Abstract
Eating disorders (anorexia nervosa, bulimia nervosa, and binge-eating disorder) are a heterogeneous class of complex illnesses marked by weight and appetite dysregulation coupled with distinctive behavioral and psychological features. Our understanding of their genetics and neurobiology is evolving thanks to global cooperation on genome-wide association studies (GWAS), neuroimaging, and animal models. Until now, however, these approaches have advanced the field in parallel, with inadequate crosstalk. This review covers overlapping advances in these key domains and encourages greater integration of hypotheses and findings to create a more unified science of eating disorders. We highlight ongoing and future work designed to identify implicated biological pathways that will inform staging models based on biology as well as targeted prevention and tailored intervention and galvanize interest in developing pharmacologic agents that target the core biology of the illnesses for which we currently have few effective pharmacotherapeutics.
Keywords: eating disorders, genomic, GWAS, metabolic, psychiatric
Eating disorders are severe psychiatric disorders that, for many, evolve into chronic or fluctuating conditions with serious adverse outcomes1. Among adolescents and young adults, the disability-adjusted life years (DALYs) for anorexia nervosa (AN) and bulimia nervosa (BN) are among the highest of all psychiatric disorders2. Binge-eating disorder (BED) and other specified feeding and eating disorders have yet to be included in estimates, but are projected to account for the majority of global disease burden of eating disorders3. Although the disorders occur in pure forms, although diagnostic crossover across the course of illness is common4. For a brief definition of the symptoms and epidemiology of the three primary eating disorders, see Box 1.
Box 1: Overview of Eating Disorder Phenotypes.
Anorexia nervosa.
The hallmark symptom of AN is low body weight, accompanied by persistent restriction of energy intake and/or increased energy expenditure, intense fear of gaining weight or persistent behavior preventing weight gain, and distorted body image1. Both a restricting and a binge-eating/purging subtype exist. The lifetime prevalence is 1.4% (0.1-3.6) for women and 0.2% (0-0.3%) for men%)131. The standardized mortality ratio of AN is >5 132 with deaths primarily attributable to illness effects and suicide133. Treatment outcome in adults is poor with only 30% achieving full recovery134. Family-based treatment for youth is recommended; multidisciplinary interventions are recommended for adults although the evidence base remains particularly weak135. No medications exist that successfully treat the disorder or are approved by the FDA135.
Bulimia nervosa.
The core symptoms of BN include binge eating (eating an unusually large amount of food in a circumscribed period of time accompanied by a sense of loss of control) and inappropriate compensatory behaviors (e.g., self-induced vomiting, abuse of laxatives, diuretics, fasting, excessive exercise), with self-evaluation being strongly influenced by shape and weight1. BN can occur at all body weights, but is diagnosed as AN binge-eating/purging subtype in the presence of AN. The lifetime prevalence is 1.9% (0.3-4.6%) for women and 0.6% (0.1-1.3%) for men131, and onset is typically in late adolescence or early adulthood. Cognitive-behavioral therapy (CBT) is the leading evidence-based treatment and fluoxetine, whose efficacy was established in placebo-controlled clinical trials in the early 1990s, is the only FDA approved medication135 BN recovery rates are ~50% at 5-10 years follow-up136.
Binge-eating disorder.
BED is marked by recurrent binge eating that causes distress, the absence of regular compensatory behaviors, and behavioral and emotional features such as eating rapidly or when not hungry, and feeling embarrassed or disgusted by one’s behavior1. The lifetime prevalence is 2.8% (0.6-5.8%) for women and 1.0% (0.3-2.0%) for men131. Onset is typically early adulthood but can be at any time and in individuals spanning the normal to obese weight ranges1. Evidence-based treatments for BED include CBT, second-generation antidepressants, and since 2015, lisdexamfetamine an FDA approved stimulant originally prescribed for the treatment of attention deficit hyperactivity disorder137. Preclinical, clinical, genetic studies (of binge eating), and neuroimaging data converged on dysfunction in systems regulating eating behavior and reward (i.e., dopaminergic and noradrenergic), and support the repositioning of lisdexamfetamine in BED138.
Biological effects within eating disorders range from the subcellular (genetic variants and their effects on gene expression and structure), through the cellular (signaling) and intercellular (neurons and neuronal circuits), to organismal effects (eating disorders and disorder-related behaviors)5. These levels of biology are interconnected, but are investigated using different scientific approaches, with limited crosstalk to date. Integrating results from different approaches is vital. A hierarchically connected research approach, reflecting the biological interconnectivity, will provide a more complete understanding of the biology of eating disorders, providing the foundations for developing much-needed novel treatments. In this review, we describe the state of the science across biological levels and approaches and conclude by discussing progress towards this more integrated understanding.
Human Genetics of Eating Disorders
Twin-based heritability.
Large-scale twin studies yield heritability estimates ranging from 0.28 to 0.74 for AN6, with imprecision reflecting varying definitions of illness and sample size, with more severe definitions associated with higher heritabilities7. Heritability estimates range from 0.55 to 0.626 for BN and 0.39 to 0.45 for BED6.
GWAS.
Candidate gene and linkage literature in eating disorders was rife with unreplicated small-sample studies and was minimally informative beyond signaling the likely complexity of their genetics6. We take as a starting point the most recent genome-wide association study (GWAS) of AN performed by the Eating Disorders Working Group of the Psychiatric Genomics Consortium (PGC-ED)8. Combining existing samples9 with the Anorexia Nervosa Genetics Initiative (ANGI)10 yielded a European ancestry dataset including 16,992 AN cases and 55,525 controls, from 17 countries, and identified 8 genome-wide significant loci, including 4 single-gene loci: CADM1, MGMT, FOXP1 and PTBP28. Analysis of tissue enrichment for AN-associated genes revealed a significant association with the central nervous system, and comparison to single-cell gene expression datasets from mice revealed significant associations with hippocampal pyramidal neurons and striatal medium spiny neurons. Song et al.11 further identified that AN-associated genes were enriched in the prefrontal cortex.
Genetic correlations implicate psychiatric, education, and metabolic factors in AN.
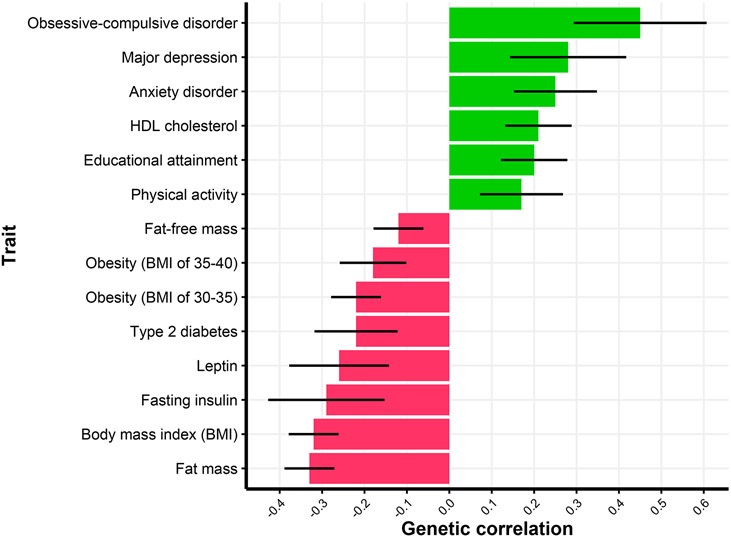
Genetic correlations (SNP-rg) between AN and psychiatric disorders such as obsessive-compulsive disorder (OCD), major depressive disorder (MDD), anxiety, and substance-related disorders 8,12 correspond with clinical observations of comorbidity13 and twin studies14-16. Building on this work, AN and OCD risk genes pinpointed through GWAS showed similar prefrontal cortex expression alterations meaning that these disorders may have similar functional pathways11. A novel positive SNP-rg with objectively measured physical activity was found in the PGC-ED GWAS8 and may relate to the driven exercise seen in AN. Significant positive SNP-rg with educational attainment was not seen for IQ (Figure 1).
Figure 1. Genetic correlations between anorexia nervosa and selected top traits.
The error bar represents the 95% confidence interval. Values retrieved from8.
Earlier results hinted at a significant negative genetic correlation between AN and body mass index (BMI)9,17. The PGC-ED GWAS revealed significant genetic correlations with metabolic, lipid, and anthropometric traits, suggesting that metabolic mechanisms may drive physiological resistance to healthy body weight in AN8. The observed correlations were not confounded by the genetics of BMI—a concern given that BMI is a component of the AN diagnosis8. Likewise, exploratory Mendelian randomization (MR) analyses in the PGC-EDs GWAS indicated a bidirectional causal relationship between AN and low BMI. The authors concluded that both metabolic and psychological factors may need to be considered to fully understand AN and that doing so may hasten the development of more effective treatments. Based on the strong associations with psychiatric traits, physical activity, educational attainment, and metabolic traits, multivariate GWAS analyses (e.g., genomic structural equation modeling) could offer novel opportunities to disentangle common and specific genetic effects and help boost power for discovery.
