Abstract
Ants provide protection to various organisms via myrmecophilous relationships. Most notably, ants and several butterfly species are involved in mainly mutualistic interactions. Previous field studies have shown that butterfly larval survival is increased in the presence of tending ants, suggesting that ants are providing protection against insect predation or parasitism. Here, we conducted a series of timed observational trials under laboratory conditions to assess larval survival and ant protection from insect predators for a myrmecophilous lycaenid butterfly. We focused on a critically endangered butterfly, the Miami blue (Cyclargus thomasi bethunebakeri) (Comstock and Huntington) (Lepidoptera: Lycaenidae), and its most common ant associate, the Florida carpenter ant (Camponotus floridanus) (Buckley) (Hymenoptera: Formicidae), to test this assumption of ant protection. We found that ants provide significant protection to Miami blue larvae, with later instar larvae receiving a higher level of protection due to differences in tending frequencies. These results will aid in informing conservation management and future organism reintroductions for this endangered butterfly.
Keywords: Myrmecophily, Lepidoptera, Lycaenidae, Formicidae
Many organisms have evolved symbiotic interactions with ants in which the ant-associated species, or myrmecophile, provides resources to the ants in exchange for protection. Among the most well-known ant protection mutualisms are found in two closely related butterfly families, Riodinidae and Lycaenidae, where at least 900 ant-associated species have been documented (Pierce et al. 2002, Espeland et al. 2018, Pierce and Dankowicz, 2022). About 75% of lycaenids (5,200 species) and 20% of riodinids (1,500 species) are closely associated with ants, primarily during the larval stage (Pierce et al. 2002, Espeland et al. 2018). Across the diversity of ant taxa that act as potential partners with lycaenids, the majority are nectarivorous and trophobiotic ants within the subfamilies Formicinae, Myrmicinae, and Dolichoderinae, primarily in the genera Crematogaster and Camponotus (Fiedler 2021). Interactions between lycaenid butterflies and ants can be obligate or facultative, and range from mutualistic to parasitic (Pierce et al. 2002). Obligate relationships involve butterfly larvae that are dependent on ants for survival, sometimes relying on ants from a single species or genus (~30% of associations) (Pierce et al. 2002). Facultative relationships involve instances where larvae are tended by multiple species of ants, and the larvae are not necessarily dependent on ants for survival but may gain some physiological or protective benefit (~45% of associations). The remaining lycaenid species may not associate with ants at all or may lack certain organs that produce ant-attracting chemicals but still have other strategies and adaptations for avoiding ant aggression (Fiedler 1989, Kaminski and Freitas 2010, Oliver and Stein 2011, Bachtold and Alves-Silva 2013).
The morphology of myrmecophilous lycaenid larvae includes one or more of the following features not present in other Lepidoptera: (1) specialized epidermal glands called perforated cupola organs (PCOs) that release a volatile substance which acts as a pheromone and may resemble ant brood signals (Pierce et al. 2002), (2) cuticular hydrocarbons (CHCs) to be recognized by tending ants or mimic ant hosts (Barbero 2016, Casacci et al. 2019), (3) a dorsal nectary organ (DNO) that secretes nitrogen-rich exudates to attract ants, (4) an extra thick cuticle to avoid damage from biting ants, (5) long setae that mechanically help protect against ant attacks (Dupont et al. 2016), (6) a pair of tentacular organs that flank the DNO and can be everted in the presence of tending ants (DeVries et al. 1986), and (7) vibroacoustic signals used to mimic or enhance communication in order to coexist with ants (Schönrogge et al. 2017). Tentacular organs were initially described as releasing volatile chemicals that elicit ‘excited runs’ in ants, but are now thought to potentially signal the presence of sugar secretions for ants (Gnatzy et al. 2017). Ant tending is positively correlated with tentacle display, delivery of DNO droplets, and simulated predator attacks (Leimar and Axen 1993, Axen et al. 1996). DNO secretions may not only provide nutrients to tending ants, but may also act to manipulate dopamine levels by enacting aggressive defense toward potential predators (Hojo et al. 2015). In many cases, the combination of chemicals released from PCOs and secretions from the DNO work in concert and play a strong role in manipulative communication with ants (Fiedler et al. 1996).
Many myrmecophiles face various costs and benefits or physiological tradeoffs related to life-history traits or to spatial distribution of the ant partner (Fraser et al. 2001, Stadler et al. 2001). Some lycaenid species may experience longer or shorter development times and changes in pupal weight due to ant tending intensity and specific species of attendant ant (Pierce et al. 1987, Robbins 1991, Wagner 1993, Fiedler and Samm 1994, Axén, 2000, Kaminski and Rodrigues 2011). There are specific developmental benefits for some lycaenid butterfly species in which sex-related differences may be observed in either larval growth, pupal weight, or female fecundity depending on ant attendance (Fiedler and Holldobler 1992, Cushman et al. 1994, Trager et al. 2013, Mizuno et al. 2019). Ant attendance can have profound effects on the length of time individuals spend in vulnerable immature stages as well as the resulting fecundity of adults.
Ant protection against predators is often assumed to be the primary benefit in ant-lycaenid associations, and several studies have experimentally demonstrated protective benefits in field settings (Pierce and Mead 1981, Pierce and Easteal 1986, Pierce et al. 1987, Peterson 1993, Weeks 2003, Kaminski et al. 2010, Forister et al. 2011, Thomas et al. 2020). Among previous research that has evaluated protection against predators, including for other myrmecophiles such as sapsucking insects (Way 1963, Buckley and Gullan 1991), most have not included ant behavior as part of the study (but see Thomas et al. 2020). To assess ant protection in a behavioral approach, we focused on the myrmecophilic relationship in the Miami blue (Cyclargus thomasi bethunebakeri) (Lepidoptera: Lycaenidae) (Comstock and Huntington 1943), a critically imperiled butterfly listed as endangered under the U.S. Endangered Species Act (USFWS 2012), and its most common ant associate, the Florida carpenter ant (Camponotus floridanus) (Buckley) (Hymenoptera: Formicidae).
