1B,C), which are similarly distributed along genes under control conditions (Supplemental Fig. S1D). These results suggest that Xrn2 colocalized with pol II in PICs stabilized by triptolide.
Figure 1.
Xrn2 localization at start sites does not require initiation or elongation. (A) Pol II and Xrn2 ChIP-seq on RPL23. Note Xrn2 localization at start sites in cells treated with 100 μM DRB for 1 h, 10 μM triptolide for 10 min, and 300 nM flavopiridol for 1 h (red arrows). (B) Metaplots (25-base bins) of overexpressed Avi-Xrn2 ChIP-seq in control (DMSO) and triptolide-treated cells, as in A. Note that in triptolide, the peak of Xrn2 (red arrow) is shifted upstream of the promoter-proximal pause site (see Supplemental Fig. S1C). (C–E) Heat maps (C) and genome browser screenshots (D,E) of pol II and overexpressed Avitag Xrn2 ChIP-seq in control and triptolide-treated cells, as in B. Note the 5′ peak of Xrn2 is maintained in triptolide (red arrows), while pol II is depleted from gene bodies (blue arrows). (F) Metaplots of endogenous Xrn2 ChIP-seq in control (DMSO), DRB-treated, and flavopiridol-treated cells, as in A. Note that in DRB and flavopiridol, Xrn2 colocalizes with pol II that accumulates at the promoter-proximal pause site downstream from the TSS (red arrows). Fifty-base bins; ChIP signals are relative to bin 1.
To confirm that Xrn2 recruitment to 5′ ends is due to early association with pol II complexes rather than potential gene looping interactions, we treated cells with the PTEFb inhibitor DRB, which caused pol II clearance from gene bodies and accumulation in a promoter-proximal peak (Fig. 1A; Supplemental Fig. S1B). In DRB, Xrn2 accumulates with pol II at 5′ ends, even when the polymerase has completely cleared from 3′ ends (Fig. 1A,F; Supplemental Fig. S1B). A different PTEFb inhibitor, flavopiridol, caused a similar accumulation of Xrn2 at 5′ ends (Fig. 1A,F). In summary, Xrn2 does not require synthesis of the nascent transcript to be recruited to start sites, and association with pol II at the promoter-proximal pause persists even when polymerase is absent from 3′ ends, showing that its 5′ localization is not a result of gene looping.
Xrn2 associates with elongation complexes throughout the length of genes
To investigate whether Xrn2 associates with pol II complexes in gene bodies, we treated cells with the topoisomerase I inhibitor camptothecin, which stalls pol II within genes (Collins et al. 2001). Consistent with previous work (Baranello et al. 2010), CPT reduced promoter-proximal pol II occupancy at GAPDH and increased occupancy within the gene body, as determined by ChIP-qPCR (Supplemental Fig. S2A). ChIP-seq revealed accumulation of both pol II and Xrn2 within gene bodies when elongation was blocked by CPT (Fig. 2A–C; Supplemental Fig. S1E). We conclude that Xrn2 accumulates with stalled elongation complexes within genes.
Figure 2.
Xrn2 appears to “travel” with transcription elongation complexes. (A) Pol II and Xrn2 ChIP-seq at ACTB in control (DMSO) and 30-min 10 μM camptothecin (CPT)-treated HEK293 cells. Note that Xrn2 accumulates with pol II stalled within the gene body in CPT (red arrows). (B,C) Metaplots of pol II and Xrn2 ChIP-seq relative frequency, as in A. Note the relative accumulation of both in gene bodies when transcription is stalled by CPT (red arrows). (D) ChIP-seq of Xrn2 and pol II at time points after washing out DRB on NEAT1. Note that parallel waves of Xrn2 and pol II appear within the gene body (red arrows) before arriving at the 3′ end. (E) Metaplots of Xrn2 and pol II ChIP-seq signals at time points after DRB washout showing parallel waves of Xrn2 and pol II moving through genes. Genes <7 kb in length were selected for visualization. (F) Xrn2/Rat1 localization throughout genes is conserved in Drosophila. Metaplots and genome browser shot of anti-Rat1 ChIP-seq in S2 cells for 15 min at 25°C and 37°C. Note that induction of Hsp70 is associated with Rat1 “spreading” from the start site throughout the length of the gene (red arrow).
To test whether Xrn2 associates with transcribing elongation complexes, we asked whether it colocalizes with the wave of pol II that progresses along genes after release from a DRB block (Singh and Padgett 2009). We observed that after DRB washout, Xrn2 tracks closely with pol II and can be seen within gene bodies before it reaches 3′ ends, though our analysis is limited to relatively short genes due to low ChIP-seq read coverage (Fig. 2D,E; Supplemental Fig. S1F,G).
To ask whether the association of Xrn2 with elongating pol II is a conserved interaction, we localized Rat1, the Drosophila homolog of Xrn2, by ChIP-seq using an anti-Rat1 antibody (Supplemental Fig. S2B). This analysis showed that, as in human cells, Rat1 localizes to the 5′ and 3′ ends of Drosophila genes and within gene bodies. Following heat shock, Rat1 levels are slightly reduced, consistent with suppression of transcription (Fig. 2F; Supplemental Fig. S2C). Notably, on heat shock genes such as Hsp70, Rat1 localized exclusively at the 5′ end at 25°C and spread throughout the gene at 37°C (Fig. 2F, bottom panel), mimicking pol II, which only elongates efficiently along these genes when they are activated (Lis and Wu 1993). We conclude that in human and Drosophila cells, Xrn2 binds pol II complexes at 5′ ends and maintains this association throughout elongation and into the 3′ flanking regions. This association could reflect stable long-lived binding to the TEC or dynamic binding and release throughout elongation.
Mapping Xrn2 entry points by 5′-PO4 Bru-seq
Xrn2 specifically degrades RNAs with 5′-PO4 ends. To identify potential substrates, we developed a method of mapping 5′-PO4 ends within nascent transcripts called “5′-PO4 Bru-seq” (see the Materials and Methods) (Fig. 3A). Total RNA was fragmented with micrococcal nuclease (MNase), and nascent transcripts were enriched by immunoprecipitation of 5-bromouridine (Bru) pulse-labeled molecules followed by 3′ end repair of 2′–3′ cyclic phosphates with T4 polynucleotide kinase, ligation of 5′ and 3′ adaptors, and PCR amplification of sequencing libraries. Only RNA fragments with 5′-PO4 ends could be incorporated into these libraries (Harigaya and Parker 2012). Unique molecular identifiers (UMIs) were included to allow removal of PCR duplicates, and results were quantified by normalization to 5′-PO4 ends of Bru-labeled mitochondrial transcripts (Supplemental Fig. S3A); Xrn2 is not present in mitochondria even at low levels, as determined by proteomic analysis in 14 human tissues (https://pubs.broadinstitute.org/mitocarta/mitocarta30-inventory-mam malian-mitochondrial-proteins-and-pathways). 5′-PO4 Bru-seq was validated by demonstrating that it mapped the 5′-PO4 ends of rRNA precursors (Fig. 3B), microprocessor cleavage sites (Fig 3C), snoRNAs (Supplemental Fig. S3B,D), and tRNA precursors (Fig. 5A; Supplemental Figs. S3F, S4D) with single-base precision.
Figure 3.
Mapping of nascent RNA 5′-PO4 ends identifies cotranscriptional Xrn2 activity at histone 3′ ends. (A) Mapping of 5′-PO4 ends in nascent RNA by 5′-PO4 Bru-seq. (B) 5′-PO4 ends of pulse-labeled rRNAs in HEK293 cells expressing WT and D235A mutant Xrn2. Note stabilization of the 47S rRNA 5′ end, a known Xrn2 substrate (Wang and Pestov 2011), by the Xrn2 mutant (blue arrow). (C) 5′-PO4 Bru-seq maps 5′-PO4 ends made by microprocessor cleavage of the MIR17A cluster (red arrows) with single-base resolution. Note these ends are not stabilized by Xrn2 inactivation. (D–F) Xrn2 D235A stabilizes 5′-PO4 ends (red arrows) several bases downstream from histone mRNA cleavage sites at positions coinciding with CPSF73 exonuclease digestion products. The stem–loop (SL), histone downstream element (HDE), and cleavage site (blue arrow) are marked. (G) Metaplots show 5′-PO4 ends stabilized by Xrn2 inactivation mapped up to 20 bases downstream from histone mRNA 3′ ends. Mean and range of three replicates. (H) Xrn2 and Pol II ChIP-seq on a histone gene in HEK293 cells expressing Avitag WT and D235A mutant Xrn2. Note that there is only a modest delay in termination caused by the Xrn2 mutant (blue arrow).