Polygenic scores.
Polygenic scores (PGS) summarize genome-wide data into a single variable of genetic liability to the phenotype—as a sum of the number of risk alleles present in the individual weighted by the SNP effects from GWAS. Higher PGS for AN derived from the 2019 PGC-ED GWAS were associated with increased odds of AN, and equally in females and males8. Further, PGS of age of onset of AN predicted age at onset in independent cohorts18. A rich body of literature applying PGS is emerging, with multiple studies reporting that BMI and psychiatric PGS influence childhood and adolescent disordered eating behaviors19-21. PGS for other phenotypes may also differentiate among AN, BN, and BED on the genomic level. UK Biobank analyses revealed strong positive associations between binge-type eating disorders (BN and BED) and anthropometric PGS (e.g., overweight and waist circumference), suggesting that binge eating may share genomic variants with overweight and obesity. In contrast, in AN, the direction of these associations was reversed22.
We anticipate increasing evidence that PGS will predict developmental phenotypic manifestations of illness (i.e., premorbid behavioral traits, course of illness) and gene-environment associations, and ultimately inform risk assessment and clinical applications. Presently, clinical utility is limited23,24.
Cross-disorder GWAS.
A cross-disorder GWAS of eight psychiatric disorders (AN, ADHD, autism spectrum disorder, bipolar disorder, MDD, OCD, schizophrenia, and Tourette’s syndrome) revealed 109 pleiotropic loci (i.e., signals for at least two disorders), of which eight had signals for AN25. The top locus (18q21.2) was associated with all disorders, has previously been implicated in MDD and neuroticism, and encodes the netrin 1 receptor gene (DCC) that regulates axon outgrowth. A joint genomic structural equation model showed that AN loaded onto a factor with OCD and Tourette’s syndrome26. These findings suggest overlapping genetic risk across disorders and that work needs to be done to separate specific from non-specific susceptibility variants, and the association of specific variants with cellular gene expression datasets to generate testable hypotheses for their ultimate impact on brain circuit function. Other cross-disorder GWAS on AN/OCD have yielded promising insights but require larger samples to pinpoint risk loci (i.e.,27).
Mendelian randomization (MR).
MR studies treat genetic variants as unconfounded proxies of an exposure of interest to evaluate the causal effect of an exposure on a phenotypic outcome. However, MR analyses investigating the causal effects of AN on other traits are presently limited in power. As stated earlier, a bidirectional causal association between AN and low BMI was found in the PGC-ED GWAS8. An MR study on the ALSPAC cohort (n = 4,473) suggested possible causal effects of genetically predicted childhood BMI on binge eating and weight and shape concern, and of binge eating and overeating on BMI28. In another study, adiponectin, a fat-tissue derived hormone, showed evidence consistent with a causal effect on eating disinhibition but not eating restraint29. Adams et al.30 found evidence consistent with a protective effect of fasting insulin level (n = 108,557) on AN (n = 72,515) (OR = 0.48, 95% CI: 0.33, 0.71), but not for fasting glucose or glycated hemoglobin (HbA1c) on AN. Future MR studies will help to disentangle the nature of pleiotropy and shed light on causal exposure-outcome effects.
Rare mutations and copy number variants.
No Mendelian forms of eating disorders have been identified and efforts to find rare genetic variants have not yet been successful. In an exome-chip based GWAS in 2,158 AN cases and 15,327 controls31, 16 independent variants were taken forward for in silico and de novo replication. No findings reached genome-wide significance. Scott-Van Zeeland et al.32 conducted a series of targeted sequencing and genotyping studies focusing on 152 candidate genes in 1,205 AN cases and 1,948 controls, implicating variants in the estrogen receptor-b (ESR2) and epoxide hydrolase 2 (EPHX2) genes. A smaller exome sequencing study of 93 AN cases reported an enrichment of rare variants in neuropeptide/neurotrophic pathways33. Combined linkage and sequencing of densely affected families with multiple AN or BN cases has been reported34,35. Results of these studies are tentative as sufficient sample sizes to identify rare point mutations have yet to be attained.
The situation is similar for genome-wide analyses of copy number variants (CNVs). Wang et al.36 found no evidence of CNV enrichment in AN cases compared to controls. Yilmaz et al.37 analyzed 1,983 female AN cases38 seeing no occurrence of well-established pathogenic CNVs for neurodevelopmental disorders (1q21.1; 2p16.3; 3q29; 7q36.3; 13q12; 15q11.2; 15q13.3; 16p11.2(1); 16p11.2(2); 17q12; or 22q11.21). Chang et al.39 reported association of a 15q11.2 microduplication with AN, but cautioned that the results require validation. Currently, there is no credible evidence that novel or known large effect size CNVs influence AN. Much larger sample sizes may be required before we can exclude a role for CNVs carrying moderate effect sizes.
Limitations of human genetic studies.
Several limitations in the field should be considered. First, to our knowledge, no GWAS of bulimia nervosa, binge-eating disorder, purging disorder, atypical AN, avoidant/restrictive food intake disorder (ARFID), pica, or rumination disorder have been published. Second, for AN, genome-wide signals presently account for a low proportion of phenotypic variance (1.7%), while gene mapping and gene expression results presently rely on limited Hi-C, eQTL, and brain cell data. Larger sample sizes are required to improve statistical power and detect more susceptibility loci. Large, systematic sequencing studies are required to assess the role of rare variants and to complement common variant GWAS. Finally, although eating disorders are likely under-detected in males, the gender ratio is definitely tipped in the direction of females. Accruing larger samples sizes of afflicted males is essential for identifying genetic sex differences and is a priority for future research.
Integration.
Genomic discovery in eating disorders is underway and early discoveries highlight new directions for deepening our understanding of some of the more perplexing facets of AN (e.g., extreme weight dysregulation, the frequent re-loss of therapeutically restored weight, compulsive exercise). The PGC is expanding genomic samples of AN, BN, and BED with efforts such as the Eating Disorders Genetics Initiative (EDGI)40. Novel biological discoveries are anticipated to accelerate therapeutic opportunities in drug discovery, the repositioning of current drugs, and our understanding of pleiotropy including opposing effects that may mitigate unintended off-target effects. The availability of biobanks with genetic and electronic health record data offer opportunities for extending genetic associations to other medical phenotypes not currently available. As with many illnesses, global cooperation and harmonization of methods boost sample sizes and accelerate science. Clearly, collaboration with researchers in other fields will increase the utility of GWAS results and integrate them into a unified science of eating disorders. This unification can be achieved through targeting dimensional phenotypes and symptoms and generating hypotheses for testing the causal impact of genomic variants on neural circuit function.
Eating disorders GWAS have focused primarily on diagnoses; however, exploring relevant dimensions or core symptoms may enhance translation to approximate phenotypes in animal models. Fine-mapping approaches are required to delve deeper into trait-associated regions to identify specific variants or genes that causally influence the target trait. Clearly describing the strength of evidence for the functional involvement of specific genes and variants in eating disorders will provide strong targets for causal validation and neurobiological exploration.
Neurobiology of genes implicated in AN GWAS.
Although too recent to have influenced neurobiological studies directly, several genes implicated by the latest AN GWAS have been studied in relation to other traits and show neurobiological relevance to eating disorders. For example, FOXP1 haploinsufficiency is a rare form of intellectual disability, related to autism spectrum disorder41. Haploinsufficiency of FOXP1 is associated with neurodevelopmental impairments, but also with feeding difficulties and gastrointestinal disturbance in humans and in mice, supporting FOXP1 in AN41. Conditional Foxp1 knockout studies in the mouse brain implicate neurogenesis and neural migration, particularly the development and functioning of medium spiny neurons and pyramidal neurons. Both cell types have been implicated as relevant neuronal cell types by systems biological analyses of the AN GWAS41.
As well as being implicated in AN, CADM1 and PTBP2 have also been implicated in GWAS of BMI42. PTBP2 encodes a neuron-specific RNA binding protein that organizes axonogenesis in the developing cortex, relevant across psychiatric disorders43. By comparison, CADM1 has not been associated with psychological or behavioral traits except BMI and AN (although family member CADM2 has been implicated in numerous traits related to impulsive behavior)44. CADM1 encodes a synaptic cell adhesion molecule. The BMI-associated variants appear to increase the expression of CADM1 in the human hypothalamus and cerebellum, and parallel experiments in mice suggest this increased expression contributes to weight gain, potentially through CADM1-positive innervation of POMC neurons45. CADM1 is also involved in the neural control of first estrous in mice, which is of particular interest given amenorrhea in AN1. MGMT, which encodes a DNA alkyltransferase, is the most well-studied of the genes implicated in the AN GWAS, as it is involved in DNA repair and protection against cancer46. The biological pathways linking DNA repair dysfunction to AN is unclear, although recent research suggests that postmitotic neurons require recurrent DNA repair that is relevant to neuronal dysfunction47,48. Assuming future GWASs support MGMT as a causal gene for AN, its role is worthy of future investigation.
