Abstract
Planet Earth has experienced many dramatic atmospheric and climatic changes throughout its 4.5‐billion‐year history that have profoundly impacted the evolution of life as we know it. Photosynthetic organisms, and specifically plants, have played a paramount role in shaping the Earth's atmosphere through oxygen production and carbon sequestration. In turn, the diversity of plants has been shaped by historical atmospheric and climatic changes: plants rose to this challenge by evolving new developmental and metabolic traits. These adaptive traits help plants to thrive in diverse growth conditions, while benefiting humanity through the production of food, raw materials, and medicines. However, the current rapid rate of climate change caused by human activities presents unprecedented new challenges to the future of plants. Here, we discuss the potential effects of modern climate change on plants, with specific attention to plant specialized metabolism. We explore potential avenues of future scientific investigations, powered by cutting‐edge methods such as synthetic biology and genome engineering, to better understand and mitigate the consequences of rapid climate change on plant fitness and plant usage by humans.
Keywords: climate change, growth‐defense balance, metabolic engineering, plant metabolism, specialized metabolism, synthetic biology
1. INTRODUCTION
Plants make up an estimated 80% of Earth's biomass, 1 and we humans rely on plants for our basic existence. From generating the air we breathe and the food we eat to supplying the raw material for the roofs over our heads and the clothes on our backs, plants and plant products are inextricable from everyday human life. Beyond these basics, the plant kingdom produces an incredible suite of specialized metabolites, also called secondary metabolites, which they use for internal and external signaling, attracting pollinators and seed dispersers, and defending against herbivores and pathogens, among a multitude of other activities. 2 , 3 , 4
Many of these specialized metabolites have been explored and subsequently repurposed by humans for our own uses. From personal grooming to medicine, the list is extensive (Figure 1A). For example, we use a plethora of characteristic flavor and fragrance compounds extracted from plants to enhance the taste and odor of a variety of dietary and consumer products. 5 , 6 , 7 , 8 Several classes of colored compounds, including flavonoids and betalains, are used as natural dyes. 9 , 10 , 11 , 12 Moreover, resveratrol from red wine, anthocyanins in berries, and catechins from tea (Camellia sinensis) exhibit a multitude of bioactivities with potential benefits for human health. 13 , 14 , 15 , 16 Most traditional medicines and a handful of modern medicines are also plant‐derived. 17 , 18 , 19 , 20 For example, willow (Salix spp.) bark is traditionally chewed to alleviate general pain, which led to the development of aspirin, an analog of the willow‐derived compound salicin. 21 , 22 Another prominent case is artemisinin, a potent antimalarial which was isolated from sweet wormwood, Artemisia annua, a Chinese medicinal plant traditionally prescribed to treat symptoms of malaria. 23 Yet another example of modern medicines derived from plant precursors is diosgenin, which is extracted from the Mexican yam, Dioscorea mexicana, at large scale and subsequently used to manufacture most modern steroidal drugs, including hormonal contraceptives and corticosteroid anti‐inflammatory agents. 24
FIGURE 1.
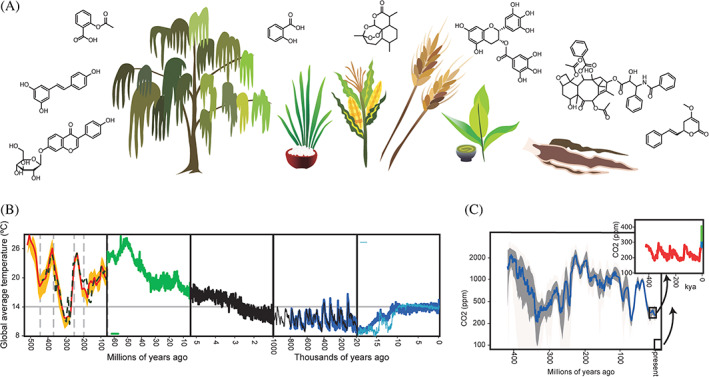
Historical and current climate change and its potential impact on plant metabolism. A, Climate change affects the output of commercially and culturally valuable plants and plant metabolites, some of which are depicted here. These metabolites are used by plants for their own immunity and growth‐defense balance, and are used by humans in food, medicine, and cosmetics, among other fields. B, Global average temperatures extrapolated from the geologic record show dramatic fluctuations in the 500 million years since the estimated rise of land plants. Vertical gray dashed lines indicate major extinction events. Plot is modified from Reference 147 with data from References 148, 149, 150, 151, 152. C, Estimates of global CO2 levels in the last 500 million years since the rise of land plants show dramatic fluctuations. Plot is adapted from Reference 153. CO2 inset shows measurements compiled from ice cores for the last 500 thousand years as well as more recent measurements taken at the Mauna Loa observatory. Plot was generated with data from References 154, 155, 156, 157, 158, 159
Human life is highly dependent upon the rich bio‐ and chemo‐diversity of plants in ways far surpassing the brief list above. However, the anthropogenic acceleration of climate change in the modern era presents a mounting threat to Earth's flora. Forest fires, which are increasing globally, not only destroy the very plants that produce oxygen, but also release a significant amount of sequestered carbon back into the atmosphere, fueling downstream environmental changes. 25 Warmer temperatures melt permafrost, leading to the appearance of massive sinkholes in the Arctic, which in turn results in the release of large amounts of sequestered methane, a more potent greenhouse gas than carbon dioxide (CO2), back into the atmosphere. 26 Atmospheric concentrations of CO2, which have remained relatively stable for the last 800 000 years in the range of 200‐300 ppm, have been creeping up since the first industrial revolution, recently passing a historic high of 400 ppm in 2015 and continuing to climb. 27 , 28 , 29 , 30 The average annual increase, which in recent years has surpassed 2 ppm/y, may seem insignificant, but is up to two orders of magnitude faster than is recorded in the geologic record (Figure 1B,C). 28 , 31 Damage to the stratospheric ozone layer has also led to elevated intensity of ultraviolet (UV) radiation and ozone levels on the ground. 32 , 33 , 34
