Abstract
Background
Impairment of cardiovascular control is common in multiple sclerosis (MS), possibly due to damage of strategic brain regions such as the insula. Aerobic training (AT) targets cardiopulmonary system and may represent a neuroprotective strategy.
Purpose
To investigate whether insular damage (T2-hyperintense lesions and volume) is associated with cardiovascular fitness (CF) and influences AT effects in MS.
Methods
Sixty-one MS patients were randomized to an AT intervention group (MS-AT) and a motor training control group (MS-C). At baseline and after training (24 sessions over 2–3 months), peak of oxygen consumption (VO2max), heart rate reserve (HRR), 6-min walk test (6MWT) and whole brain and insula MRI data were collected. Two healthy control (HC) groups were enrolled for CF and MRI data analysis.
Results
At baseline, MS patients vs HC showed impaired VO2max, HRR and 6MWT (p < 0.001) and widespread gray matter atrophy, including bilateral insula. In MS patients, left insula T2-lesion volume correlated with HRR (r = 0.27, p = 0.042). After training, MS-AT, especially those without insular T2-hyperintense lesions, showed 6MWT improvement (p < 0.05) and a stable insular volume, whereas MS-C showed left insular volume loss (p < 0.001).
Conclusions
By increasing 6MWT performance, our results suggest that AT may improve walking capacity and submaximal measure of CF in MS patients. Such beneficial effect may be modulated by insula integrity.
Keywords: Multiple sclerosis, Exercise, Insula, Magnetic resonance imaging, Aerobic training
Introduction
Multiple sclerosis (MS) is one of the most common causes of neurological disability in young adults and its incidence and socioeconomic impact are increasing worldwide [1]. Typical clinical manifestations include locomotor, sensory, visual and cognitive impairment; however many other symptoms and signs can occur [2]. Dysfunction of the autonomic system with impairment of cardiovascular function regulation, including attenuated increases in hearth rate (HR) and systolic blood pressure during exercise, has been described in up to 60% of MS patients [3].
Among the brain regions potentially implicated in modulating autonomic system function, the insula represents a key area involved in the regulation of cardiac functions, most likely through its direct projections to the lateral hypothalamic area, the parabrachial nucleus, and the nucleus of the solitary tract [4]. The insula is anatomically and functionally divided into a larger anterior and a smaller posterior lobule by the central sulcus [5]. However, the role of insular sub-regions involved in this autonomic control shows large variations across studies [5–8]. Moreover, some evidence suggests a certain degree of insular lateralization in cardiac representation [7, 8]. Cardiac acceleration has been correlated with the right insula function, while cardiac deceleration with the left one [6].
Aerobic training (AT) directly targets cardiovascular and pulmonary systems [9]. In healthy controls (HC), AT promotes improvements in cardiopulmonary functions, including lowering HR at rest and increasing maximal oxygen consumption (VO2max) [9]. In MS patients, AT may ameliorate aerobic capacity, fatigue, quality of life and emotional aspects [10]. Some studies have suggested that AT may exert neuroprotective effects in aging [11] and several neurological diseases [12], including MS [13], possibly due to the creation of a better environment for CNS-resident cells [14].
In MS, focal demyelinating lesions and irreversible tissue loss frequently affect the insula and are clinically relevant [15, 16]. A higher T2-hyperintense WM lesion volume (LV) in the left insula was associated with a shift of sympathetic–parasympathetic cardiac modulation toward sympathetic predominance, with increased sympathetic blood pressure modulation [15] and prolongation of QT interval duration [16]. Since the insula modulates the function of the autonomic system, it is tempting to speculate that the accumulation of structural damage in this region in these patients may have a detrimental impact on the regulation of cardiovascular functions and on the beneficial effects promoted by AT.
By evaluating a quite large cohort of MS patients who were randomized to perform AT or a motor training not influencing the cardiopulmonary system, we explored whether focal WM lesions and volume of the insula were associated with cardiovascular functions and whether insula involvement modulated improvements on cardiovascular fitness (CF) and walking performances promoted by AT.
Materials and methods
Subjects
In this secondary analysis of an interventional ongoing study, 61 MS patients (20 relapsing–remitting and 41 progressive) enrolled between 2017 and 2021 were retrospectively analyzed. To be included they had to be between 18 and 65 years old, right-handed, without additional neurologic, psychiatric, orthopedic or rheumatologic diseases and not engaged in structured physical activity for more than 3 times a week. MS patients had to have an Expanded Disability Status Scale (EDSS) < 7.0, be relapse- and steroid free for at least 3 months and on a stable treatment for at least 1 month. Data from two groups of HC without any neurologic diseases or systemic disorders potentially affecting the CNS recruited at our Unit for ongoing studies were also evaluated. The first group (“HC-clinic”) (n = 20) was selected to analyze baseline cardiopulmonary data, whereas the second one (“HC-MRI”) (n = 60) was selected for MRI analysis. Two different HC groups were selected since subjects included in the HC-clinic group did not perform the MRI acquisition, while subjects of the HC-MRI group did not have data on CF.
Clinical evaluation
At the beginning and immediately after the training period (see below for details), MS patients underwent clinical evaluation with rating of Expanded Disability Status Scale (EDSS) score, disease duration, clinical phenotype, body mass index (BMI), and cardiopulmonary exercise testing to assess peak of VO2max, maximal peak of HR (HRmax), heart rate reserve (HRR), 6-min walk test (6MWT) and timed 25-foot walk (T25FW). Relapses and adverse events were recorded during the whole training period. At baseline, HC-clinic group underwent the same cardiopulmonary evaluation of patients.
