Abstract
One representative recombinant clone encoding Klebsiella pneumoniae O5-antigen lipopolysaccharide (LPS) was found upon screening for serum resistance in a cosmid-based genomic library of K. pneumoniae KT769 (O5:K57) introduced into Escherichia coli DH5α. A total of eight open reading frames (wbO5 gene cluster) were necessary to produce K. pneumoniae O5-antigen LPS in E. coli K-12. The enzymatic activities proposed for the wbO5 gene cluster are in agreement with the activities proposed for the biosynthesis of K. pneumoniae O5-antigen LPS. Using the complete DNA sequence of the K. pneumoniae wbO5 gene cluster, we obtained (by single or double recombination) genetically well-characterized mutants devoid only of this O5-antigen LPS. Finally, using these O5− mutants and the corresponding wild-type strains or complemented mutants with the wbO5 gene cluster (O5+ strains), we found that the presence of K. pneumoniae O5-antigen LPS is essential for some pathogenic features like serum resistance, adhesion to uroepithelial cells, and colonization (experimental infections) of the urinary tract in rats.
The O antigen is the most external component of lipopolysaccharide (LPS) and consists of a polymer of oligosaccharide repeating units. Another interesting feature is the high chemical variability shown by the O antigen, leading to a similar genetic variation in the genes involved in O-antigen biosynthesis, the so called wb (rfb) cluster (for reviews, see references 35 and 45). The genetics of O-antigen biosynthesis have been intensively studied in the family Enterobacteriaceae, and it has been shown that the wb clusters usually contain genes involved in biosynthesis of activated sugars, glycosyl transferases, O-antigen polymerases, and O-antigen export (35, 45).
Escherichia coli DH5α and other K-12-derived strains are rough, unable to produce O-antigen LPS (O−) and serum sensitive. As we and other authors have previously shown for different gram-negative bacteria (10, 24), the presence of O-antigen LPS (smooth phenotype) is a determinant for serum resistance. We used this characteristic to clone O-antigen LPSs from different bacteria in E. coli DH5α.
In a recent study of the prevalence of the O serogroups among clinical Klebsiella isolates from different sources and countries, serogroup O5 represented 9% of the isolates (13). The chemical structure of the Klebsiella O5-antigen LPS was reported (20) to be a homopolymer of mannose: → 3-d-Manp1α → 2-d-Manp1α → 3-d-Manp1α → 2-d-Manp1α → 2-d-Manp1α →. Despite the similarity in chemical composition to the Klebsiella O3-antigen LPS, also a homopolymer of mannose (5), no cross-reactivity was observed for O5- and O3-antigen LPS with specific antibodies (13), in spite of the high heterogeneity of the O3 serogroup strains of this bacterium (13).
In this work, we cloned and sequenced the wbO5 gene cluster of Klebsiella pneumoniae to obtain genetically well-characterized mutants devoid of this O5-antigen LPS. Finally, using these O5− mutants and their corresponding wild-type strains or complemented mutants with the wbO5 gene cluster (O5+ strains), we studied some pathogenic features of the K. pneumoniae O5-antigen LPS.
MATERIALS AND METHODS
Bacterial strains, plasmids and growth conditions.
The bacterial strains, cosmids, and plasmids used are listed in Table 1. Bacteria were grown in Luria-Bertani (LB)-Miller broth and LB-Miller agar (26). The LB media were supplemented with ampicillin (100 μg/ml), chloramphenicol (25 μg/ml), kanamycin (30 μg/ml), tetracycline (20 μg/ml), or rifampin (100 μg/ml) when needed.
TABLE 1.
Bacterial strains, cosmids and plasmids used in this study
| Strain, cosmid, or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F−endA hsdR17 (rk− mk+) supE44 thi-1 recA1 gyr-A96 φ80lacZ | 12 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F′ proAB lacIqZ ΔM15 Tn10) | Stratagene |
| MC1061 | thi thr1 leu6 proA2 his4 argE2 lacY1 galK2 ara14 xyl5 supE44, λ pir | 30 |
| SM10 | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu, Kmr, λpir | 30 |
| CLM4 | lacZ trp Δ(sbcB-rfb) upp rel rpsL ΔrecA | 22 |
| K. pneumoniae strains | ||
| KT769 | Wild type, serotype O5:K57 | I. Ørskov |
| KT769 Rifr | Rifr mutant derived from KT769 | This work |
| KT769-1 | wbdC insertion KT769 Rifr mutant obtained with pJT80 | This work |
| KT769-6 | wzm wzt deletion KT769 Rifr mutant obtained with pJT86 | This work |
| KT769-1C | KT769-1 mutant complemented with CosKT4 | This work |
| KT769-6C | KT769-6 mutant complemented with CosKT4 | This work |
| Plasmids | ||
| pLA2917 | Tcr Kmr | 1 |
| CosKT4 | pLA2917 with 20-kb chromosomal KT679 Sau3A insert | This work |
| pFS100 | pGP704 suicide plasmid, λpir dependent, Kmr | 30 |
| pJT80 | pFS100 with an internal fragment (697 bp) of wbdC | This work |
| pKO3 | Cmr, temperature sensitive for replication; sacB | 21 |
| pJT86 | pKO3 with an internal fragment (1,101 bp) of wzm wzt | This work |
General DNA methods.