Many plants, including some producers of valuable specialized metabolites, are sensitive to their growing conditions and are therefore threatened by rapid climate change. For example, important commercial crops such as coffee (Coffea arabica and C canephora), tea, wine grape (Vitis spp.), sugar cane (Saccharum spp.), rubber trees (Hevea brasiliensis) and oil palms (Eleis spp.) have undergone artificial selection by humans to favor certain growth traits at the expense of defense and stress responses. 35 , 36 , 37 Other plants that are the sources of various commercial materials, such as mangroves (Rhizophora spp.), have adapted to niche environments, and therefore face habitat loss. 38 Climate change can influence plant growth, thus affecting the quantity and quality of the specialized metabolites produced. For example, flowering time has been shown to be extremely responsive to environmental stress, potentially via a signaling pathway mediated by simple sugars such as sucrose and glucose. 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 Additionally, the flavor of tea can vary widely depending on growth conditions. 47 , 48 Similarly, the richness of the flavor profile of coffee correlates strongly with altitude, but the ideal altitude ranges have been shown to be changing and becoming more inconvenient for farmers in recent years. 49 , 50 , 51 , 52 , 53 , 54 Kava, Piper methysticum, a medicinal plant native to the Polynesian islands, produces a bouquet of bioactive kavalactones, and its root is used for preparing a beverage with relaxing effects. 55 The growth of kava plants is highly sensitive to soil composition, temperature and humidity. The rapidly changing climate therefore raises concerns about the continued viability of kava among other important crops which have served as primary sources of valuable specialized metabolites.
In the estimated 470 million years of land plant evolution, 56 plants have tolerated and overcome more extreme challenges than present‐day temperatures and CO2 levels (Figure 1B,C). For example, atmospheric CO2 levels at the time when plants first transitioned from water to land were about 4000 ppm, an order of magnitude higher than today (Figure 1C). 57 , 58 , 59 Notably, plants, as carbon‐fixing organisms, can grow more robustly in the presence of higher CO2 levels. 60 However, many plants may fail to compensate for the accompanying changes such as elevated temperature, humidity, ground ozone, and UV radiation. 28 As seen in the geologic record, dramatic climate changes such as these often precede mass extinctions (Figure 1C). 61 Any potential catastrophic loss of floral diversity and accompanying metabolic traits could in turn lead to an existential threat for humanity. However, while the rapid advance of human industry in the past two centuries has contributed in large part to the current looming climate crisis, it has also yielded a wealth of scientific and technological advances that could be harnessed to protect the combined future of plant and human life.
Below, we provide our perspectives on the ways in which climate change directly and indirectly affects plant metabolism, productivity, and other relevant growth traits, and how we could use modern technologies to ameliorate these effects.
2. ENVIRONMENTAL FACTORS INFLUENCE PLANT SPECIALIZED METABOLISM
Many specialized metabolic processes in plants are regulated by environmental factors, as demonstrated by an extensive body of research in the past decades. 62 For example, light induces a subset of phenylpropanoid production in plants, including flavonoids and hydroxycinnamoyl glycosides known to be involved in UV protection and defense. 63 , 64 Meanwhile, in response to heightened light intensity, the indole alkaloid camptothecin increases in leaves and decreases in roots in Camptotheca acuminata. 65 Temperature also influences some specialized metabolites: some phenylpropanoids and flavonoids are more abundant at lower growth temperatures, 66 , 67 , 68 while some other compounds, such as the alkaloids conferring bitter flavor in carrots (Daucus carota), accumulate at higher temperature. 69 Other climate‐change‐related parameters, such as elevated ozone and UV radiation, lead to enhanced production of flavonoids, including rutin and quercetin, in soybean (Glycine max) plants, 70 but suppresses terpene production in peaches (Prunus persica) and Asterids, among others. 71 , 72 , 73 For terpenes, in addition to environment‐induced changes in terpene biosynthesis, accumulation, and related gene expression, elevated ground‐level ozone is hypothesized to also directly react with many terpenes to generate a host of gaseous and particulate oxygenated compounds, which negatively affect both plant fitness and human health. 74
While the above studies inform the impact of individual environmental stressors on specific metabolic pathways, they lack the context of complex real‐world climate changes that involve simultaneous variations of multiple environmental factors over dynamic time scales. To this end, Mikkelsen et al recently investigated the effect of varying multiple climate‐change‐related environmental parameters on the accumulation of specialized metabolites in barley (Hordeum vulgare), and its relationship with plant pathogen resistance. 75 They found that elevated CO2, ozone, and temperature each increased the resistance of barley to powdery mildew (Blumeria graminis f. sp. hordei.) infection, but collectively negated any additional resistance. The authors further examined metabolites related to cell wall maintenance, since the route of infection of powdery mildew is through the cell wall. They noticed that changing environmental factors delayed the production of defense metabolites to reinforce the cell wall. Future studies of how various plant metabolic systems respond to complex multifactorial environmental changes, as well as a deeper mechanistic understanding of the integrated genetic circuits underlying these responses, are urgently needed and will enlighten future efforts to engineer desirable plant metabolic traits for the rapidly changing climate.