Identifying conserved, eating disorder-relevant genes is currently limited by underpowered phenotype-driven analyses in humans and rodents. Neuroscience typically focuses on a different level of the biological hierarchy than statistical genetics. As such, deeper insight may be gained through examining the neuronal circuitry implicated directly through neuroscientific approaches and indirectly through GWAS-based approaches. GWAS-based approaches use information on cell-type specific genome biology, such as epigenetic markers49 or gene expression50. These methods show that genes associated with AN are on average specifically expressed in brain tissues, especially in medium spiny neurons and CA1 hippocampal pyramidal neurons8. Results from these methods implicate the same cell types in other psychiatric disorders and are supported by functional studies in rodents. Differential vulnerability to ABA in female C57BL/6J mice was associated with GABAergic inhibition of hippocampal CA1 pyramidal cells51. Prolonged binge-like consumption of sucrose has been shown to alter the morphology of medium spiny neurons in the nucleus accumbens of rats52. However, beyond broad support for brain tissues in general, most circuits implicated in neurobiological studies of mice and of humans are poorly supported by these GWAS-based approaches, most likely due to limitations of power and insufficient data from appropriate tissues. For example, in contrast to the neurobiological evidence supporting a role for DA circuitry in AN, there is no such evidence from AN GWAS8. However, evidence for genetic variants affecting the DA system in eating disorders may emerge as the power of eating disorder GWAS increases. Both of these limitations are being overcome through increasing GWAS sample sizes and via collaborative brain mapping initiatives like the PsychENCODE project53.
Human Neuroimaging in Eating Disorders
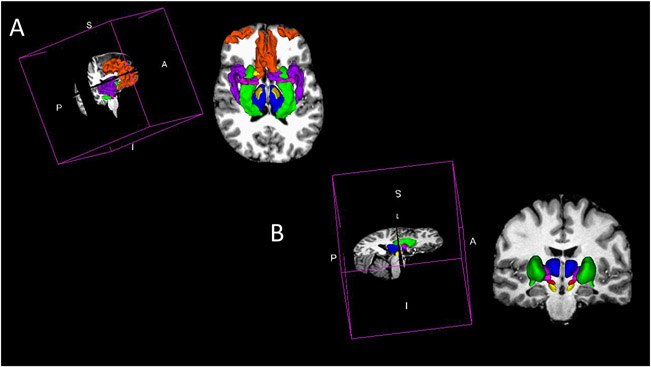
Neuroimaging research in eating disorders has been motivated by (1) prior work in animal models which study microcircuitry involved in homeostatic and hedonic eating pathways and (2) genetic/GWAS studies that have identified shared functional networks that exhibit overlapping phenotypes (cortico-striatal-thalamo-cortical pathways) (Figure 2).
Figure 2.
Human neuroimaging circuitry involved in eating disorders. (A) Represents the structures comprising two major dopaminergic pathways, mesolimbic and mesocortical pathways, supported by prior work in animal models. Both originate in the ventral tegmental area (red); mesolimbic pathways project to the nucleus accumbens, and is part of the complex circuit involving the amygdala (pink), hippocampus (green), and the bed nucleus of the stria terminalis (yellow). The mesocortical pathway projects primarily to the prefrontal cortex (orange) and insula (purple). (B) Represents sub-structures involved in the cortico-striatal-thalamo-cortical (CSTC) pathway that are supported by recent genetic/GWAS studies with shared functional networks that exhibit overlapping phenotypes. The CSTC pathway is a multi-synaptic neuronal circuit that connects the cortex with the striatum and thalamus. The striatum (green) receives glutamatergic input from the cortex and the thalamus (blue) sends out GABAergic inputs to the sub-thalamic nucleus (pink, purple, red).
Hedonic reward pathways - Mesocorticolimbic and mesolimbic networks.
The brain reward system is well-defined and plays a central role in the drive to eat. Mesocorticolimbic and mesolimbic pathways project from the ventral tegmental area (VTA) to the cerebral cortex (frontal, cingulate, and entorhinal cortex) and limbic structures (ventral striatum, hippocampus, and amygdala), respectively. Importantly, these systems are largely intertwined and overlapping54, and assigning specific brain regions to one clinical phenotype (e.g., restriction) or characteristic (e.g., cognitive control) is unhelpful as they act in unison. Collectively, mesocorticolimbic and mesolimbic pathways are responsible for cognitive functions, reward, emotion, and motivation, which may represent transdiagnostic factors underlying AN, BN, and BED55,56.
Early studies focusing on understanding the neurobiology of eating disorders suggested that there were pathological alterations in monoamine neurotransmitter systems, specifically DA and 5-HT56. These align with other works that have strongly implicated the reward system in pathological changes in hedonic eating and decision making. The VTA comprises a cluster of dopamine (DA) producing neurons that play a key role in positive and negative reinforcement, decision making, working memory, incentive salience, and aversion. Dopaminergic VTA neurons innervate corticolimbic regions via the mesocortical pathway, forming the mesocorticolimbic reward network. A subpopulation of midbrain DA neurons projecting to the striatum co-release glutamate and GABA onto their target neural substrates57. Understanding the structural and functional connectivity of these reward circuits in eating disorders has been a central focus for neuroimaging investigations highlighted below.
Functional neuroimaging studies suggest altered processing of rewarding and aversive food stimuli in acute and recovered AN58,59. In binge-type eating disorders (BN, BED), functional neuroimaging findings are less consistent60,61. Positron emission tomography (PET) data in individuals recovered from AN show increased striatal dopamine receptor (D2/D3) binding, which was also related to striatal responses during monetary choices and self-reported trait anxiety62. Although, two PET studies focusing on individuals with acute63 and weight restored AN64 compared to healthy controls, found no difference in DA receptor binding. PET studies in individuals with BN have identified decreased striatal dopamine transporter availability65. In patients with BN, increases in glutamate signaling receptors (metabotropic glutamate receptor subtype 5; mGlu5) were higher in the anterior cingulate cortex (ACC), subgenual prefrontal cortex, and straight gyrus compared with controls66. Structural neuroimaging studies in AN and BN reveal volumetric abnormalities in the insula67. Further, lower white matter measures of axonal integrity (measured via fractional anisotropy) and increased structural white matter connectivity between the insula and orbital frontal cortex suggest that altered processing of taste perception may be present across eating disorders. The insular cortex extends beyond taste function and is a center of body awareness, integrating autonomic, cognitive, and affective processing68 of the homeostatic state of the body. Both structural and functional neuroimaging studies highlight deficits in the insula and abnormal interoceptive activity in AN69,70 and BN71.
Cognitive control and habitual responding—cortico-striatal-thalamo-cortical pathways.
The cortico-striatal-thalamo-cortical (CTSC) pathway is a brain circuit that controls movement execution, habit formation, and reward, all of which have been hypothesized to be relevant to eating disorders. Hyperactivity throughout the CTSC circuits is believed to underlie OCD72, increasingly relevant given the strong positive genetic correlation between OCD and AN8. Overlapping CTSC loops, including lateral PFC, ACC, dorsal striatum, the presupplementary motor area, insula, and parietal regions, are involved in these processes.
In patients with BN, there are limited neuroimaging data to suggest that functional and structural alterations in control circuits occur early in the course of BN and may contribute to the disorder’s persistence over time73,74. In patients with AN, maladaptive excessive self-control is commonly described although the differences in underlying neurobiological correlates (i.e., structures) involving cognitive control are inconsistent in imaging studies75,76. Both AN and BN patients show reduced gray matter volume in caudate nucleus, ACC, and insula77; however, these results may normalize in AN and BN following successful treatment78.
Limitations.
Deficits in cognitive control, executive functioning, reward and affective processing are commonly reported across multiple psychiatric disorders, with similar neurobiological correlates reported in the dorsal lateral prefrontal cortex, insula, and dorsal anterior cingulate cortex79-81. A limited number of studies compare neuroimaging across psychiatric disorders (e.g.,82,83) or use meta-analytic techniques to do so (e.g.,79,80). Those that have been conducted have not included eating disorder patients despite evidence of psychiatric multimorbidity (including mood, substance use, and anxiety disorders)84. Eating disorder exclusion may occur because of state-specific neurobiological alterations due to malnourishment or dehydration stemming from key eating disorder specific behaviors (e.g., caloric restriction, purging, excessive exercise). Failure to account for state-specific effects has stymied human neuroimaging research in eating disorders. Eating disorder specific recommendations from experts in the fields of eating disorders and neuroimaging85 have been proposed to account for malnutrition effects. Importantly, novel experimental designs are essential to disaggregate the extent to which any observed neurobiological alterations in AN are truly related to disease etiology or more accurately attributed to the impact of prolonged malnutrition/starvation. Accordingly, re-evaluation of prior eating disorder neuroimaging findings accounting for effects of malnourishment (e.g., white-matter alterations in AN86 and BN87) has led to improved understanding of state vs. trait effects in the brain.