Figure 5.
tRNA 3′ end processing sites and pre-mRNA 5′ ends are cotranscriptional Xrn2 substrates. (A,B). Xrn2 D235A stabilizes 5′-PO4 ends relative to WT (red arrows) at RNase Z cleavage sites at the 3′ ends of tRNA genes. At some tRNA genes, the 5′ end of the primary tRNA transcript prior to RNase P cleavage is also modestly stabilized in the Xrn2 mutant (blue arrows). Three replicate 5′-PO4 Bru-seq data sets are shown. See Supplemental Figure S4, D and E. (C). Metaplots show 5′-PO4 ends stabilized by Xrn2 inactivation mapped within two bases of the 3′ ends of tRNAs. (D) Xrn2 ChIP-seq on a tRNA gene cluster in cells expressing Avitag WT and D235A Xrn2. Note Xrn2 localization at multiple tRNA genes. (E,F). Xrn2 D235A stabilizes 5′-PO4 ends relative to WT (red arrows) near the TSSs of RPS27 and RPS12 and the intron 1 5′ splice site of RPS27 (blue arrows). Three replicate 5′-PO4 Bru-seq data sets are shown.
To identify Xrn2 substrates, we compared 5′-PO4 ends of nascent transcripts in cells with doxycycline-inducible wild-type (WT) or exonuclease-dead Xrn2 (D235A), which are equivalently overexpressed (Fong et al. 2015). The advantage of this dominant-negative mutant over Xrn2 depletion by a degron is that its phenotype is specifically due to the protein's enzymatic activity. 5′-PO4 ends that are entry points for Xrn2 are expected to be specifically stabilized by the mutant. As validation of this approach, the 5′ end of the 47S rRNA precursor, a known Xrn2 substrate (Wang and Pestov 2011), was specifically stabilized by Xrn2(D235A) (Fig. 3B, blue arrow), whereas other rRNA 5′ ends and products of microprocessor cleavage at the miR-17∼92 cluster (Du et al. 2015) were unaffected (Fig. 3C, red arrows). The latter result is consistent with the fact that we did not observe Xrn2 enrichment by ChIP-seq at miR-17∼92 (Supplemental Fig. S3C) or other intronic miRNAs (miR330 or miR25) (data not shown). The Xrn2 mutant had little effect on the 5′-PO4 ends of snoRNAs, tRNAs, or uncapped snRNAs (Supplemental Fig. S3D–F), suggesting that these species are not common substrates.
Xrn2 loads onto RNA downstream from histone mRNA 3′ cleavage sites
It is unclear whether Xrn2 plays a role at the 3′ ends of replication-dependent histone genes that make polyA− mRNAs (Fong et al. 2015; Eaton et al. 2018). Using 5′-PO4 Bru-seq, we found that Xrn2(D235A) strongly stabilized 5′-PO4 ends at multiple positions downstream from histone mRNA 3′ ends within the histone downstream element (HDE) complementary to U7 snRNA (Fig. 3D–G). These novel Xrn2 substrates coincide closely with 5′ ends produced by the 5′–3′ exonuclease activity of CPSF73 acting on the downstream cleavage product in in vitro processing reactions (Gick et al. 1986; Streit et al. 1993; Walther et al. 1998; Yang et al. 2009, 2020). The stabilization of these 5′-PO4 ends is consistent with the localization of Xrn2 at the 3′ ends of histone genes (Fig. 3H; Supplemental Fig. S4A; Fong et al. 2015), which suggests it is acting cotranscriptionally. Our results differ from a report that Xrn2 degron depletion did not stabilize 5′ ends at histone 3′ processing sites (Sousa-Luís et al. 2021). This apparent discrepancy could reflect different 5′ end mapping methods or different effects of Xrn2 depletion versus inactivation by active site mutation. These results support the existence in vivo of CPSF73 exonuclease products previously identified in vitro and suggest that the CPSF73 and Xrn2 exonucleases act sequentially. First, CPSF73 degrades the spacer between the cleavage site and the HDE, where it may be blocked by U7snRNP (Cho et al. 1995; Walther et al. 1998). Subsequently, Xrn2 further degrades these products either after U7snRNP dissociation or perhaps in a concerted way that facilitates dissociation.
Despite the clear evidence of cotranscriptional recruitment and loading onto nascent transcripts downstream from histone mRNA 3′ processing sites, the exonuclease-dead Xrn2 had only a minor effect on transcription termination downstream from histone genes (Fig. 3H, blue arrow), in agreement with previous work (Eaton et al. 2018; Sousa-Luís et al. 2021). We conclude that while Xrn2 engages histone 3′ processing sites, it does not act as a strong terminator at these genes under normal conditions.
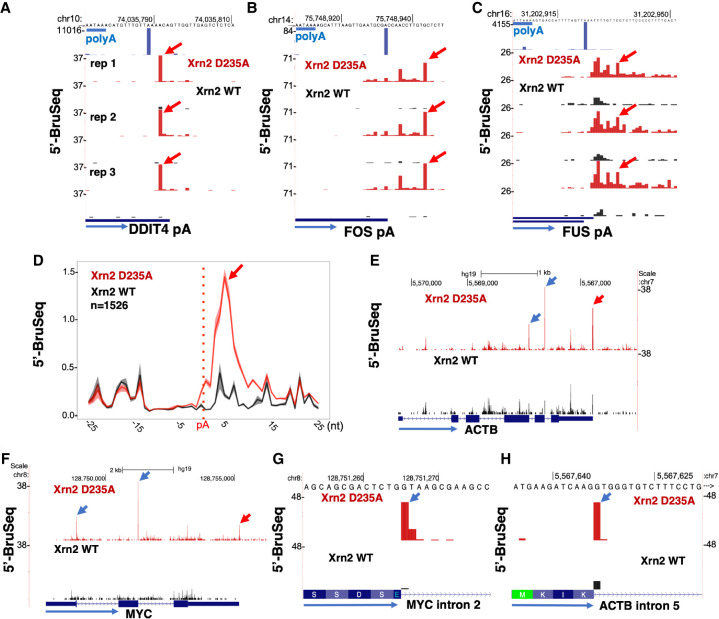
The Xrn2 entry point is often 3′ of the polyA cleavage site
The torpedo model proposes that the 5′-PO4 created at the polyA cleavage site serves as the entry point for Xrn2. We used 5′-PO4 Bru-seq to test this key aspect of the model. The rationale is that 5′-PO4 ends stabilized near polyA sites by the Xrn2(D235A) mutant will correspond to entry points where the Xrn2 torpedo engages the nascent transcript. PolyA cleavage sites in HEK293 cells were mapped at single-base resolution by 3′ READS (Hoque et al. 2013), with the limitation that cleavage within runs of As in the genomic sequence cannot be distinguished from post-transcriptionally synthesized polyA tails. Consistent with the torpedo model, we identified cases where 5′ ends stabilized by Xrn2 inactivation mapped within two bases of the cleavage site (Fig. 4A), but in most cases they were further downstream at multiple positions two to 20 bases 3′ of cleavage sites (Fig. 4B,C; Supplemental Fig. S4B,C). The stabilization of 5′-PO4 ends downstream from polyA sites is evident from inspection of individual genes and from metaplots of several thousand genes (Fig. 4D). The 5′-PO4 RNA ends that we observed in vivo downstream from polyA cleavage sites strongly resemble the products of 5′–3′ trimming of the 3′ half of products of polyA site cleavage reported in vitro (Sheets et al. 1987). The heterogeneity of 5′ ends at polyA sites is in contrast to the unique 5′ ends detected at microprocessor cleavage sites (Fig. 3C) and 5′ splice sites (Fig. 4G,H) in the presence of WT and mutant Xrn2. The heterogeneous 5′-PO4 ends therefore do not result from imprecision of the 5′-PO4 Bru-seq method or nonprocessive exonucleolytic digestion by the D235A mutant. We conclude that at genes encoding polyA+ mRNAs and polyA− histone mRNAs, CPSF73 cleavage is followed by limited 5′–3′ exonucleolytic degradation that precedes handoff to Xrn2 for further degradation. Hence, in most cases, the entry point of the Xrn2 torpedo for polyA site-dependent termination is not the 5′ end created by endonucleolytic cleavage, but rather a cluster of 5′ ends created by exonucleolytic digestion. At histone genes, this exonuclease is CPSF73 itself (Yang et al. 2009, 2020), and it seems likely that this is also the case at polyA sites (Sheets et al. 1987).
Figure 4.
Xrn2 loads several bases downstream from polyA sites. (A–C) Xrn2 D235A stabilizes 5′-PO4 ends (red arrows) relative to WT at the DDIT4 polyA site mapped by 3′ READS (top track, A) and several bases downstream from the FOS and FUS polyA sites (B,C). PolyA consensus is underlined in blue. Three replicate 5′-PO4 Bru-seq data sets are shown. (D) Metaplots of 5′-PO4 ends near polyA sites show Xrn2 engages a few bases downstream from the cleavage site. Reads were aligned to HEK293 polyA sites mapped by 3′ READS. Mean and range of three replicates. (E,F) Xrn2 inactivation stabilizes 5′-PO4 ends near 5′ splice sites (blue arrows) and polyA sites (red arrows). (G,H). Xrn2 degradation of debranched introns, suggested by stabilization of 5′-PO4 ends at the first base of the intron (blue arrow) in Xrn2 mutant cells.
