Abstract
Vemurafenib (PLX4032), a selective inhibitor of Braf, has been FDA-approved for the treatment of unresectable or metastatic melanoma in patients with BrafV600E mutations. Many patients treated with vemurafenib initially display dramatic improvement, with decreases in both risk of death and tumor progression. Acquired resistance, however, rapidly arises in previously sensitive cells. We attempt to overcome this resistance by targeting the Stat3-PAX3 signaling pathway, which is upregulated, due to FGF2 secretion or increased kinase activity, with the BrafV600E mutation. We found that activation of Stat3 or overexpression of PAX3 induced resistance to vemurafenib in melanoma cells. Additionally, PAX3 or Stat3 silencing inhibited the growth of melanoma cells with acquired resistance to vemurafenib. Furthermore, treatment with the Stat3 inhibitor, WP1066, resulted in growth inhibition in both vemurafenib-sensitive and -resistant melanoma cells. Significantly, vemurafenib stimulation induced FGF2 secretion from keratinocytes and fibroblasts, which might uncover, at least in part, the mechanisms underlying targeting Stat3-PAX3 signaling to overcome the acquired resistance to vemurafenib. Our results suggest that Stat3-targeted therapy is a new therapeutic strategy to overcome the acquired resistance to vemurafenib in the treatment of melanoma.
Introduction
Braf is a serine–threonine specific protein kinase and an isoform of Raf. Raf proteins (Raf-1, Araf and Braf) are intermediates in the Ras and MAPK signaling pathways and affect cell proliferation (Fecher et al., 2007). Braf mutations are the most prevalent somatic genetic events in human cutaneous melanoma, occurring in 40% to 60% of metastatic melanoma (Chin et al., 2006). Most Braf melanoma mutations are within the kinase domain, with a single substitution (V600E), accounting for 80% of the Braf melanoma mutations (Brose et al., 2002; Davies et al., 2002; Pollock and Meltzer, 2002). The mutant BrafV600E protein possesses 10.7-fold increased kinase activity, compared to wild-type Braf (Davies et al., 2002).
Currently, selective inhibitors of Braf, such as vemurafenib (Bollag et al.; Chapman et al., 2011; Flaherty et al., 2010) and GSK2118436 (Flaherty and McArthur, 2010), have demonstrated remarkable clinical activity in patients with melanoma, and vemurafenib has recently been FDA-approved for the treatment of patients with unresectable or metastatic melanoma with BrafV600E mutations (Chapman et al., 2011). Vemurafenib was associated with a 63% relative reduction in the risk of death and a 74% reduction in the risk of tumor progression in patients with previously untreated, unresectable stage IIIC or stage IV melanoma with BrafV600E mutations (Chapman et al., 2011). Although the initial response to these drugs is profound, it is temporary. Drug resistance frequently appears after only 6 to 9 months of therapy (Poulikakos and Rosen, 2011). This type of “acquired resistance” develops after the melanomas were originally sensitive to vemurafenib. Acquired resistance has emerged as the major hurdle, preventing vemurafenib from having a truly transformative impact on patients with unresectable or metastatic melanoma with BrafV600E mutations.
One potential solution to overcome the acquired resistance of vemurafenib in patients with melanoma is to develop combination-targeted therapies. One possible approach is targeting a frequently altered pathway together with one of the essential pathways in normal pigment cells. This strategy could simplify patient selection, because the status of only one molecularly altered pathway is required for choosing the most appropriate therapy. In addition, this therapy strategy would reduce the potential toxicities, as lower drug doses could be utilized to target the essential pathway. One prospective candidate of the essential pathway in melanocytes is the paired box homeotic gene 3 (PAX3) signaling cascade.
PAX3 is essential for maintaining melanocytic progenitor cells (Blake and Ziman, 2005; Scholl et al., 2001; Steingrimsson et al., 2005). A chromosomal deletion, a splice-site mutation and an amino acid substitution within PAX3 cause Splotch-retarded, Splotch and Splotch-delayedgenetic mouse mutants, respectively (Tassabehji et al., 1994). Splotch-delayed homozygous mice survive to birth, compared to Splotch mutant mice which die at E13 due to neural tube defects (Moase and Trasler, 1992). Heterozygous Splotch-delayed (Splotch/+) mice display pigmentation abnormalities characterized by abdominal white patches due to defective neural crest-derived melanocyte development (Epstein et al., 1993). PAX3 mutations result in humans to produce type I and type III Waardenburg syndromes (Read and Newton, 1997; Tassabehji et al., 1992), conditions characterized by melanocyte deficiencies in the skin and inner ear.
Our previous work has demonstrated that Stat3 binds to the PAX3 upstream regulatory regions to transactivate the PAX3 promoter, resulting in constitutive PAX3 expression in melanocytes in vivo and increased melanocyte numbers (Dong et al., 2012). Although no small molecules are currently available to directly target PAX3 transcription factor, Stat3-targeted therapies are being evaluated in clinical trials for several types of tumors (Darnell, 2005).We therefore investigated whether Stat3 inhibition could be used to overcome the acquired resistance to vemurafenib in melanoma.