DNA manipulations were carried out essentially as previously described (33). DNA restriction endonucleases, T4 DNA ligase, E. coli DNA polymerase (Klenow fragment), and alkaline phosphatase were used as recommended by the suppliers. Recombinant clones were selected on LB-Miller agar plates containing the appropriate antibiotics.
Construction of a K. pneumoniae KT769 genomic library.
K. pneumoniae KT769 genomic DNA was isolated and partially digested with Sau3A as described by Sambrook et al. (33). Cosmid pLA2917 (1) was digested with BglII, dephosphorylated, and ligated to Sau3A genomic DNA fragments. DNA packaging by using Gigapack Gold III (Stratagene) and infection of E. coli DH5α were carried out as previously described (11). Recombinant clones were selected on LB-Miller agar plates supplemented with tetracycline (20 μg/ml).
Construction of mutant strains KT769-1 (wbdC) and KT769-6 (wzm-wzt).
Two different mutant strains of K. pneumoniae KT769 were constructed. To obtain mutant KT769-1 (insertion in the wbdC gene), a method based on suicide plasmid pFS100 was used which renders two incomplete copies of the gene (30). A wbdC internal DNA fragment (697 bp) was amplified from CosKT4 using oligonucleotides 5′-CACTCGGATATTGTGAAAC-3′ and 5′-TCTTCAAAACGACGACGC-3′; isolated; ligated to EcoRV-digested, blunt-ended, and dephosphorylated pSF100; and transformed into E. coli MC1961(pir) to generate plasmid pJT80. Plasmid pJT80 was isolated, transformed into E. coli SM10(pir), and transferred by conjugation to a KT769 rifampin-resistant (Rifr) mutant (from our laboratory collection) as previously described (30).
To obtain mutant KT769-6, the method of Link et al. (21) was used to create an in frame deletion encompassing both the wzm and wzt genes. Briefly, CosKT4 and primer pairs A (5′-CGCGGATCCCAGGAAGACGCCATTTACGG-3′) plus B (5′-TGTTTAAGTTTAGTGGATGGGTGTAAAACGAGCCATAACGCG-3′) and C (5′-CCCATCCACTAAACTTAAACAGTCGTTAACACGGAACAACAAG-3′) plus D(5′-CGCGGATCCAGGTCCCGACGCTTACATTC-3′) were used in two sets of asymmetric PCRs to amplify DNA fragments of 567 bp (AB) and 555 bp (CD). DNA fragments AB and CD were annealed at their overlapping region and amplified by PCR as a single fragment, using primers A and D (1,101 bp). The fusion product was purified, BamHI digested, ligated into BamHI-digested and phosphatase-treated pKO3 vector (21), electroporated into E. coli DH5, and plated on chloramphenicol-containing plates at 30°C to obtain plasmid pJT86. The PCR amplification procedures and mutant construction by gene replacement, using plasmid pJT86, were exactly as described by Link et al. (21).
DNA sequencing.
Double-stranded DNA sequencing was performed by using the Sanger dideoxy-chain termination method (34) with the ABI Prism dye terminator cycle-sequencing kit (Perkin-Elmer). Primers used for DNA sequencing were purchased from Pharmacia LKB Biotechnology. Primers 5′-GACTGGGCGGTTTTATGG-3′ and 5′-CCATCTTGTTCAATCATGCA-3′, designed by us from the known sequence in our laboratory of cosmid pLA2917, were used to sequence the inserts in the BglII restriction site on pLA2917.
DNA and protein sequence analysis.
The DNA sequence was translated in all six frames, and all open reading frames (ORFs) greater than 100 bp were inspected. The deduced amino acid sequences were compared with those of DNA translated in all six frames from nonredundant GenBank and EMBL databases by using the BLAST network service at the National Center for Biotechnology Information (2). Multiple sequence alignments were carried out using the Clustal W program (39). Possible terminator sequences were identified by using the Terminator program from the Genetics Computer Group (Madison, Wis.) package in a VAX 4300. Hydropathy profiles were calculated by the method of Kyte and Doolitle (17).
Cell surface isolation and analysis.
Cell envelopes were prepared by lysis of whole cells in a French press at 16,000 lb/in2. Unbroken cells were removed by centrifugation at 10,000 × g for 10 min, and the envelope fraction was collected by centrifugation at 100,000 × g for 2 h. Cytoplasmic membranes were solubilized twice with sodium N-lauroyl sarcosinate, and the outer membrane (OM) fraction was collected as described above. OM proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the Laemmli procedure (18). Protein-containing gels were fixed and stained with Coomassie blue. LPS was purified by the method of Westphal and Jann (44). For screening purposes, LPS was obtained after proteinase K digestion of whole cells by the procedure of Darveau and Hancock (6). SDS-PAGE was performed, and LPS bands were detected by the silver-staining method of Tsai and Frasch (42).
Antisera.
Anti-K. pneumoniae O5 LPS serum was obtained using purified K. pneumoniae O5 LPS, adsorbed by a K. pneumoniae rough mutant (KT141) (13), and assayed as previously described for other LPSs (24, 40).
Western immunoblotting.