3. THE PLANT GROWTH‐DEFENSE BALANCE COULD BE UPSET BY RAPID CLIMATE CHANGE
Plants grow less when under biotic or abiotic stresses. This tradeoff response is intuitive: plants have access to a finite pool of resources and must decide whether to allocate those resources toward growth or defense. This “growth‐defense balance” evolved over millions of years as an essential survival mechanism in wild plants. However, in human‐cultivated varieties, this trait is likely to have experienced intense artificial selection to maximize biomass production under relatively stable growing environments. The current pace of anthropogenic climate change is faster than has been seen before and may exceed the rate at which evolution can compensate. As such, these new changes may profoundly cripple this fine balance for many wild and crop plants, resulting in significant negative consequences for yields.
Examples of the growth‐defense balance can easily be seen in the field and have been quantified using seed and biomass production and various other measures. For example, drought stress, either alone or in concert with temperature or nutrient stress, decreases seed yield in soybean, pea, and other plants. 76 , 77 , 78 In terms of temperature, an increasing average global temperature is accompanied by a coincident increase in extreme temperature swings. 79 High temperatures stunt plant growth and pose a dual challenge to plant immunity: not only does the plant's own ability to generate an immune response decrease, but also the virulence of pathogens, represented in one case study by the bacterium Pseudomonas syringae, can increase. 80 In addition, high temperatures in wet areas lead to high humidity, which has been shown to compromise plant immune responses to bacterial pathogens. 81 Other biotic and abiotic stresses, including pathogen infection, herbivory, drought and UV irradiation, have been shown to adversely affect crop productivity in various plants. 70 , 82 , 83 , 84 In contrast, the rising atmospheric CO2 level alone was shown to not only promote growth, crop productivity and plant water‐use efficiency, 85 , 86 but also prime plant defense against biotic stresses, 87 illustrating one positive outcome of increasing global CO2 levels for plant growth.
However, a recent modeling study predicts that these positive influences on plant growth would be compromised by other aspects of climate change, subsequently affecting overall productivity and imposing carbon penalties on nutrient content. 88 , 89
The growth‐defense balance is mediated by complex coordinated actions of several phytohormones and their downstream signaling pathways. 90 , 91 For instance, elevated atmospheric CO2 levels induce plant stomatal closure through abscisic acid (ABA) signaling, contributing to enhanced water‐use efficiency. 92 On the other hand, CO2‐induced defense priming is partly orchestrated by the plant defense hormone salicylic acid (SA), and is additionally linked to the redox signaling pathway. 87 A current effort in the field focuses on identifying key regulators that facilitate decoupling of beneficial plant stress responses and their associated negative impacts on plant productivity. For example, the plant defense hormone jasmonate (JA) plays an important role in regulating growth‐defense balance by promoting the production of diverse defense compounds and simultaneously inhibiting growth. This is achieved through the JASMONATE ZIM‐DOMAIN (JAZ)‐MYC transcriptional module. 93 By generating quintuple and decuple mutants of the 13 JAZ proteins in Arabidopsis, Guo et al recently showed that JAZ family members promote biomass accumulation by repressing constitutive immune responses. 94 Most interestingly, although higher‐order mutants accumulated less biomass at maturity, a quintuple mutant grew at the same relative growth rate as wild‐type plants while exhibiting enhanced defense against insects. Developing mechanistic understandings of the growth‐defense balance under various environmental assaults will be crucial for improving the growth robustness of plants against more variable environments.