Results
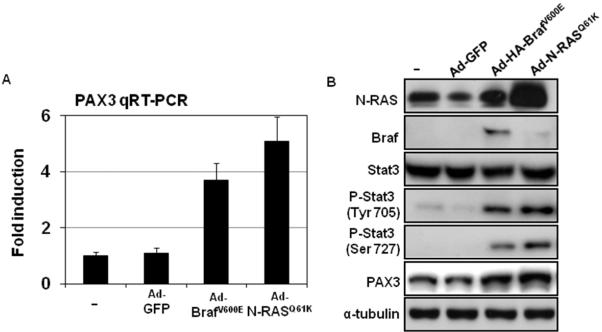
Stat3-PAX3 signaling is activated by BrafV600E or N-RASQ61K in melanoma cells
In the case of vemurafenib resistance, certain N-RAS mutations have been implicated (Nazarian et al., 2010). Thus, a plethora of additional agents are needed to continue therapy. To characterize the connection of vemurafenib resistance and Stat3-PAX3 signaling in melanocytes, we introduced N-RASQ61K or BrafV600E mutations into genetically modified human melanocytes (hTERT/CDK4(R24C)/p53DD melanocytes) (Garraway et al., 2005) and measured phospho-Stat3 protein as well as PAX3 mRNA and protein levels at 48 hr after introduction of N-RASQ61K or BrafV600E. We observed a nearly 5-fold induction of PAX3 mRNA, accompanied by a marked induction of phospho-Stat3 and PAX3 protein expression after the infection with N-RASQ61K virus or BrafV600E virus (Figure 1). These results suggest that activation of Stat3 and upregulation of-PAX3 are downstream targets of mutant N-RASQ61K or BrafV600E in melanoma cells.
Figure 1. Introduction of BrafV600E or N-RASQ61K activates Stat3-PAX3 signaling in melanocytes.
Genetically engineered human melanocytes (hTERT/p53DD/CDK4(R24C)) were infected with Ad-N-RASQ61K, Ad-BrafV600E or Ad-GFP. RNA and protein were collected at 24 hr after infections. (A) The mRNA expression of PAX3 was measured by quantitative RT-PCR and normalized to GAPDH. Results are expressed as the mean of the experiment done in triplicate ± SEM. Induction is calculated relative to PAX3 levels in vehicle-treated cells. (B) Protein expressions of Stat3, phospho-Stat3 and PAX3 were analyzed by western blot along with tubulin, which served as a loading control.
Vemurafenib resistance in melanoma cell lines
To identify whether Stat3-PAX3 targeted therapy is an effective strategy to overcome the acquired resistance to vemurafenib, we generated vemurafenib-resistant A375 (A375R) and UACC62 (UACC62R) melanoma cells. A375 and UACC62 melanoma cells both harbor the BrafV600E mutation, and both are sensitive to vemurafenib treatment (Supplementary Figure 1). Drug-resistant A375 and UACC62 cells were generated through treatment with increasing concentrations of vemurafenib (Nazarian et al., 2010; Villanueva et al., 2010) (Supplementary Figure 1). The IC50's of parental A375 and UACC62 were 70nM and 90nM, respectively. As expected, we found that the vemurafenib-resistant cells required higher doses of vemurafenib for partial growth inhibition; the IC50's of A375R and UACC62R were 4.4μM and 5.1μM, respectively (Supplementary Figure 1B & C). Cell cycle analysis demonstrated that treatment with 90nM of vemurafenib induced G0/G1 cell cycle arrest after 24 hr in parental cells, but this level of vemurafenib did not effect in the vemurafenib-resistant cells (Supplementary Figure 1C).
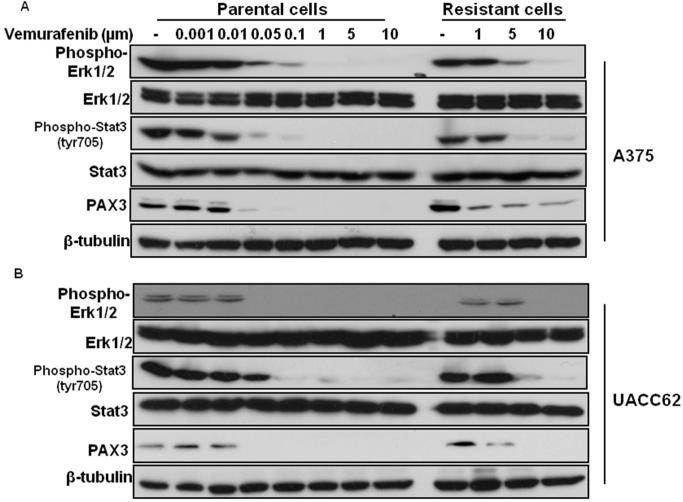
Vemurafenib treatment represses Stat3-PAX3 signaling in vemurafenib-sensitive melanoma cells
The activation of Stat3-PAX3 signaling is induced upon infection with Ad-RASQ61K or Ad-BrafV600E adenoviral vectors (Figure 1). Next, we examined the effect of vemurafenib on Stat3-PAX3 signaling in vemurafenib-sensitive and -resistant melanoma cells. The expression of phospho-Stat3 and PAX3 was detected in vemurafenib-stimulated parental and resistant A375 and UACC62 melanoma cells (Figure 2). We found that the activation of Stat3 and the protein expression of PAX3 were both repressed in vemurafenib sensitive (parental) A375 and UACC62 melanoma cells, but higher vemurafenib concentration were required in resistant A375 and UACC62 melanoma cells. Specifically, vemurafenib at 1μM inhibited the expression of phospho-Stat3 in sensitive cells, but not in resistant cells (Figure 2). These results suggest that inhibition of Stat3 signaling represents a potential therapeutic strategy to overcome the acquired resistance to vemurafenib in melanoma cells.
Figure 2. Stat3 is activated in melanoma cells with acquired resistance to vemurafenib.
Sensitive and resistant A375 and UACC62 melanoma cells were stimulated by different doses of vemurafenib as indicated. Total protein was collected 6 hr after stimulation. Protein expressions of phospho--ERK1/2, Stat3, phospho-Stat3 and PAX3 were analyzed by western blot along with tubulin, which served as a loading control.