Xrn2 loading at 5′ splice sites
Exonuclease-dead Xrn2 also specifically stabilized the 5′ ends of some introns (Fig. 4E–H). This observation indicates that at least some debranched introns are Xrn2 substrates, consistent with results in yeast (Petfalski et al. 1998). We do not understand why some intron 5′ ends are stabilized much more than others even within the same gene (Fig. 4E,F), but it could be related to different extents of cotranscriptional splicing. While the degradation of debranched introns is most likely a post-transcriptional activity of Xrn2, we cannot exclude the possibility of cotranscriptional degradation of debranched intron–exon step 1 intermediates resulting from failure to complete exon ligation, as previously observed in yeast (Harigaya and Parker 2012).
Cotranscriptional Xrn2 degradation of tRNA 3′ trailer RNAs
Unexpectedly, Xrn2 also targets nascent tRNA transcripts made by pol III. Xrn2 inactivation specifically stabilized the 5′-PO4 ends of 3′ tRNA trailer RNAs cleaved by RNase Z, a member of the β-CASP family of endonucleases that includes CPSF73 and IntS11 (Späth et al. 2007). Xrn2 entry at RNase Z cleavage sites is evident from inspection of individual tRNA genes and a metaplot of several hundred genes (Fig. 5A–C; Supplemental Fig. S4D,E). RNase Z-generated substrates of Xrn2 include the 5′ end of the tRNA-derived miRNA4521 (Supplemental Fig. S4E; Pekarsky et al. 2016). We also noted a modest but reproducible stabilization of the 5′-PO4 ends of some tRNA primary transcripts when Xrn2 was inactivated (Fig. 5A; Supplemental Fig. S4D, blue arrows), suggesting that if these ends are not removed by RNase P they may become substrates of Xrn2. Consistent with a direct effect, Xrn2 was detected by ChIP at many tRNA genes (Fig. 5D), suggesting that it is working cotranscriptionally on pol III elongation complexes. As expected, the Xrn2 mutant had no effect on termination of tRNA transcription, as determined by Bru-seq nascent RNA sequencing (data not shown). In summary, these results demonstrate that Xrn2 can cotranscriptionally degrade cleaved RNAs made by pol III as well as pol II.
Xrn2 mediates nascent transcript degradation within genes under normal conditions
In addition to Xrn2 entry at 3′ end processing sites, we also observed 5′-PO4 ends stabilized by mutant Xrn2 near the TSSs of some mRNA coding genes (Fig. 5E,F; Supplemental Fig. S4F). This result suggests that Xrn2, present near 5′ ends (Figs. 1, 2), is actually active in cotranscriptional degradation of uncapped RNAs. To investigate this possibility further, we asked whether Xrn2 inactivation increases active transcription within genes as well as in termination zones downstream from genes. Synthesis of nascent transcripts was measured by pulse labeling with bromouridine for 30 min in cells overexpressing WT Xrn2 or Xrn2(D235A). Labeled RNA was immunopurified and sequenced (Bru-seq) (Paulsen et al. 2014), and signals were quantified relative to mitochondrial transcripts, which served as an internal control (Fig. 6A). qPCR confirmed equivalent mitochondrial copy number in these two cell lines (Supplemental Fig. S5A). If Xrn2 D235A expression affected mitochondrial RNA metabolism, then this normalization would introduce bias. However, this possibility is unlikely for two reasons: (1) Xrn2 is not present in mitochondria even at low levels, as mentioned above, and (2) comparison of mitochondrial Bru-seq or 5′PO4 Bru-seq reads between WT and mutant Xrn2 cells shows no differential effects between genes (coding and noncoding) or individual 5′-PO4 ends (Fig. 6A; Supplemental Fig. S3A). Therefore, if the Xrn2 mutant were altering mitochondrial gene expression, it must do so by affecting two independent processes—transcription (measured by Bru-seq) and 5′-PO4 turnover (measured by 5′-PO4 Bru-seq)—without any apparent specificity.
Figure 6.
Xrn2 inactivation enhances nascent RNA transcription within genes and 3′ flanking regions. (A) Bru-seq nascent RNA sequencing in cells expressing WT and D235A mutant Xrn2. Data were normalized to mitochondrial signal, and overlayed plots of the mitochondrial genome are shown. (B–E) Overlayed plots of nascent RNA Bru-seq, as in A. Note the enhanced transcription within pol II transcribed genes in cells expressing mutant Xrn2(D235A). (F) Metaplots of Bru-seq signal normalized to mitochondrial reads in cells expressing WT and D235A mutant Xrn2. In the Xrn2 mutant, note enhanced sense transcription downstream from genes and within genes (blue arrow) as well as antisense transcription (negative values) upstream of genes (green arrow). (G) Metaplots of eNET-seq signal in 3′ flanking regions downstream from polyA sites (mean and ranges of three replicates). Note enhanced transcription due to impaired termination in the Xrn2 mutant (red arrow). (H) eNET-seq at MYC in cells expressing WT and D235A mutant Xrn2. Reads correspond to 3′-OH ends of nascent pol II-associated transcripts. Note enhanced transcription at the 5′ end and 3′ flank in Xrn2 mutant relative to WT (arrows). Mapped reads for WT and mutant Xrn2 data sets (three replicates) were equalized by subsampling.
As expected, Xrn2(D235A) elevated levels of Bru-labeled nascent transcripts downstream from polyA sites due to impaired termination (Fig. 6B–D). Xrn2 inactivation also enhanced divergent antisense transcription upstream of genes (Fig. 6F, green arrow). Remarkably, inhibition of Xrn2 activity also markedly enhanced nascent transcript synthesis within gene bodies, as evidenced by inspection of individual genes and metaplots of many genes (Fig. 6B–F; Supplemental Fig. S5B–D). The effect of Xrn2(D235A) on nascent pol II transcript synthesis was detectable both at 5′ ends and within gene bodies on short and long genes (Supplemental Fig. S5B–D). The fold effect of Xrn2 inactivation on the amount of nascent RNA synthesized within genes is substantial when compared with the effect in termination zones (Fig. 6B,C,F; Supplemental Fig. S5B–D), where Xrn2 is thought to terminate almost all pol II complexes. This observation suggests that a sizeable fraction of all nascent transcripts on most genes are cotranscriptionally turned over by the Xrn2 exonuclease under normal conditions. Our results using the Xrn2(D235A) mutant with Bru pulse labeling differ from those obtained using an Xrn2 degron with nascent RNA analysis by POINT-seq (Sousa-Luís et al. 2021). The latter study reported that Xrn2 depletion enhanced transcription of 3′ flanking regions but unexpectedly diminished transcription within protein-coding genes by an almost equivalent amount. This discrepancy could be due to an effect of the C-terminal degron fusion or to the fact that our results were normalized to mitochondrial transcripts as opposed to RPKM normalization. Mitochondrial normalization is expected to reveal global effects on nuclear nascent transcripts, whereas RPKM normalization will show only relative changes. In summary, the Bru-seq experiments suggest that nascent RNA degradation by Xrn2 associated with pol II (Figs. 1, 2) significantly suppresses RNA synthesis within genes under normal conditions in a manner that requires exonuclease activity. Cotranscriptional nascent RNA degradation by Xrn2 within genes could potentially trigger premature termination by a torpedo mechanism, as it does in 3′ flanking regions.
Xrn2 mediates premature termination of sense and antisense transcription at 5′ ends of genes
To investigate the possibility of Xrn2-dependent premature termination, we quantified transcriptionally engaged pol II within genes by enhanced NET-seq (eNET-seq) in cells overexpressing WT and exonuclease-dead Xrn2. eNET-seq (Fong et al. 2022) is a modification of mNET-seq (Nojima et al. 2015), which precisely localizes pol II by mapping of protected 3′-OH ends of nascent transcripts immunoprecipitated with pol II following MNase digestion of nuclei. eNET-seq specifically captures transcriptionally engaged pol II that is either paused or elongating and is therefore well suited to quantifying potential changes in pol II occupancy resulting from altered premature termination. eNET-seq uses decapping to permit inclusion of 5′-terminal transcripts in RNA-seq libraries and UMIs to eliminate PCR duplicates. We performed eNET-seq in triplicate on HEK293 cells expressing doxycycline-inducible codon-optimized WT and exonuclease-dead (D235A) Xrn2. The epitope-tagged WT and mutant Xrn2 proteins were approximately equally well recruited to genes, as determined by ChIP-seq (Supplemental Fig. S5E). Mapped reads corresponding to the 3′-OH ends of the nascent RNAs in Xrn2 WT and mutant eNET-seq data sets were equalized by subsampling to allow direct comparison. The lengths of eNET-seq reads were equivalent for both cell types (Supplemental Fig. S5F). If Xrn2 exonuclease-dependent transcription termination occurs, then the D235A mutant is predicted to increase the eNET-seq signal relative to WT. As expected, the Xrn2 mutant specifically elevated eNET-seq signal downstream from genes, consistent with inhibition of polyA site-dependent termination (Fig. 6G,H, red arrows). The inhibition of termination caused by Xrn2 inactivation resulted in a 1.29-fold average increase (n = 3) in eNET-seq signal in the 5-kb region downstream from polyA sites (Fig. 6G).