Integrating human genetics and neuroimaging.
Imaging and genetics target distant levels of the biological hierarchy, and so integration is challenging. Genetically informed models of eating disorders hold the potential to generate construct-valid neurobiological disease models. Direct examination of the effect of genetic variation on brain structure and function is underway through the ENIGMA consortium88. Further insights could be obtained by examining intermediate levels of the biological hierarchy. Cellular-level data can be inferred from eating disorder GWAS—this has implicated hippocampal pyramidal neurons and striatal medium spiny neurons as the cell types that express identified genes in AN8. However, this approach remains in its infancy—larger sample sizes for GWAS and for gene expression datasets in neuronal and non-neuronal cells (e.g., microglia, oligodendrocytes) are needed to provide sufficient power for the parallel implication of brain circuits from neuroimaging and from GWAS.
Animal Models for Eating Disorders
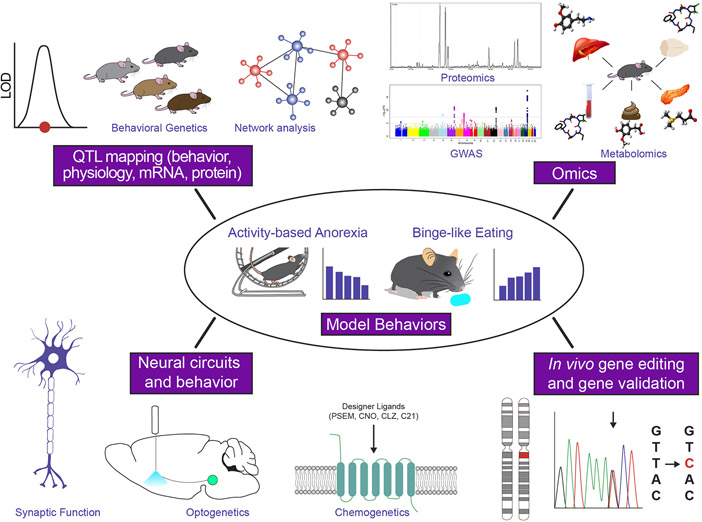
As eating disorders GWAS become larger and more robust, their ability to interconnect with animal models will increase. Animal models offer speed in achieving power, control over allele frequency and genetic background, environmental exposure, and recording and collection of the appropriate cell types and tissues at appropriate time points to study the dynamic genetic architecture and genomic adaptations across eating disorder progression and recovery. Two popular animal models for eating disorder are the activity-based anorexia (ABA) model and the binge-like eating (BLE) model (see Box 2). Genes, variants, and circuit mechanisms gleaned from animal studies can be discovered and validated first within the same species and genetic background and then in humans. Likewise, identified genes from human GWAS and proposed circuitry from human neuroimaging studies can also be tested for causality and function in animal models, potentially on multiple genetic backgrounds (Figure 3).
Box 2: Animal Models for Eating Disorders.
Activity-based anorexia (ABA).
AN has some characteristics that cannot be modeled in rodents (drive for thinness, body image distortion, intense fear of weight gain). However, key behavioral components of AN as well as traits commonly comorbid with AN can be modeled in mice (high physical activity, anxiety, depression, social anxiety, obsessive-compulsive-like behavior). Perhaps most well-known is the activity-based anorexia (ABA) model that starts with daily unlimited chow availability but for only 2h per day or less followed by introduction of a running wheel which leads to compulsive-like running, appetite suppression, voluntary restriction of food intake, decreased anxiety-like behavior, severe weight loss, and death without intervention139. ABA induces other AN-like effects on physiology, including hypothermia, loss of estrus, increased HPA axis activity, anhedonia, ulcers, and humoral, CNS, cardiovascular, and GI dysfunction140—a credible model for the behavioral and physiological components of AN.
Binge-like eating (BLE).
Binge eating shares several features with substance use disorders—tolerance, withdrawal, relapse, loss of control, and compulsive intake. Binge-like eating (BLE) has been studied in animals141, defined by increased intake of palatable food (PF; high caloric) versus chow and escalation of intake over time142. Compulsive-like eating refers to habitual, often increased effortful intake despite potential harm (aversive stimulus) that can relieve negative affect during withdrawal143. Home cage chow restriction, stress exposure, and limited, intermittent access to PF can all promote BLE144. Intermittent BLE models induce the most robust binge-like eating, yet do not typically induce obesity, as rodents voluntarily restrict chow intake in anticipation of PF which in turn, increases PF reinforcing efficacy145,146. BLE is a reasonable animal approximation of the behavioral components of dysregulated eating characteristic of human binge eating.
Figure 3.
Forward and backward translation of eating disorder-relevant traits at different levels of biological hierarchy. Activity-based anorexia (ABA) and binge-like eating (BLE) are behavioral models for restrictive eating in AN and for binge eating in BN and BED. Within the use of animal models, there are four primary approaches that seek to elucidate the fundamental biology of eating disorders: QTL mapping, Omics, neural circuit manipulations, and in vivo gene editing. QTL mapping is used to discover genetic loci and ultimately candidate causal genes and variants that regulate phenotypic traits at the molecular, cellular, or behavioral level. QT mapping capitalizes on natural variation across different strains or substrains of laboratory models like mice. Omics investigations are carried out at the genomic, transcriptomic(not shown), proteomic, microbiomic, or metabolomic levels and can reveal novel biological pathways that regulate feeding and/or metabolism. In vivo gene editing research can be used to establish causality for candidate genomic variants identified from rodent QTL/GWAS or in silico studies. Neural circuit approaches are also used to establish causal molecular, cellular, or circuit elements that drive eating behavior and metabolism. They span the range of observing neural activity and synaptic function, manipulating those circuits in real time (e.g., via optogenetic. chemogenetic, or pharmacological approaches), and layering these experiments with pathological models.
Phenotype-driven genetics of ABA.
Various mouse strains exhibit differential susceptibility to ABA. Specifically, DBA/2J female mice show greater wheel running activity, greater weight loss, less food intake, and severe hypoleptinemia compared to C57BL/6J89. C57BL/6J showed greater ABA than A/J and multiple chromosome substitution strains90. A genetic correlation between baseline physical activity (wheel running, locomotor) and ABA across inbred strains91 identified physical activity as a potential premorbid trait that predicts the development of ABA. Despite inbred strain differences in ABA, causal genetic factors have yet to be found.
Phenotype-driven genetics of BLE.
Phenotypic differences in BLE between inbred rodent strains implicate underlying causal genes92,93. In an intermittent, limited access paradigm for BLE of sweetened PF, cytoplasmic FMR-interacting protein 2(Cyfip2) was mapped and validated in a cross between C57BL/6 substrains. Cyfip2+/− mice showed a decrease in compulsive BLE of PF but not chow94. Cyfip1+/− mice also showed modulation of BLE, depending on parent-of-origin, genetic background, and sex95. CYFIP1 CNV is implicated in autism, ADHD, psychosis96, and, possibly, AN although lab validation of the CNV calling failed39. The study of BLE with increased genetic diversity, additional diets (high fat diet), and environmental variables will increase gene identification. In support, a recent study involving a cross between the BLE-prone DBA/2J strain with the BLE-resistant C57BL/6J strain92 identified multiple QTLs influencing eating behavior and body weight, including a sex-combined QTL containing the candidate gene Lcorl that influences initial consumption of palatable food, a female-selective locus containing the candidate gene Zeb1 underlying changes in body weight during BLE, and a male-selective locus for escalation in palatable food intake that contains the candidate genes Adipor2 and Plxnd197.
Given the diverse array of mouse crosses and populations that are now available to accelerate gene mapping, forward genetic studies of ABA and BLE in rodents provide the opportunity for novel gene/pathway identification. Such studies are sorely lacking. Nonetheless, CYFIP1 and CYFIP2 have homologs to study in humans. Although neither of these genes has yet been associated with AN through GWAS8, ongoing GWAS of BED and BN will be more relevant for assessing the association between CYFIP genes and binge eating in humans.
Omic studies of ABA.
Omics analysis has been applied to both brain tissue and gut microbiota to improve our understanding of molecular adaptations associated with ABA and BLE. Proteomic studies in ABA models have identified hypothalamic mitochondrial and autophagy processes98, deficits in energy metabolism in colonic mucosa99, and an increase in ATP-producing glycolytic enzymes in gut microbiota100. These results implicate an adaptive energy source in the gut that could influence brain function and feeding behavior in AN. Opposing changes in energy utilization between the hypothalamus and gut mucosa during ABA suggest that restoration of energy homeostasis between the CNS and periphery could improve treatment of AN in humans.