4. HARNESSING WORLD PLANT DIVERSITY TO BUILD MORE RESILIENT CROP PLANTS
Despite ongoing warnings about significant changes in climatic parameters, plants have persisted through more dramatic changes on Earth over the past hundreds of millions of years and will continue to adapt to new environmental conditions at their own pace. However, human reliance on plants and plant products drives us to seek solutions to maintain plant biodiversity and productivity in light of the changing climate. We may consider searching for solutions among plant extremophiles. Take, for example, desert plants, which grow under extreme temperatures, higher light intensity, and low water conditions, or plants which grow at high altitudes where the air is thinner and oxygen is less abundant (Figure 2A,B). 95 , 96 , 97 Further temperature tolerance mechanisms may be gleaned from Antarctic plants, or plants that have survived dramatic events such as forest fires and prolonged flood. 98 , 99 , 100 Some plant extremophiles have evolved to accumulate certain specialized metabolites at very high levels as part of their unique adaptive strategies, with a few already harnessed for human uses, including UV protection, food, and beverage. 101 , 102 , 103 The rich genetic and biochemical bases underlying each case of these amazing adaptations await discovery.
FIGURE 2.
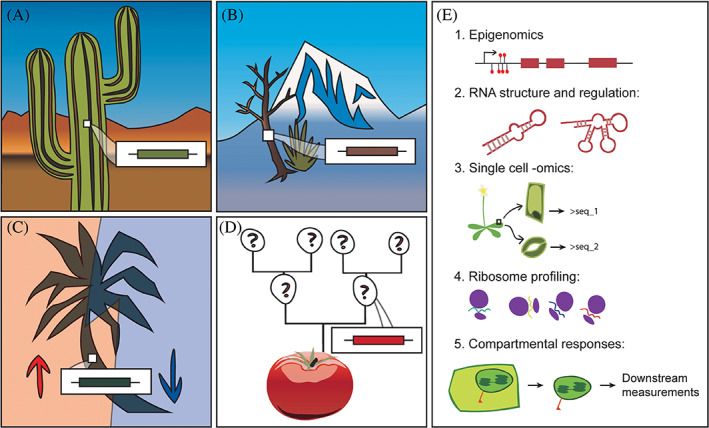
Several approaches to understand and engineer stress tolerance in plants amid climate change. Understanding the ways in which extant plants can respond to abiotic and biotic stresses can guide future work to develop plants which are more resilient to rapidly changing growing conditions. Populations of plants which are potentially interesting to focus on include those that grow in extreme environments (A, B), single species that show differential gene expression in different environments (C), and wild varieties of commercial cultivars (D). (E) The application of modern sequencing and other novel omics technologies to study epigenomes, RNA structures, single cells, ribosomes, and subcellular compartments may provide deeper insights into mechanisms of stress tolerance in diverse plants
By investigating various mechanisms of how plant extremophiles respond to and defend against various environmental stressors, we may use modern genetic engineering techniques to transplant some of the mechanisms into other plants to gain desirable resistance traits. 104 , 105 Temperature sensitivity may be a good place to start, illustrated by the following three examples. First, recent research has revealed a new temperature‐sensing mechanism in plants: the rate of conversion of the phytochrome photoreceptors between the red and far‐red light‐absorbing forms. 106 , 107 , 108 Therefore, plant temperature resistance can potentially be augmented by developing crops with variable phytochrome conversion rates, although more careful investigation of the interplay between light and temperature sensing must be conducted. Second, in plants that evolved to withstand the extreme cold of winter, various ice‐binding proteins (IBPs) have been discovered which suppress the formation of ice crystals in cells. 109 , 110 Third, in Selaginella lepidophylla, colloquially called the resurrection plant and one of the only few Selaginellaceae species that evolved the ability to revive from an entirely dried state, accumulation of high levels of drought‐resistant sugars, such as trehalose, as well as several other antioxidant metabolites, has been attributed to the plant's ability to “resurrect” from complete desiccation. 111 , 112 Plant IBPs and specific anti‐drought metabolite pathways from plant extremophiles thus present tools for engineering plant resilience under harsh environments.