Intervention
MS patients were randomly allocated to two groups: intervention group (MS-AT, n = 31) and control group (MS-C, n = 30). Both groups performed 24 training sessions of 30–40 min for 2–3 times a week with constant monitoring of HR over a period of 2–3 months. MS-AT performed moderate AT with HR between 55 and 75% of HRmax, while MS-C performed a non-specific motor training in which subjects performed lower limb stretching, passive–active mobilization and balance exercises without a direct involvement of cardiopulmonary system established as no increase of HR during physical effort.
MRI acquisition
Using a 3.0 Tesla Philips Ingenia CX scanner (Philips Medical Systems) and standardized procedures for subjects positioning and repositioning, the following brain MRI sequences were acquired at baseline and immediately after the training for all MS subjects and at baseline for HC-MRI group: (a) sagittal 3D fluid attenuation inversion recovery (FLAIR), FOV = 256 × 256 mm, pixel size = 1 × 1 mm, 192 slices, 1 mm thick, matrix = 256 × 256, TR = 4800 ms, TE = 270 ms, inversion time (TI) = 1650 ms, echo train length (ETL) = 167, acquisition time (TA) = 6.15 min; (b) sagittal 3D T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE), FOV = 256 × 256, pixel size = 1 × 1 mm, 204 slices, 1 mm thick, matrix = 256 × 256, TR = 7 ms, TE = 3.2 ms, TI = 1000 ms, flip angle = 8°, TA = 8.53 min.
MRI analysis
Focal T2-hyperintense WM lesions were identified by a fully automated approach using co-registered 3D FLAIR and 3D T1-weighted images as inputs [17]. T2-hyperintense WM LVs were obtained for each patient from their lesion masks, after a careful visual check of the results provided by the automatic segmentation from expert physicians.
At baseline, normalized brain volume (NBV), normalized GM volume (NGMV) and normalized WM volume (NWMV) were measured on 3D T1-weighted images, after lesion-filling, using the FSL-SIENAx software.
Three masks of bilateral, right and left insula were created merging the six bilateral sub-regions defined by the Human Brainnetome Atlas (http://atlas.brainnetome.org) [18]. T2-hyperintense WM LVs and volumes of right and left insula were extracted by non-linearly registering the insular masks on both FLAIR and 3D T1-weighted images of each subject, respectively.
At follow-up, new T2-hyperintense WM lesions were identified by estimating the difference of lesion mask between follow-up and baseline (after co-registration between the two time-points). Longitudinal percentage brain volume change (PBVC) was also obtained from 3D T1-weighted images using SIENA toolbox (FSL version 5.0.9).
Voxel-based morphometry (VBM) was applied on T1-weighted images to obtain a voxel-based distribution of regional GM atrophy. VBM was performed using SPM12 (Matlab version 2017) and the Diffeomorphic Anatomic Registration using Exponentiated Lie algebra (DARTEL) toolbox [19]. First, T1-weighted images were segmented into GM, WM and cerebrospinal fluid. An initial template was obtained by aligning GM and WM images for all subjects, then refined by non-linear registration including the DARTEL toolbox. Finally, images were spatially normalized to the MNI space, modulated and smoothed with an 8-mm FWHM Gaussian kernel.
Tensor-based morphometry (TBM), as implemented in SPM12, was used to map longitudinal regional volume changes within and between patient groups [20]. The method produces a mid-point average template image after group-wise alignment of each subject’s scans [21]. The rate of volume change was quantified from images of the Jacobian determinants as the ratio between volume differences and the volume in the mid-point average template, which was used as reference: negative values indicate tissue volume loss, positive values volume increase.
Statistical analysis
Baseline demographic, clinical and MRI measures and their within-group and between-group longitudinal changes were compared using the χ2 Pearson test for categorical variables and Mann–Whitney or t test for continuous variables. In MS patients, correlations between baseline clinical and MRI measures were assessed using Spearman’s partial correlations (age- and sex adjusted). T2-hyperintense WM LVs were log-transformed before statistical analysis. Longitudinal changes of global variables were expressed as baseline normalized percentages.
Longitudinal differences of clinical variables between MS-AT and MS-C groups were assessed by a 2 × 2 (group by time) repeated-measures analysis of variance.
To explore the impact of insular lesions on baseline CF, MS patients were dichotomized according to the presence (MS with insular lesions [MS-WL]) or absence (MS without insular lesions [MS-WOL]) of T2-hyperintense lesions.
To understand the role of insular damage on AT effects, differences of cardiopulmonary variables over time between MS patients with and without baseline insular lesions of the two study groups were assessed by a 2 × 2 (group by time) repeated-measures analysis of variance. Such an analysis was limited to those cardiopulmonary variables that changed after AT or that showed a correlation at baseline with insular damage.
All previous analyses were performed using SPSS software (version 25.0) with p-value < 0.05.
Using a general linear model and the theory of Gaussian fields [22], voxel-wise between-group comparisons of baseline GM volume as well as within-group and between-group longitudinal changes of GM volumes were performed using SPM12 and an analysis of covariance (ANCOVA), including age, sex, and the intracranial volume as covariates. To limit the analysis to the GM, masks obtained from the GM DARTEL templates, transformed to standard space, smoothed and thresholded at 0.25, were used.
Using SPM12, Spearman’s partial correlations (age- and sex adjusted) were performed between baseline clinical and MRI measures as well as between clinical and MRI longitudinal changes.
VBM and TBM results were assessed at a threshold of p < 0.05, family-wise-error (FWE) corrected for multiple comparisons and at a threshold of p < 0.001 uncorrected (cluster extension kE ≥ 10).