Animal models can be used to longitudinally measure the impact of early life factors like stress, diet, or genetics on eating disorders. In a mouse model involving chronic, variable mild stress in pregnant dams, prenatal stress (PNS) induced transcriptomic indices of hypothalamic HPA and metabolic dysfunction associated with obesity and protected against ABA in adolescent female mice. PNS protection was associated with increased DNA methylation and placental miR-340 downregulation and upregulation of miR-340 targets101. Low miR-340 and ABA resistance were also associated with increased expression of SLC nutrient transporters (amino acids, glucose), and growth factors that are potentially regulated by miR-340 targets. Placental overexpression of miR-340 recapitulated fetal and adolescent hypothalamic and circadian dysfunction101, supporting a role for placental miR-340 in regulating nutrient availability that could influence eating disorder susceptibility.
Omics of BLE.
Transcriptome analysis of the striatum found a BLE-induced downregulation of myelination genes94, supporting reports of decreased white matter integrity with increased BE in individuals with BN102. Gut microbiota from male rat feces following intermittent access to energy-rich “cafeteria” showed changes in microbial flavonoid, bile acid, d-arginine, d-ornithine, fatty acid, and geraniol biosynthetic pathways that correlated with body weight, adipose tissue, glucose, leptin, and insulin103. Although similar studies are needed in humans, these data suggest that microbial pathways could be targeted to normalize eating and metabolic function in BE.
To summarize, omics analysis of relevant tissues at appropriate time points in animal models for eating disorders offer distinct, complementary advantages to human studies and will continue providing unique insights into the hedonic and homeostatic adaptations that could inform therapeutics. Furthermore, combining omics with phenotype-driven forward genetic studies can further inform mechanisms of gene dysfunction and the consequent genomic adaptations that drive and sustain disordered eating. Expanding these latter approaches would provide valuable triangulation of omics studies in humans.
Viral overexpression and neural circuit studies of ABA.
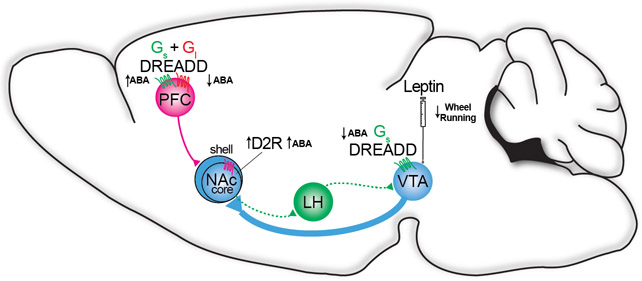
Contemporary circuit approaches to understand feeding suppression and stimulation have been reviewed elsewhere (e.g.,104-106). Here, we distinguish our discussion from the physiology of homeostatic meal termination—adaptive anorexigenic responses—and focus on related studies involving ABA that more holistically model AN pathology and behavioral phenotypes. These studies provide more direct evidence as to the associative and causal neurobiological factors of AN. Viral overexpression of the D2 dopamine receptor in D2-containing mouse neurons of the NAc core induced hyperactivity, increased food intake, and enhanced ABA-induced wheel running and weight loss in females107. Only females showed severe weight loss during scheduled fasting alone, even without a change in food intake or hyperactivity. More recently, chemogenetic activation inhibition of the projection from medial PFC to NAc shell decreased cognitive flexibility and increased ABA whereas inhibition prevented ABA weight loss and increased cognitive flexibility108. Zhang and Dulawa propose that projections from the NAc shell to the lateral hypothalamus (LH) and in turn, from the LH to the VTA, could link ABA-induced mesocorticolimbic reward dysfunction with ABA-induced changes in metabolism109 (Figure 4).
Figure 4. Mesocorticolimbic reward dysfunction in activity-based anorexia.
VTA: ventral tegmental area; NAC: nucleus accumbens; LH: lateral hypothalamus; mPFC: medial prefrontal cortex; DREADD = designer receptor exclusively activated by designer drugs; Gi = G inhibitory DREADDS; Gs = G stimulatory DREADDS; D2 = D2 dopamine receptor overexpression. Green indicates excitation of cell type and pathway. Red indicates inhibition of pathway. Purple indicates overexpression. Overexpression of D2 dopamine receptors in medium spiny neurons of NAc core increased ABA phenotypes and combined with scheduled fasting alone (no wheel running), was sufficient to induce weight loss and glucose intolerance in females without affecting food intake107. Chemogenetic activation of the mPFC->NAc shell pathway decreased cognitive flexibility and increased ABA; inhibition had the opposite effect108. Chemogenetic activation of VTA neurons decreased ABA and increased survival110. Leptin injections into the VTA decreased wheel running112. Blue arrows indicate pathway proposed by Zhang and Dulawa109 to mediate mesocorticolimbic reward modulation of energy expenditure and metabolism in ABA. Additional work is necessary to delineate the circuits, neurotransmitters, and hormones that link ABA reward dysfunction with increased energy expenditure.
The NAc receives strong input from VTA dopamine-producing neurons, and specific chemogenetic activation of NAc-projecting VTA DA neurons increases food intake, food anticipatory activity, and survival in the ABA model110. These results implicate female-specific metabolic dysfunction induced by NAc D2 overexpression107 and suggest that NAc D2 receptor density and signaling could affect risk for AN and predispose individuals toward excessive exercise, potentially as a means to alleviate underlying reward deficiency—although further studies are needed to support this hypothesis. Combined with genetic studies implicating striatal neurons that express either D1 or D2 and human imaging studies, we propose that striatal circuits are a biological risk hub that warrant further investigation.
Moreover, animal, clinical, and genetic findings implicate leptin in the risk and maintenance of AN. Hyperactivity, eating restraint, and earlier weight loss after inpatient refeeding are correlated with lower leptin in AN patients111, implicating a loss of leptin signaling in VTA underlies ABA hyperactivity. In ABA rats, leptin treatment reduced hyperactivity via the VTA region112, implicating a loss of leptin signaling in VTA underlies ABA hyperactivity. GWAS-based genetic correlations suggest an overlap in the biological regulation of AN, leptin, and physical activity8. A case report suggested that leptin treatment may reduce hyperactivity and eating disorder-related cognitions in AN113. Although it has not been studied in AN, human neuroimaging studies in healthy individuals demonstrate that leptin regulates mesolimbic dopamine systems under stress114. Accordingly, leptin is an important hormone in linking genetics with alterations in the DA reward system.
Optogenetic and chemogenetic analysis of BLE.
Optogenetic and chemogenetic approaches seek to identify and manipulate specific cell types and fibers to test proposed circuitry in feeding115, see reviews (e.g., 104). Here, we focus on studies employing BLE paradigms directly or indirectly associated with the mesolimbic reward pathway.
Several studies have identified that activation of inputs to VTA dopamine (DA) neurons from the lateral hypothalamus, bed nucleus of the stria terminalis, or the dorsal raphe nucleus produces positive valence, appetitive behavior, and can increase compulsive-like food or liquid reward consumption104. Broadly, these studies support a physiological role for these brain circuits in shaping motivation and food consumption by transient phasic activation of VTA DA neurons. Conversely, persistent activation of VTA DA neurons using chemogenetics or via the 5HT2C receptor agonist lorcaserin resulted in a reduction in binge eating116. Of note, direct stimulation of VTA DA neurons does not stimulate eating per se117. These data suggest that the observed contribution of VTA DA neurons to BLE is complex and sensitive to the time scales of experimental manipulations. These findings also underscore the critical need to understand how VTA DA neuron activity is altered across multiple binge eating episodes. Finally, the incorporation of pathological BLE models that pair food consumption with aversive consequences (e.g., footshock) will be useful in distinguishing between patterns of VTA neuron activity that accompany adaptive hedonic food consumption versus repetitive compulsive-like BLE that is insensitive to negative reinforcement.
Studies of BLE have also revealed important changes in neural circuits receiving robust dopamine inputs and how dopamine receptors modulate neural activity in these sites. The VTA and substantia nigra pars compacta DA neurons project to the ventral (NAc) and dorsal striatum. Released DA then shapes ongoing neural activity in medium spiny neurons primarily via modulation of downstream PKA-dependent signaling cascades. In the NAc, a multiday course of binge eating increase delta band oscillations of local field potentials (LFPs) and single unit activity in anticipation of palatable food intake118. Palatable food consumption was specifically disrupted via targeted stimulation during anticipatory periods, a phenomenon that may require activation of D2 receptors119. Interestingly, delta band oscillations in the ventral striatum were observed in humans118. Anticipatory activity, or ramps, are also visible in the dorsal striatum at the level of single neurons or bulk calcium transients during food approach 120. These ramps rapidly terminate during food consumption and their functional requirement to binge eating is unknown.