Notably, exonuclease-dead Xrn2 also specifically increased the abundance of pol II complexes engaged in sense and divergent antisense transcription at the 5′ ends of genes measured by eNET-seq, consistent with the Bru-seq results (Fig. 6F). This effect is evident from inspection of individual genes and in metaplots, showing that it is widespread (Fig. 7A–D, blue arrows). Note that the distinct profiles seen in metaplots of Bru-seq (Fig. 6F; Supplemental Fig. S5B) and eNET-seq (Fig. 7B) reads reflect detection of different populations of polymerases. While Bru-seq only detects polymerases actively incorporating nucleotides, eNET-seq additionally detects paused polymerases that specifically accumulate in the promoter-proximal region. In contrast to most protein-coding genes, Xrn2 inactivation had little or no effect on transcription of snRNA (Fig. 7E) and histone genes (Supplemental Fig. S5G), consistent with previous studies (Fong et al. 2015; Eaton et al. 2018, 2020; Sousa-Luís et al. 2021). In addition to its effects at 5′ ends of genes, the Xrn2 mutant also specifically augmented eNET-seq signal at enhancers, implicating the exonuclease in limiting bidirectional eRNA transcription (Fig. 7F).
Figure 7.
Extensive Xrn2-dependent premature termination of sense and antisense pol II transcription. (A) eNET-seq in cells expressing WT and D235A mutant Xrn2 (three replicates). Reads correspond to 3′-OH ends of pol II-associated transcripts showing enhanced transcription at 5′ ends (blue arrows) in cells expressing mutant Xrn2. (B) Metaplots of eNET-seq signal, as in A (3′-OH ends, mean and range of three replicates), show widespread elevation of sense and antisense (above and below, respectively) transcription at 5′ ends in the Xrn2 mutant. (C) Metaplots are as in B for sense transcripts zoomed in on the 5′ region. (D) Metaplots of divergent antisense transcripts upstream of start sites show enhanced transcription in the Xrn2 mutant. 5′ ends of eNET-seq reads are mapped (mean and range of three replicates). (E) Xrn2 does not affect transcription of UsnRNA genes. Metaplots show 5′ ends of eNET-seq reads for the 100 highest-expressed pol II transcribed genes (mean and range of three replicates). (F) Metaplots of eNET-seq reads (3′-OH ends; plus and minus strands above and below, respectively) show elevated divergent transcription at enhancers in the Xrn2 mutant (arrows). Mean signal and range of three replicates. (G) Xrn2 digestion of nascent transcripts occurs very close to cap sites. Metaplots are as in B and C but showing the 5′ ends of eNET-seq reads. Genes shown are a subset of those in B and C. Note that the Xrn2 mutant affects nascent transcripts within five bases of the TSS. (H) Model of cotranscriptional Xrn2 activity with handoff from β-CASP endonucleases within genes (top) and at 3′ ends (bottom).
The effect of Xrn2 on the abundance of pol II complexes was greatest in the region within 200 bases upstream of and downstream from the TSS (Fig. 7B–D). This finding is consistent with the peak of Xrn2 near promoters (Fig. 1) and suggests that the nuclease is active on short nascent transcripts soon after transcription initiates. To determine how close to the start site Xrn2 can attack the nascent transcript, we mapped the 5′ ends of the short nascent RNAs captured by eNET-seq. Xrn2 inactivation most strongly elevates nascent RNA 5′ ends within one to five bases of the cap site, and the magnitude of this effect declines between the TSS and +100 (Fig. 7G). This observation implies that Xrn2 can attack 5′-PO4 ends created at or very close to the start site. The fold effect of Xrn2 inactivation on the abundance of pol II complexes engaged in the region between −50 and +100 from the TSS, as measured by the areas under the curves (n = 3) in Figure 7G, is 1.65-fold compared with a 1.29-fold effect in the termination zone within 5 kb downstream from genes (Fig. 6G). This result suggests that comparable amounts of Xrn2-dependent termination occur over several kilobases in 3′ flanking regions and within ∼100 bases of the start site. The implication is that Xrn2 is responsible for extensive premature termination of transcription in promoter-proximal regions under normal physiological conditions. In theory, an alternative interpretation of elevated eNET-seq signal at 5′ ends of genes is that rather than inhibiting premature termination, the exonuclease-dead Xrn2 enhances promoter-proximal pausing. We think this is unlikely because it is inconsistent with increased metabolic labeling of nascent transcripts in the presence of mutant Xrn2 and would mean that inactivation of the same enzymatic activity affects transcription by two different mechanisms at 5′ and 3′ ends. Furthermore, while there is a strong mechanistic basis for inhibition of termination by the exonuclease-dead mutant, there is no such basis for how it would affect polymerase pausing.
Discussion
In this report, we investigated association of the RNA 5′–3′ exonuclease Xrn2 with pol II throughout the transcription cycle and mapped RNA 5′-PO4 entry sites for degradation of nascent transcripts, which can lead to transcription termination. The salient results are as follows:
Xrn2 is a component of pol II complexes engaged on genes at all phases of the transcription cycle from before initiation to termination. It associates with pol II PICs at promoters in a way that does not require transcription initiation and also with the TEC at the promoter-proximal pause, during elongation through gene bodies, and in termination zones downstream from polyA sites.
Identification of Xrn2 entry points in nascent RNA by mapping 5′-PO4 ends stabilized by exonuclease-dead Xrn2 showed that the enzyme acts at 3′ processing sites of polyA+ mRNAs, polyA− histone mRNAs, and tRNAs that are cleaved by CPSF73 and RNase Z, members of the same β-CASP subfamily of endonucleases. We suggest that following endonucleolytic cleavage at all mRNA 3′ ends, the CPSF73 5′–3′ exonuclease trims the downstream cleavage product, and the resulting heterogeneous ends are where Xrn2 loads (Fig. 7H). Further degradation by Xrn2 is required for torpedo termination downstream from polyA sites, but at histone genes this termination mechanism plays only a minor role.
Analysis of transcription in cells expressing WT and exonuclease-dead Xrn2 by metabolic labeling and eNET-seq confirmed the role of this exonuclease in termination downstream from polyA sites and furthermore demonstrated its importance in termination of divergent transcripts at enhancers and promoters. Most surprisingly, these experiments implicate the Xrn2 exonuclease torpedo in PTT close to TSSs under normal physiological conditions at a scale similar to that of termination downstream from genes.
Points 2 and 3 above are based on the phenotype of an overexpressed exonuclease-dead Xrn2 D235A mutant that could have indirect as well as direct effects. However, phenotypes that are directly attributable to loss of enzymatic activity (i.e., stabilization of RNA 5′PO4 ends and inhibition of transcription termination by the torpedo mechanism that requires exonuclease activity) are most economically accounted for by direct effects, especially when the Xrn2 protein is localized at the site of action by ChIP.
Xrn2 accompanies pol II throughout the length of human and Drosophila genes, but the molecular basis for this association is unclear. Xrn2 association with pol II complexes does not require transcription initiation (Fig. 1), though it could be stabilized by RNA interaction. In addition, colocalization with pol II does not appear to involve interaction with the pol II CTD based on its interactome (Ebmeier et al. 2017). This observation is consistent with the fact that Xrn2 does not exclusively colocalize with pol II but also colocalizes with pol I (El Hage et al. 2008) and pol III (Fig. 5D). Unexpectedly, we identified the 3′ trailer products of RNase Z cleavage downstream from tRNAs (Späth et al. 2007) as novel substrates stabilized by mutant Xrn2 (Fig. 5A–C; Supplemental Fig. S4D,E), and this activity is likely to operate cotranscriptionally, as Xrn2 localized to tRNA genes (Fig. 5D). It follows that RNase Z processing of tRNA trailers must also work cotranscriptionally.
The torpedo model (Connelly and Manley 1988) elegantly accounts for the polyA site dependence of termination by proposing that cleavage of the nascent transcript at this site provides an entry point for a 5′–3′ exonuclease. To test this central piece of the model, we mapped 5′-PO4 ends in nascent RNA that were stabilized by exonuclease-dead Xrn2. These experiments confirm the basic idea of the torpedo model but show that in fact Xrn2 loading usually occurs two to 20 bases downstream from the CPSF73 cleavage site where the polyA tail is added (Fig. 4B–D). Our results suggest that a 5′–3′ exonuclease, which is probably CPSF73, carries out limited degradation after cleavage and then hands off to Xrn2, which degrades the nascent transcript further and eventually triggers termination. A similar handoff appears to occur at histone mRNA 3′ processing sites where Xrn2 inactivation stabilized multiple 5′ ends within the HDE (Fig. 3D–G) that coincide closely with the products of exonucleolytic digestion by CPSF73 in vitro (Yang et al. 2009, 2020).