We experimentally evaluated ant protection of Miami blue butterfly larvae in a series of laboratory trials designed to assess ant behavior, predator behavior, and larval survival. Specifically, we asked three focused research questions related to the function, conditionality, and potential mechanisms of ant protection in this system: (1) Do ants increase survival for Miami blue larvae in the presence of a generalist predator?; (2) Does developmental stage (i.e. larval instar) and predator presence affect ant tending behavior?; and (3) Are ants more aggressive towards a predator when a Miami blue larva is present? Experimental evaluation of these questions improves our understanding of the potential effects of ant mutualists on Miami blue conservation, as well as adds to our broader knowledge of the range of interactions between ants and lycaenids.
Materials & Methods
Study System
The Miami blue butterfly is endemic to southern coastal Florida (Carroll and Loye 2006). Although there are many reasons for the decline of this once common taxon, its initial range reduction coincided with urban development in the first half of the 20th century, and by the late 20th century was restricted to a few locations in extreme south Florida and the Florida Keys. As reports of documented sightings rapidly declined, the Miami blue was presumed to be extirpated by the early 1990s. However, a small breeding population was rediscovered in Bahia Honda State Park in 1999, which led to an emergency listing of the taxon under the Endangered Species Act (Calhoun et al. 2002). Additional populations were subsequently discovered in the Lower Florida Keys, within the Key West National Wildlife Refuge (KWNWR) in 2006 (Cannon et al. 2010). In 2010, the population at Bahia Honda State Park went extinct for unknown reasons. Current experimental reintroduction efforts are underway on conservation lands in South Florida within the butterfly’s historic range with the goal of re-establishing self-sustaining breeding populations, as well as better understanding the factors that help facilitate colony establishment and persistence.
The larvae of Miami blue are similar in appearance to other ant-tended lycaenid larvae: they have a sluglike shape with prominent ant organs, including tentacular organs and a DNO, for recruiting attendant ants (Saarinen and Daniels 2006). Within wild populations, reintroduction sites, or in the laboratory, Miami blue larvae are known to interact with at least 17 different ant species (Carroll and Loye 2006, Saarinen and Daniels 2006, Trager and Daniels 2009). The most common ant species associated with Miami blue larvae is Camponotus floridanus and C. planatus (Roger) (Hymenoptera: Formicidae). Under laboratory conditions, larvae that are reared with C. floridanus will selectively pupate in ant harborages with ants (Trager and Daniels 2009), which has been documented in other facultatively ant-tended lycaenid species (Wagner 1995).
The benefits to Miami blue butterflies of ant tending have been shown to be sex-dependent. Ant-tended female larvae and pupae increased in size compared to untended larvae, whereas in ant-tended male larvae, maximum mass decreased and pupal size was unaffected (Trager et al. 2013). Female development time decreases in association with higher frequencies of ant tending, but male development time increases with ant tending. An additional benefit of ant tending for female Miami blues is increased adult egg production from larger ant-tended females (Trager et al. 2013). Such studies that focused on the costs and benefits of ant-tending are crucial for understanding the life history of the Miami blue. However, the protective benefits that ants may provide during development have not been demonstrated. While insect predation is unlikely to be the sole driver for the Miami blue’s decline, it is critical to understand all aspects of the butterfly’s ecology – including the importance of tending ants – for informing conservation management and recovery decisions.
Methods
We conducted controlled experimental trials in a laboratory setting to evaluate tending frequency and protective benefits of ants. Healthy Miami blue larvae were selected from an existing captive breeding population at the Florida Museum of Natural History’s McGuire Center for Lepidoptera and Biodiversity for these trials. Health was evaluated by visual assessment before trials and further validated by frass produced 24 hr posttrial; unhealthy larvae (i.e., little to no frass produced, or larvae appeared yellowish) were excluded from data analysis. A single Miami blue larva was placed on fresh cut terminal growth of gray nicker (Guilandina bonduc) (Roxburgh) (Fabales: Fabaceae) (a known larval host), that was secured in a floral tube containing water. Gray nicker host cuttings were approximately 25 cm tall and 10 cm wide. We placed the floral tube in a 0.34 kg paper cup and the lid was secured so that ants could not crawl underneath. The paper cup was placed in a 0.71 kg plastic food storage container with fluon on the inside so that ants could not escape. This entire setup was placed in a mesh flight cage (34 × 61 cm) (Supp Fig. 1 [online only]). Trials consisted of four treatments: Treatment 1 (T1) = predator + Miami blue butterfly larva (MBBL), Treatment 2 (T2) = ants + MBBL, Treatment 3 (T3) = ants + MBBL + predator, and Treatment 4 (T4) = ants + predator.
New Miami blue larvae and gray nicker cuttings were used for each replicate, and each individual larva and predator were assigned a unique number. MBBL total body length was measured (head to last abdominal segment) daily using Adoric electronic digital calipers. Three repeated measurements were taken, and the mean recorded. For a full description of the life history protocol, see Daniels et al. (2020). Miami blue larvae were separated into two age classes (early and late instar) based on the mean size of all larval instars (5.97 mm): larvae smaller than 6 mm were classified as ‘early’ and larvae larger than 6 mm were classified as ‘late’.