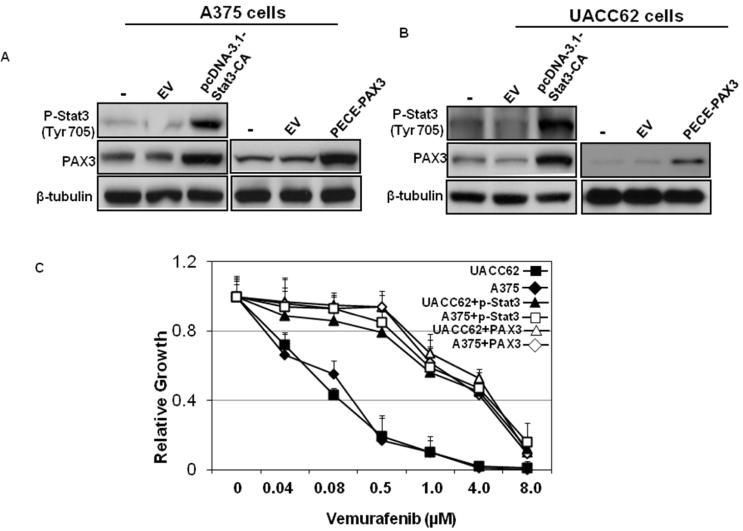
PAX3 or Stat3 overexpression inhibits the sensitivity of melanoma cells to vemurafenib
To further characterize the connection of vemurafenib resistance and Stat3-PAX3 signaling in melanoma cells, we introduced pcDNA-3.1-Stat3-CA (Ginsberg et al., 2007) or PECE-PAX3 plasmids into A375 and UACC62 parental cells and then measured the response of these cells to vemurafenib. Introduction of pcDNA-3.1-Stat3-CA results in overexpression of constitutively active Stat3 due to cysteine residues at A661 and N663 of Stat3 (Dai et al., 2011; Ginsberg et al., 2007). The IC50's of A375 sensitive cells with Stat3 or phospho-PAX3 overexpression were 2.3μM and 3.7μM, respectively. The IC50's of UACC62 sensitive cells with phospho-Stat3 or PAX3 overexpression were 3.6μM and 4.7μM, respectively (Figure 3C). These results indicate that A375 and UACC62 cells were resistant to vemurafenib treatment after phospho-Stat3 or PAX3 introduction.
Figure 3. Overexpression of PAX3 or Stat3 inhibits the response of melanoma cells to vemurafenib.
A375 and UACC62 parental cells with pcDNA-3.1-Stat3-CA plasmids or PECE-PAX3 plasmids introduction were stimulated with different doses of vemurafenib as indicated. Total protein was collected 6 hr after stimulation. Protein expressions of Stat3 and PAX3 were analyzed by western blot along with tubulin, which served as a loading control. (A) A375 cells and (B) UACC62 cells. (C) Melanoma cell growth was assessed by MTT assays. Relative growth (RG) was calculated as the ratio of treated to untreated cells at each dose for each replicate.
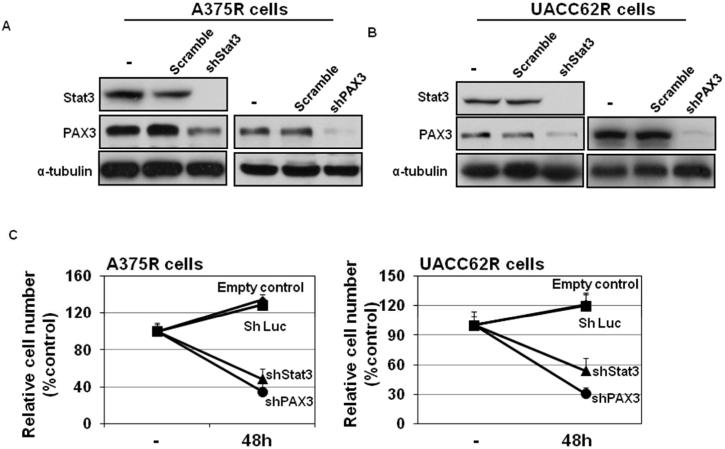
PAX3 or Stat3 silencing inhibits the growth of resistant melanoma cells
To examine whether inhibition of Stat3-PAX3 signaling could be a therapeutic strategy to overcome the acquired resistance to vemurafenib in melanoma cells, Stat3 or PAX3 expression was silenced in A375 and UACC62 vemurafenib-resistant cells (Figure 4A & B). We found that knockdown of PAX3 and Stat3 expression significantly inhibited melanoma cell growth (Figure 4C). Taken together, the phospho-Stat3 and PAX3 expression studies and the Stat3 and PAX3 silencing experiments both indicate that Stat3-PAX3 signaling represents a target for overcoming acquired vemurafenib resistance in melanoma cells.
Figure 4. Resistant melanoma cellular growth was inhibited after silencing PAX3 or Stat3 and vemurafenib treatment.
A375 and UACC62 resistant cells stably expressing control shRNA (shScr), shStat3 or shPAX3 were stimulated with different doses of vemurafenib as indicated. Total protein was collected 6 hr after stimulation. Protein expressions of Stat3 and PAX3 were analyzed by western blot along with tubulin, which served as a loading control. (A) A375R cells and (B) UACC62R cells. (C) Melanoma cells were infected with shPAX3, shStat3 or control virus and after 48 hrs, cells were subjected to MTT assays to evaluate the relative cell numbers. Relative growth (RG) was calculated as the ratio of treated to untreated cells at each dose for each replicate.