In contrast to polyA sites, Xrn2 digestion of histone downstream cleavage products does not effectively terminate transcription (Fig. 3H; Supplemental Fig. S5G; Fong et al. 2015; Eaton et al. 2018, 2020; Sousa-Luís et al. 2021). This distinction between polyA site-dependent termination and histone stem–loop-dependent termination could be because of reduced Xrn2 exonucleolytic activity and/or failure of pol II to decelerate and become a suitable target for the torpedo at histone 3′ ends compared with polyA sites (Cortazar et al. 2019). It is also possible that the CPSF73 exonuclease activity contributes to termination at histone 3′ ends.
In addition to its role in polyA site-dependent termination at 3′ ends, we identified active Xrn2-mediated termination of sense and antisense pol II transcription within 150 bases of transcription start sites, which is unlikely to depend on cleavage/polyadenylation. Notably, enhanced Bru incorporation in Xrn2 mutant cells is evident at 5′ ends and persists throughout the length of genes (Fig. 6F; Supplemental Fig. S5B–D), consistent with the idea that Xrn2-dependent premature termination eliminates a substantial fraction of polymerases early in elongation. This conclusion is supported by eNET-seq, which showed that Xrn2 inactivation preferentially increases the density of transcriptionally engaged pol II within 150 bases upstream of and downstream from start sites (Fig. 7A–D,G). Remarkably, the rescue of pol II elongation complexes by mutant Xrn2 at 5′ ends is similar to 3′ flanking regions where the exonuclease is thought to terminate almost all transcription. The implication is that a major fraction of pol II that engages the gene at 5′ ends is culled by Xrn2-dependent termination within the first 150 bases. This high level of PTT suggests that establishment of productive elongation complexes is surprisingly inefficient, consistent with previous studies of pol II turnover at 5′ ends (Darzacq et al. 2007; Krebs et al. 2017; Erickson et al. 2018; Steurer et al. 2018; Forero-Quintero et al. 2021). We speculate that polymerases that initiate and terminate soon thereafter might rapidly exchange with local pools of pol II corresponding to the dynamic clusters evident from single-gene imaging (Pombo et al. 2000; Cisse et al. 2013; Forero-Quintero et al. 2021).
Xrn2-mediated termination requires a 5′-PO4 as a substrate for the exonuclease. At polyA sites, these substrates are created by CPSF73 cleavage and exonuclease activity. We speculate that at 5′ ends, Xrn2 substrates may be created by the IntS11 subunit of integrator, a homolog of CPSF73 with relatively non-sequence-specific endonuclease activity (Baillat and Wagner 2015; Tatomer et al. 2019). Consistent with this model, integrator localizes to the 5′ ends of genes (Gardini et al. 2014; Elrod et al. 2019), where Xrn2-dependent termination takes place. Furthermore, multiple studies show that integrator facilitates PTT (Elrod et al. 2019; Tatomer et al. 2019; Beckedorff et al. 2020; Lykke-Andersen et al. 2021). The strongest effects of integrator in attenuation of gene expression have been reported in Drosophila (Elrod et al. 2019; Tatomer et al. 2019). Notable in this regard is our finding that Drosophila Rat1 colocalizes with pol II at 5′ ends, where it could cooperate with integrator to evoke PTT.
Integrator contacts pol II via the CTD (Baillat et al. 2005; Ebmeier et al. 2017) as well as the Spt5 and NELF elongation factors and cleaves nascent transcripts ∼20 bases upstream of the 3′ end very close to where RNA exits the polymerase (Fianu et al. 2021). Further experiments will be required to determine whether products of IntS11 cleavage serve as Xrn2 substrates. Our results show that Xrn2 digestion of nascent transcripts can occur within five bases of the 5′ end (Fig. 7G). Integrator cleavage close to the 5′ end is consistent with its binding to the promoter-proximal paused complexes prior to release of NELF (Fianu et al. 2021). Whether integrator can cleave nascent transcripts before cap addition and whether capping affects cleavage are unknown. It is also possible that 5′-PO4 ends are created near start sites by CPSF73 cleavage (Chiu et al. 2018), by decapping (Brannan et al. 2012), or by processing of uncapped ends. Whether capping antagonizes Xrn2-mediated PTT is an open question.
In sum, our results suggest that together with integrator and/or other factors that generate 5′-PO4 ends, Xrn2 functions globally to limit pol II transcription by inducing PTT near 5′ ends of genes. Integrator-facilitated PTT is controlled in a gene-specific way in Drosophila, but the basis for this regulation is poorly understood (Elrod et al. 2019; Tatomer et al. 2019). Factors that stimulate or inhibit the Xrn2 exonuclease could function as general attenuators or amplifiers of transcription. In this regard, it is interesting to note that PTEFb phosphorylation of Xrn2 stimulates the exonuclease (Sansó et al. 2016).
Together, these findings point to a recurring theme of nascent RNA cleavage by members of the β-CASP family of nucleases followed by handoff to Xrn2 for further 5′–3′ degradation that in some cases leads to transcription termination (Fig. 7H). We suggest that this theme applies to RNase Z at tRNAs, CPSF73 at polyA sites and histone 3′ ends, and Integrator-associated IntS11 at 5′ ends of most pol II transcription units.
Materials and methods
Cell lines
Flp-In-293 TREX cells (female; Invitrogen) expressing inducible Xrn2-HA6 have been described (Fong et al. 2015) and were used for Bru-seq analysis. Mitochondrial copy number was assayed with PCR primers Mt fw1 (AGTCATTCTCATAATCGCCC) and Mt rev 1 (GGAGTAGAGTTTGAAGTCCTT).
Flp-In-293 TREX cells expressing codon-optimized human Xrn2 WT and D235A with a C‐terminal 2× Avitag tag were generated through integration of pcDNA5/FRT/Xrn2-Avi2 by Flp recombinase-mediated site-specific recombination and were used for ChIP-seq, 5′-PO4 Bru-seq, and eNET-seq. These cells were infected with lentivirus pLK0.1-expressing shRNA to the Xrn2 3′ UTR (TRCN0000293640 target sequence GTGTATTCTAGATCATCTAAG)
The cells were maintained in DMEM supplemented with 10% FBS, 200 µg/mL hygromycin B, 6.5 µg/mL blasticidin, 2.0 µg/mL puromycin, and penicillin/streptomycin. Xrn2 expression was induced with 2 µg/mL doxycycline for 24 h.
Drosophila S2 cells were maintained in SFX insect medium (Hyclone), penicillin/streptomycin, and 10% heat-inactivated FBS at 25°C and heat-shocked for 15 min at 37°C. dsRNA was transfected with Effectene (Qiagen). Rat1 dsRNA was transcribed from the PCR amplicon DRSC02044 provided by the Drosophila Research and Screening Center–Biomedical Technology Research Resource at Harvard University.
Antibodies
Rabbit anti-pol II pan-CTD (Schroeder et al. 2000) and antihuman Xrn2 (Fong et al. 2015) have been described. Anti-Avitag was made by immunizing rabbits with the peptide NH2-GLNDIFEAQKIEWHEC-COOH. Anti-Drosophila Rat1 was made by immunizing rabbits with GST-Rat1(404–517) and affinity-purified. Anti-BrdU monoclonal antibodies were 3D4 (Pharmingen 555627) and B44 (Gratzner 1982).
Immunoblotting
Immunoblots were developed with HRP-conjugated swine antirabbit secondary antibody (DAKO P0217) and ECL Plus (Perkin Elmer NEL103E001EA).
ChIP-seq
ChIP has been described previously (Glover-Cutter et al. 2008). Extracts were sonicated using a Covaris ultrasonicator or Bioruptor. One milligram of each sonicated lysate was precleared with 30 µL of protein A Sepharose beads (Life Technologies 101042) for 1 h at 4°C. Libraries were sequenced on an Illumina Hi-Seq 4000 (1X50) or NovaSeq 6000 (2X150). Adapters were trimmed using CutAdapt version 1.16, and reads were mapped to the hg19 UCSC human genome (February 2009) with Bowtie2 version 2.3.2. PCR duplicates were removed using Picard Tools version 2.18.7. Read coordinates were collapsed to a single base pair and centered, assuming a fragment size of 180 bp.
ChIP-seq metaplots
Mean ChIP-seq reads per bin were normalized to bin length and divided by the number of millions of mapped reads using deepTools (Ramírez et al. 2016), bamCoverage, and computeMatrix. To make metaplots of whole genes (separated by 1 kb of human or 0.1 kb of Drosophila), we used 100-base bins in the 5′ region from −1.5 kb to +0.5 kb relative to the TSS and the 3′ region −0.5 kb to +3.5 kb relative to the polyA site. Gene body regions between +500 relative to the TSS and −500 relative to the polyA site were divided into 20 variable-length bins. The most upstream TSS and most downstream polyA site coordinates from RefSeq were used. Metaplots included all genes in common between the data sets for which at least one read was obtained in the 5′, gene body, and 3′ regions. Relative frequency was calculated as total read counts for each bin within a gene divided by the sum total of read counts in all bins for that gene. Means of relative frequencies for gene groups were plotted. The Y-axis of these plots represents the proportion of counts contained in each bin, and the area below each curve is equal. Heat maps were made with plotHeatmap from deepTools (Ramírez et al. 2016) using the default settings and the binned files used for metaplots.