To elucidate unknown adaptive mechanisms underpinning plant environmental tolerance, particularly in nonmodel plants, we may consider comparing closely related species or ecotypes of the same species that have adapted to different environments (Figure 2C). For example, a recent study compared seedling growth of three related cactus species under differential light and humidity conditions and found significantly different growth performance across them. 113 Similarly, various ecotypes of the biomass crop Arundo donax, commonly known as the giant reed, exhibit different productivities under drought stress. 114 Stemming from these initial findings, it may be possible to identify stress‐tolerance‐related candidate genes and pathways by correlating differential gene expression with variable phenotypic performances under specific stress conditions across a diversity panel. Moreover, more effort could also be directed to research in those so‐called “wild” relatives of commercial crops (Figure 2D). Domesticated plants have undergone artificial selection to favor traits that promote growth or prolong shelf life, often at the expense of stress resistance and other “soft” traits, such as flavor. 115 By studying wild cousins of modern crop plants, we could identify those lost resilience traits and reintroduce them back into crops. Some of these traits indeed involve production of specialized metabolites playing roles in biotic or abiotic defenses, which in turn may improve or diversify those “soft” qualities in derived plant products. 116
5. EPIGENETIC MECHANISMS CONTRIBUTE TO PLANT ADAPTATION UNDER CLIMATE CHANGE
In addition to plant adaptation through genetic changes, epigenetic mechanisms may also play an important role in plant phenotypic variation under rapid climate change (Figure 2E). Epigenetic chromatin and DNA modifications have been shown to influence plant metabolism and stress tolerance. 117 , 118 , 119 , 120 , 121 For instance, in clonally propagated white clover Trifolium repens, 122 apomictic dandelion Taraxacum officinale 123 and alligator weed Alternanthera philoxeroides, 124 epigenetic marks arising from stress are inherited across multiple generations. Whereas sexual reproduction effectively erases most of the drought‐induced epigenome changes in subsequent generations of Arabidopsis, 125 parental exposure to pathogen attack led to enhanced pathogen resistance in the immediate next generation, likely through transgenerational inheritance of specific DNA and histone epi‐marks. 126 Recent development of CRISPR/Cas9‐based epigenome‐editing tools therefore affords a new avenue to alter crop metabolic traits or stress resistance without changing the DNA sequence. 127
Another epigenetic mechanism relevant to climate change is RNA secondary structure dynamics. 128 , 129 In particular, we may expect RNA secondary structures to be influenced by environmental factors such as temperature, leading to differential functional outputs. 130 In fact, it has been shown that mRNA structure is involved in the cold shock response in bacteria. 131 In plants, early study of the maize (Zea mays) transcriptional activator Lc uncovered a role of mRNA secondary structure in regulating anthocyanin biosynthesis through translational repression.132 A more recent in vivo genome‐wide survey of RNA secondary structure in Arabidopsis further revealed that, while genes involved in fundamental cellular maintenance display more confined and predictable mRNA secondary structures, stress‐induced genes contain more plastic mRNA secondary structures likely associated with regulatory functions under different environmental conditions. 133 , 134 Understanding the structure‐function relationship of the RNA secondary structures in regulating gene function in response to environmental changes can provide additional tools for precision engineering of certain traits tailored to specific growth conditions.
6. OUTLOOK
Global and regional climate changes over the past 470 million years have profoundly shaped the evolution of the Earth's flora. Although all extant plant species have adapted to historical climate changes, including those catastrophic periods that led to mass extinction events, 135 rapid anthropogenic climate change in the past two centuries may present another unprecedented challenge. Certainly, new cycles of natural selection will continue to select the fittest plants to survive the new environments, but humans have now become a driving force in shaping future plant evolution, both through our desire to retain valuable plant traits, and our increasing capability to modify plants.
Many novel technologies can be readily deployed to help expand our understanding of how diverse plants respond to complex and rapid climate changes, including single‐cell RNA sequencing (scRNA‐seq), 136 ribosome profiling, 137 and single‐cell proteomics and metabolomics (Figure 2E. 138 , 139 Moreover, the ability to quantitatively assess metabolic states of various cellular compartments in defined tissues 140 , 141 will further advance our knowledge on plant organellar responses to environmental changes. 142 Armed with the growing toolsets of genome editing, 143 synthetic biology, 144 and ethically aware regulation of technology, 145 we can, and shall responsibly manipulate plants to provide commercial, medicinal, or other specialized values with built‐in resilience in the face of climate change. New knowledge and engineered plants arising from these studies may be further applied to mitigate the increasing demand for plant biomass resulting from the current rapid expansion of the human population. 146 Considering that a few crop species have arisen as the most successful terrestrial plants due to human selection and facilitation, it is foreseeable that new generations of engineered plants with various desirable traits—either bred, edited, or even created from scratch—will arise to accompany future human life on Earth. Existing, preliminary, planned and future efforts to understand and augment our repertoire of plants and their mechanisms of resilience will continue to serve humanity into future generations.
CONFLICT OF INTEREST
J.K.W. is a co‐founder, a member of the Scientific Advisory Board and a shareholder of DoubleRainbow Biosciences, which develops biotechnologies related to natural products.
AUTHOR CONTRIBUTIONS
Sophia Xu: Conceptualization; writing‐original draft; writing‐review and editing. Jing‐Ke Weng: Conceptualization; writing‐original draft; writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/ggn2.10022.
Supporting information
Transparent‐Peer‐Review‐Record
ACKNOWLEDGMENT
This work was supported in part by grants from the National Science Foundation (grant no. CHE‐1709616) and the Keck Foundation.