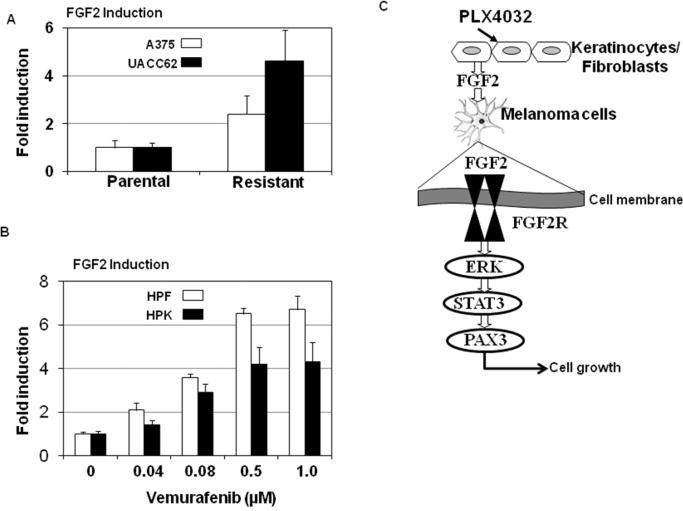
Vemurafenib treatment induces FGF2 secretion from melanoma cells and stromal cells
A recent report demonstrates that tumor drug resistance is caused, at least in part, by factors secreted by the tumor micro-environment (Straussman et al., 2012). Secreted fibroblast growth factor (FGF2) signaling plays an important role in the activation of Stat3-PAX3 signaling in melanocytes (Dong et al., 2012). To examine whether FGF2 secretion is induced by vemurafenib treatment in melanoma cells, FGF2 levels were monitored in growth media from cultured parental and vemurafenib-resistant melanoma cells. Cells were grown for 48 hr at 80% confluence in serum-free media and FGF2 was assayed in growth media, using an enzyme-linked immunosorbent assay (ELISA) FGF2 kit (F4210-19, US Biologic Marblehead, MA)). We found that secreted FGF2 levels were higher in vemurafenib-resistant melanoma cells compared to vemurafenib-sensitive (parental) cells (Figure 5A). In addition, both primary keratinocytes and fibroblasts were treated with different doses of vemurafenib. Culture media was collected and FGF2 levels were determined by ELISA (Figure 5B). We found that FGF2 secretion was induced by vemurafenib treatment in a dose dependent manner. FGF2 has well–documented proliferative activities and transduces signals via FGF receptors (Jaye et al., 1992). Multiple studies have shown that FGF2 treatment can induce proliferation of melanocytes in vitro (Halaban et al., 1988; Imokawa et al., 1992) as well as in pigmented lesions in grafts (Berking et al., 2001). Our previous work demonstrated that secreted FGF2 from keratinocytes activates Stat3 in melanocytes. Mechanistically, Stat3 binds to PAX3 upstream regulatory sequences resulting in constitutive PAX3 expression and increased numbers of melanocytes (Dong et al., 2012). In this study, we found higher levels of secreted FGF2 in vemurafenib-resistant melanoma cells compared to vemurafenib-sensitive melanoma cells. In addition, vemurafenib stimulation induced FGF2 secretion from both keratinocytes and fibroblasts. We postulate that Stat3-PAX3-mediated vemurafenib resistance involves FGF2 secreted by the tumor micro- environment (Figure 5C).
Figure 5. Vemurafenib induces FGF2 secretion.
(A) Levels of FGF2 were measured in resistant melanoma cells. FGF2 levels in growth media of cultured parental and resistant melanoma cells, growing at 80% confluence in serum-free medium were examined by ELISA. FGF2 is induced by vemurafenib treatment in vitro. (B) Human primary keratinocytes and human primary fibroblast were stimulated by different doses of vemurafenib as indicated. FGF2 levels were measured by ELISA in culture media 24 hr after stimulation. Results are expressed as the mean of the experiment done in triplicate ± SEM. Induction is calculated relative to FGF2 levels in vehicle-treated cell media. (C) Schematic diagram of the FGF2-Stat3-PAX3 signaling pathway.
Stat3 targeted therapy overcomes the acquired resistance to vemurafenib in melanomas
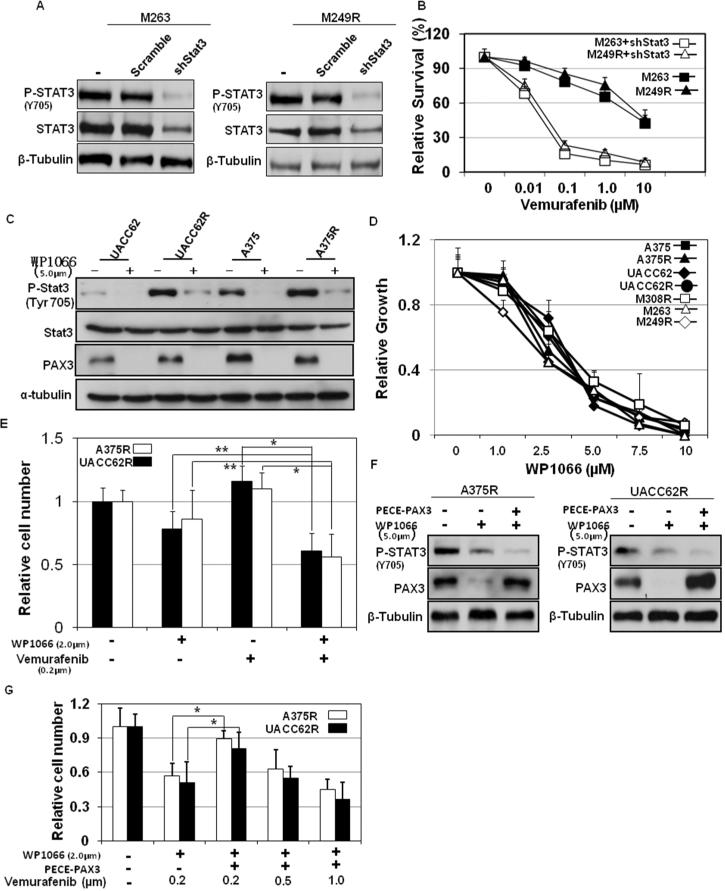
We have demonstrated that silencing Stat3-PAX3 signaling inhibits the growth of vemurafenib-resistant cells (Figure 4). This suggests that Stat3-PAX3 targeted treatment could be a new therapeutic strategy to overcome the acquired resistance to vemurafenib. To date, small molecules that target the PAX3 transcription factor are not available. In contrast, Stat3-targeted therapies are well established (Levy and Inghirami, 2006) and, in fact, several small molecules targeting Stat3 are currently being evaluated for head and neck tumors in Phase I/II clinical trials (clinicaltrials.gov), including the reagent WP1066 (Verstovsek et al., 2008). To examine whether Stat3-targeting reagents can overcome melanoma acquired resistance to vemurafenib, we tested the effect of WP1066 on vemurafenib sensitivity of melanoma. We found that WP1066 blocked Stat3 signal activation by inhibiting the phosphorylation of Stat3 and repressing of PAX3 protein expression in both parental and vemurafenib-resistant melanoma cells (A375 and UACC62; Figure 6A). Consistent with this, Stat3-PAX3 inhibition by WP1066 resulted in growth inhibition (Figure 6B) in parental and resistant A375 and UACC62 melanoma cells. The IC50's of parental A375 and UACC62 cells were 2.5μM and 3.4μM, respectively. The IC50's of resistant A375 and UACC62 cells were 2.5μM and 3.5μM, respectively, which are similar to the IC50's of parental A375 and UACC62 cells. We next evaluated the growth inhibitory potential of combining vemurafenib and WP1066 in vemurafenib-resistant cells. As shown in Figure 6C, cell growth was more significantly inhibited by combining WP1066 (2μM) and vemurafenib (200nM) treatments together, compared to either agent on its own. Thus, combination therapy with WP1066 and vemurafenib can overcome the acquired resistance to vemurafenib.