Results
Baseline demographic, clinical and MRI findings
MS patients and HC clinic did not differ for age (p = 0.08) and sex (p = 0.53), whereas MS patients had impaired VO2max, HRR, 6MWT and T25FW (p < 0.001 for all comparisons) (Table 1).
Table 1.
Baseline demographic, clinical and MRI variables among MS and HC groups and between MS subgroups
| MS (n = 61) | HC-clinic (n = 20) | HC-MRI (n = 60) | p* | p° | MS-AT (n = 31) | MS-C (n = 30) | p | MS-WL (n = 27) | MS-WOL (n = 34) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age Mean (SD) [years] |
49.72 (7.57) |
45.67 (11.61) |
48.57 (8.97) |
0.081 | 0.448 |
49.06 (7.78) |
50.40 (7.42) |
0.901 |
51.78 (5.97) |
48.09 (8.36) |
0.058 |
| Sex: male/female | 23/38 | 6/14 | 20/40 | 0.532 | 0.615 | 11/20 | 12/18 | 0.716 | 11/23 | 12/15 | 0.427 |
|
BMI Median (IQR) [Kg/m2] |
22.9 (19.4;15.5) |
22.2 (20.5;24.8) |
– | 0.954 | – |
22.7 (19.5;25.9) |
20.1 (19.36;25.9) |
0.241 |
22.89 (20.2;24.9) |
22.74 (19.3;27.1) |
0.959 |
|
EDSS Median (IQR) |
4.5 (3.0;6.5) |
– | – | – | – |
4.3 (3.0;6.5) |
4.0 (3.0;6.5) |
0.611 |
5.5 (4.0;6.5) |
4.0 (2.5;6.0) |
0.044 |
|
Disease duration Median (IQR) [years] |
18.0 (10.9;25.8) |
– | – | – | – |
15.9 (10.9;25.6) |
19.0 (11.0;25.8) |
0.682 |
23.0 (13.5;27.0) |
15.8 (9.5;21.0) |
0.053 |
| Phenotype: RR/P | 20/41 | – | – | – | – | 8/23 | 12/18 | 0.238 | 21/6 | 20/14 | 0.171 |
| Disease-modifying treatments: no/first line/second line [n] | 7/21/33 | – | – | – | – | 4/8/19 | 3/13/14 | 0.354 | 1/8/18 | 6/13/15 | 0.117 |
|
VO2max* Median (IQR) [mL/Kg/min] |
15.4 (11.9;18.5) |
28.3 (25.6;31.0) |
– | < 0.001 | – |
15.7 (11.8;18.7) |
16.1 (11.8;18.6) |
0.831 |
13.1 (11.6;17.9) |
16.45 (11.9;18.9) |
0.066 |
|
HRR* Median (IQR) [bpm] |
51 (32;63) |
14 (9;23) |
– | < 0.001 | – |
53 (34;64) |
46 (34;63) |
0.171 |
54 (34;65) |
47 (32;63) |
0.257 |
|
6MWT* Median (IQR) [meters] |
324 (185;420) |
602 (564;659) |
– | < 0.001 | – |
314 (175;420) |
367 (180;420) |
0.163 |
225 (165;414) |
351 (188;420) |
0.050 |
|
T25FW* Median (IQR) [sec] |
6.67 (5.32;10.2) |
4.30 (3.85;4.57) |
– | < 0.001 | – |
7.44 (5.31;10.45) |
6.16 (5.31;10.34) |
0.262 |
7.45 (5.48;10.77) |
6.15 (5.25;9.92) |
0.080 |
|
T2-hypeintense WM LV* Mean (SD) [mL] |
6.30 (2.82;12.1) |
– |
0.02 (0.00;0.05) |
– | < 0.001 |
5.69 (2.76;12.07) |
6.45 (2.79;12.12) |
0.885 |
11.81 (6.70;20.80) |
2.91 (1.20;6.40) |
< 0.001 |
|
NBV* Mean (SD) |
1474 (60) |
– |
1550 (42) |
– | < 0.001 |
1481 (1433;1518) |
1467 (1431;1517) |
0.369 |
1448 (47) |
1494 (62) |
0.002 |
|
NGMV* Mean (SD) [mL] |
821 (46) |
– |
859 (35) |
– | < 0.001 |
826 (801;850) |
816 (800;848) |
0.404 |
800 (47) |
837 (38) |
0.002 |
|
NWMV* Mean (SD) [mL] |
653 (34) |
– |
691 (28) |
– | < 0.001 |
655 (631;674) |
651 (631;673) |
0.643 |
648 (31) |
657 (36) |
0.283 |
|
Left Insula T2-hyperintense LV* Median (IQR) [mL] |
0.00 (0.00;1.00) |
– |
0.00 (0.00;0.01) |
– | < 0.001 |
0.00 (0.00;1.50) |
0.00 (0.00;1.25) |
0.499 |
2.00 (0.00;18.50) |
0.00 (0.00;0.00) |
< 0.001 |
|
Right Insula T2-hyperintense LV* Median (IQR) [mL] |
0.00 (0.00;3.00) |
– |
0.00 (0.00;0.01) |
– | < 0.001 |
0.00 (0.00;2.00) |
0.00 (0.00;3.25) |
0.089 |
5.00 (0.00;11.50) |
0.00 (0.00;0.00) |
< 0.001 |
|
Left Insula volume* Mean (SD) [mL] |
6.07 (0.96) |
– |
7.06 (0.75) |
– | < 0.001 |
6.64 (5.53;6.74) |
6.45 (5.50;6.69) |
0.604 |
5.70 (0.90) |
6.37 (0.96) |
0.007 |
|
Right Insula volume* Mean (SD) [mL] |
5.54 (0.87) |
– |
6.38 (0.70) |
– | < 0.001 |
6.07 (4.87;6.16) |
5.81 (4.87;6.05) |
0.387 |
5.22 (0.83) |
5.79 (0.82) |
0.009 |
Statistically significant comparisons are shown in bold
BMI body mass index, bpm beats per minute, EDSS Expanded Disability Status Scale, HC-clinic healthy control group for baseline cardiopulmonary and clinical comparison, HC-MRI healthy control group for baseline magnetic resonance imaging comparison, HRR heart rate reserve, IQR interquartile range, Kg kilograms, min minutes, LV lesion volume, mL milliliters, MRI magnetic resonance imaging, MS multiple sclerosis, MS-A MS group that performed aerobic training, MS-C MS group that performed motor control training without direct involvement of cardiopulmonary system, MS-WL MS patients with baseline insula lesions, MS-WOL MS patients without baseline insula lesions, NBV normalized brain volume, NGMV normalized gray matter volume, NWMV normalized white matter volume, P progressive, p p-value, RR relapsing–remitting, SD standard deviation, T25FW timed 25-foot walk test, VO2max maximal oxygen consumption, 6MWT six-minute walk test
Compared to HC-MRI, MS patients were comparable for age (p = 0.45) and sex (p = 0.62), whereas they showed significant higher brain and insular T2-hyperintense WM LV as well as lower NBV, NGMV, NWMV and bilateral insular volume (p < 0.001 for all comparisons) (Table 1).