Bru-seq
Nascent RNA sequencing by Bru-seq (Paulsen et al. 2014) was as described (Cortazar et al. 2019). HEK293 Xrn2 WT or D235A cells with doxycycline induction at 2 μg/mL for 24 h were incubated with 2 mM bromouridine in fresh medium plus doxycycline for 30 min. Labeled RNA was fragmented and then immunopurified with B44 monoclonal anti-BrdU (Gratzner 1982) immobilized on protein A Dynabeads with rabbit antimouse IgG. Immunoprecipitation of 30–50 μg of labeled RNA was performed in DEPC-treated 1× PBS, 0.2% (v/v) Triton X-100, and 1 mM DTT supplemented with RiboLock RNase inhibitor (EO0382). Beads were washed four times in the same buffer, and RNA was eluted in RNase-free water for 3 min at 95°C. RNA-seq libraries were made with the KAPA Biosciences stranded RNA-seq kit (KK8401). After filtering out rRNA, reads were mapped to the hg19 UCSC human genome (February 2009) with Bowtie2 version 2.3.2.
5′-PO4 Bru-seq
HEK293 cells expressing WT and D235A Xrn2 were doxycycline-induced for 24 h and then pulse-labeled with 2 mM Bru for 30 min. RNA (250 μg) was fragmented in a 500-μL reaction with 1000 U/mL micrococcal nuclease (NEB) in 1× MNase buffer (NEB) plus 250 mM NaCl for 1 min at 37°C in a Thermoshaker, and the reaction was stopped with 10 mM EGTA. One milliliter of PBS, 0.05%Triton X-100, and 1 M DTT were added plus1 µL of RNase inhibitor, and Bru-labeled RNA was immunoprecipitated with 4 μg of 3D4 monoclonal anti-BrdU and washed three times in 500 µL of PBS Triton buffer. 3′ ends were dephosphorylated in a 50-µL reaction with 1× Thermo buffer A and 10 U of PNK for 30 min at 30°C, and then RNA was purified by Trizol extraction and Zymo column and converted into sequencing libraries with 10-base UMIs using the QIAseq miRNA library kit (Qiagen 331502). 5′-PO4 ends were identified as sites of ligation to the 5′ adapter. Transcripts with 5′ ends other than a monophosphate, including cap structures, were excluded because they could not be ligated to the adaptor. PCR duplicates were removed using UMI-tools (v0.5.4), and reads were mapped to the hg19 UCSC human genome (February 2009) with Bowtie2 version 2.3.2. Read coordinates were collapsed to a single base corresponding to the RNA 5′-PO4 end.
Bru-seq and 5′-PO4 Bru-seq and eNET-seq metaplots
Mean Bru-seq and 5′-PO4 Bru-seq reads per bin were normalized to bin length and divided by the number of millions of reads mapped to the mitochondrial genome. Bru-seq metaplots were created by defining the 5′ (−1.0 kb to the TSS; 20 bins), body (TSS to the pA site; 30 bins of variable length), and 3′ (pA site to +5.0 kb; 20 bins) regions for annotated RefSeq genes. Bru-seq metaplots included all genes in common between the data sets for which at least one read was obtained in each of the 5′, gene body, and 3′ regions. For 5′-PO4 Bru-seq metaplots, 1-bp bins were used, and genes included had at least one read in the region.
eNET-seq data sets were downsampled so that all libraries being compared had the same number of aligned and filtered reads. Mean reads per bin were calculated after normalizing to bin length and dividing by the number of millions of mapped reads.
eNET-seq
eNET-seq was modified from mNET-seq (Nojima et al. 2015) as described (Fong et al. 2022).
eNET-seq was performed using ∼3 × 107 to 9 × 107 HEK293 Xrn2 WT and D235A cells induced with doxycycline. Nuclei were extracted in 20 mM HEPES (pH 7.6), 300 mM NaCl, 0.2 mM EDTA, 7.5 mM MgCl2, 1% NP40, and 1 M urea prior to solubilization by MNase digestion (40,000 U/mL) in 50 mM Tris (pH 7.9), 5 mM CaCl2, and 250 mM NaCl for 2 min at 37°C in a Thermomixer. Immunoprecipitation was with 40 μL of antipan pol II CTD antiserum coupled to protein A Dynabeads. After washing the IPs, on-bead decapping and phosphorylation were performed in a 30-μL reaction with 5 U of T4 PNK 3′phosphatase minus (NEB), 2.25 μg of GST-Dcp1–Edc1–Dcp2, and 1 μL of murine RNase inhibitor in 50 mM Tris HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 0.01% NP40 for 30 min at 30°C in a Thermomixer. RNA was extracted in 200 µL of Trizol, and libraries with UMIs were generated using the QIAseq miRNA library kit (Qiagen 331501).
eNET-seq read processing
eNET-seq libraries were sequenced on an Illumina NovaSeq 6000 (2X150). Adapters were trimmed using CutAdapt (v2.3), and reads were aligned to the hg19 human genome using Bowtie2 (v2.3.2). PCR duplicates were removed using UMI-tools (v0.5.4), and read coordinates were collapsed to a single base pair coordinate corresponding to the RNA 3′ or 5′ end. Reads were filtered to only include those with a mapping quality score of ≥10 and to remove reads that did not align within 5 kb of a protein-coding gene or that aligned to a snoRNA gene.
Enhancer RNA metaplots
Enhancer midpoints used in Figure 7F were derived from NET-CAGE 5′ ends (∼21,000) (Hirabayashi et al. 2019) that intersected with sites of bidirectional transcription in HEK293 cells, defined as nonzero coverage on both strands within 300 bases of the midpoint in published mNET-seq data (GEO GSM3173834) (Tellier et al. 2020). Enhancers included in Figure 7F had eNET-seq signal in at least one bin in the −1000:+1000 region in all six samples in both directions.
Data availability
Sequencing data sets are available at GEO under accession number GSE217033.
Supplementary Material
Acknowledgments
We thank N. Mukherjee, M. Taliaferro, S. Ramachandran, H. Shenasa, and T. Stasevitch for valuable suggestions; Z. Moqtaderi for 3′ READS mapping; and B. Gao and the University of Colorado at Denver Sequencing Facility for sequencing. M.A.C. was a scholar of the University of Colorado at Denver RNA Bioscience Initiative. This work was supported by a Fondation Santé Research Grant (2022) to E.N. and National Institutes of Health grant R35GM118051 to D.L.B.
Author contributions: M.A.C., S.J.P., and D.L.B. designed the experiments. M.A.C., B.E., N.F., and S.J.P. performed the experiments and computational analysis. E.N. performed informatics. M.A.C. and D.L.B. wrote the manuscript.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.350004.122.
Competing interest statement
The authors declare no competing interests.