Figure 6. Inhibition of Stat3 overcomes the acquired resistance to vemurafenib in resistant melanoma cells.
(A) M263R and M249R melanoma cells stably expressing control shRNA (shScr), shStat3 or shPAX3 were stimulated different doses of vemurafenib as indicated. (C) Sensitive and resistant A375 and UACC62 melanoma cells were stimulated with 5.0μm WP1066. Total protein was collected at 6 hr after stimulation. Protein expression of Stat3 and phospho-Stat3 were analyzed by western blot along with tubulin, which served as a loading control. A375R and UACC62R melanoma cells were stimulated with different doses of vemurafenib (B), WP1066 (D) or both (E) as indicated. Melanoma cell growth was assessed by MTT assays. Relative growth (RG) was calculated as the ratio of treated to untreated cells at each dose for each replicate (*p< 0.01; **p<0.05). A375 and UACC62 resistant cells with PECE-PAX3 plasmids were stimulated with vemurafenib and/or WP1066 as indicated. (F) Total protein was collected 6 hr after stimulation. Protein expression of phospho-Stat3 and PAX3 were analyzed by western blot along with tubulin, which served as a loading control. (G) Cells were subjected to MTT assays to evaluate the relative cell numbers. Relative growth (RG) was calculated as the ratio of treated to untreated cells at each dose for each replicate (*p< 0.01).
We also investigated the effects of Stat3 inhibition on cell growth in three other vemurafenib-resistant cells: M249R, M263R and M308R (Nazarian et al., 2010). Naturally occurring vemurafenib resistance arose in both M263R and M308R cells and a specific N-RAS mutation gave rise to vemurafenib resistance in M249R cells (Nazarian et al., 2010). To confirm that inhibition of Stat3 is a new therapeutic strategy to overcome the acquired resistance to vemurafenib in melanoma cells, we first silenced the expression of Stat3 in M263R and M249R cells (Figure 6D). The IC50's of vemurafenib in M263R resistant cells with stable shStat3 was 46nM and the IC50 of vemurafenib in M249R cells with stable shStat3 was 58nM (Figure 6E). These results indicate that PAX3 or Stat3 silencing inhibits the growth of M263R and M249R cells. We also found that WP1066 treatment resulted in growth inhibition in M263R, M249R and M308R cells (Figure 6B). The vemurafenib IC50's in M249R, M263R and M308R cells were 2.3μM, 2.3μM and 3.9μM, respectively. These results are consistent with the effects of WP1066 on parental and vemurafenib-resistant A375 and UACC62 melanoma cells and suggest that inhibition of Stat3-PAX3 signaling by WP1066 is an effective therapeutic strategy to overcome acquired resistance to vemurafenib.
To identify whether PAX3 repression is required in WP1066-induced growth inhibition in resistant melanoma cells, we introduced PECE-PAX3 plasmids into A375 and UACC62 resistant cells and then evaluated the growth inhibitory potential of combining vemurafenib and WP1066. As shown in Figure 6 F & G, cell growth was inhibited by combining WP1066 (2 μ M) and vemurafenib (200nM) treatment together in A375 and UACC62 resistant melanoma cells, but higher vemurafenib concentration were required (500nm) in resistant A375 and UACC62 melanoma cells with PAX3 overexpression.
Discussion
Vemurafenib received FDA approval for the treatment of late-stage melanoma on August 17, 2011. Unfortunately, de novo and acquired resistance to vemurafenib are common (Chapman et al., 2011). Therefore, it is important to understand vemurafenib resistance mechanisms and identify potential therapeutic strategies that could overcome this resistance. In the case of vemurafenib resistance, PDGFRβ up-regulation, N-RAS mutation (Nazarian et al., 2010) and increased MAP3K8/COT activity (Johannessen et al., 2010; Wagle et al., 2011) have all been implicated.