Predators
Preliminary trials suggested that attack frequency by potential predators varied by Miami blue larval size. We used milkweed assassin bug nymphs (Zelus longipes) (Linnaeus) (Hemiptera: Reduviidae), a recently documented predator of Miami blue larvae, for the early instar age class for T1 and T3 trials. For the late instar age class, wheel bug (Arilus cristatus) (Linnaeus) (Hempitera: Reduviidae) nymphs and adults were used as the primary generalist predator. Both Z. longipes and A. cristatus are assassin bugs (Reduviidae) and as ambush predators, use a very similar hunting style to track down and capture their prey. These two species were selected based on their abundance and potential to prey on Miami blues in the wild. We minimized the number of times individual predators were used for multiple trials, but some were reused for 2–4 different trials.
Ant Mutualist
Eight C. floridanus workers from two queenright colonies (6 minors, 2 majors) were used for each replicate (T2 and T3). Colonies consisted of approximately 100 individual workers, and individual workers were randomly selected for each trial. Measures were taken to ensure that each trial was conducted with new workers by separating the ants used in prior trials from the colony. If trials ran for consecutive days, colonies were alternated between days to minimize disturbance.
Observational Trials and Data Collection
For each replicate, one Miami blue larva was placed on the host and allowed to acclimate for thirty minutes. For T2 and T3 (ants present), ants acclimated with each larva for approximately one hour before trials commenced. Trials ran for ninety minutes and observations (tending, number of workers tending, ant attacks on predator, and predation on Miami blue) were taken at 30 three-minute intervals. The resulting observations were used to calculate behavioral metrics for tending frequency, ant aggression, and predation.
Tending was defined as ants drumming their antennae on the back of the larva, specifically focusing on the posterior end where the DNO is located (Fig. 1). If tending occurred, the total number of workers tending was also recorded. Ant attacks on the predator were recorded at the intervals when the ant workers used their mandibles to bite the predator. Predation on Miami blue larvae was recorded at the interval when the predator first attacked the larvae, but trials continued for the full 90 min.
Fig. 1.
Camponotus floridanus worker tending a late instar Miami blue larva. Inset shows everted tentacular organs and a droplet from the dorsal nectary organ.
For T1 and T3, the predator was gently placed on the plant using a small paintbrush, and the trial began three minutes after placement to allow for a brief acclimation period. For T1, predation and time were recorded as soon as predator first attacked a Miami blue larva since chances for survival after initial attack are extremely low.
We conducted all statistical analyses in RStudio Version 1.1.463 (R Core Team 2016). We used the tidyr and dplyr packages for data manipulation and organization, and ggplot2 for all graphs. A time interval where tending occurred was categorized as a positive tending interval. The average tending frequency per trial was calculated by dividing the total number of positive tending intervals by the total number of intervals observed per trial (typically 30 intervals but some trials were missing data for one or two intervals). We quantified proportion of ant attacks as the total number of attacks occurring in a trial, divided by the total number of observations. To avoid bias from using different predators between the two age classes, a two-way contingency and Pearson’s chi-squared test were conducted separately on both early and late instar age classes. Generalized linear models (GLM) were used to test the best predictors for larval survival (binomial response variable) and tending frequency (gaussian response variable) using the glm function from the car package. To test whether life-stage influences ant tending, we conducted a two-sample t-test on early and late instar age classes. Two sample t-tests were also used to evaluate the effect of predator presence on tending frequency (T2 vs. T3), which was analyzed separately for the two age classes. Data for a fourth treatment (T4 = ants + predator, n = 12) were analyzed to determine whether ants were more aggressive toward predators in the presence of a Miami blue larva as well as to test whether ants tended Miami blue larvae more in the presence of predators. We used a t-test to examine the difference in number of attacks between T3 (late instar) and T4 (only using Arilus cristalus).
Results
We found that ant presence increased survival for late instar Miami blue larvae in the presence of predators (late instar group: chi-squared = 39.05, df = 1, p < 0.001; early instar group: chi-squared = 3.72, df = 1, p = 0.054). For late instar larvae, predation did not occur when tending ants were present (Table 1, Fig. 2). By contrast, all but one late instar larva was predated when ants were absent. In the early instar age class (Table 2, Fig. 2), the largest effect is seen when ants are present and no predation on larvae occurred (36.1%). In the absence of ants, 26.2% of larvae were predated by Z. longipes, which could be due to the fact that smaller larvae are difficult to detect. Predation of early instars was lowest (14.8%) when ants were present.
Table 1.
Two-way contingency for late instar larvae. Results refer to number of larvae
| Ants absent | Ants present | |
|---|---|---|
| No predation | 1 (23.26%) | 17 (39.53%) |
| Predation | 25 (58.14%) | 0 (0%) |
Fig. 2.
Bar plot showing proportions of survival against predators for early and late instar larvae in the presence and absence of ants.
Table 2.
Two-way contingency for early instar larvae. Results refer to number of larvae
| Ants absent | Ants present | |
|---|---|---|
| No predation | 14 (22.95%) | 22 (36.07%) |
| Predation | 16 (26.23%) | 9 (14.75%) |
Results from a generalized linear model showed that there are effects of both larval length (a proxy for age) and tending frequency on survival (Table 3, GLM, Chi-square = 66.47, df = 104, AIC = 87.85, p < 0.001). These effects were not highly correlated with each other. We found no interaction between larval length and tending frequency on death as the response variable, so we analyzed the effect of length and tending separately. Proportion of observation intervals in which ants attacked the predator was not a significant predictor for larval survival (Table 3, GLM, p = 0.95).
Table 3.