At baseline, demographic, clinical, cardiopulmonary and MRI measures did not differ between MS-AT and MS-C. In addition, the type of disease-modifying treatments was equally distributed between the two groups of patients (p ≥ 0.163) (Table 1).
Compared to MS-WOL, MS-WL showed higher EDSS score (p = 0.044), lower NBV (p = 0.002), lower NGMV (p = 0.002), and lower bilateral insular volumes (p ≤ 0.009). They also showed a trend for older age (p = 0.058), longer disease duration (p = 0.053), lower VO2max (p = 0.066), shorter 6MWT distance (p = 0.050) and longer T25FW (p = 0.080) (Table 1).
Baseline VBM findings
Compared to HC, MS patients had a widespread pattern of GM atrophy, including sensorimotor, cerebellar, occipital areas, bilateral insula and deep GM nuclei (p < 0.05, FWE) (Fig. 1). No significant differences were found between MS-AT and MS-C or between MS-WL and MS-WOL.
Fig. 1.
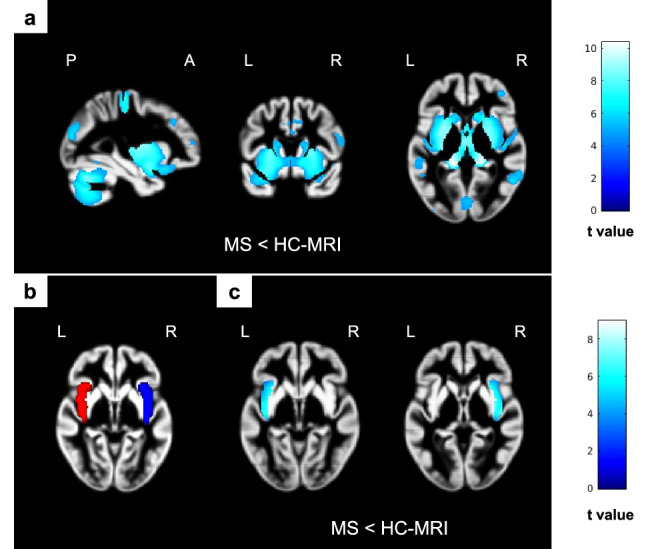
Baseline voxel-based morphometry results. SPM analysis showing significant differences in GM volume (lower volume encoded in blue/light blue color-coded, p < 0.05 family-wise corrected, cluster extent = 10) superimposed on the custom GM template. (a) Clusters showing widespread pattern of lower GM volume in MS patients compared to HC-MRI. (b) The insula masks used for local VBM analysis (left mask in red and right mask in blue), (c) clusters showing lower GM volume within bilateral insula areas in MS compared to HC-MRI. GM gray matter, HC-MRI healthy control group for baseline magnetic resonance imaging comparison, MS multiple sclerosis, SPM statistical parametric mapping
Correlations between baseline clinical and MRI findings
In MS patients, a higher left insular T2-hyperintense WM LV correlated with a higher HRR (r = 0.27, p = 0.042).
A trend towards a correlation between higher right insular T2-hyperintense WM LVs and higher HRR (r = 0.23, p = 0.083) was also found. No other significant correlations among clinical variables and MRI measures were found (Table 2).
Table 2.