Drug combination therapies involving 1) Braf inhibitors and MEK inhibitors (Basse et al., 2010; Bollag et al., 2010; Joseph et al., 2010; Paraiso et al., 2010; Villanueva et al., 2010), 2) PI3K/AKT/mTOR inhibitors (Dankort et al., 2009; Shi et al., 2011; Villanueva et al., 2010) and 3) different immunotheraptic reagents (Hodi et al., 2010; Tsao et al., 2004) have all been studied in vitro. However, these combination therapies would theoretically only be effective in specific group of patients. In our present study, we demonstrate that vemurafenib treatment represses Stat3-PAX3 signaling in vemurafenib-sensitive melanoma cells, but higher vemurafenib concentration are required in vemurafenib-resistant melanoma cells. In addition, we show that inhibition of Stat3-PAX3 signaling inhibits cellular growth in melanoma cells with the acquired resistance to vemurafenib.
PAX3 is essential for maintaining melanocytic progenitor cells (Blake and Ziman, 2005; Schollet al., 2001; Steingrimsson et al., 2005) and PAX3 overexpression is also frequently detected in melanomas (Abrahams et al., 2008; Barr et al., 1999; Carreira et al., 1998; Plummer et al., 2008; Rodriguez et al., 2008; Scholl et al., 2001; Vachtenheim and Novotna, 1999). Approximately 30-70% of primary melanoma specimens and 77% of cultured primary melanoma cells displayed PAX3 overexpression (Barr et al., 1999; Plummer et al., 2008; Scholl et al., 2001; Yang et al., 2008). However, the role of PAX3 in acquired resistance to vemurafenib in melanoma is not known. Here, we demonstrate that PAX3 silencing inhibits growth in melanomas with acquired resistance to vemurafenib.
The Jak2-Stat3 pathway is emerging as a target of interest for melanoma (Kortylewski et al., 2005; Krasilnikov et al., 2003; Smalley and Herlyn, 2005). In malignant cells, Stat3 functions in regulating cell proliferation, angiogenesis and inhibition of apoptosis (Amin et al., 2004; Catlett-Falcone et al., 1999; Zushi et al., 1998). Importantly, activation of Stat3 signaling is a negative prognostic factor in human cutaneous melanoma (Lee et al., 2012; Wang et al., 2007). In addition, the anti-tumor effects of tyrosine isomers were mediated in part by both inhibition of the MAP/ERK pathway and inactivation of Stat3 signaling (Ruggiero et al., 2012). Here we confirm previous reports and demonstrate that vemurafenib treatment repressed the activation of Stat3 in melanoma cells. We also show that inhibition of Stat3 signaling inhibits cellular growth in melanoma cells with acquired resistance to vemurafenib. A previous report demonstrated that tyrosine phosphorylation of Stat3/5 and of Jak2 was induced upon treatment of LU1205 melanoma cells with the MEK inhibitor PD98059 (Krasilnikov et al., 2003). To further identify the connection between MEK and Stat3 in melanoma cells, we silenced MEK1/2 expression in B16-F10 cells and found that tyrosine phosphorylation of Stat3 was repressed upon MEK1/2 silencing in B16-F10 cells (Supplementary Figure 2). One possibility is that the PD98059-mediated induction and activation of Stat3 signaling in LU1205 melanoma cells is a cell and time-point specific.
WP1066 is a cell-permeable, AG 490 tyrphostin analog that effectively inhibits the Jak2-Stat3 pathway (Hussain et al., 2007) and subsequently inhibits the growth of malignant glioma cells (Hussain et al., 2007), acute myelogenous leukemia cells (Ferrajoli et al., 2007) and melanoma cells (Kong et al., 2008). Previous reports have demonstrated that WP1066 inhibits melanoma cell growth and melanoma metastasis (Kong et al., 2008), and enhances T-cell cytotoxicity against melanoma by inhibiting regulatory T cells (Kong et al., 2009). A recent report shows that WP1066 enhanced the antitumor activity of cyclophosphamide (CTX) in a xenograft melanoma mouse models (Hatiboglu et al., 2012). In this study, we demonstrate that WP1066 reduced cell proliferation, and induced apoptosis and cell cycle arrest in melanoma cells both with and without the acquired resistance to vemurafenib. The IC50's of WP1066 for cancer cells, including the melanoma cells used here, range from 1.5-5μmols (Kong et al., 2008; Kong et al., 2009). Although WP1066 is currently being evaluated for head and neck tumors and lymphoma in Phase I/II clinical trials (clinicaltrials.gov), these high IC50 values would limit its potential in a clinical setting. Therefore, new, more-sensitive small molecules that target Stat3 need to be developed and evaluated. However, these intriguing findings should encourage the identification of other Stat3-targeting reagents that could be useful in addressing the acquired resistance to vemurafenib in melanoma.
Material and Methods
Cell lines and reagents
Primary keratinocytes and human foreskin fibroblasts were isolated from normal discarded foreskins as described (Dunham et al., 1996; Horikawa et al., 1996). Human primary keratinocytes were cultured in keratinocyte serum-free medium (SFM) (Invitrogen Corporation, USA). A375 and UACC62 melanoma cells were generously provided by Dr. David Fisher (MGH, Harvard Medical School). M249R, M263R and M308R were generously provided by Dr. Roger S. Lo and Dr. Antoni Ribas (UCLA) (Nazarian et al., 2010). All cells were cultured in DMEM medium plus 10% FBS. Immortal human melanocytes (hTERT/p53DD/CDK4(R24C)) (Garraway et al., 2005) were cultured in glutamine containing Ham's F12 media supplemented with 7% FBS, 0.1mM IBMX, 50ng/mL TPA, 1 μ M Na3VO4 and 1 μ M dbcAMP. Vemurafenib (PLX4032) was purchased from Selleckchem (Houston, TX). WP1066 was purchased from Santa Cruz (Santa Cruz, CA). pRetro-shStat3, pRetro-shPAX, pRetro-shMEK1 and pRetro-shMEK2 silencing vectors, were purchased from Cellogenetics (Ijamsville, MD). Plasmids pcDNA-3.1-Stat3-CA was generously provided by Dr. James E. Darnell (The Rockefeller University, New York) (Ginsberg et al., 2007). PECE-PAX3 plasmids were generously provided by Dr. Michel Goossens. Lipofectamine™ 2000 (Invitrogen, Inc) was used in transfection. FGF2 ELISA kits were purchased from Invitrogen.