Generalized linear model. Dependent variable: death
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Est. | S.E. | p | Est. | S.E. | p | Est. | S.E. | p | |
| (Intercept) | −1.22 | 0.63 | 0.05. | −1.21 | 0.60 | 0.04* | −0.18 | 0.49 | 0.71 |
| MB.length | 0.43 | 0.13 | 0.001 | 0.43 | 0.13 | <0.001*** | 0.09 | 0.06 | 0.16 |
| Tending | -7.33 | 1.65 | <0.001*** | -7.30 | 1.55 | <0.001*** | |||
| Attacks | 0.20 | 2.91 | 0.95 | −12.96 | 4.81 | 0.01** | |||
| N | 106 | 107 | 106 | ||||||
| AIC | 89.83 | 87.85 | 136.59 | ||||||
| BIC | 100.48 | 95.87 | 144.58 | ||||||
| Pseudo R2 | 0.61 | 0.62 | 0.19 | ||||||
*** p < 0.001;
** p < 0.01;
* p < 0.05.
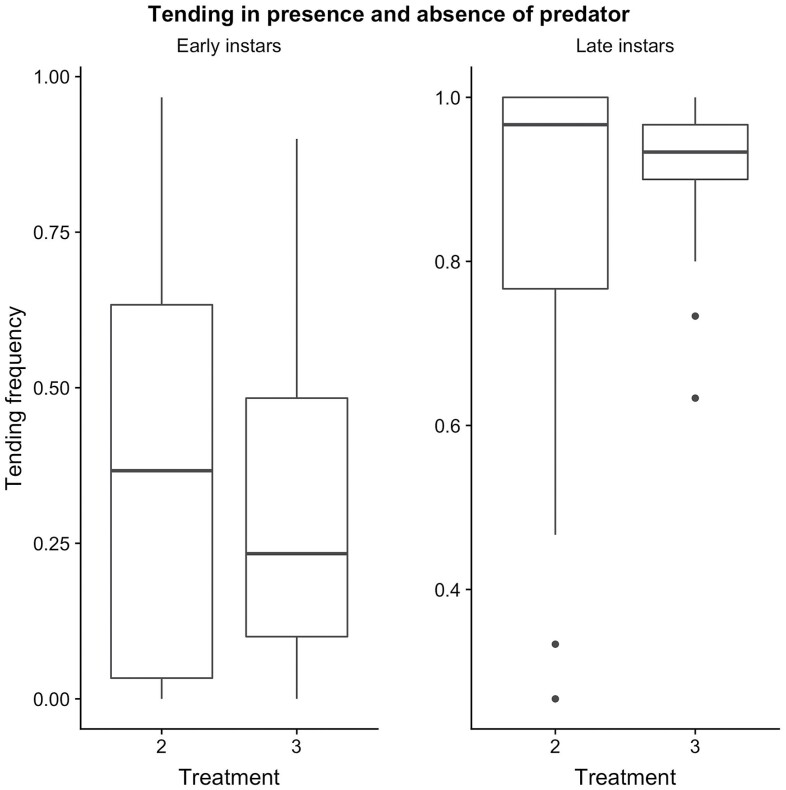
To evaluate the effect of predator presence and larval length on tending frequency, a separate generalized linear model was used. Larval length was a significant predictor of tending frequency (Table 4, GLM, p < 0.0001), whereas predator presence was not (GLM, p = 0.93). To test the age classes separately, two sample t-tests also demonstrated that predator presence was not a significant predictor for tending frequency in the early instar group (Fig. 3, t = 0.513, df = 57.86, p = 0.61), nor for the late instar group (Fig. 3, t = −1.323, df = 44.526, p = 0.193). Larval length had a significant positive effect on ant tending behavior; late instar larvae tended twice as much as early instar larvae (85% compared to 37% of observations) in the absence of a predator (Fig. 4). Thus, there is a significant difference between tending in age classes (t = −6.92, df = 48.4, p < 0.0001). To test whether ants were more aggressive towards Arilus cristalus when a Miami blue larva was present, we used a two-sample t-test to compare T3 (late instar) and T4. Proportion of ant attacks on predators was significantly higher when a larva was present (Fig. 5, t = 2.523, df = 18.157, p-value = 0.0211).
Table 4.
Generalized linear model. Dependent variable: tending frequency. Treatment: T3 (ants + MBBL + predator)
| Coefficients | Est. | S.E. | p |
|---|---|---|---|
| (Intercept) | 0.05 | 0.06 | 0.34 |
| MB.length | 0.08 | 0.01 | <0.001*** |
| Treatment | −0.02 | 0.05 | 0.74 |
| prop.attack | 0.06 | 0.23 | 0.79 |
| N | 107 | ||
| AIC | 4.76 | ||
| BIC | 18.12 | ||
| Pseudo R2 | 1.04 |
*** p < 0.001;
** p < 0.01;
* p < 0.05.
Fig. 3.
Box plots showing the effect of predator presence on ant tending in early and late instar larvae.
Fig. 4.
Violin plot shows difference in tending frequency between early and late age classes. On average, early instar larvae are tended by ants 37% of the time, whereas late instar larvae are tended 85% of the time in the absence of predators.
Fig. 5.
Box plot showing the difference between number of ant attacks on a predator when a Miami blue larva is present; only late age class was analyzed from Treatment 3.
Discussion
Our results indicate that for Miami blue butterfly caterpillars, carpenter ants were effective protectors against predator attacks, which significantly improved butterfly larvae survivorship. Ant tending frequency and larval development status were important factors that affected the likelihood of predator attack in experimental trials, and therefore may also influence the survival of wild Miami blue larvae. For early instar larvae, individuals that were tended by ants at higher frequencies typically survived whereas larvae that were tended at lower frequencies were more likely to suffer mortality by predation (Figs. 6 and 7), but those differences were not significant at α = 0.05 in our study. These findings echo other studies involving facultative myrmecophilous lycaenids in North America, which have also quantified the effects of larval survival in the wild and have found that mortality is twice as high, or higher, when attendant ants are excluded (Pierce and Eastel 1986, Savignano 1994, Weeks 2003, Thomas et al. 2020).