References
- Aoi Y, Smith ER, Shah AP, Rendleman EJ, Marshall SA, Woodfin AR, Chen FX, Shiekhattar R, Shilatifard A. 2020. NELF regulates a promoter-proximal step distinct from RNA Pol II pause-release. Mol Cell 78: 261–274.e5. 10.1016/j.molcel.2020.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austenaa LMI, Barozzi I, Simonatto M, Masella S, Della Chiara G, Ghisletti S, Curina A, de Wit E, Bouwman BAM, de Pretis S, et al. 2015. Transcription of mammalian cis-regulatory elements is restrained by actively enforced early termination. Mol Cell 60: 460–474. 10.1016/j.molcel.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Austenaa LMI, Piccolo V, Russo M, Prosperini E, Polletti S, Polizzese D, Ghisletti S, Barozzi I, Diaferia GR, Natoli G. 2021. A first exon termination checkpoint preferentially suppresses extragenic transcription. Nat Struct Mol Biol 28: 337–346. 10.1038/s41594-021-00572-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillat D, Wagner EJ. 2015. Integrator: surprisingly diverse functions in gene expression. Trends Bicochem Sci 40: 257–264. 10.1016/j.tibs.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillat D, Hakimi M-A, Näär AM, Shilatifard A, Cooch N, Shiekhattar R. 2005. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123: 265–276. 10.1016/j.cell.2005.08.019 [DOI] [PubMed] [Google Scholar]
- Baranello L, Bertozzi D, Fogli MV, Pommier Y, Capranico G. 2010. DNA topoisomerase I inhibition by camptothecin induces escape of RNA polymerase II from promoter-proximal pause site, antisense transcription and histone acetylation at the human HIF-1α gene locus. Nucleic Acids Res 38: 159–171. 10.1093/nar/gkp817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckedorff F, Blumenthal E, daSilva LF, Aoi Y, Cingaram PR, Yue J, Zhang A, Dokaneheifard S, Valencia MG, Gaidosh G, et al. 2020. The human integrator complex facilitates transcriptional elongation by endonucleolytic cleavage of nascent transcripts. Cell Rep 32: 107917. 10.1016/j.celrep.2020.107917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan K, Bentley DL. 2012. Control of transcriptional elongation by RNA polymerase II: a retrospective. Genet Res Int 2012: 1–5. 10.1155/2012/170173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan K, Kim H, Erickson B, Glover-Cutter K, Kim S, Fong N, Kiemele L, Hansen K, Davis R, Lykke-Andersen J, et al. 2012. mRNA decapping factors and the exonuclease Xrn2 function in widespread premature termination of RNA polymerase II transcription. Mol Cell 46: 311–324. 10.1016/j.molcel.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer-Jensen P, Wilson CB, Abernethy J, Mollison L, Card S, Searles LL. 2015. Suppressor of sable [Su(s)] and Wdr82 down-regulate RNA from heat-shock-inducible repetitive elements by a mechanism that involves transcription termination. RNA 22: 139–154. 10.1261/rna.048819.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamcharla VR, Folco HD, Dhakshnamoorthy J, Grewal SIS. 2015. Conserved factor Dhp1/Rat1/Xrn2 triggers premature transcription termination and nucleates heterochromatin to promote gene silencing. Proc Natl Acad Sci 112: 15548–15555. 10.1073/pnas.1522127112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AC, Suzuki HI, Wu X, Mahat DB, Kriz AJ, Sharp PA. 2018. Transcriptional pause sites delineate stable nucleosome-associated premature polyadenylation suppressed by U1 snRNP. Mol Cell 69: 648–663.e7. 10.1016/j.molcel.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DC, Scharl EC, Steitz JA. 1995. Decreasing the distance between the two conserved sequence elements of histone pre-messenger RNA interferes with 3′ processing in vitro. RNA 1: 905–914. [PMC free article] [PubMed] [Google Scholar]
- Cisse II, Izeddin I, Causse SZ, Boudarene L, Senecal A, Muresan L, Dugast-Darzacq C, Hajj B, Dahan M, Darzacq X. 2013. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 341: 664–667. 10.1126/science.1239053 [DOI] [PubMed] [Google Scholar]
- Collins I, Weber A, Levens D. 2001. Transcriptional consequences of topoisomerase inhibition. Mol Cell Biol 21: 8437–8451. 10.1128/MCB.21.24.8437-8451.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly S, Manley JL. 1988. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev 2: 440–452. 10.1101/gad.2.4.440 [DOI] [PubMed] [Google Scholar]
- Cortazar M, Sheridan RM, Erickson B, Fong N, Glover-Cutter K, Brannan KW, Bentley DL. 2019. Control of RNA pol II speed by PNUTS-PP1 and Spt5 dephosphorylation facilitates termination by a ‘sitting duck torpedo’ mechanism. Mol Cell 76: 896–908.e4. 10.1016/j.molcel.2019.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugusi S, Mitter R, Kelly GP, Walker J, Han Z, Pisano P, Wierer M, Stewart A, Svejstrup JQ. 2022. Heat shock induces premature transcript termination and reconfigures the human transcriptome. Mol Cell 82: 1573–1588.e10. 10.1016/j.molcel.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH. 2007. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 14: 796–806. 10.1038/nsmb1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Kerr A, West S. 2012. Co-transcriptional degradation of aberrant pre-mRNA by Xrn2. EMBO J 31: 2566–2578. 10.1038/emboj.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di C, So BR, Cai Z, Arai C, Duan J, Dreyfuss G. 2019. U1 snRNP telescripting roles in transcription and its mechanism. CSH Symp Quant Biol 84: 115–122. 10.1101/sqb.2019.84.040451 [DOI] [PubMed] [Google Scholar]
- Dominski Z, Yang XC, Marzluff WF. 2005. The polyadenylation factor CPSF-73 is involved in histone–pre-mRNA processing. Cell 123: 37–48. 10.1016/j.cell.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Du P, Wang L, Sliz P, Gregory Richard I. 2015. A biogenesis step upstream of microprocessor controls miR-17∼92 expression. Cell 162: 885–899. 10.1016/j.cell.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JD, Davidson L, Bauer DLV, Natsume T, Kanemaki MT, West S. 2018. Xrn2 accelerates termination by RNA polymerase II, which is underpinned by CPSF73 activity. Genes Dev 32: 127–139. 10.1101/gad.308528.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JD, Francis L, Davidson L, West S. 2020. A unified allosteric/torpedo mechanism for transcriptional termination on human protein-coding genes. Genes Dev 34: 132–145. 10.1101/gad.332833.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier CC, Erickson B, Allen BL, Allen MA, Kim H, Fong N, Jacobsen JR, Liang K, Shilatifard A, Dowell RD, et al. 2017. Human TFIIH kinase CDK7 regulates transcription-associated chromatin modifications. Cell Rep 20: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A, Koper M, Kufel J, Tollervey D. 2008. Efficient termination of transcription by RNA polymerase I requires the 5′ exonuclease Rat1 in yeast. Genes Dev 22: 1069–1081. 10.1101/gad.463708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod ND, Henriques T, Huang KL, Tatomer DC, Wilusz JE, Wagner EJ, Adelman K. 2019. The Integrator complex attenuates promoter-proximal transcription at protein-coding genes. Mol Cell 76: 738–752.e7. 10.1016/j.molcel.2019.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B, Sheridan RM, Cortazar M, Bentley DL. 2018. Dynamic turnover of paused Pol II complexes at human promoters. Genes Dev 32: 1215–1225. 10.1101/gad.316810.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estell C, Davidson L, Steketee PC, Monier A, West S. 2021. ZC3H4 restricts non-coding transcription in human cells. Elife 10: e67305. 10.7554/eLife.67305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fianu I, Chen Y, Dienemann C, Dybkov O, Linden A, Urlaub H, Cramer P. 2021. Structural basis of integrator-mediated transcription regulation. Science 374: 883–887. 10.1126/science.abk0154 [DOI] [PubMed] [Google Scholar]
- Flynn RA, Almada AE, Zamudio JR, Sharp PA. 2011. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc Natl Acad Sci 108: 10460–10465. 10.1073/pnas.1106630108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N, Brannan K, Erickson B, Kim H, Cortazar MA, Sheridan RM, Nguyen T, Karp S, Bentley DL. 2015. Effects of transcription elongation rate and Xrn2 exonuclease activity on RNA polymerase II termination suggest widespread kinetic competition. Mol Cell 60: 256–267. 10.1016/j.molcel.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N, Sheridan RM, Ramachandran S, Bentley DL. 2022. The pausing zone and control of RNA polymerase II elongation by Spt5: implications for the pause-release model. Mol Cell 82: 3632–3645.e4. 10.1016/j.molcel.2022.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero-Quintero LS, Raymond W, Handa T, Saxton MN, Morisaki T, Kimura H, Bertrand E, Munsky B, Stasevich TJ. 2021. Live-cell imaging reveals the spatiotemporal organization of endogenous RNA polymerase II phosphorylation at a single gene. Nat Commun 12: 3158. 10.1038/s41467-021-23417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini A, Baillat D, Cesaroni M, Hu D, Marinis JM, Wagner EJ, Lazar MA, Shilatifard A, Shiekhattar R. 2014. Integrator regulates transcriptional initiation and pause release following activation. Mol Cell 56: 128–139. 10.1016/j.molcel.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gick O, Krämer A, Keller W, Birnstiel ML. 1986. Generation of histone mRNA 3′ ends by endonucleolytic cleavage of the pre-mRNA in a snRNP-dependent in vitro reaction. EMBO J 5: 1319–1326. 10.1002/j.1460-2075.1986.tb04362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover-Cutter K, Kim S, Espinosa J, Bentley DL. 2008. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol 15: 71–78. 10.1038/nsmb1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner HG. 1982. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science 218: 474–475. 10.1126/science.7123245 [DOI] [PubMed] [Google Scholar]