MTT Assay of Cell Numbers
Ten thousand cells per well were plated in a 96-well plate. Vemurafenib (PLX4032) or WP1066 was added to the growth media after overnight culture and MTT solution was added 72 hrs later. Optical density was read at 550 nm, and background was subtracted at 690 nm.
Flow cytometry
Cells were fixed in ice-cold 70% ethanol before DNA staining with 50μg/ml propidium iodide (Sigma Aldrich) in PBS containing 0.1 mg/ml RNase (Amersham). DNA content was analyzed by flow cytometry (Becton Dickinson FACSCalibur).
Total RNA isolation, protein isolation, Real-Time RT-PCR, Western Blotting and Enzyme Immunoassay
Total RNA was isolated from melanocytes using TRIzol reagent (Invitrogen). cDNA was synthesized with the SuperScript first strand system (Invitrogen) using 2 μg of total RNA as template and oligo(dT) as primer. Total protein was extracted by in DOC buffer (Pierces protein assay kit).
For quantitative RT-PCR, total RNA was converted into cDNA using the SuperScript™ III reverse transcriptase kit (Invitrogen). cDNA expression was measured using the QuantiTect Probe RT-PCR kit (Qiagen, Valencia, CA) and the ICycler detector (BioRad, Hercules, CA). Gene-specific primers are as described (Dong et al., 2012).
Western blotting was performed using the following antibodies: anti-PAX3 (The Developmental Studies Hybridoma Bank at the University of Iowa); anti-p-Stat3 (Tyr705) (Cell Signaling); anti-p-Stat33 (Ser727) (Cell Signaling); anti-Stat3 (9D8, Thermo Scientific); anti-p-ERK1/2 (Cell Signaling); and anti-tubulin (Sigma). Enzyme immunoassay was performed using FGF2 ELISA kit (F4210-19, US Biologic Marblehead, MA).
Statistical Analysis
Experiments were independently carried out at least three times and one representative data set out of the three independent experiments was presented where appropriate. The results were evaluated for statistical significance by student t-test or two-sample t-test. Error bars were marked as the standard deviation (SD) of the mean. p-values less than 0.05 were regarded as significant.
Supplementary Material
Acknowledgments
The PAX3 monoclonal antibody developed by Dr. Charles P. Ordahl was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This work was supported by National Institutes of Health (7RO1CA137098 for RC), American Cancer Society (RSG-09-022-01-CNE), and The Harry J. Lloyd Charitable Trust (RC). RC is an American Cancer Society Research Scholar. No potential conflicts of interest exist.
References
- Abrahams A, Mowla S, Parker MI, Goding CR, Prince S. UV-mediated regulation of the anti-senescence factor Tbx2. J Biol Chem. 2008;283:2223–2230. doi: 10.1074/jbc.M705651200. [DOI] [PubMed] [Google Scholar]
- Amin HM, McDonnell TJ, Ma Y, Lin Q, Fujio Y, Kunisada K, et al. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene. 2004;23:5426–5434. doi: 10.1038/sj.onc.1207703. [DOI] [PubMed] [Google Scholar]
- Barr FG, Fitzgerald JC, Ginsberg JP, Vanella ML, Davis RJ, Bennicelli JL. Predominant expression of alternative PAX3 and PAX7 forms in myogenic and neural tumor cell lines. Cancer Res. 1999;59:5443–5448. [PubMed] [Google Scholar]
- Basse B, Joseph WR, Marshall ES, Baguley BC. Analysis of radiation-induced changes to human melanoma cultures using a mathematical model. Cell Prolif. 2010;43:139–146. doi: 10.1111/j.1365-2184.2010.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking C, Takemoto R, Satyamoorthy K, Elenitsas R, Herlyn M. Basic fibroblast growth factor and ultraviolet B transform melanocytes in human skin. Am J Pathol. 2001;158:943–953. doi: 10.1016/S0002-9440(10)64041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JA, Ziman MR. Pax3 transcripts in melanoblast development. Dev Growth Differ. 2005;47:627–635. doi: 10.1111/j.1440-169X.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- Carreira S, Dexter TJ, Yavuzer U, Easty DJ, Goding CR. Brachyury-related transcription factor Tbx2 and repression of the melanocyte-specific TRP-1 promoter. Mol Cell Biol. 1998;18:5099–5108. doi: 10.1128/mcb.18.9.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- Dai B, Meng J, Peyton M, Girard L, Bornmann WG, Ji L, et al. STAT3 mediates resistance to MEK inhibitor through microRNA miR-17. Cancer Res. 2011;71:3658–3668. doi: 10.1158/0008-5472.CAN-10-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dong L, Li Y, Cao J, Liu F, Pier E, Chen J, et al. FGF2 regulates melanocytes viability through the STAT3-transactivated PAX3 transcription. Cell Death Differ. 2012;19:616–622. doi: 10.1038/cdd.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham WR, Klein SB, Rhodes LM, Marcelo CL. Oleic acid and linoleic acid are the major determinants of changes in keratinocyte plasma membrane viscosity. J Invest Dermatol. 1996;107:332–335. doi: 10.1111/1523-1747.ep12363174. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Vogan KJ, Trasler DG, Gros P. A mutation within intron 3 of the Pax-3 gene produces aberrantly spliced mRNA transcripts in the splotch (Sp) mouse mutant. Proc Natl Acad Sci U S A. 1993;90:532–536. doi: 10.1073/pnas.90.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007;25:1606–1620. doi: 10.1200/JCO.2006.06.0442. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, et al. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;67:11291–11299. doi: 10.1158/0008-5472.CAN-07-0593. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010;116:4902–4913. doi: 10.1002/cncr.25261. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- Ginsberg M, Czeko E, Muller P, Ren Z, Chen X, Darnell JE., Jr. Amino acid residues required for physical and cooperative transcriptional interaction of STAT3 and AP-1 proteins c-Jun and c-Fos. Mol Cell Biol. 2007;27:6300–6308. doi: 10.1128/MCB.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, et al. Paracrine stimulation of melanocytes by keratinocytes through basic fibroblast growth factor. Ann N Y Acad Sci. 1988;548:180–190. doi: 10.1111/j.1749-6632.1988.tb18805.x. [DOI] [PubMed] [Google Scholar]