After SDS-PAGE, immunoblotting was carried out by transfer to polyvinylidene difluoride membranes (Millipore Corp., Bedford, Mass.) at 1.3 A for 1 h in the buffer of Towbin et al. (41). The membranes were then incubated sequentially with 1% bovine serum albumin, specific anti-O antiserum (1:500), alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G, and 5-bromo-4-chloro-indolylphosphate disodium-nitroblue tetrazolium. Incubations were carried out for 1 h, and washing steps with 0.05% Tween 20 in phosphate-buffered saline (PBS) were included after each incubation step. Colony blotting was performed using K. pneumoniae O5 antiserum as indicated above.
Serum killing.
The survival of exponential-phase bacteria in nonimmune human serum was measured as previously described in a 90% serum in PBS after 3 h of incubation at 37°C, taking samples for viable counts every 30 min (24), or by a microtiter plate-based assay for screening (43).
Cell surface hydrophobicity.
Cell surface hydrophobicity was determined by two different methods. The first method used was hydrophobic interaction chromatography (HIC) on phenyl-Sepharose as previously described (14). Briefly, bacteria were resuspended in 10 mM PBS (pH 7.4) to an optical density at 470 nm (OD470) of 1.0, applied to a phenyl-Sepharose column, and eluted with 4 M NaCl. The eluate was collected, and its OD470 was determined.
The second method used was the bacterial adherence to hydrocarbons (BATH) method, as previously described (29). Briefly, cells were washed twice in phosphate-urea-magnesium buffer (pH 7.1), suspended in the same buffer at an OD400 of 1.0, and vortexed with various volumes of hydrocarbon. The OD400 of the aqueous phase was expressed as a percentage of the OD400 of a standard volume of untreated cells.
Bacterial surface charge.
The bacterial surface charge was determined by measurement of the zeta potential using a Zetasizer II (Malvern Instruments, Malvern, United Kingdom).
Bacterial adherence assay.
The assay measuring the adherence of K. pneumoniae strains to uroepithelial cells (UEC) was done as described by Falkowski et al. (7) and Merino et al. (25). Briefly, samples containing bacteria and UEC (100:1) were incubated for 1 h at 37°C and filtered under vacuum through a 5-μm-pore-size filter. The filters were solubilized to lyse the UEC, and the adherent bacteria were counted by viable plate count determination. In some cases, the adherence was also examined by direct visualization of Gram-stained filters, in which a minimum of 40 UEC were examined.
Urinary tract infections in rats.
The bacterial strains used to establish infection were grown overnight in LB-Miller agar (supplemented with antibiotics when needed) and gently suspended in PBS to the appropriate concentration. In each experiment, 12 female Wistar rats (weighing 200 to 250 g) of strain CFHB (Interfauna UK, Huntington, United Kingdom) were used. Ten animals were infected transurethrally in the bladder after voiding urine by gentle compression of the bladder through the external abdominal wall, and two were used as controls. The infections were quantified as previously described (3).
Nucleotide sequence accession number.
The nucleotide sequence of the genes described here have been assigned GenBank accession no. AF189151.
RESULTS AND DISCUSSION
Cloning of the K. pneumoniae (O5:K57) O5-antigen LPS genomic region that confers serum resistance to E. coli K-12.
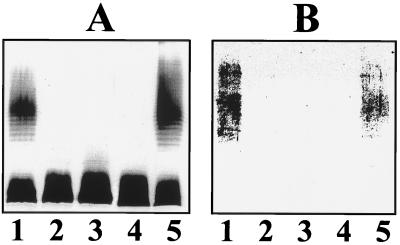
K. pneumoniae (O5:K57) strain KT769 is characterized, like other encapsulated and smooth strains, by being serum resistant (24), while E. coli K-12 strains like DH5α are serum sensitive. A cosmid-based genomic library of Klebsiella strain KT769 chromosomal DNA was constructed and introduced into E. coli DH5α, and recombinants were selected on LB-Miller agar plus tetracycline. Several serum-resistant clones were isolated using a microtiter plate-based assay (43); CosKT4 was one of the clones which conferred the highest serum resistance to E. coli DH5α (data not shown). DH5α harboring CosKT4 was characterized by analysis of the OM protein and LPS profile on SDS-PAGE. No major differences were found in the OM protein pattern, but cosmid CosKT4 conferred K. pneumoniae O5-antigen LPS production to E. coli DH5α. Of course, no antigen was detected on DH5α with or without the cosmid pLA2917 (Fig. 1A). CosKT4 was cured from the recipient strain DH5α by serial growth without antibiotics, single-colony isolation, and testing for antibiotic sensitivity and lack of the plasmid DNA. The cured strains lacked the O5-antigen LPS in gels (Fig. 1A) and became serum sensitive, like DH5α (with or without cosmid pLA2917). When CosKT4 was transferred to E. coli CLM4 (a strain with the wb cluster deleted [22]), it was able to confer O5-antigen LPS production to this strain. This result suggested that CosKT4 contains all the genetic information necessary for O5-antigen production (Fig. 1).
FIG. 1.
Silver-stained polyacrylamide gel (A) and Western immunoblot (B) of LPS reacted with K. pneumoniae O5-specific antiserum. Antisera were obtained and assayed as described in Materials and Methods. LPS samples were prepared from E. coli strains by the method of Darveau and Hancock (6). Lanes: 1, DH5α harboring CosKT4; 2, DH5α; 3, cured strain from lane 1; 4, CLM4 (Δwb); 5, CLM4 harboring CosKT4.