Xu SY, Weng J‐K. Climate change shapes the future evolution of plant metabolism. Advanced Genetics. 2020;1:e10022. 10.1002/ggn2.10022
Funding information Division of Chemistry, Grant/Award Number: CHE‐1709616; W. M. Keck Foundation
REFERENCES
- 1. Bar‐On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci U S A. 2018;115(25):6506‐6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kortbeek RWJ, van der Gragt M, Bleeker PM. Endogenous plant metabolites against insects. Eur J Plant Pathol. 2019;154(1):67‐90. [Google Scholar]
- 3. Singh B, Sharma RA. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3. Biotechnology. 2015;5(2):129‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H, Ban Z, Qin H, Ma L, King AJ, Wang G. A heteromeric membrane‐bound prenyltransferase complex from hop catalyzes three sequential aromatic prenylations in the bitter acid pathway. Plant Physiol. 2015;167(3):650‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oladokun O, Tarrega A, James S, Smart K, Hort J, Cook D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem. 2016;205:212‐220. [DOI] [PubMed] [Google Scholar]
- 7. Baldwin IT. Plant volatiles. Curr Biol. 2010;20(9):R392‐R397. [DOI] [PubMed] [Google Scholar]
- 8. Muhlemann JK, Klempien A, Dudareva N. Floral volatiles: from biosynthesis to function. Plant Cell Environ. 2014;37(8):1936‐1949. [DOI] [PubMed] [Google Scholar]
- 9. Asen S, Norris KH, Stewart RN. Absorption spectra and color of aluminiun‐cyanidin 3‐glucoside complexes as influenced by pH. Phytochemistry. 1969;8(3):653‐659. [Google Scholar]
- 10. Strack D, Vogt T, Schliemann W. Recent advances in betalain research. Phytochemistry. 2003;62(3):247‐269. [DOI] [PubMed] [Google Scholar]
- 11. Stafford HA. Anthocyanins and betalains: evolution of the mutually exclusive pathways. Plant Sci. 1994;101(2):91‐98. [Google Scholar]
- 12. Prabhu KH, Bhute AS. Plant based natural dyes and mordants: a review. J Nat Prod Plant Resour. 2012;2(6):649‐664. [Google Scholar]
- 13. Filip V, Plocková M, Šmidrkal J, Špičková Z, Melzoch K, Schmidt Š. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem. 2003;83(4):585‐593. [Google Scholar]
- 14. Kähkönen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem. 2003;51(3):628‐633. [DOI] [PubMed] [Google Scholar]
- 15. Einbond LS, Reynertson KA, Luo XD, Basile MJ, Kennelly EJ. Anthocyanin antioxidants from edible fruits. Food Chem. 2004;84(1):23‐28. [Google Scholar]
- 16. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89‐143. [DOI] [PubMed] [Google Scholar]
- 17. Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Traditional herbal remedies for primary health care. c 2010, World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/206024. Accessed November 19, 2019 [Google Scholar]
- 19. Gu S, Pei J. Innovating Chinese herbal medicine: from traditional health practice to scientific drug discovery. Front Pharmacol. 2017;8:381. 10.3389/fphar.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benzie IFF, Wachtel‐Galor S, eds. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Boca Raton, FL: CRC Press/Taylor & Francis; 2011. [PubMed] [Google Scholar]
- 21. Kenstaviciene P, Nenortiene P, Kiliuviene G, Zevzikovas A, Lukosius A, Kazlauskiene D. Application of high‐performance liquid chromatography for research of salicin in bark of different varieties of Salix. Medicina (Kaunas). 2009;45(8):644‐651. [PubMed] [Google Scholar]
- 22. Vlachojannis J, Magora F, Chrubasik S. Willow species and aspirin: different mechanism of actions. Phytother Res. 2011;25(7):1102‐1104. 10.1002/ptr.3386. [DOI] [PubMed] [Google Scholar]
- 23. Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217‐1220. [DOI] [PubMed] [Google Scholar]
- 24. American Chemical Society . The “Marker Degradation” and Creation of the Mexican Steroid Hormone Industry, 1938‐1945: An International Historic Chemical Landmark. University Park: American Chemical Society; 1999. [Google Scholar]
- 25. Abatzoglou JT, Williams AP. Impact of anthropogenic climate change on wildfire across western US forests. Proc Natl Acad Sci U S A. 2016;113(42):11770‐11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moskvitch K. Mysterious Siberian crater attributed to methane. Nature. 2014;31. 10.1038/nature.2014.15649. [DOI] [Google Scholar]
- 27. Parrenin F, Masson‐Delmotte V, Köhler P, et al. Synchronous change of atmospheric CO2 and Antarctic temperature during the last deglacial warming. Science. 2013;339(6123):1060‐1063. 10.1126/science.1226368. [DOI] [PubMed] [Google Scholar]
- 28. Bereiter B, Eggleston S, Schmitt J, et al. Revision of the EPICA Dome C CO2 record from 800 to 600 kyr before present. Geophys Res Lett. 2014;42(2):542‐549. 10.1002/2014GL061957. [DOI] [Google Scholar]
- 29. Kahn B. We just breached the 410 ppm threshold for CO2. Sci Am [Internet]; 2017. https://www.scientificamerican.com/article/we-just-breached-the-410-ppm-threshold-for-co2/. Accessed February 10, 2020
- 30. Tans P and Keeling R. Monthly average Mauna Loa CO2. NOAA: Earth System Research Laboratory Global Monitoring Division; c 2020. https://www.esrl.noaa.gov/gmd/ccgg/trends/mlo.html. Accessed February 10, 2020.