- Gregersen LH, Mitter R, Ugalde AP, Nojima T, Proudfoot NJ, Agami R, Stewart A, Svejstrup JQ. 2019. SCAF4 and SCAF8, mRNA anti-terminator proteins. Cell 177: 1797–1813.e18. 10.1016/j.cell.2019.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzechnik P, Tan-Wong SM, Proudfoot NJ. 2014. Terminate and make a loop: regulation of transcriptional directionality. Trends Bicochem Sci 39: 319–327. 10.1016/j.tibs.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah N, Murakami S, Nagari A, Danko CG, Kraus WL. 2013. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res 23: 1210–1223. 10.1101/gr.152306.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya Y, Parker R. 2012. Global analysis of mRNA decay intermediates in Saccharomyces cerevisiae. Proc Natl Acad Sci 109: 11764–11769. 10.1073/pnas.1119741109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi S, Bhagat S, Matsuki Y, Takegami Y, Uehata T, Kanemaru A, Itoh M, Shirakawa K, Takaori-Kondo A, Takeuchi O, et al. 2019. NET-CAGE characterizes the dynamics and topology of human transcribed cis-regulatory elements. Nat Genet 51: 1369–1379. 10.1038/s41588-019-0485-9 [DOI] [PubMed] [Google Scholar]
- Hoque M, Ji Z, Zheng D, Luo W, Li W, You B, Park JY, Yehia G, Tian B. 2013. Analysis of alternative cleavage and polyadenylation by 3′ region extraction and deep sequencing. Nat Methods 10: 133–139. 10.1038/nmeth.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno-González S, Haaning LL, Malagon F, Jensen TH. 2010. The yeast 5′–3′ exonuclease Rat1p functions during transcription elongation by RNA polymerase II. Mol Cell 37: 580–587. 10.1016/j.molcel.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Kamieniarz-Gdula K, Proudfoot NJ. 2019. Transcriptional control by premature termination: a forgotten mechanism. Trends Genet 35: 553–564. 10.1016/j.tig.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, Buratowski S. 2004. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432: 517–522. 10.1038/nature03041 [DOI] [PubMed] [Google Scholar]
- Kolev NG, Yario TA, Benson E, Steitz JA. 2008. Conserved motifs in both CPSF73 and CPSF100 are required to assemble the active endonuclease for histone mRNA 3′-end maturation. EMBO Rep 9: 1013–1018. 10.1038/embor.2008.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs AR, Imanci D, Hoerner L, Gaidatzis D, Burger L, Schübeler D. 2017. Genome-wide single-molecule footprinting reveals high RNA polymerase II turnover at paused promoters. Mol Cell 67: 411–422.e4. 10.1016/j.molcel.2017.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J, Wu C. 1993. Protein traffic on the heat shock promoter: parking, stalling and trucking along. Cell 74: 1–4. 10.1016/0092-8674(93)90286-Y [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen S, Žumer K, Molska ES, Rouvière JO, Wu G, Demel C, Schwalb B, Schmid M, Cramer P, Jensen TH. 2021. Integrator is a genome-wide attenuator of non-productive transcription. Mol Cell 81: 514–529.e6. 10.1016/j.molcel.2020.12.014 [DOI] [PubMed] [Google Scholar]
- Nojima T, Gomes T, Grosso AR, Kimura H, Dye MJ, Dhir S, Carmo-Fonseca M, Proudfoot NJ. 2015. Mammalian NET-seq reveals genome-wide nascent transcription coupled to RNA processing. Cell 161: 526–540. 10.1016/j.cell.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntini E, Järvelin AI, Bornholdt J, Chen Y, Boyd M, Jørgensen M, Andersson R, Hoof I, Schein A, Andersen PR, et al. 2013. Polyadenylation site–induced decay of upstream transcripts enforces promoter directionality. Nat Struct Mol Biol 20: 923–928. 10.1038/nsmb.2640 [DOI] [PubMed] [Google Scholar]
- Paulsen MT, Veloso A, Prasad J, Bedi K, Ljungman EA, Magnuson B, Wilson TE, Ljungman M. 2014. Use of Bru-seq and BruChase-seq for genome-wide assessment of the synthesis and stability of RNA. Methods 67: 45–54. 10.1016/j.ymeth.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson EL, Moore CL. 2013. Dismantling promoter-driven RNA polymerase II transcription complexes in vitro by the termination factor Rat1. J Biol Chem 288: 19750–19759. 10.1074/jbc.M112.434985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Balatti V, Palamarchuk A, Rizzotto L, Veneziano D, Nigita G, Rassenti LZ, Pass HI, Kipps TJ, Liu CG, et al. 2016. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc Natl Acad Sci 113: 5071–5076. 10.1073/pnas.1604266113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petfalski E, Dandekar T, Henry Y, Tollervey D. 1998. Processing of the precursors to small nucleolar RNAs and rRNAs requires common components. Mol Cell Biol 18: 1181–1189. 10.1128/MCB.18.3.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo A, Jones E, Iborra FJ, Kimura H, Sugaya K, Cook PR, Jackson DA. 2000. Specialized transcription factories within mammalian nuclei. Crit Rev Eukaryot Gene Expr 10: 21–29. 10.1615/CritRevEukarGeneExpr.v10.i1.40 [DOI] [PubMed] [Google Scholar]
- Proudfoot NJ. 2016. Transcriptional termination in mammals: stopping the RNA polymerase II juggernaut. Science 352: aad9926. 10.1126/science.aad9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dündar F, Manke T. 2016. Deeptools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44: W160–W165. 10.1093/nar/gkw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansó M, Levin RS, Lipp JJ, Wang VY-F, Greifenberg AK, Quezada EM, Ali A, Ghosh A, Larochelle S, Rana TM, et al. 2016. P-TEFb regulation of transcription termination factor Xrn2 revealed by a chemical genetic screen for Cdk9 substrates. Genes Dev 30: 117–131. 10.1101/gad.269589.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SC, Schwer B, Shuman S, Bentley D. 2000. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev 14: 2435–2440. 10.1101/gad.836300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MD, Stephenson P, Wickens MP. 1987. Products of in vitro cleavage and polyadenylation of simian virus 40 late pre-mRNAs. Mol Cell Biol 7: 1518–1529. 10.1128/mcb.7.4.1518-1529.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Padgett RA. 2009. Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol 16: 1128–1133. 10.1038/nsmb.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Luís R, Dujardin G, Zukher I, Kimura H, Weldon C, Carmo-Fonseca M, Proudfoot NJ, Nojima T. 2021. POINT technology illuminates the processing of polymerase-associated intact nascent transcripts. Mol Cell 81: 1935–1950.e6. 10.1016/j.molcel.2021.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späth B, Canino G, Marchfelder A. 2007. tRNase Z: the end is not in sight. Cell Mol Life Sci 64: 2404–2412. 10.1007/s00018-007-7160-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steurer B, Janssens RC, Geverts B, Geijer ME, Wienholz F, Theil AF, Chang J, Dealy S, Pothof J, van Cappellen WA, et al. 2018. Live-cell analysis of endogenous GFP-RPB1 uncovers rapid turnover of initiating and promoter-paused RNA polymerase II. Proc Natl Acad Sci 115: E4368–E4376. 10.1073/pnas.1717920115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Koning TW, Soldati D, Melin L, Schümperli D. 1993. Variable effects of the conserved RNA hairpin element upon 3′ end processing of histone pre-mRNA in vitro. Nucleic Acids Res 21: 1569–1575. 10.1093/nar/21.7.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang Y, Aik WS, Yang X-C, Marzluff WF, Walz T, Dominski Z, Tong L. 2020. Structure of an active human histone pre-mRNA 3′-end processing machinery. Science 367: 700–703. 10.1126/science.aaz7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatomer DC, Elrod ND, Liang D, Xiao MS, Jiang JZ, Jonathan M, Huang KL, Wagner EJ, Cherry S, Wilusz JE. 2019. The Integrator complex cleaves nascent mRNAs to attenuate transcription. Genes Dev 33: 1525–1538. 10.1101/gad.330167.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier M, Zaborowska J, Caizzi L, Mohammad E, Velychko T, Schwalb B, Ferrer-Vicens I, Blears D, Nojima T, Cramer P, et al. 2020. CDK12 globally stimulates RNA polymerase II transcription elongation and carboxyl-terminal domain phosphorylation. Nucleic Acids Res 48: 7712–7727. 10.1093/nar/gkaa514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uájvári A, Pal M, Luse DS. 2002. RNA polymerase II transcription complexes may become arrested if the nascent RNA is shortened to less than 50 nucleotides. J Biol Chem 277: 32527–32537. 10.1074/jbc.M201145200 [DOI] [PubMed] [Google Scholar]
- Wagschal A, Rousset E, Basavarajaiah P, Contreras X, Harwig A, Laurent-Chabalier S, Nakamura M, Chen X, Zhang K, Meziane O, et al. 2012. Microprocessor, Setx, Xrn2, and Rrp6 co-operate to induce premature termination of transcription by RNAPII. Cell 150: 1147–1157. 10.1016/j.cell.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TN, Koning THW, Schümperli D, Müller B. 1998. A 5′–3′ exonuclease activity involved in forming the 3′ products of histone pre-mRNA processing in vitro. RNA 4: 1034–1046. 10.1017/S1355838298971771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Pestov DG. 2011. 5′-end surveillance by Xrn2 acts as a shared mechanism for mammalian pre-rRNA maturation and decay. Nucleic Acids Res 39: 1811–1822. 10.1093/nar/gkq1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S, Gromak N, Proudfoot NJ. 2004. Human 5′ → 3′ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature 432: 522–525. 10.1038/nature03035 [DOI] [PubMed] [Google Scholar]
- Yang X-C, Sullivan KD, Marzluff WF, Dominski Z . 2009. Studies of the 5′ exonuclease and endonuclease activities of CPSF-73 in histone pre-mRNA processing. Mol Cell Biol 29: 31–42. 10.1128/MCB.00776-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-C, Sun Y, Aik WS, Marzluff WF, Tong L, Dominski Z. 2020. Studies with recombinant U7 snRNP demonstrate that CPSF73 is both an endonuclease and a 5′–3′ exonuclease. RNA 26: 1345–1359. 10.1261/rna.076273.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data sets are available at GEO under accession number GSE217033.