Subcloning and sequencing of CosKT4.
We tried to subclone the genes responsible for the biosynthesis of O5-antigen LPS from CosKT4 using different restriction enzymes and plasmid vectors commonly used in other cases, such as K. pneumoniae O1 or O8 antigen-LPS, but these attempts were unsuccessful. For this reason, we used oligonucleotides flanking the BglII pLA2917 cosmid site (see Materials and Methods). This sequence rendered a high degree of homology to hisIE genes in one of the ends, and the presence of wb O5 genes in CosKT4 was confirmed by using the oligonucleotide primers from the boundary region of the K. pneumoniae O5 wb (wbdC) and his genes described by Sugiyama et al. (37). Other sequence-derived oligonucleotides were used to complete the nucleotide sequence. A total of eight complete ORFs were determined, and their characteristics are shown in Table 2. Upstream of each ORF, putative ribosomal binding sequence were found. On the other hand, no Rho-independent transcription termination similar sequences were found among the eight ORFs. This feature, plus the overlap between the ORF5 stop codon and the ORF6 initial codon and the short spacing (no spacing among ORF3, ORF4, and ORF5) between the eight ORFs, strongly suggested that these ORFs are part of a transcriptional unit. The last (truncated) ORF, ORF9, was found to be similar to hisI from several members of the Enterobacteriaceae and was unrelated to the wbO5 cluster. In other wb gene clusters, a similar situation was found, with a hisI gene transcribed into opposite direction from the wb operon.
TABLE 2.
K. pneumoniae KT769 (O5) wb gene cluster
| Locus | Base positions | % G+C | Protein encoded (kDa) | pIb | GRAVYc |
|---|---|---|---|---|---|
| manC (ORF1) | 1–1416 | 61.6 | 52.8 | 5.38 | − 0.273 |
| manB (ORF2) | 1436–2812 | 62.5 | 50.4 | 5.27 | − 0.236 |
| wzm (ORF3) | 2918–3607 | 46.3 | 26.0 | 9.17 | + 0.842 |
| wzt (ORF4) | 3607–4821 | 47.3 | 44.9 | 6.09 | − 0.172 |
| wbbD (ORF5) | 4821–6116 | 46.6 | 49.4 | 9.12 | − 0.310 |
| wbdA (ORF6) | 6100–9741 | 49.5 | 135.4 | 5.47 | − 0.124 |
| wbdB (ORF7) | 9809–10954 | 54.7 | 43.8 | 7.11 | − 0.212 |
| wbdC (ORF8) | 11255–12079 | 54.7 | 31.1 | 6.31 | − 0.247 |
| hisI (ORF9)a | 12451–12731 | NDd | ND | ND | ND |
Truncated ORF.
Isoelectric point of the protein, calculated using ProtParam at the Expassy server.
Grand average hydropathicity of the protein, calculated using the Kyte and Doolitle method (17).
ND, not determined.
Analysis of the ORF deduced amino acid sequence.
The analysis of the ORF deduced amino acid sequences showed that the ORF1 and ORF2 products are highly similar to two enzymes involved in the biosynthesis of the mannose (Table 3). Accordingly we suggest that ORF1 and ORF2 correspond respectively to the manC and manB genes, encoding GDP-mannose pyrophosphorylase or mannose-1-phosphate guanyltransferase and phosphomannomutase, respectively (36). The ORF3 and ORF4 products are similar to the ATP binding cassette 2 (ABC-2)-type transport system integral membrane and ATP binding proteins, respectively (Table 3). Exporter systems similar to the ORF3-ORF4 system are involved in export of O antigen, except for ATP binding protein AbcA, which is involved in A-protein expression (4). The putative exporter component (the ORF3 product) showed a 96% level of amino acid similarity to the corresponding Wzm protein involved in E. coli O9a antigen export, while the putative ATP-binding component showed 45% similarity to the ATP binding protein AbcA involved in Aeromonas salmonicida A-protein expression (4) and 35 to 40% similarity to several ATP binding proteins (Wzt) involved in capsule or LPS biosynthesis. Hydrophobicity analysis and identification of putative transmembrane domains of Wzm protein (amino acid residues 29 to 51, 76 to 98, 112 to 134, 145 to 167, and 197 to 218) by the method of Klein et al. (16) suggested that this protein is indeed an integral membrane protein. On the other hand, the sequence GINGAGKS (residues 58 to 65) from Wzt was found to correspond to box A, a motif present in ATP binding proteins, as well as the ABC transporter family signature YSSGMQVRLAFSVAT (residues 146 to 160). Thus, ORF3 and ORF4 have been named the wzm and wzt genes, respectively.
TABLE 3.