- 31. Royer DL. Climate sensitivity in the geologic past. Annu Rev Earth Planet Sci. 2016;44:277‐293. 10.1146/annurev-earth-100815-024150. [DOI] [Google Scholar]
- 32. Marenco A, Gouget H, Nédélec P, Pagés J‐P, Karcher F. Evidence of a long‐term increase in tropospheric ozone from Pic du Midi data series: consequences: positive radiative forcing. J Geophys Res. 1994;99:16617. 10.1029/94jd00021. [DOI] [Google Scholar]
- 33. Morgan PB, Ainsworth EA, Long SP. How does elevated ozone impact soybean? A meta‐analysis of photosynthesis, growth and yield. Plant Cell Environ. 2003;26(8):1317‐1328. 10.1046/j.0016-8025.2003.01056.x. [DOI] [Google Scholar]
- 34. van der Leun JC, Tang X, Tevini M. Environmental effects of ozone depletion: 1998 assessment. J Phytochem Photobiol B Biol. 1998;4:ix. 10.1016/s1011-1344(98)00195-x. [DOI] [Google Scholar]
- 35. Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Q Rev Biol. 1992;67(3):283‐335. [Google Scholar]
- 36. Chen YH, Gols R, Benrey B. Crop domestication and its impact on naturally selected trophic interactions. Annu Rev Entomol. 2015;60:35‐58. [DOI] [PubMed] [Google Scholar]
- 37. Gaillard MDP, Glauser G, Robert CAM, Turlings TCJ. Fine‐tuning the “plant domestication‐reduced defense” hypothesis: specialist vs. generalist herbivores. New Phytol. 2018;217(1):355‐366. 10.1111/nph.14757. [DOI] [PubMed] [Google Scholar]
- 38. Dahibhate NL, Saddhe AA, Kumar K. Mangrove plants as a source of bioactive compounds: a review. Nat Prod J. 2018;9(2):86‐97. [Google Scholar]
- 39. Springer CJ, Ward JK. Flowering time and elevated atmospheric CO2 . New Phytologist. 2007;176(2):243‐255. 10.1111/j.1469-8137.2007.02196.x. [DOI] [PubMed] [Google Scholar]
- 40. Kazan K, Lyons R. The link between flowering time and stress tolerance. J Exp Bot. 2016;67(1):47‐60. [DOI] [PubMed] [Google Scholar]
- 41. Cui LG, Shan JX, Shi M, Gao JP, Lin HX. The miR156‐SPL9‐DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014;80(6):1108‐1117. [DOI] [PubMed] [Google Scholar]
- 42. Becklin KM, Walker SM, Way DA, Ward JK. CO2 studies remain key to understanding a future world. New Phytol. 2017;214(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walker SM, Ward JK. Interactions between rising CO2 and temperature drive accelerated flowering in model plants under changing conditions of the last century. Oecologia. 2018;187(4):911‐919. [DOI] [PubMed] [Google Scholar]
- 44. Yu S, Cao L, Zhou CM, et al. Sugar is an endogenous cue for juvenile‐to‐adult phase transition in plants. Elife. 2013;2:e00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang L, Xu M, Koo Y, He J, Poethig RS. Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. Elife. 2013;2:e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wahl V, Ponnu J, Schlereth A, et al. Regulation of flowering by trehalose‐6‐phosphate signaling in Arabidopsis thaliana . Science. 2013;339(6120):704‐707. [DOI] [PubMed] [Google Scholar]
- 47. Ahmed S, Griffin TS, Kraner D, et al. Environmental factors variably impact tea secondary metabolites in the context of climate change. Front Plant Sci. 2019;10:939. 10.3389/fpls.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahmed S, Stepp JR, Orians C, et al. Effects of extreme climate events on tea (Camellia sinensis) functional quality validate indigenous farmer knowledge and sensory preferences in tropical China. PLoS One. 2014;9(10):e109126. 10.1371/journal.pone.0109126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Avelino J, Barboza B, Araya JC, et al. Effects of slope exposure, altitude and yield on coffee quality in two altitude terroirs of Costa Rica, Orosi and Santa María de Dota. J Sci Food Agric. 2005;85:1869‐1876. 10.1002/jsfa.2188. [DOI] [Google Scholar]
- 50. Jonsson M, Raphael IA, Ekbom B, Kyamanywa S, Karungi J. Contrasting effects of shade level and altitude on two important coffee pests. J Pest Sci. 2015;88(2):281‐287. 10.1007/s10340-014-0615-1. [DOI] [Google Scholar]
- 51. Telwala Y, Brook BW, Manish K, Pandit MK. Climate‐induced elevational range shifts and increase in plant species richness in a himalayan biodiversity epicentre. PLoS One. 2013;8(2):e57103. 10.1371/journal.pone.0057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322(5899):258‐261. 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- 53. Chala D, Brochmann C, Psomas A, et al. Good‐bye to tropical alpine plant giants under warmer climates? Loss of range and genetic diversity in Lobelia rhynchopetalum. Ecol Evol. 2016;6(24):8931‐8941. 10.1002/ece3.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lamprecht A, Semenchuk PR, Steinbauer K, Winkler M, Pauli H. Climate change leads to accelerated transformation of high‐elevation vegetation in the Central Alps. New Phytol. 2018;220(2):447‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pluskal T, Torrens‐Spence MP, Fallon TR, De Abreu A, Shi CH, Weng J‐K. The biosynthetic origin of psychoactive kavalactones in kava. Nat Plants. 2019;5:867‐878. [DOI] [PubMed] [Google Scholar]