Baseline correlations between MRI measures and clinical cardiopulmonary variables in MS patients
| VO2max | HRR | HRrest | MWT6 | T25FW | ||
|---|---|---|---|---|---|---|
| T2-hyperintense WM LV | r-value | 0.057 | − 0.010 | − 0.162 | − 0.0204 | 0.094 |
| p-value | 0.668 | 0.941 | 0.220 | 0.125 | 0.506 | |
| NGMV | r-value | 0.245 | 0.052 | 0.170 | 0.259 | − 0.164 |
| p-value | 0.077 | 0.714 | 0.199 | 0.061 | 0.240 | |
| Left Insula T2-hyperintense LV | r-value | − 0.219 | 0.268 | − 0.079 | − 0.028 | 0.038 |
| p-value | 0.098 | 0.042 | 0.551 | 0.837 | 0.791 | |
| Right Insula T2-hyperintense LV | r-value | 0.074 | 0.230 | − 0.123 | 0.081 | 0.112 |
| p-value | 0.579 | 0.083 | 0.354 | 0.547 | 0.430 | |
| Left Insula volume | r-value | − 0.480 | 0.239 | 0.047 | − 0.40 | 0.148 |
| p-value | 0.719 | 0.071 | 0.723 | 0.768 | 0.294 | |
| Right Insula volume | r-value | − 0.069 | 0.207 | 0.054 | − 0.046 | 0.148 |
| p-value | 0.606 | 0.119 | 0.687 | 0.743 | 0.294 |
Statistically significant comparisons are shown in bold
bpm beats per minute, HRrest heart rate at rest, HRR heart rate reserve, IQR interquartile range, Kg kilograms, min minutes, mL milliliters, MS-A Multiple sclerosis (MS) patients that performed aerobic training, MS-C MS group that performed motor control training without direct involvement of cardiopulmonary system, MS-WL-A MS patients with baseline insula lesions that performed aerobic training, MS-WOL-A MS patients without baseline insula lesions that performed aerobic training, MS-WL-C MS patients with baseline insula lesions that performed motor control training, MS-WOL-C MS patients without baseline insula lesions that performed motor control training, WM white matter, NGMV normalized gray matter volume, T25FW timed 25-foot walk test, VO2max maximal oxygen consumption, 6MWT six-minute walk test
The GM volume of clusters in fronto-temporo-parietal cortices and the cerebellum, but not in the insula, was significantly correlated with cardiopulmonary variables (r values ranging from 0.434 to 0.504) (Table 3).
Table 3.
Baseline correlations between voxel-based morphometry results and clinical data (p < 0.001 uncorrected, cluster extension kE ≥ 10)
| Variable | Side | Area | BA | MNI (x y z) | kE | T-value | R-value |
|---|---|---|---|---|---|---|---|
| VO2max | L | Cerebellum (Crus2) | NA | − 34 − 80 − 34 | 724 | 3.98 | 0.504 |
| L | Middle frontal gyrus | 46 | − 39 56 14 | 26 | 3.83 | 0.489 | |
| R | Postcentral gyrus | 43 | 57 − 14 36 | 74 | 3.73 | 0.480 | |
| R | Fusiform gyrus | 37 | 33 − 57 − 9 | 22 | 3.64 | 0.471 | |
| R | Superior temporal pole | 48 | 60 4 3 | 33 | 3.59 | 0.465 | |
| R | Middle temporal gyrus | 21 | 57 − 46 − 3 | 17 | 3.54 | 0.461 | |
| L | Rolandic operculum° | 48 | − 57 12 4 | 21 | 3.5 | 0.457 | |
| Vermis (7) | 18 | − 3 − 75 − 18 | 16 | 3.38 | 0.444 | ||
| HRR | L | Middle frontal gyrus | 46 | − 32 20 38 | 43 | 3.31 | 0.437 |
| R | Hippocampus | 20 | 34 − 12 − 22 | 16 | 3.21 | 0.425 | |
| 6MWT | R | Postcentral gyrus | 43 | 60 − 10 33 | 310 | 4.08 | 0.503 |
| R | Rolandic operculum | 48 | 62 8 4 | 81 | 3.97 | 0.492 | |
| L | Inferior parietal lobule | 2 | − 51 − 28 48 | 56 | 3.52 | 0.449 | |
| L | Postcentral gyrus | 2 | − 44 − 33 50 | 3.38 | 0.434 | ||
| L | Rolandic operculum | 48 | − 57 9 4 | 22 | 3.48 | 0.444 | |
| R | Superior parietal lobule | 7 | 26 − 63 54 | 48 | 3.46 | 0.442 | |
| T25FW | R | Inferior frontal gyrus (Orb) | 11 | 27 30 − 24 | 35 | 3.93 | − 0.496 |
| R | Inferior parietal lobule | 40 | 32 − 46 54 | 43 | 3.49 | − 0.451 |
BA Brodmann area, kE cluster extension, L left, MNI Montreal Neurological Institute, R right, T25FW timed 25-foot walk test, VO2max maximal oxygen consumption, 6MWT six-minute walk test
Longitudinal clinical and MRI findings
All MS patients completed the training period. No relapses or adverse events were recorded during the training period. Table 4 summarizes longitudinal clinical changes in MS subgroups.
Table 4.