Percent identity and similarity of the amino acid sequences proteins encoded K. pneumoniae KT769 ORF1 through ORF8 to the most significant other proteins
| Protein | No. of amino acids | % Similaritya | % Identitya | Accession no. |
|---|---|---|---|---|
| ORF1 (ManC) | ||||
| K. pneumoniae KT769 ManC | 471 | AF189151 | ||
| K. pneumoniae O3 ManC | 452 | 100 | 99 | Q48462 |
| E. coli O9 | 471 | 100 | 99 | Q59427 |
| ORF2 (ManB) | ||||
| K. pneumoniae KT769 ManB | 458 | AF189151 | ||
| E. coli O9a ManB | 458 | 99 | 98 | Q66229 |
| E. coli O9 | 460 | 99 | 98 | Q59428 |
| ORF3 (Wzm) | ||||
| K. pneumoniae KT769 Wzm | 229 | AF189151 | ||
| E. coli O9a Wzm | 264 | 96 | 92 | Q66230 |
| E. coli O9 | 261 | 78 | 63 | Q47590 |
| ORF4 (Wzt) | ||||
| K. pneumoniae KT769 Wzt | 404 | AF189151 | ||
| A. salmonicida AbcA | 308 | 65 | 45 | Q07698 |
| E. coli KpsT (polysialic capsule) | 359 | 59 | 40 | P23888 |
| ORF5 (WbdD) | ||||
| K. pneumoniae KT769 WbdD | 431 | AF189151 | ||
| E. coli O9a | 425 | 96 | 92 | O66232 |
| ORF6 (WbdA) | ||||
| K. pneumoniae KT769 WbdA | 1,213 | AF189151 | ||
| E. coli O9a | 704 | 96 | 93 | O66234 |
| ORF7 (WbdB) | ||||
| K. pneumoniae KT769 WbdB | 381 | AF189151 | ||
| E. coli O9 | 381 | 100 | 99 | Q47594 |
| ORF8 (WbdC) | ||||
| K. pneumoniae KT769 WbdC | 274 | AF189151 | ||
| E. coli O9a | 274 | 100 | 98 | O24713 |
The percentages were obtained from pairwise comparisons using the Gap program with the following settings: gap weight, 12; length weight, 12.
The ORF5 product was found to be practically identical to WbdD of E. coli O9a, with unknown function, and we used the same name (WbdD) for our ORF5 product. ORF6 to ORF8 encoded products with very high levels of amino acid similarity to mannosyl transferase A (α→2-d-Manp1), B (α→3-d-Manp1) and C (initial, α→3-d-Manp1 depending on wecA), respectively (15). The high levels of amino acid identity strongly suggest that these ORFs encoded the same enzymes in the K. pneumoniae O5 biosynthesis, which prompted to us to name the ORF6 to ORF8 products WbdA to WbdC, respectively. The enzymatic activities proposed for the wbO5 cluster are in agreement with those proposed for the biosynthesis of K. pneumoniae O5-antigen LPS.
The final truncated ORF9 deduced amino acid sequence showed a high level of similarity to the product of the hisI gene of E. coli, located at 45.2 min on the E. coli map (a bifunctional enzyme related to histidine biosynthesis).
Characterization of mutant strains KT769-1 (wbdC) and KT769-6 (wzm wzt).
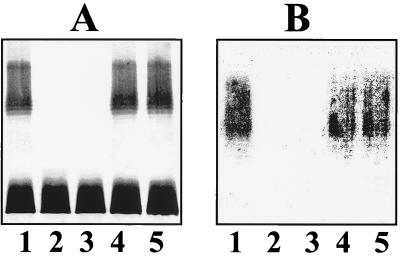
Both mutants KT769-1 and KT769-6 were devoid of the O5-antigen LPS in LPS gels and Western blots (Fig. 2), while no other major differences were observed in their other OM molecules. These mutants contained capsular polysaccharide, which reacts with K57 antiserum like the wild-type strain and the rifampin-resistant mutant. However, there are some differences among the mutants; while KT769-1 is unable to form O5-antigen LPS, KT769-6 is able to form this antigen but is unable to transport it to the OM (instead, it is accumulated in the inner membrane) (data not shown). This situation for wzm wzt mutants was described previously (32). When CosKT4 was transferred by mating, it was able to complement both mutants, rendering them able to biosynthesize the O5-antigen LPS, as in LPS gels and Western blots (Fig. 2).
FIG. 2.
Silver stained polyacrylamide gel (A) and Western immunoblot (B) of LPS reacted with K. pneumoniae O5-specific antiserum. LPS samples were prepared from K. pneumoniae strains by the method of Darveau and Hancock (6). Lanes: 1, KT769 (O5:K57, wild type); 2, KT769-1 (wbdC mutant); 3, KT769-6 (wzm-wzt mutant); 4, KT769-1C (KT769-1 mutant complemented with CosKT4); 5, KT769-6C (KT769-6 mutant complemented with CosKT4).
Contribution of the O5-antigen LPS to pathogenic features.
Taking advantage of the isogenic mutants obtained lacking only the O5-antigen LPS (KT769-1 and KT769-6) and the complementation of these mutants by CosKT4 (KT769-1C and KT769-6C, showing a complete O5-antigen LPS), we decide to investigate the contribution of this molecule to pathogenic features. Some of these pathogenic features have been previously described in studies of K. pneumoniae O1-antigen LPS using spontaneous phage resistance mutants, but they have not genetically characterized.