- Hatiboglu MA, Kong LY, Wei J, Wang Y, McEnery KA, Fuller GN, et al. The tumor microenvironment expression of p-STAT3 influences the efficacy of cyclophosphamide with WP1066 in murine melanoma models. Int J Cancer. 2012;131:8–17. doi: 10.1002/ijc.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa T, Norris DA, Zekman T, Morelli JG. Effective elimination of fibroblasts in cultures of melanocytes by lowering calcium concentration in TPA depleted medium following geneticin treatment. Pigment Cell Res. 1996;9:58–62. doi: 10.1111/j.1600-0749.1996.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–9636. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- Jaye M, Schlessinger J, Dionne CA. Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta. 1992;1135:185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–5768. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LY, Wei J, Sharma AK, Barr J, Abou-Ghazal MK, Fokt I, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- Lee I, Fox PS, Ferguson SD, Bassett R, Kong LY, Schacherer CW, et al. The expression of p-STAT3 in stage IV melanoma: risk of CNS metastasis and survival. Oncotarget. 2012;3:336–344. doi: 10.18632/oncotarget.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A. 2006;103:10151–10152. doi: 10.1073/pnas.0604042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moase CE, Trasler DG. Splotch locus mouse mutants: models for neural tube defects and Waardenburg syndrome type I in humans. J Med Genet. 1992;29:145–151. doi: 10.1136/jmg.29.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer RS, Shea CR, Nelson M, Powell SK, Freeman DM, Dan CP, et al. PAX3 expression in primary melanomas and nevi. Mod Pathol. 2008 doi: 10.1038/modpathol.3801019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell. 2002;2:5–7. doi: 10.1016/s1535-6108(02)00089-2. [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Rosen N. Mutant BRAF melanomas--dependence and resistance. Cancer Cell. 2011;19:11–15. doi: 10.1016/j.ccr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Read AP, Newton VE. Waardenburg syndrome. J Med Genet. 1997;34:656–665. doi: 10.1136/jmg.34.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Aladowicz E, Lanfrancone L, Goding CR. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008;68:7872–7881. doi: 10.1158/0008-5472.CAN-08-0301. [DOI] [PubMed] [Google Scholar]
- Ruggiero RA, Bruzzo J, Chiarella P, Bustuoabad OD, Meiss RP, Pasqualini CD. Concomitant tumor resistance: the role of tyrosine isomers in the mechanisms of metastases control. Cancer Res. 2012;72:1043–1050. doi: 10.1158/0008-5472.CAN-11-2964. [DOI] [PubMed] [Google Scholar]
- Scholl FA, Kamarashev J, Murmann OV, Geertsen R, Dummer R, Schafer BW. PAX3 is expressed in human melanomas and contributes to tumor cell survival. Cancer Res. 2001;61:823–826. [PubMed] [Google Scholar]
- Shi H, Kong X, Ribas A, Lo RS. Combinatorial Treatments That Overcome PDGFR{beta}-Driven Resistance of Melanoma Cells to V600EB-RAF Inhibition. Cancer Res. 2011;71:5067–5074. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Herlyn M. Targeting intracellular signaling pathways as a novel strategy in melanoma therapeutics. Ann N Y Acad Sci. 2005;1059:16–25. doi: 10.1196/annals.1339.005. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocyte stem cell maintenance and hair graying. Cell. 2005;121:9–12. doi: 10.1016/j.cell.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Newton VE, Leverton K, Turnbull K, Seemanova E, Kunze J, et al. PAX3 gene structure and mutations: close analogies between Waardenburg syndrome and the Splotch mouse. Hum Mol Genet. 1994;3:1069–1074. doi: 10.1093/hmg/3.7.1069. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Read AP, Newton VE, Harris R, Balling R, Gruss P, et al. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992;355:635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- Vachtenheim J, Novotna H. Expression of genes for microphthalmia isoforms, Pax3 and MSG1, in human melanomas. Cell Mol Biol (Noisy-le-grand) 1999;45:1075–1082. [PubMed] [Google Scholar]
- Verstovsek S, Manshouri T, Quintas-Cardama A, Harris D, Cortes J, Giles FJ, et al. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 2008;14:788–796. doi: 10.1158/1078-0432.CCR-07-0524. [DOI] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Edington HD, Rao UN, Jukic DM, Land SR, Ferrone S, et al. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res. 2007;13:1523–1531. doi: 10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y, et al. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell. 2008;32:554–563. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S, Sugimachi M, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.