Longitudinal clinical and cardiopulmonary results in MS subgroups, focus on lesion-based groups in those variables where longitudinal changes or baseline correlations were statistically significant
| Group (n) | Timepoint | p-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Delta % | Group | Time | Interaction | ||
|
VO2max Median (IQR) [mL/Kg/min] |
MS-AT (31) | 15.7 (11.8;18.7) | 14.6 (11.3;19.0) | 5.5 (− 17.5;15.7) | 0.91 | 0.39 | 0.64 |
| MS-C (30) | 16.1 (11.8;18.6) | 15.3 (12.0;15.3) | 4.7 (− 12.9;13.3) | ||||
|
HRR Median (IQR) [bpm] |
MS-AT (31) | 53 (34;64) | 52 (38;67) | − 2.8 (− 21.4;24.8) | 0.93 | 0.29 | 0.44 |
| MS-C (30) | 46 (34;63) | 49 (37;61) | 11.7 (− 9.9;37.8) | ||||
|
6MWT Median (IQR) [meters] |
MS-AT (31) | 314 (175;420) | 317 (134;424) | 5.7 (− 3.3;13.7) | 0.01 | 0.89 | 0.05 |
| MS-C (30) | 367 (180;420) | 328 (215;443) | 1.4 (− 13.0;6.1) | ||||
|
T25FW Median (IQR) [seconds] |
MS-AT (31) | 7.44 (5.31;10.45) | 6.62 (5.50;11.36) | − 2.0 (− 9.6;8.8) | 0.40 | 0.67 | 0.09 |
| MS-C (30) | 6.16 (5.31;10.34) | 6.81 (5.15;9.71) | 0.4 (− 6.3;10.2) | ||||
|
6MWT Median (IQR) [meters] |
MS-WL-AT (13) | 252 (162;417) | 210 (85;340) | 3.1 (− 3.4;13.5) | 0.04 | 0.36 | 0.05 |
| MS-WOL-AT (18) | 347 (188;420) | 385 (191;471) | 6.4 (3.2;14.9) | ||||
|
6MWT Median (IQR) [meters] |
MS-WL-C (14) | 377 (197;414) | 340 (193;363) | − 5.7 (− 14.6;1.8) | 0.15 | 0.15 | 0.06 |
| MS-WOL-C (16) | 351 (185;420) | 370 (267;460) | 4.6 (− 5.8;6.1) | ||||
|
HRR Median (IQR) [bpm] |
MS-WL-A (13) | 64 (37;65) | 60 (41;73) | − 6.3 (− 10.1;23.0) | 0.67 | 0.64 | 0.49 |
| MS-WOL-A (18) | 46 (34;64) | 49 (36;56) | 1.1 (− 21.8;24.8) | ||||
|
HRR Median (IQR) [bpm] |
MS-WL-C (14) | 39 (26;57) | 41 (38;56) | 2.9 (− 8.9;41.9) | 0.47 | 0.84 | 0.42 |
| MS-WOL-C (16) | 46 (32;63) | 51 (31;64) | − 0.8 (− 8.5;20.4) | ||||
Statistically significant comparisons are shown in bold. Delta% = [(follow-up) – (baseline)/(baseline)] × 100
bpm beats per minute, HRR heart rate reserve, IQR interquartile range, Kg kilograms, min minutes, mL milliliters, MS-AT Multiple sclerosis (MS) patients that performed aerobic training, MS-C MS group that performed motor control training without direct involvement of cardiopulmonary system, MS-WL-AT MS patients with baseline insula lesions that performed aerobic training, MS-WOL-AT MS patients without baseline insula lesions that performed aerobic training, MS-WL-C MS patients with baseline insula lesions that performed motor control training, MS-WOL-C MS patients without baseline insula lesions that performed motor control training, T25FW timed 25-foot walk test, VO2max maximal oxygen consumption, 6MWT six-minute walk test
At follow-up, all clinical and cardiovascular measures showed a substantial improvement in MS-AT, even though only the 6MWT distance increase was statistically significant (median 6MWT delta = + 5.7%, in MS-AT), confirmed by group (p < 0.01) and time-group interaction significance level (p < 0.05). Within MS-AT, only 6MWT distance showed a significant improvement for MS-WOL (median 6MWT delta = + 6.4%) compared to MS-WL (median 6MWT delta = + 3.1%) patients (p < 0.05) (Table 4).
At follow-up, no new T2-hyperintense WM lesions were found in MS patients and PBVC was − 0.19% ± 0.76%. PBVC did not differ between MS-AT (− 0.91% ± 0.82%) and MS-C (− 0.31% ± 0.47%) nor between MS-WL-AT and MS-WL-C (p ≥ 0.133). Within MS-WOL, MS-AT showed a lower brain atrophy (mean PBVC = 0.08% ± 1.01%) compared to MS-C patients (− 0.46% ± 0.54%), although not significant (p = 0.067). Insular volume change did not differ within and between MS-AT and MS-C groups (p ≥ 0.231).
TBM findings
At follow-up, MS-AT showed increased GM volume in the right middle frontal gyrus and decreased GM volume in cerebellar, temporal and occipital areas. MS-C showed increased GM volume in few clusters within cerebellar and frontal areas and decreased GM volume in several clusters of occipital–temporal, cerebellar and frontal areas as well as bilateral insula (p < 0.01, uncorrected). Compared to MS-C, MS-AT showed significant GM volume increase in frontotemporal regions and GM volume decrease in parieto-occipital areas (Fig. 2).
Fig. 2.
Longitudinal tensor-based morphometry results. SPM analysis showing significant GM volume changes (increased volumes are encoded in red–yellow, while decreased volumes are encoded in blue–light blue, p < 0.001 uncorrected, cluster extent = 10). (a) Clusters showing GM volume increase within MS-AT group after training. (b) Clusters showing GM volume increase within MS-C group after training. (c) Clusters showing GM atrophy within MS-AT group after training. (d) Clusters showing GM atrophy within MS-C group after training. (e) Clusters showing greater GM volume increase in MS-AT compared to MS-C. (f) Clusters showing greater GM atrophy in MS-AT compared to MS-C. GM gray matter, MS multiple sclerosis, MS-AT MS group that performed aerobic training, MS-C MS group that performed motor control training without direct involvement of cardiopulmonary system, SPM statistical parametric mapping. The arrow in (d) points out the atrophy process experienced by MS-C within left insula area
Correlations between longitudinal clinical and MRI findings
In MS-AT, greater improvement in 6MWT distance was correlated with more limited left anterior insula volume loss (r = 0.651, p < 0.001 uncorrected). No other correlations were found.
Discussion
By evaluating cardiopulmonary and MRI data from a cohort of MS patients who underwent rehabilitation, we investigated the role of insular damage in modulating CF and training responses in this condition.