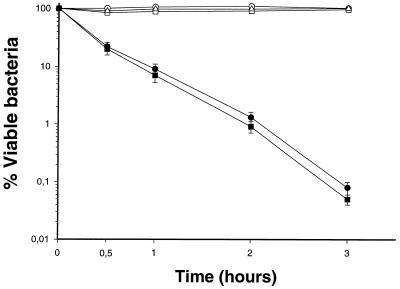
As observed in Fig. 3, mutant strains KT769-1 and KT769-6 are sensitive to the bactericidal activity of nonimmune human serum, while the wild-type strain as well as the CosKT4-complemented mutants (KT769-1C and KT769-6C, respectively) are resistant to this activity. Because the mutants showed a complete LPS core, we demonstrated that the K. pneumoniae O5-antigen LPS, as with O1-antigen LPS from the same bacterium (24) or from other members of the Enterobacteriaceae, is critical for complement resistance (8, 38). We suggested that the reasons for complement resistance in K. pneumoniae O5+ strains are the same as we described for O1+ strains (24). The mutants that lacked the O5-antigen LPS also showed an increase (less electronegative) in their surface charge as measured in millivolts (−40.6 ± 0.4 mV) in comparison with the wild-type strain or the strains that recovered the O5-antigen LPS with plasmid CosKT4 (−52.8 ± 0.5 mV). This surface charge increase is explained by the loss of negative surface molecules like the O5-antigen polysaccharide chains. The change in the surface charge leads to changes in the surface hydrophobicity of the O5− mutants. As shown in Table 4, the surface hydrophobicity of O5− mutants, as measured by several methods, is increased (more hydrophobic) with respect to that of the O5+ strains (wild type or COS-KT4 complemented mutants). Also, the reason for this change is the loss of a hydrophilic molecule, as with the O5-antigen LPS. However, HIC seems to be a more sensitive technique to assay the surface hydrophobicity on these mutants than is BATH, which seems to be more useful with n-xylene than with hexadecane (Table 4), perhaps because the n-xylene is a more penetrating agent than the hexadecane and these mutants are only devoid of the O5-antigen LPS but are encapsulated (K57).
FIG. 3.
Survival of K. pneumoniae strains in nonimmune serum. (▵) KT769 (O5:K57 wild type), (●) KT769-1 (wbdC defined insertion mutant, O5−), (■) KT769-6 (wzm-wzt deletion mutant, O5−), (○) KT769-1C (KT769-1 mutant complemented with CosKT4, O5+), (□) KT769-6C (KT769-6 mutant complemented with CosKT4, O5+). The results are the averages of at least three independent experiments (values are means and standard deviations).
TABLE 4.
Surface hydrophibicity of K. pneumoniae strains measured by different methods
| Strain | % Surface hydrophobicitya measured by:
|
||
|---|---|---|---|
| HICb | BATHc
|
||
| Xylene | Hexadecane | ||
| KT769 (O5:K57, wild type) | 1.5 ± 0.3 | 74 ± 3.4 | 89 ± 5.1 |
| KT769-1 (O5−; wbdC defined insertion KT769 mutant) | 28.4 ± 0.7 | 69 ± 2.8 | 78 ± 4.4 |
| KT769-6 (O5−; wzm wzt KT769 deletion mutant) | 29.3 ± 0.6 | 66 ± 2.6 | 74 ± 4.2 |
| KT769-1C (O5+, KT769-1 complemented with CosKT4) | 1.7 ± 0.2 | 75 ± 3.0 | 88 ± 4.9 |
| KT769-6C (O5+, KT769-6 complemented with CosKT4) | 1.6 ± 0.3 | 74 ± 3.6 | 85 ± 2.7 |
Mean ± standard deviation of three independent determinations.
Percentage of bacteria retained in the gel.
Percent absorbance of the aqueous phase after treatment with hydrocarbon (0.2 ml) relative to initial absorbance.
The physicochemical properties of the bacterial surface are basic to the interactions (association or adhesion) between the bacteria and the eukaryotic cells of the host tissues. When we measured the adhesion of these strains to UEC (Table 5), we found that mutants lacking the O5-antigen LPS showed approximately a threefold reduction in their ability to adhere to UEC in comparison with the O5+ strains (wild type or CosKT4-complemented mutants). From these experiments, we can conclude that the O5-antigen LPS in K. pneumoniae is an important adhesion factor, and we suggested that this is because alterations in the surface charge and hydrophobicity clearly interfere with the bacterial association, a primary step in bacterial adhesion (23). Finally, changes in adhesion should be critical for bacterial colonization. As observed in Table 6, mutants lacking the O5-antigen LPS showed a drastic reduction in their ability to infect the urinary tract in rats, as measured either by the number of animals infected or by the viable bacterial counts found in the kidneys, bladder, or urine, in this experimental infection with respect to the O5+ strains (wild type or Cos-KT4-complemented mutants). Then, the O5-antigen LPS of K. pneumoniae is an important colonization factor (at least for urinary tract infections), and this is based at least in part on their role as an adhesion to UEC. It seems clear that adhesion is a critical step for bacterial colonization (infection), as previously described for different bacterium-host interactions (9, 28).
TABLE 5.
Adhesion of different K. pneumoniae strains to UEC
| Strain | % of bacteria adhereda |
|---|---|
| KT769 (O5:K57, wild type) | 43.8 ± 3.9 |
| KT769-1 (O5−; wbdC defined insertion KT769 mutant) | 17.3 ± 3.5 |
| KT769-6 (O5−; wzm wzt KT769 deletion mutant) | 16.7 ± 3.2 |
| KT769-1C (O5+, KT769-1 complemented with CosKT4) | 42.6 ± 3.4 |
| KT769-6C (O5+, KT769-6 complemented with CosKT4) | 43.2 ± 4.2 |
The percentage of bacteria adhering to UEC cells ± standard deviation was calculated as previously described (25). All the assays were done at least in triplicate. Student's t test, P < 0.01.