Fig. 6.
Linear model evaluating larval length as a predictor for tending frequency in early instar larvae in Treatment 3; blue line represents larvae that survived, and red line shows larvae that were predated.
Fig. 7.
Linear models for early and late instar larvae. Grey lines represent Treatment 2 (larva and ants) and black lines represent Treatment 3 (larva, ants, and predator).
While our understanding of how ant-caterpillar interactions influence survival and fitness across a diversity of partners is still developing, it is evident that many species of lycaenid butterflies associate with ants through exclusive, species-specific symbioses, as well as more diffuse interactions involving suites of potential partners, in relationships that range from mutualism to parasitism (Mizuno et al. 2019, Fiedler 2021, Pierce and Dankowitz 2022). This study explored interactions between Miami blue butterflies and Camponotus floridanus, the ant species most commonly observed to interact with this butterfly, but it is not yet clear whether this species may also benefit from the protection of other ants. Ants in the hyper-diverse genus Camponotus are well-known for their association with extra-floral nectaries of plants and with trophobionts, including honeydew-producing hemipteran insects such as aphids. Research on Camponotus associates suggests that these ants can engage in facultative mutualisms, tending to many species who offer them nutritional benefits in exchange for protection (Stadler and Dixon 2008), but also have some exclusive relationships where they preferentially tend to certain taxa (Fiedler 2021). Several species of Camponotus have been documented as important partners and effective protectors to various lycaenid species (Fiedler 2001, Watanabe and Hagiwara 2009, Kaminski et al. 2010, Kaminski and Rodrigues 2011), suggesting that this may be a guide for how to view this myrmecophilous system. This genus is one of the most well-known symbiotic partners of myrmecophilous butterflies and its attractiveness may be due, in part, to large colony sizes and ready recruitment to defend resources (Clark and Singer 2018), making Camponotus species dominant in some habitats.
While there are limitations to conducting an experiment ex situ, this study allowed us to assess ant and predator behavior in a controlled laboratory setting, although it should be noted that insect behaviors can be altered in the lab even if allowed to acclimate before experimental observations (Hoffman and Ross 2018). At the time of this study, Miami blue field sites were difficult to access to conduct field experiments. In the future, it would be beneficial to conduct similar ant protection studies at Miami blue release sites to compare results. Furthermore, additional ant colonies may be used for the trials if supplementary queenright colonies are accessible for collection. Other non-queenright colonies were initially collected but not included in the experiments to limit behavioral changes due to lacking a queen. For each trial, we ensured that fresh ant workers were used from the queenright colonies.
There was no relationship between predator presence and tending behavior (Table 4, p = 0.74). The best predictor for tending was larval length (Table 4, p < 0.0001). The general trend was that as larvae increase in size, tending frequency increases. This was seen in early instar and late instar larvae in the absence of a predator, and in early instar larvae in the presence of a predator. In late instar larvae in the presence of a predator, tending was unrelated to variation in larval size. We hypothesized that this was due to ants leaving the larva to act aggressively toward the predator, but we did not see a significant correlation between ant tending and attacks in late instar larvae (Supp Fig. 2 [online only]). This is potentially due to a smaller range in size variation of larval length for this group. Life stage greatly influences ant tending behavior, which is consistent with a finding by Pierce et al. (1987) where the mean number of ants for each individual larva increased as a function of larval age. In our study, late instar larvae were tended more than twice as much as early instar larvae. This finding could be explained by the increasing development of ant organs as larvae molt and increase in size. Larger, later instar larvae are likely producing more DNO secretions, which would increase ant attendance and tending frequency.
Ant aggression, as measured by the proportion of attacks on the predator, was not a significant predictor of larval survival. This could be an artifact of the specific predator used, or of the fact that the ants are obtaining more DNO secretions with late instar larvae, which in turn may change their behavior and make them more aggressive or sensitive to threats or other stimuli (Hojo et al. 2015). While this study did not utilize larval behavior as a predictor for ant recruitment, various studies have demonstrated that DNO drop emission and TOs influence ant tending and behavior, especially in the cases of simulated predator attacks where larval secretions increase with the level of ant attendance (Leimar and Axén 1993, Fiedler and Hummel 1995, Axen et al. 1996). Additionally, it is interesting to note that larvae classified as unhealthy were rarely tended by C. floridanus. This finding is one that is not documented in the literature and should be studied further, especially as it related to ex situ conservation activities.
Furthermore, it would be beneficial to test if DNO secretion rates vary based on the host plant species or plant part utilized by larvae. Several studies have assessed the effects of diet influences on larval secretions and have found that flower-fed or seed pod-fed larvae had significantly higher DNO secretion rates compared to foliage-fed individuals (Pierce and Easteal 1986, Burghardt and Fiedler 1996). Also, myrmecophilous lycaenid butterflies may select for host plants with high nitrogen content (Pierce 1985, Pellissier et al. 2012). Some herbaceous plants may be more nutrient-rich than others, and it would be interesting to test all known Miami blue host plants for possible variation related to DNO secretion volume, quality, and influence on ant attendance levels.