At baseline, compared to HC, MS patients showed significantly lower VO2max and 6MWT, higher HRR, especially in the presence of insular lesions, as well as widespread GM atrophy, including the bilateral insula. After training, MS-AT patients showed greater improvement in 6MWT compared to MS-C, especially in MS patients without insular lesions, and a significant association between a longer 6MWT distance and a more limited left anterior insula volume loss.
Our study showed that, compared to HC, MS patients had significantly lower VO2max and 6MWT, higher HRR and a widespread pattern of GM atrophy, suggesting that the disease is associated with worse aerobic fitness and walking capacity [23] together with irreversible neurodegeneration [24].
Consistently with previous literature [15, 16], our study shows a crucial role of insular damage on CF, despite the fact that no correlation has been found with HR at rest, but only with HRR, a measure of heart function during maximal physical effort. In MS patients, higher T2-hyperintense WM LV in the left insula, but not global T2-hyperintense WM LV or volumetric data, was significantly associated with higher HRR, indicating worst cardiopulmonary performance. Moreover, although not reaching the statistical significance, MS patients with insular lesions tended to have lower insular volumes, lower VO2max and shorter 6MWT distance, suggesting that insular damage may negatively influence maximal and submaximal indicators of aerobic fitness. Our results should be interpreted cautiously, since MS patients with lesions had more severe disability and structural brain damage that may also contribute to worse CF. However, they may explain, at least partially, the attenuated cardiovascular system response to exercise that is typically seen in MS patients compared to HC [3].
Disappointingly, insular volume was not associated with CF. The inclusion of MS patients with heterogeneous disease course and severity and the small sample size may contribute to explain this negative finding. However, it is tempting to speculate that insular focal WM lesions may be more relevant than atrophy in influencing CF. A disconnection of the insula from other brain regions involved in cardiac autonomic control may be one of the underlying phenomena. This process has been described in other neurological conditions, such as Parkinson disease [25], and it has been shown to contribute to cognitive impairment in MS [26].
Our analysis of the effects of AT confirmed that this rehabilitation strategy may significantly ameliorate aerobic and walking capacity in MS patients [27]. Indeed, MS-AT patients showed a significant improvement in 6MWT compared to MS-C and a trend toward improvement in all the other variables. It is noteworthy that the insula showed a key role in modulating the effects of AT. In particular, MS patients without insular lesions experienced a greater improvement in cardiopulmonary and walking capacity (i.e., 6MWT) after AT compared to those having insular lesions. It is also interesting that in patients who did not perform AT the presence of insular lesions led to a faster process of cardiopulmonary deconditioning over a period of three months (6MWT: − 5.7% vs + 4.6%, HRR: + 2.9% vs − 0.8%). Accordingly, the presence of insular lesions seems to impair the physiological improvement of cardiovascular fitness promoted by AT and may worsen CF in those MS patients not performing aerobic activity.
Consistently with previous studies [28], following rehabilitation both groups of MS patients showed dynamic volume modifications of GM areas involved in sensorimotor control, visual processing, and cognitive functions. Several physiological processes, such as synaptic sprouting and plasticity, angiogenesis, as well as changes at axonal, myelin, and non-neuronal cell levels, may concur to explain these volumetric changes following rehabilitative trainings [29]. In line with previous studies [23, 27], these longitudinal MRI results endorse the possible neuroprotective role of AT with a reduced atrophy progression rate in MS patients who performed this type of training.
Noteworthy, longitudinal volumetric analysis further supported the relevant interplay between insular structural integrity, CF modulation and response to AT. MS patients who performed AT did not experience insular atrophy and their greater improvement in 6MWT distance was correlated with a more limited progression of insular volume loss. Conversely, MS patients who performed motor training not involving the cardiopulmonary system showed a significant progression of left insular atrophy.
The prevailing role of the left compared to the right insula is in line with previous literature [30, 31] showing a stronger link between left rather than right insular lesions after a stroke and cardiovascular dysfunction in human and experimental models, supporting a lateralization of functions in the insula [6]. Moreover, the dominant hemisphere might be more vulnerable to the accumulation of damage for increased excitability and consequent overuse [32].
Our study is not without limitations. We evaluated a relatively small number of MS patients with some heterogeneities in the training protocol, thus possibly limiting our statistical power. However, given the nature of the study, the concomitant COVID-19 pandemic and the time commitment required, it was difficult to enroll a desirable larger number of subjects. For these reasons, our exploratory results should be confirmed on a larger sample. The lack of clinical and MRI longitudinal comparisons from matched HC did not allow to explore the potential effect of different training on HC’s CF and brain structure. Finally, although cortical lesions have been consistently reported in the insular cortex, a specific MRI sequence for their assessment was not available for our patients.
In conclusion, this study provides evidence that AT is an effective tool to improve cardiovascular function and walking capacity and to exert neuroprotective effects in MS patients. The insula, especially the left one, can modulate CF and AT responses in MS patients, thus its integrity should be taken into account to optimize the efficacy of AT treatments. Determining insula integrity using MRI can be informative of the possible results the patient can expect from moderate AT. However, further studies are needed to determine the best tailored treatment for patients also taking into account the insula status.
Acknowledgements
None.
Funding
This study has been supported by grant from Italian Ministry of Health (GR-2019–12369599).
Data availability
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
M. Albergoni has nothing to disclose. L. Storelli declared the receipt of grants and contracts from FISM—Fondazione Italiana Sclerosi Multipla—within a fellowship program (cod. 2019/BR/009). P. Preziosa received speaker honoraria from Roche, Biogen, Novartis, Merck Serono, Bristol Myers Squibb and Genzyme. He has received research support from Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. M.A. Rocca received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Merck Healthcare Germany, Merck Serono SpA, Novartis, Roche, and Teva. She receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders. M. Filippi is Editor in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Ethics approval
Approval was received from the institutional ethical standards committee on human experimentation of IRCCS Ospedale San Raffaele for any experiments using human subjects (Protocol number 59/INT/2015). Written informed consent was obtained from all subjects prior to study participation according to the Declaration of Helsinki.