TABLE 6.
Experimental urinary tract infection of rats by different K. pneumoniae strains
| Strain | Infectious dose (CFU/rat) | Sample | Infectiona measured by:
|
|
|---|---|---|---|---|
| % of infected ratsb | Viable countsc | |||
| KT769 (O5:K57, wild type) | 1.2 × 109 | Kidney | 100 | 6.8 ± 0.7 |
| Bladder | 100 | 6.5 ± 0.5 | ||
| Urine | 100 | 8.6 ± 0.5 (6)a | ||
| KT769-1 (O5−; wbdC defined insertion KT769 mutant | 0.9 × 109 | Kidney | 30 | 3.8 ± 0.3 |
| Bladder | 20 | 3.6 ± 0.5 | ||
| Urine | 20 | 5.8 ± 0.4 (2) | ||
| KT769-6 (O5−; wzm wzt KT769 deletion mutant | 1.1 × 109 | Kidney | 30 | 3.9 ± 0.4 |
| Bladder | 30 | 3.2 ± 0.7 | ||
| Urine | 20 | 5.6 ± 0.2 (2) | ||
| KT769-1C (O5+, KT769-1 complemented with CosKT4) | 1.0 × 109 | Kidney | 90 | 6.9 ± 0.6 |
| Bladder | 90 | 6.3 ± 0.5 | ||
| Urine | 100 | 8.4 ± 0.8 (4) | ||
| KT769-6C (O5+, KT769-6 complemented with CosKT4) | 1.2 × 109 | Kidney | 100 | 7.0 ± 0.8 |
| Bladder | 100 | 6.5 ± 0.3 | ||
| Urine | 100 | 8.3 ± 0.6 (6) | ||
A total of 20 kidneys and 10 bladders were studied in each group. Numbers in parentheses represent numbers of urine samples studied.
Percentage of cultures that were positive. The smallest number of organisms detectable by the method was 50 CFU per g (kidney or bladder) or per ml (urine). The values for the O5− mutants correspond to the percentage of animals infected.
Values represent the mean log10 CFU per gram or per milliliter ± standard deviation of the positive cultures. All the assays were done at least in triplicate.
In summary, all these roles in pathogenicity that we attributed to the K. pneumoniae O5-antigen LPS are supported by the behavior of genetically well-characterized isogenic mutants, with no alterations in other surface molecules. It is also clear that some of the roles described here for this O5-antigen LPS have been previously described for other O-antigen LPS from the same bacterium (3, 24, 25) and other members of the Enterobacteriaceae (19, 27, 31), but to our knowledge this is the first time this has been done using well-characterized isogenic mutants and cloned wb cluster genes for complementation of these isogenic mutants.
ACKNOWLEDGMENTS
This work was supported by grants from DGICYT and Plan Nacional de I+D (Ministerio de Educación y Cultura, Madrid, Spain). L.I., M.M.N., and M.A. were supported by fellowships from the Ministerio de Educación y Cultura, Generalitat de Catalunya, and University of Barcelona, respectively.
We thank Maite Polo for her technical assistance.
REFERENCES
- 1.Allen L N, Hanson R S. Construction of broad-host-range cosmid cloning vector: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985;161:955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Camprubí S, Merino S, Tomás J M. The role of the O-antigen lipopolysaccharide and capsule on an experimental Klebsiella pneumoniae infection of the rat urinary tract. FEMS Microbiol Lett. 1993;111:9–14. doi: 10.1016/0378-1097(93)90175-2. [DOI] [PubMed] [Google Scholar]
- 4.Chu S, Trust T J. An Aeromonas salmonicida gene which influences A-protein expression in Escherichia coli encodes a protein containing an ATP-binding casette and maps beside the surface array protein gene. J Bacteriol. 1993;175:3105–3114. doi: 10.1128/jb.175.10.3105-3114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curvall M, Lindberg B, Lönngren J, Nimmich W. Structural studies on the Klebsiella O group 3 lipopolysaccharide. Acta Chem Scand. 1973;27:2645–2649. doi: 10.3891/acta.chem.scand.27-2645. [DOI] [PubMed] [Google Scholar]
- 6.Darveau R P, Hancock R E W. Procedure for the isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983;155:831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falkowski W, Edwards M, Schaeffer A J. Inhibitory effect of substituted aromatyc hydrocarbons on adherence of Escherichia coli to human epithelial cells. Infect Immun. 1986;52:863–866. doi: 10.1128/iai.52.3.863-866.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa J E, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank M M. Complement in host defense against bacterial infections. In: Ayoub E M, Cassell G H, Branche W C Jr, Henry P J, editors. Microbial determinants of virulence and host response. Washington, D.C.: American Society for Microbiology; 1990. pp. 305–317. [Google Scholar]
- 11.Guasch J F, Piqué N, Climent N, Ferrer S, Merino S, Rubires X, Tomás J M, Regué M. Cloning and characterization of two Serratia marcescens genes involved in core lipopolysaccharide biosynthesis. J Bacteriol. 1996;178:5741–5747. doi: 10.1128/jb.178.19.5741-5747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 13.Hansen D S, Mestre F, Albertí S, Hernández-Allés S, Alvarez D, Domenech-Sanchez A, Gil J, Merino S, Tomás J M, Benedí V J. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J Clin Microbiol. 1999;37:56–62. doi: 10.1128/jcm.37.1.56-62.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Källenius G, Korkhonen T K, Väisänen-Rhen V, Svenson S B. Adherence assays. In: Korkhonen T K, Dawes E A, Mäkelä P H, editors. Enterobacterial surface antigens. Amsterdam, The Netherlands: Elsevier; 1985. pp. 321–331. [Google Scholar]