Recovery actions for the Miami blue butterfly include identifying predators and parasitoids of all life stages, evaluating the impact of predators on Miami blue populations, and evaluating its symbiotic relationship with ants. Before this study, specific predators of Miami blue larvae and pupae had not been documented in the literature. Due to their abundance and potential to feed on Miami blues in their habitat, wheel bugs and milkweed assassin bugs were selected as the primary predators for our experiment. However, we documented predation on larvae from several other species, which include: Pseudomyrmex gracilis (Fabricius) (Hymenoptera: Formicidae), Hippodamia convergens (Guérin-Méneville) (Coleoptera: Coccinellidae), Tetramorium bicarinatum (Nylander) (Hymenoptera: Formicidae), P. pallidus (Smith) (Hymenoptera: Formicidae), Solenopsis molesta complex cf. tenuis subgroup (Hymenoptera: Formicidae), Anolus sp., and Iguana iguana (Linnaeus) (Squamata: Iguanidae). These species were not used in ant trials but should be investigated in the future to assess the level of ant protection benefits across species and groups. The impact of potential predators and overall predation on extant populations remains poorly known. While predation may not be a significant threat to the overall status of the Miami blue, it may have the potential to affect smaller populations in specific sites. Predation on vulnerable life stages, especially eggs and pupae, has the potential to impact the Miami blue’s continued survival, given its few remaining populations, limited range, and low abundance in certain sites.
Our research helps to supplement existing data and fill research gaps for understanding the relationship of attendant ants and this critically endangered butterfly. It offers a baseline and protocol for other researchers to carry out similar behavioral experiments under controlled settings to test the possible effects of ant protection on butterfly larval survival. As organism reintroductions continue, it will be important to select release sites where Camponotus species and other attendant ant taxa are present. Furthermore, we need to understand how important other common native attendant ants are for lycaenid butterflies in facultative myrmecophilous relationships, especially for species with rapidly declining populations.
Supplementary Material
Acknowledgments
We thank the following colleagues for their support and feedback on this study: Lauren Cirino, Ginny Greenway, Emily Khazan, Nich Martin, Javiera Rudolph, Ummat Somjee, Yash Sondhi, Sarah Steele Cabrera, and Colette St. Mary. We also thank Gail Dewsbury, Jacob Hornfeldt, Kristin Rossetti, and Matt Standridge for rearing larvae and ants and assisting with the life history portion of this study, and the Lucky Lab and the Urban Lab for their assistance with ant colony collection. All research was conducted under Federal Fish and Wildlife Permit No. TE86220A-2. This work was supported by grants from the U.S. Fish and Wildlife Service’s Cooperative Recovery Initiative (F17AP00467) and the Disney Conservation Fund.
Contributor Information
Geena M Hill, Florida Natural Areas Inventory, Florida State University, 1018 Thomasville Road, #200-C, Tallahassee, FL 32303, USA.
Matthew D Trager, US Forest Service, 325 John Knox Road, STE F-100, Tallahassee, FL 32303, USA.
Andrea Lucky, Entomology and Nematology Department, University of Florida, 1881 Natural Area Drive, Gainesville, FL 32611-0620, USA.
Jaret C Daniels, Entomology and Nematology Department, University of Florida, 1881 Natural Area Drive, Gainesville, FL 32611-0620, USA; Florida Museum of Natural History, University of Florida, 3215 Hull Road, Gainesville, FL 32611, USA.
Author Contributions
Conceptualization: GMH and JCD; Data curation: GMH; Formal analysis: GMH; Funding acquisition: JCD; Investigation: GMH; Methodology: GMH, MDT, AL and JCD; Project administration: GMH and JCD; Resources: JCD; Supervision: MDT, AL and JCD; Validation: GMH; Visualization: GMH; Writing – original draft: GMH; Writing – review & editing: MDT, AL, and JCD.
References Cited
- Axén, A. H. 2000. Variation in behavior of lycaenid larvae when attended by different ant species. Evol. Ecol. 14: 611–625. [Google Scholar]
- Axén, A. H., Leimar O., and Hoffman V.. . 1996. Signalling in a mutualistic interaction. Anim. Behav. 52: 321–333. [Google Scholar]
- Bachtold, A., and Alves-Silva E.. . 2013. Behavioral strategy of a lycaenid (Lepidoptera) caterpillar against aggressive ants in a Brazilian savanna. Acta Ethol. 16: 83–90. [Google Scholar]
- Barbero, F. 2016. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 17: 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, R. C., and Gullan P. J.. . 1991. More aggressive ant species (Hymenoptera: Formicidae) provide better protection for soft scales and mealybugs (Homoptera: Coccidae, Pseudococcidae). Biotropica. 23: 282–286. [Google Scholar]
- Burghardt, F., and Fiedler K.. . 1996. The influence of diet on growth and secretion behaviour of myrmecophilous Polyommatus icarus caterpillars (Lepidoptera: Lycaenidae). Ecol. Entomol. 21: 1–8. [Google Scholar]
- Calhoun, J. V., Slotten J. R.Salvato M. H.. . 2002. The rise and fall of tropical blues in Florida: Cyclargus ammon and Cyclargus thomasi bethunebakeri (Lepidoptera: Lycaenidae). Holarct. Lepid. 7: 13–20. [Google Scholar]