References
- 1.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 2.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 3.Huang M, Jay O, Davis SL. Autonomic dysfunction in multiple sclerosis: implications for exercise. Auton Neurosci. 2015;188:82–85. doi: 10.1016/j.autneu.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogolla N. The insular cortex. Curr Biol. 2017;27(12):R580–R586. doi: 10.1016/j.cub.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheimer S, Cechetto D. The insular cortex and the regulation of cardiac function. In: Terjung R, editor. Comprehensive physiology. Hoboken: John Wiley & Sons, Inc.; 2016. pp. 1081–1133. [DOI] [PubMed] [Google Scholar]
- 6.Chouchou F, Mauguière F, Vallayer O, Catenoix H, Isnard J, Montavont A, et al. How the insula speaks to the heart: cardiac responses to insular stimulation in humans. Hum Brain Mapp. 2019;40(9):2611–2622. doi: 10.1002/hbm.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24(83):1–262. [PubMed] [Google Scholar]
- 8.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi: 10.1212/WNL.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 9.Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135. doi: 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017;17(1):185. doi: 10.1186/s12883-017-0960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen FT, Hopman RJ, Huang CJ, Chu CH, Hillman CH, Hung TM, et al. The effect of exercise training on brain structure and function in older adults: a systematic review based on evidence from randomized control trials. J Clin Med. 2020;9:4. doi: 10.3390/jcm9040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay CP, Kuys SS, Brauer SG. The effect of aerobic exercise on brain-derived neurotrophic factor in people with neurological disorders: a systematic review and meta-analysis. Neural Plast. 2017;2017:4716197. doi: 10.1155/2017/4716197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negaresh R, Motl RW, Zimmer P, Mokhtarzade M, Baker JS. Effects of exercise training on multiple sclerosis biomarkers of central nervous system and disease status: a systematic review of intervention studies. Eur J Neurol. 2019;26(5):711–721. doi: 10.1111/ene.13929. [DOI] [PubMed] [Google Scholar]
- 14.Consorti A, Di Marco I, Sansevero G. Physical exercise modulates brain physiology through a network of long- and short-range cellular interactions. (1662–5099 (Print)). [DOI] [PMC free article] [PubMed]
- 15.Winder K, Linker RA, Seifert F, Wang R, Lee D-H, Engelhorn T, et al. Cerebral lesion correlates of sympathetic cardiovascular activation in multiple sclerosis. Hum Brain Mapp. 2019;40(17):5083–5093. doi: 10.1002/hbm.24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turri G, Calabrese M, Pancheri E, Monaco S, Gajofatto A, Marafioti V. QTc interval in patients with multiple sclerosis: an inference from the insula of Reil? (1468–1331 (Electronic)). [DOI] [PubMed]
- 17.Valverde S, Cabezas M, Roura E, Gonzalez-Villa S, Pareto D, Vilanova JC, et al. Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage. 2017;155:159–168. doi: 10.1016/j.neuroimage.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 20.Leow AD, Klunder AD, Jack CR, Jr, Toga AW, Dale AM, Bernstein MA, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage. 2006;31(2):627–640. doi: 10.1016/j.neuroimage.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 23.Dalgas U. Exercise therapy in multiple sclerosis and its effects on function and the brain. Neurodegen Dis Manag. 2017;7(6s):35–40. doi: 10.2217/nmt-2017-0040. [DOI] [PubMed] [Google Scholar]
- 24.Andravizou A, Dardiotis E, Artemiadis A, Sokratous M, Siokas V, Tsouris Z, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Auto Immun Highlights. 2019;10(1):7. doi: 10.1186/s13317-019-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fathy YY, Hepp DH, de Jong FJ, Geurts JJG, Foncke EMJ, Berendse HW, et al. Anterior insular network disconnection and cognitive impairment in Parkinson's disease. Neuroimage Clin. 2020;28:102364. doi: 10.1016/j.nicl.2020.102364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302–317. doi: 10.1016/S1474-4422(14)70250-9. [DOI] [PubMed] [Google Scholar]
- 27.Rampello A, Franceschini M, Piepoli M, Antenucci R, Lenti G, Olivieri D, et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther. 2007;87(5):545–555. doi: 10.2522/ptj.20060085. [DOI] [PubMed] [Google Scholar]
- 28.Rocca MA, Meani A, Fumagalli S, Pagani E, Gatti R, Martinelli-Boneschi F, et al. Functional and structural plasticity following action observation training in multiple sclerosis. Mult Scler. 2019;25(11):1472–1487. doi: 10.1177/1352458518792771. [DOI] [PubMed] [Google Scholar]
- 29.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min J, Farooq MU, Greenberg E, Aloka F, Bhatt A, Kassab M, et al. Cardiac dysfunction after left permanent cerebral focal ischemia: the brain and heart connection. Stroke. 2009;40(7):2560–2563. doi: 10.1161/STROKEAHA.108.536086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6(3):131–140. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 32.Preziosa P, Pagani E, Mesaros S, Riccitelli GC, Dackovic J, Drulovic J, et al. Progression of regional atrophy in the left hemisphere contributes to clinical and cognitive deterioration in multiple sclerosis: a 5-year study. Hum Brain Mapp. 2017;38(11):5648–5665. doi: 10.1002/hbm.23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.