Modulation of the dorsal and ventral striatum also occurs from prefrontal and insular cortex (IC) inputs. The IC integrates taste, interoception, and motivation to regulate feeding, and food-predictive cues reliably activate IC neurons121,122. Chemogenetic activation of the anterior IC as a whole decreases palatable food intake and cue reactivity in a rat model of binge eating123, and optogenetic activation of the right anterior IC in mice similarly reduces food intake124. Conversely, in a model of compulsive binge eating in rats, pathway-specific inhibition of the insula cortex (IC) to NAc decreased appetitive behavior to receive palatable food intermittently paired with foot shock125. These studies indicate a complex role of the insula in reinforcement and compulsive BLE. In the prefrontal cortex (PFC), activation of NAc shell projecting neurons reduced food intake in a binge-eating model in rats predisposed to high impulsivity, a trait observed in a previous study126. Optogenetic activation of inhibitory VIP-expressing interneurons of infralimbic and prelimbic mPFC in male mice decreased BLE of a high caloric diet127. These findings implicate reduced function of the PFC->NAc circuit in impulsivity and BLE. Further study of these circuits across the acquisition of BLE behavior (first episode vs last) is necessary to investigate the dynamics and link to pathology more precisely. Nonetheless, these findings demonstrate that BLE can be inhibited in real time through neural circuit manipulations demonstrating that ongoing activity of specific cortical and limbic circuits is required for BLE.
Animal Model Limitations.
One of the primary limitations in the use of animal models is their inability to capture complex psychological constructs important to the etiology of eating disorders like a drive for thinness, body dysmorphia, or intense fear of weight gain in the case of AN and a loss of control for BLE. However, emerging technologies in markerless pose estimation and deep learning provide basic scientists the opportunity to extract novel behavioral phenotypes that may be associated with these constructs128. The other major limitation of animal models is that, to date, animal models have not yet been used to test the causal nature of a sum of polygenic risk-associated common variants from an eating disorder group. This is an important technical and conceptual factor to consider as we move closer to testing causality of disease-associated variants.
Integration.
Circuit approaches in animal models provide increasing specificity with regard to cell types and their connections underlying ABA and BLE. Convergent evidence across studies suggests that longitudinal assessment of neuronal function in mesolimbic and nigrostriatal circuits, combined with single cell omics will advance our understanding of the central neuronal adaptations as well as peripheral cell type-specific adaptations that drive maladaptive feeding. These assessments should incorporate hypotheses informed by emergent GWAS and human imaging data and can in turn contextualize and inform studies in humans.
Genetic, neuroimaging, and animal model research in eating disorders have largely represented independent disciplines. These sciences of eating disorders have now matured adequately that cross-communication is both possible and essential in order to strengthen causal inferences and translate observations into biological understanding and novel therapeutics.
Conclusions and Future Directions.
As human GWAS and neuroimaging studies grow and diversify and novel animal models are developed, efforts to bridge disciplines must expand. Ongoing GWAS of BED and BN will give context to findings from mouse BLE models. Gene-driven approaches in neurobiology and behavior should increasingly incorporate evidence for association of target genes from human GWAS. Interdisciplinary efforts will benefit studies investigating the main effects of genes and variants, as well as gene-gene and gene-environment interactions. Exquisite control over environmental factors in animal models will allow rigorous testing of putative interactions from genome-wide observational epidemiology in eating disorders. Finally, eating disorder research must be proactive in embracing other disciplines. For example, the utility and diversity of induced pluripotent stem cell models has increased rapidly, and the application of these models to eating disorder research could yield valuable new insights129. Our hope is to accelerate target identification by applying robust statistical genetic and pathway analysis methods, large-scale brain and neurodevelopmental systems biology approaches, and innovative chemoinformatics130. In order to do this, eating disorders should seek to communicate across disciplines, including establishing meetings dedicated to translational eating disorders research. Ultimately, the goal is to translate genetic and neuroscience findings directly into the clinic to enable biologically informed tailored treatment selection and delivery and to eliminate morbidity and mortality from these debilitating illnesses.
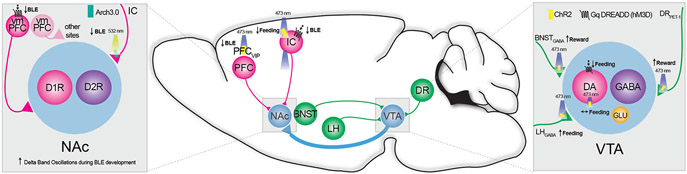
Figure 5. Mesolimbic-centered neural circuits that modulate binge-like eating (BLE).
Converging on the VTA to NAc dopaminergic circuit, behavioral neuroscientists have used circuit-level techniques like chemogenetics and optogenetics to study BLE, normal feeding, and reward-like behavior. Inputs to the VTA from the DR, BNST, and LH modulate reward and food consumption as shown by pathway-specific optogenetics. Within the VTA, chemogenetic activation of VTA DA neuron reduces BLE and direct optogenetic activation has no impact on feeding. Within the NAc, pathway specific optogenetic inhibition of the inputs from the insular cortex reduces BLE. Similarly, chemogenetic activation of the NAc-projecting input cells from the vmPFC or VIP-expressing neurons in the prelimbic and infralimbic PFC also reduces BLE. Abbreviations: BLE – binge-like eating; BNST – bed nucleus of the stria terminalis; ChR2 – Channelrhodopsin2; DA – dopamine; D1R – dopamine D1 receptor; D2R – dopamine D2 receptor; DR – dorsal raphe; GABA – gamma aminobutyric acid; GLU – glutamate; IC – insular cortex; LH – lateral hypothalamus; NAc – nucleus accumbens; PET-1 - PC12 ETS Domain-Containing Transcription Factor; PFC – prefrontal cortex; VIP – vasoactive intestinal polypeptide; vmPFC – ventromedial prefrontal cortex; VTA – Ventral tegmental area.
Acknowledgements:
CMB is supported by NIMH (R01MH120170; R01MH124871; R01MH119084; R01MH118278; R01 MH124871); Brain and Behavior Research Foundation Distinguished Investigator Grant; Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864); Lundbeck Foundation (Grant no. R276-2018-4581). JRIC and GB acknowledge that paper represents independent research part funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. JAH is supported by K01DK115902; CDB is supported by U01DA050243. LB is supported by T32MH112485, Harvard Medical School Livingston Fellowship, and the International OCD Foundation. GB is also supported by UK Medical Research Council (MR/V012878/1; MR/V03605X/1; MR/R024804/1). And Charlotte's Helix.
Footnotes
Competing interests: CM Bulik reports: Shire (grant recipient, Scientific Advisory Board member); Idorsia (consultant); Pearson (author, royalty recipient); Equip Health Inc. (Clinical Advisory Board Member) G Breen reports: Otsuka Pharma (advisory board), Illumina Inc. (grant recipient, conference sponsorship).
Data availability:
No data were generated associated with this review.
Code availability:
No code was generated associated with this review.