- Cannon, P., Wilmers T., and Lyons K.. . 2010. Discovery of the imperiled Miami Blue Butterfly (Cyclargus Thomasi Bethunebakeri) on Islands in the Florida Keys National Wildlife Refuges, Monroe County. Southeast. Nat. 9: 847–853. [Google Scholar]
- Carroll, S. P., and Loye J.. . 2006. Invasion, colonization, and disturbance; historical ecology of the endangered Miami blue butterfly. J. Insect Conserv. 10: 13–27. [Google Scholar]
- Casacci, L. P., Bonelli S., Balletto E., and Barbero F.. . 2019. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Environ. 7: 454. [Google Scholar]
- Clark, R. E., and Singer M. S.. . 2018. Keystone mutualism strengthens top-town effects by recruiting large-bodied ants. Oecologia. 186: 601–610. [DOI] [PubMed] [Google Scholar]
- Comstock, W. P., and Huntington E. I.. . 1943. Lycaenidae of the Antilles (lepidoptera, Rhopalocera). Ann. N. Y. Acad. Sci. 45: 49–130. [Google Scholar]
- Cushman, J. H., Rashbrook V. K., and Beattie A. J.. . 1994. Assessing benefits to both participants in a Lycaenid-Ant Association. Ecology. 75: 1031–1041. [Google Scholar]
- Daniels, J. C., Hill G. M., Rossetti K. A., Sanchez S. J., and Hornfeldt J. A.. . 2020. At-risk butterfly captive propagation programs to enhance life history knowledge and effective ex situ conservation techniques. J. Vis. Exp. 156: 1–17. [DOI] [PubMed] [Google Scholar]
- DeVries, P. J., Harvey D. J., and Kitching I. J.. . 1986. The ant associated epidermal organs on the larva of the lycaenid butterfly Curetis regula Evans. J. Nat. Hist. 20: 621–633. [Google Scholar]
- Dupont, S. T., Zemeitat D. S., Lohman D. J., and Pierce N. E.. . 2016. The setae of parasitic Liphyra brassolis butterfly larvae form a flexible armour for resisting attack by their ant hosts (Lycaenidae: Lepidoptera). Biol. J. Linn. Soc. 117: 607–619. [Google Scholar]
- Espeland, M., Breinholt J., Willmott K. R., Warren A. D., Vila R., Toussaint E. F. A., Maunsell S. C., Aduse-Poku K., Talavera G., Eastwood R., . et al. 2018. A comprehensive and dated phylogenomic analysis of butterflies. Curr. Biol. 28: 770–778.e5 [DOI] [PubMed] [Google Scholar]
- Fiedler, K. 1989. Differences in the behaviour of ants towards two larval instars of Lycaena tityrus (Lep., Lycaenidae). Dtsch. Entomol. Z. 36: 267–271. [Google Scholar]
- Fiedler, K. 2001. Ants that associate with Lycaenidae butterfly larvae: diversity, ecology, and biogeography. Biodivers. Res. 7: 45–60. [Google Scholar]
- Fiedler, K. 2021. The ant associates of Lycaenidae butterfly caterpillars–revisited. Nota Lepidopterol. 44: 159–174. [Google Scholar]
- Fiedler, K., and Holldobler B.. . 1992. Ants and Polyommatus icarus Immatures (lycaenidae) - sex-related developmental benefits and costs of ant attendance. Oecologia. 91: 468–473. [DOI] [PubMed] [Google Scholar]
- Fiedler, K., and Hummel V.. . 1995. Myrmecophily in the brown argus butterfly, Aricia agestis (Lepidoptera: Lycaenidae): effects of larval age, ant number and persistence of contact with ants. Zoology. 99: 128–137. [Google Scholar]
- Fiedler, K., and Samm C.. . 1994. Does ant-attendance influence development in 5 European Lycaenidae butterfly species? (Lepidoptera). Nota Lepidopterol. 17: 5–24. [Google Scholar]
- Fiedler, K., Hölldobler B., and Seufert P.. . 1996. Butterflies and ants: the communicative domain. Experientia. 52: 14–24. [Google Scholar]
- Forister, M. L., Gompert Z., Nice C. C., Forister G. W., and Fordyce J. A.. . 2011. Ant association facilitates the evolution of diet breadth in a lycaenid butterfly. Proc. R. Soc. B Biol. Sci. 278: 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, A. M., Axén A. H., and Pierce N. E.. . 2001. Assessing the quality of different ant species as partners of a myrmecophilous butterfly. Oecologia. 129: 452–460. [DOI] [PubMed] [Google Scholar]
- Gnatzy, W., Jatho M., Kleinteich T., Gorb S. N., and Hustert R.. . 2017. The eversible tentacle organs of Polyommatus caterpillars (Lepidoptera, Lycaenidae): morphology, fine structure, sensory supply and functional aspects. Arthropod Struct. Dev. 46: 788–804. [DOI] [PubMed] [Google Scholar]
- Hoffman, A. A., and Ross P. A.. . 2018. Rates and patterns of laboratory adaptation in (mostly) insects. J. Econom. Entom. 111: 501–509. [DOI] [PubMed] [Google Scholar]
- Hojo, M. K., Pierce N. E., and Tsuji K.. . 2015. Lycaenid Caterpillar secretions manipulate attendant ant behavior. Curr. Biol. 25: 2260–2264. [DOI] [PubMed] [Google Scholar]
- Kaminski, L. A., and Freitas A. V. L.. . 2010. Natural history and morphology of immature stages of the butterfly Allosmaitia strophius (Godart) (Lepidoptera: Lycaenidae) on flower buds of Malpighiaceae. Stud. Neotrop. Fauna Environ. 45: 11–19. [Google Scholar]
- Kaminski, L. A., and Rodrigues D.. . 2011. Species-specific levels of ant attendance mediate performance costs in a facultative myrmecophilous butterfly. Physiol. Entomol. 36: 208–214. [Google Scholar]
- Kaminski, L. A., Freitas A. V. L., and Oliveira P. S.. . 2010. Interaction between mutualisms: ant-tended butterflies exploit enemy-free space provided by ant-treehopper associations. Am. Nat. 176: 322–334. [DOI] [PubMed] [Google Scholar]