- 15.Kido N, Torgov V I, Sugiyama T, Uchiya K, Sugihara H, Komatsu T, Kato N, Jann K. Expression of the O9 polysaccharide of Escherichia coli: sequencing of the E. coli O9 rfb gene cluster, characterization of mannosyl transferases, and evidence for an ATP-binding casette transport system. J Bacteriol. 1995;177:2178–2187. doi: 10.1128/jb.177.8.2178-2187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein P, Kanehisa M, DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985;815:468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- 17.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Licht T R, Krogfelt K A, Cohen P S, Poulsen L K, Urbance J, Molin S. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect Immun. 1996;64:3811–3817. doi: 10.1128/iai.64.9.3811-3817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindberg B, Lönngren J, Nimmich W. Structural studies on Klebsiella O group 5 lipopolysaccharides. Acta Chem Scand. 1972;26:2231–2236. doi: 10.3891/acta.chem.scand.26-2231. [DOI] [PubMed] [Google Scholar]
- 21.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marolda C L, Valvano M A. Identification, expression, and DNA sequence of the GDP-mannose biosynthesis region encoded by the O7 rfb gene cluster of strain VW187 (Escherichia coli O7:K1) J Bacteriol. 1993;175:148–158. doi: 10.1128/jb.175.1.148-158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall K C. Glossary. In: Marshall K C, editor. Microbial adhesion and aggregation. New York, N.Y: Springer-Verlag; 1984. pp. 397–399. [Google Scholar]
- 24.Merino S, Camprubí S, Albertí S, Benedí V J, Tomás J M. Klebsiella pneumoniae resistance mechanisms to complement-mediated killing. Infect Immun. 1992;60:2529–2535. doi: 10.1128/iai.60.6.2529-2535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merino S, Rubires X, Aguilar A, Tomás J M. The role of O1-antigen in the adhesion to uroepithelial cells of Klebsiella pneumoniae grown in urine. Microb Pathog. 1997;23:49–53. doi: 10.1006/mpat.1997.0132. [DOI] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 27.Nevola J J, Laux D C, Cohen P S. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant. Infect Immun. 1987;55:2884–2990. doi: 10.1128/iai.55.12.2884-2890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid G, Sobel J D. Bacterial adherence in the pathogenesis of urinary tract infections: a review. Rev Infect Dis. 1984;9:470–487. doi: 10.1093/clinids/9.3.470. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measure cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 30.Rubirés X, Saigí F, Piqué N, Climent N, Merino S, Albertí S, Tomás J M, Regué M. A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J Bacteriol. 1997;179:7581–7586. doi: 10.1128/jb.179.23.7581-7586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo T A, Brown J J, Jodush S T, Johnson J R. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence of an extraintestinal isolate of Escherichia coli. Infect Immun. 1996;64:2343–2348. doi: 10.1128/iai.64.6.2343-2348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saigi F, Climent N, Piqué N, Sanchez C, Merino S, Rubirés X, Aguilar A, Tomás J M, Regué M. Genetic analysis of the Serratia marcescens N28b O4 antigen gene cluster. J Bacteriol. 1999;181:1883–1891. doi: 10.1128/jb.181.6.1883-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnaitman C L, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugiyama T, Kido N, Komatsu T, Otha M, Jann K, Jann B, Saeki A, Kato N. Genetic analysis of Escherichia coli O9 rfb: identification and DNA sequence of phosphomannomutase and GDP-mannose pyrophosphorylase genes. Microbiology. 1994;140:59–71. doi: 10.1099/13500872-140-1-59. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama T, Kido N, Kato Y, Koide N, Yoshida T, Yokochi T. Evolutionary relationship among rfb gene clusters synthesizing mannose homopolymer as O-specific polysaccharides in Escherichia coli and Klebsiella. Gene. 1997;198:111–113. doi: 10.1016/s0378-1119(97)00300-4. [DOI] [PubMed] [Google Scholar]
- 38.Taylor P W. Bacterial resistance to complement. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C.: American Society for Microbiology; 1988. pp. 107–120. [Google Scholar]
- 39.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomás J M, Camprubí S, Merino S, Davey M R, Williams P. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun. 1991;59:2006–2011. doi: 10.1128/iai.59.6.2006-2011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 43.Vukajlovich S W. Antibody-independent activation of the classical pathway of human serum complement by lipidA is restricted to Re-chemotype lipopolysaccharide and purified lipidA. Infect Immun. 1986;53:480–485. doi: 10.1128/iai.53.3.480-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 45.Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 1995;3:178–185. doi: 10.1016/s0966-842x(00)88917-9. [DOI] [PubMed] [Google Scholar]