References
- 1.American Psychiatric, A. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). (American Psychiatric Publishing, 2013). [Google Scholar]
- 2.Erskine HE, Whiteford HA & Pike KM The global burden of eating disorders. Curr Opin Psychiatry 29, 346–353 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Santomauro DF et al. The hidden burden of eating disorders: an extension of estimates from the Global Burden of Disease Study 2019. Lancet Psychiatry 8, 320–328 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaumberg K et al. Patterns of diagnostic flux in eating disorders: a longitudinal population study in Sweden. Psychol Med 49, 432–450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Southern J et al. Multi-scale computational modelling in biology and physiology. Prog Biophysics Mol Biol 96, 60–89 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yilmaz Z, Hardaway J & Bulik C Genetics and epigenetics of eating disorders. Adv Genom Genet 5, 131–150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellava JE, Thornton LM, Lichtenstein P, Pedersen NL & Bulik CM Impact of broadening definitions of anorexia nervosa on sample characteristics. J Psychiatr Res 45, 691–698 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson H et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 51, 1207–1214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan L et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry 174, 850–858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornton L et al. The Anorexia Nervosa Genetics Initiative (ANGI): Overview and methods. Contemp Clin Trials 74, 61–69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Wang W, Yu S & Lin GN Dissection of the genetic association between anorexia nervosa and obsessive-compulsive disorder at the network and cellular levels. Genes (Basel) 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn-Chernoff MA et al. Shared genetic risk between eating disorder- and substance-use-related phenotypes: Evidence from genome-wide association studies. Addict Biol 26, e12880 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye W et al. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry 161, 2215–2221 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Cederlöf M et al. Etiological overlap between obsessive-compulsive disorder and anorexia nervosa: A longitudinal cohort, family and twin study. World Psychiatry 14, 333–338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton LM, Welch E, Munn-Chernoff MA, Lichtenstein P & Bulik CM Anorexia nervosa, major depression, and suicide attempts: Shared genetic factors. Suicide Life Threat Behav 46, 525–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellava J, Kendler K & Neale M Generalized anxiety disorder and anorexia nervosa: evidence of shared genetic variation. Depress Anxiety 28, 728–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulik-Sullivan B et al. An atlas of genetic correlations across human diseases and traits. Nat Genetics 47, 1236–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson HJ et al. Common genetic variation and age at onset of anorexia nervosa. Biol Psychiatry:GSO, doi: 10.1016/j.bpsgos.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solmi F, Mascarell MC, Zammit S, Kirkbride JB & Lewis G Polygenic risk for schizophrenia, disordered eating behaviours and body mass index in adolescents. Br J Psychiatry 215, 428–433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilmaz Z, Gottfredson N, Zerwas S, Bulik C & Micali N Developmental premorbid body mass index trajectories of adolescents with eating disorders in a longitudinal population cohort. J Am Acad Child Adol Psychiatry 58, 191–199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdulkadir M et al. Polygenic score for body mass index is associated with disordered eating in a general population cohort. J Clin Med 9, 1187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hübel C et al. One size does not fit all. Genomics differentiates among anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int J Eat Disord 54, 785–793, doi: 10.1002/eat.23481 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray GK et al. Could polygenic risk scores be useful in psychiatry?: A review. JAMA Psychiatry 78, 210–219 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Wray N et al. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry 55, 1068–1087 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Lee PH et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482. e1411 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JE, Namkoong K & Jung Y-C Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. Neurosci Lett 651, 95–101 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz Z et al. Examination of the shared genetic basis of anorexia nervosa and obsessive-compulsive disorder. Mol Psychiatry 25, 2036–2046 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed ZE, Micali N, Bulik CM, Davey Smith G & Wade KH Assessing the causal role of adiposity on disordered eating in childhood, adolescence, and adulthood: a Mendelian randomization analysis. Am J Clin Nutr 106, 764–772 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrrell J et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA 315, 1129–1140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams DM, Reay WR, Geaghan MP & Cairns MJ Investigating the effect of glycaemic traits on the risk of psychiatric illness using Mendelian randomisation. Neuropsychopharmacol 46, 1093–1102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huckins LM et al. Investigation of common, low-frequency and rare genome-wide variation in anorexia nervosa. Mol Psychiatry 23, 1169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott-Van Zeeland AA et al. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Molr Psychiatry 19, 724–732 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutter M et al. Novel and ultra-rare damaging variants in neuropeptide signaling are associated with disordered eating behaviors. PloS one 12, e0181556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui H et al. Eating disorder predisposition is associated with ESRRA and HDAC4 mutations. J Clin Invest 123, 4706–4713, doi: 10.1172/JCI71400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi L et al. Anorexia nervosa is associated with Neuronatin variants. Psychiatric Genetics 29, 103–110 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Wang K et al. A genome-wide association study on common SNPs and rare CNVs in anorexia nervosa. Mol Psychiatry 16, 949–959 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz Z et al. Exploration of Large, Rare CNVs Associated with Psychiatric and Neurodevelopmental Disorders in Individuals with Anorexia Nervosa. Psychiatr Genet 27, 152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boraska V et al. A genome-wide association study of anorexia nervosa. Mol Psychiatry 19, 1085–1094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang X et al. Microduplications at the 15q11. 2 BP1–BP2 locus are enriched in patients with anorexia nervosa. J Psychiatr Res 113, 34–38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulik CM et al. The Eating Disorders Genetics Initiative (EDGI): Study protocol. BMC Psychiatry 21, 234 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Co M, Anderson AG & Konopka G FOXP transcription factors in vertebrate brain development, function, and disorders. Wiley Interdiscip Rev Dev Biol 9, e375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yengo L et al. Meta-analysis of genome-wide association studies for height and body mass index in~ 700000 individuals of European ancestry. Hum Mol Genet 27, 3641–3649 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M et al. Axonogenesis is coordinated by neuron-specific alternative splicing programming and splicing regulator PTBP2. Neuron 101, 690–706. e610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arends RM et al. Associations between the CADM2 gene, substance use, risky sexual behavior, and self-control: A phenome-wide association study. Addict Biol, e13015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathjen T et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat Neurosci 20, 1096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerson SL MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer 4, 296–307 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Reid DA et al. Incorporation of a nucleoside analog maps genome repair sites in postmitotic human neurons. Science 372, 91–94 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu W et al. Neuronal enhancers are hotspots for DNA single-strand break repair. Nature, 593, 440–444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finucane H et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nature Genetics 47, 1228–1235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skene NG et al. Genetic identification of brain cell types underlying schizophrenia. Nat Genet 50, 825–833 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chowdhury TG et al. Voluntary wheel running exercise evoked by food-restriction stress exacerbates weight loss of adolescent female rats but also promotes resilience by enhancing gabaergic inhibition of pyramidal neurons in the dorsal hippocampus. Cerebral Cortex 29, 4035–4049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klenowski PM et al. Prolonged consumption of sucrose in a binge-like manner, alters the morphology of medium spiny neurons in the nucleus accumbens shell. Front Behav Neurosci 10, 54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D et al. Comprehensive functional genomic resource and integrative model for the human brain. Science 362, eaat8464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossi MA & Stuber GD Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 27, 42–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank GK, Shott ME & DeGuzman MC Recent advances in understanding anorexia nervosa. F1000Research 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaye WH, Wagner A, Fudge JL & Paulus M Neurocircuity of eating disorders. Curr Top Behav Neurosci, 37–57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lammel S, Lim BK & Malenka RC Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacol 76, 351–359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cowdrey FA, Park RJ, Harmer CJ & McCabe C Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry 70, 736–743 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Kaye WH et al. Neural insensitivity to the effects of hunger in women remitted from anorexia nervosa. Am Jf Psychiatry 177, 601–610 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bohon C & Stice E Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord 44, 585–595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon JJ et al. Neural signature of food reward processing in bulimic-type eating disorders. Soc Cogn Affect Neurosci, 11, 1393–1401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailer UF et al. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry 61, 1090–1099 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Broft A et al. Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Res: Neuroimaging 233, 380–387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bailer UF et al. Amphetamine induced dopamine release increases anxiety in individuals recovered from anorexia nervosa. Int J Eat Disord 45, 263–271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broft A et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int J Eat Disord 45, 648–656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mihov Y et al. Metabotropic glutamate receptor 5 in bulimia nervosa. Sci Reps 10, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank GK, Shott ME, Hagman JO & Mittal VA Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry 170, 1152–1160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craig AD & Craig A How do you feel--now? The anterior insula and human awareness. Nat Revs Neurosci 10 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Kerr KL et al. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacol 41, 521–528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zucker NL et al. The clinical significance of posterior insular volume in adolescent anorexia nervosa. Psychosom Med 79, 1025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berner LA et al. Altered anticipation and processing of aversive interoceptive experience among women remitted from bulimia nervosa. Neuropsychopharmacol 44, 1265–1273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ting JT & Feng G Neurobiology of obsessive–compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Curr Opin Neurobiol 21, 842–848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marsh R et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry 168, 1210–1220 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skunde M et al. Neural signature of behavioural inhibition in women with bulimia nervosa. J Psychiatry Neurosci: JPN 41, E69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wierenga C et al. Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PloS one 9, e92017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oberndorfer TA, Kaye WH, Simmons AN, Strigo IA & Matthews SC Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. Int J Eat Disord 44, 1–8 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Finch JE, Palumbo IM, Tobin KE & Latzman RD Structural brain correlates of eating pathology symptom dimensions: A systematic review. Psychiatry Res Neuroimaging 317, 111379 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Geisler D et al. Altered white matter connectivity in young acutely underweight patients with anorexia nervosa. J Am Acad Child Adolesc Psychiatry 61, 331–340 (2022). [DOI] [PubMed] [Google Scholar]