- Leimar, O., and Axen A.. . 1993. Strategic behavior in an interspecific mutualism - Interactions between lycaenid larvae and ants. Anim. Behav. 46: 1177–1182. [Google Scholar]
- Mizuno, T., Hagiwara Y., and Akino T.. . 2019. Varied effects of tending ant species on the development of facultatively myrmecophilous lycaenid butterfly larvae. Insects. 10: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. C., and Stein L. R.. . 2011. Evolution of influence: signaling in a lycaenid-ant interaction. Evol. Ecol. 25: 1205–1216. [Google Scholar]
- Pellissier, L., Rasmann S., Litsios G., Fiedler K., Dubuis A., Pottier J., and Guisan A.. . 2012. High host-plant nitrogen content: a prerequisite for the evolution of ant-caterpillar mutualism? J. Evol. Biol. 25: 1658–1666. [DOI] [PubMed] [Google Scholar]
- Peterson, M. A. 1993. The nature of ant attendance and the survival of larval Icaria acmon (Lycaenidae). J. Lepid. Soc. 47: 8–16. [Google Scholar]
- Pierce, N. E. 1985. Lycaenid butterflies and ants: selection for nitrogen-fixing and other protein-rich food plants. Am. Nat. 125: 888–895. [Google Scholar]
- Pierce, N. E., and Dankowicz E.. . 2022. Behavioral, ecological, and evolutionary mechanisms underlying caterpillar-ant symbioses. Curr. Opin. Insect Sci. 51: 100898. [DOI] [PubMed] [Google Scholar]
- Pierce, N. E., and Easteal S.. . 1986. The selective advantage of attendant ants for the larvae of a Lycaenid butterfly, Glaucopsyche lygdamus. J. Anim. Ecol. 55: 451. [Google Scholar]
- Pierce, N. E., and Mead P. S.. . 1981. Parasitoids as selective agents in the symbiosis between lycaenid butterfly larvae and ants. Science. 211: 1185–1187. [DOI] [PubMed] [Google Scholar]
- Pierce, N. E., Kitching R. L., Buckley R. C., Taylor M. F. J., and Benbow K. F.. . 1987. The costs and benefits of cooperation between the Australian lycaenid butterfly, Jalmenus evagoras, and its attendant ants. Behav. Ecol. Sociobiol. 21: 237–248. [Google Scholar]
- Pierce, N. E., Braby M. F., Heath A., Lohman D. J., Mathew J., Rand D. B., and Travassos M. A.. . 2002. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 47: 733–771. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2016. R: A language and environment for statistical computing.Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org. [Google Scholar]
- Robbins, R. K. 1991. Cost and evolution of a facultative mutualism between ants and Lycaenid Larvae (Lepidoptera). Oikos. 62: 363. [Google Scholar]
- Saarinen, E. V., and Daniels J. C.. . 2006. Miami blue butterfly larvae (Lepidoptera: Lycaenidae) and ants (Hymenoptera: Formicidae): new information on the symbionts of an endangered taxon. Fla. Entomol. 89: 69–74. [Google Scholar]
- Savignano, D. A. 1994. Benefits to Karner blue butterfly larvae from association with ants. InAndow D. A., Baker R. J. and Lane C. P. (eds.), Karner blue butterfly: a symbol of vanishing landscape. University of Minnesota, Minneapolis. [Google Scholar]
- Schönrogge, K., Barbero F., Casacci L. P., Settele J., and Thomas J. A.. . 2017. Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim. Behav. 134: 249–256. [Google Scholar]
- Stadler A. B., and Dixon F. G.. . 2008. Mutualism: ants and their insect partners. Cambridge University Press, Cambridge. [Google Scholar]
- Stadler, B., Fiedler K., Kawecki T. J., and Weisser W. W.. . 2001. Costs and benefits for phytophagous myrmecophiles: when ants are not always available. Oikos. 92: 467–478. [Google Scholar]
- Thomas, C. C., Tillberg C. V., and Schultz C. B.. . 2020. Facultative mutualism increases survival of an endangered ant-tended butterfly. J. Insect Conserv. 24: 385–395. [Google Scholar]
- Trager, M. D., and Daniels J. C.. . 2009. Ant tending of Miami blue butterfly larvae (Lepidoptera: Lycaenidae): partner diversity and effects on larval performance. Fla. Entomol. 92: 474–482. [Google Scholar]
- Trager, M. D., Thom M. D., and Daniels J. C.. . 2013. Ant-related oviposition and larval performance in a myrmecophilous lycaenid. Int. J. Ecol. 2013: 152139. [Google Scholar]
- USFWS [U.S. Fish and Wildlife Service]. 2012. Endangered and threatened wildlife and plants; listing of the Miami blue butterfly as endangered throughout its range; listing of the Cassius blue, Ceraunus blue, and nickerbean blue butterflies as threatened due to similarity of appearance to the Miami blue butterfly in coastal south and central Florida. Federal Register 77: 20948–20986. [Google Scholar]
- Wagner, D. 1993. Species-specific effects of tending ants on the development of lycaenid butterfly larvae. Oecologia. 96: 276–281. [DOI] [PubMed] [Google Scholar]
- Wagner, D. 1995. Pupation site choice of a North American lycaenid butterfly: the benefits of entering ant nests. Ecol. Entomol. 20: 384–392. [Google Scholar]
- Watanabe, M., and Hagiwara Y.. . 2009. A newly observed form of symbiotic relationship between Reverdin’s blue Lycaeides argyrognomon praeterinsularis (Verity), (Lycaenidae) and Camponotus japonicus Mayr (Formicidae). J. Res. Lep. 41: 70–75, 2002. [Google Scholar]
- Way, M. J. 1963. Mutualism between ants and honeydew-producing Homoptera. Annu. Rev. Entomol. 8: 307–344. [Google Scholar]
- Weeks, J. A. 2003. Parasitism and ant protection alter the survival of the lycaenid Hemiargus isola. Ecol. Entomol. 28: 228–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.