- 79.Goodkind M et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McTeague LM et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry 177, 411–421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shanmugan S et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry 173, 517–526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S et al. Neurobiological commonalities and distinctions among 3 major psychiatric disorders: a graph theoretical analysis of the structural connectome. J Psychiatry Neurosci: JPN 45, 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia M et al. Shared and distinct functional architectures of brain networks across psychiatric disorders. Schiz Bull 45, 450–463 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hudson JI, Hiripi E, Pope HG Jr. & Kessler RC The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61, 348–358 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frank GK, Favaro A, Marsh R, Ehrlich S & Lawson EA Toward valid and reliable brain imaging results in eating disorders. Int J Eat Disord 51, 250–261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaufmann L-K et al. Fornix under water? Ventricular enlargement biases forniceal diffusion magnetic resonance imaging indices in anorexia nervosa. Biol Psychiatry: Cogn Neurosci Neuroimaging 2, 430–437 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Gaudio S, Carducci F, Piervincenzi C, Olivo G & Schiöth HB Altered thalamo–cortical and occipital–parietal–temporal–frontal white matter connections in patients with anorexia and bulimia nervosa: a systematic review of diffusion tensor imaging studies. J Psychiatry Neurosci: JPN 44, 324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson PM et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transll Psychiatry 10, 1–28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gelegen C et al. Difference in susceptibility to activity-based anorexia in two inbred strains of mice. Eur Neuropsychopharmacol 17, 199–205 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Gelegen C et al. Chromosomal mapping of excessive physical activity in mice in response to a restricted feeding schedule. Eur Neuropsychopharmacoly 20, 317–326 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Pjetri E et al. Identifying predictors of activity based anorexia susceptibility in diverse genetic rodent populations. PLoS One 7, e50453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Babbs RK et al. Genetic differences in the behavioral organization of binge eating, conditioned food reward, and compulsive-like eating in C57BL/6J and DBA/2J strains. Physiol Behav 197, 51–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Newmyer BA, Whindleton CM, Srinivasa N, Jones MK & Scott MM Genetic variation affects binge feeding behavior in female inbred mouse strains. Sci Rep 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirkpatrick SL et al. Cytoplasmic FMR1-interacting protein 2 is a major genetic factor underlying binge eating. Biol Psychiatry 81, 757–769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Babbs RK et al. Cyfip1 Haploinsufficiency Increases Compulsive-Like Behavior and Modulates Palatable Food Intake in Mice: Dependence on Cyfip2 Genetic Background, Parent-of Origin, and Sex. G3: Genes Genom Genet 9, 3009–3022 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farrell M et al. Treatment-resistant psychotic symptoms and the 15q11. 2 BP1–BP2 (Burnside-Butler) deletion syndrome: case report and review of the literature. Transl Psychiatry 10, 1–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao EJ et al. Systems genetic analysis of binge-like eating in a C57BL/6J x DBA/2J-F2 cross. Genes Brain Behav, e12751 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nobis S et al. Alterations of proteome, mitochondrial dynamic and autophagy in the hypothalamus during activity-based anorexia. Sci Rep 8, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nobis S et al. Colonic mucosal proteome signature reveals reduced energy metabolism and protein synthesis but activated autophagy during anorexia‐induced malnutrition in mice. Proteomics 18, 1700395 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Breton J et al. Proteome modifications of gut microbiota in mice with activity-based anorexia and starvation: Role in ATP production. Nutr 67, 110557 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Schroeder M et al. Placental miR-340 mediates vulnerability to activity based anorexia in mice. Nat Comms 9, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He X, Stefan M, Terranova K, Steinglass J & Marsh R Altered white matter microstructure in adolescents and adults with bulimia nervosa. Neuropsychopharmacol 41, 1841–1848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaakoush NO et al. Alternating or continuous exposure to cafeteria diet leads to similar shifts in gut microbiota compared to chow diet. Mol Nutr Food Res 61, 1500815 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Sweeney P & Yang Y Neural circuit mechanisms underlying emotional regulation of homeostatic feeding. Trends Endocrinol Metab 28, 437–448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Webber ES, Bonci A & Krashes MJ The elegance of energy balance: Insight from circuit‐level manipulations. Synapse 69, 461–474 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Andermann ML & Lowell BB Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welch AC et al. Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol Psychiatry, 26, 3765–3777 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milton LK et al. Suppression of corticostriatal circuit activity improves cognitive flexibility and prevents body weight loss in activity-based anorexia in rats. Biol Psychiatry 90, 819–828 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Zhang J & Dulawa SC The utility of animal models for studying the metabo-psychiatric origins of anorexia nervosa. Front Psychiatry 12, 711181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foldi CJ, Milton LK & Oldfield BJ The role of mesolimbic reward neurocircuitry in prevention and rescue of the activity-based anorexia (ABA) phenotype in rats. Neuropsychopharmacology 42, 2292–2300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hebebrand J, Muller T, Holtkamp K & Herpertz-Dahlmann B The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 12, 23–35 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Verhagen LA, Luijendijk MC & Adan RA Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur Neuropsychopharmacol 21, 274–281 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Antel J et al. Rapid amelioration of anorexia nervosa in a male adolescent during metreleptin treatment including recovery from hypogonadotropic hypogonadism. Eur Child Adolesc Psychiatry, doi: 10.1007/s00787-021-01778-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burghardt PR et al. Leptin regulates dopamine responses to sustained stress in humans. J Neurosci 32, 15369–15376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu Q, Han Y & Tong Q in Neural Regulation of Metabolism (eds Wu Q & Zheng R) 211–233 (Springer, 2018). [Google Scholar]
- 116.Xu P et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol Psychiatry 81, 737–747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marino RAM et al. Control of food approach and eating by a GABAergic projection from lateral hypothalamus to dorsal pons. Proc Natl Acad Sci U S A 117, 8611–8615, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu H et al. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc Natl Acad Sci U S A 115, 192–197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Halpern CH et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci 33, 7122–7129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.London TD et al. Coordinated ramping of dorsal striatal pathways preceding food approach and consumption. J Neurosci 38, 3547–3558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Livneh Y et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546, 611–616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A & Bonci A Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A 112, 1190–1195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Price AE, Stutz SJ, Hommel JD, Anastasio NC & Cunningham KA Anterior insula activity regulates the associated behaviors of high fat food binge intake and cue reactivity in male rats. Appetite 133, 231–239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Y et al. The anterior insular cortex unilaterally controls feeding in response to aversive visceral stimuli in mice. Nat Commun 11, 640 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spierling S et al. Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food. Neuropsychopharmacol 45, 579–588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anastasio NC et al. Convergent neural connectivity in motor impulsivity and high-fat food binge-like eating in male Sprague-Dawley rats. Neuropsychopharmacol 44, 1752–1761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Newmyer BA et al. VIPergic neurons of the infralimbic and prelimbic cortices control palatable food intake through separate cognitive pathways. JCI insight 5, e126283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mathis A et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 129.Maussion G, Demirova I, Gorwood P & Ramoz N Induced pluripotent stem cells: New tools for investigating molecular mechanisms in anorexia nervosa. Frontiers in Nutrition 6, 118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Breen G et al. Translating genome-wide association findings into new therapeutics for psychiatry. Nat Neurosci 19, 1392–1396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galmiche M, Déchelotte P, Lambert G & Tavolacci MP Prevalence of eating disorders over the 2000–2018 period: a systematic literature review. Am J Clin Nutr 109, 1402–1413 (2019). [DOI] [PubMed] [Google Scholar]
- 132.Papadopoulos F, Ekbom A, Brandt L & Ekselius L Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry 194, 10–17 (2009. ). [DOI] [PubMed] [Google Scholar]
- 133.Yao S et al. Familial liability for eating disorders and suicide attempts: Evidence from a population registry in Sweden. JAMA Psychiatry 73, 284–291 (2016). [DOI] [PubMed] [Google Scholar]
- 134.Fichter MM, Quadflieg N, Crosby RD & Koch S Long-term outcome of anorexia nervosa: Results from a large clinical longitudinal study. Int J Eat Disord 50, 1018–1030 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Berkman N et al. Management of Eating Disorders. Evidence Report/Technology Assessment No. 135., (AHRQ Publication No. 06-E010. (Prepared by the RTI International-University of North Carolina Evidence-Based Practice Center under Contract No. 290-02-0016.), Rockville, MD, 2006). [Google Scholar]
- 136.Keski-Rahkonen A et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 164, 1259–1265 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Brownley K et al. Binge-eating disorder in adults: A systematic review and meta-analysis. Ann Intern Med 165, 409–420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fornaro M et al. Lisdexamfetamine in the treatment of moderate-to-severe binge eating disorder in adults: systematic review and exploratory meta-analysis of publicly available placebo-controlled, randomized clinical trials. Neuropsychiatr Dis Treat 12, 1827–1836, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hall JF, Smith K, Schnitzer SB & Hanford PV Elevation of activity level in the rat following transition from ad libitum to restricted feeding. J Compar Physiol Psychol 46, 429 (1953). [DOI] [PubMed] [Google Scholar]
- 140.Schalla MA & Stengel A Activity based anorexia as an animal model for anorexia nervosa–a systematic review. Front Nutr 6, 69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Novelle MG & Diéguez C Food addiction and binge eating: lessons learned from animal models. Nutrients 10, 71 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Corwin RL & Babbs RK Rodent models of binge eating: are they models of addiction? ILAR J 53, 23–34 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Moore CF, Sabino V, Koob GF & Cottone P Pathological overeating: emerging evidence for a compulsivity construct. Neuropsychopharmacol 42, 1375–1389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rospond B, Szpigiel J, Sadakierska-Chudy A & Filip M Binge eating in pre-clinical models. Pharmacol Rep 67, 504–512 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Cottone P, Sabino V, Steardo L & Zorrilla EP Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol -Regul 295, R1066–R1076 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardaway JA et al. Nociceptin receptor antagonist SB 612111 decreases high fat diet binge eating. Behav Brain Res 307, 25–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated associated with this review.
No code was generated associated with this review.