- 56. Lenton TM, Dahl TW, Daines SJ, et al. Earliest land plants created modern levels of atmospheric oxygen. Proc Natl Acad Sci U S A. 2016;113(35):9704‐9709. 10.1073/pnas.1604787113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eggleton T. A Short Introduction to Climate Change. Cambridge, MA: Cambridge University Press; 2012. [Google Scholar]
- 58. Rothman DH. Atmospheric carbon dioxide levels for the last 500 million years. Proc Natl Acad Sci U S A. 2002;99(7):4167‐4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berner RA, Kothavala Z. Geocarb III: a revised model of atmospheric CO2 over phanerozoic time. Am J Sci. 2001;301(2):182‐204. [Google Scholar]
- 60. Zhu Z, Piao S, Myneni RB, et al. Greening of the Earth and its drivers. Nat Clim Change. 2016;6:791‐795. [Google Scholar]
- 61. McElwain JC, Beerling DJ, Woodward FI. Fossil plants and global warming at the Triassic‐Jurassic boundary. Science. 1999;285(5432):1386‐1390. [DOI] [PubMed] [Google Scholar]
- 62. Yang L, Wen KS, Ruan X, Zhao YX, Wei F, Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23(4):762. 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Camm EL, McCallum J, Leaf E, Koupai‐Abyazani MR. Cold‐induced purpling of Pinus contorta seedlings depends on previous daylength treatment. Plant Cell Environ. 1992;16:761‐764. 10.1111/j.1365-3040.1993.tb00497.x. [DOI] [Google Scholar]
- 64. Hemm MR, Rider SD, Ogas J, Murry DJ, Chapple C. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 2004;38(5):765‐778. [DOI] [PubMed] [Google Scholar]
- 65. Liu Z, Carpenter SB, Constantin RJ. Camptothecin production in Camptotheca acuminata seedlings in response to shading and flooding. Can J Bot. 1997;75(2):368‐373. [Google Scholar]
- 66. Bhatia C, Pandey A, Gaddam SR, Hoecker U, Trivedi PK. Low temperature‐enhanced flavonol synthesis requires light‐associated regulatory components in Arabidopsis thaliana . Plant Cell Physiol. 2018;59(10):2099‐2112. [DOI] [PubMed] [Google Scholar]
- 67. Janas KM, Cvikrová M, Pałagiewicz A, Szafranska K, Posmyk MM. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002;164(2):369‐373. 10.1016/s0168-9452(02)00136-x. [DOI] [Google Scholar]
- 68. Christie PJ, Alfenito MR, Walbot V. Impact of low‐temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194(4):541‐549. 10.1007/bf00714468. [DOI] [Google Scholar]
- 69. Rosenfelt HJ, Aaby K, Lea P. Influence of temperature and plant density on sensory quality and volatile terpenoids of carrot (Daucus carota L.) root. J Sci Food Agric. 2002;82(12):1384‐1390. [Google Scholar]
- 70. Mao B, Yin H, Wang Y, et al. Combined effects of O3 and UV radiation on secondary metabolites and endogenous hormones of soybean leaves. PLoS One. 2017;12(8):e0183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu H, Cao X, Liu X, et al. UV‐B irradiation differentially regulates terpene synthases and terpene content of peach. Plant Cell Environ. 2017;40(10):2261‐2275. [DOI] [PubMed] [Google Scholar]
- 72. Zavala JA, Ravetta DA. The effect of solar UV‐B radiation on terpenes and biomass production in Grindelia chiloensis (Asteraceae), a woody perennial of Patagonia, Argentina. Plant Ecol. 2002;161(2):185‐191. [Google Scholar]
- 73. Escobar‐Bravo R, Klinkhamer PG, Leiss KA. Interactive effects of UV‐B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front Plant Sci. 2017;8:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nørgaard AW, Kofoed‐Sørensen V, Mandin C, et al. Ozone‐initiated terpene reaction products in five European offices: replacement of a floor cleaning agent. Environ Sci Technol. 2014;48(22):13331‐13339. [DOI] [PubMed] [Google Scholar]
- 75. Mikkelsen BL, Olsen CE, Lyngkjær MF. Accumulation of secondary metabolites in healthy and diseased barley, grown under future climate levels of CO2, ozone and temperature. Phytochemistry. 2015;118:162‐173. [DOI] [PubMed] [Google Scholar]
- 76. Jumrani K, Bhatia VS. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol Mol Biol Plants. 2018;24(1):37‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Henriet C, Aimé D, Térézol M, et al. Water stress combined with sulfur deficiency in pea affects yield components but mitigates the effect of deficiency on seed globulin composition. J Exp Bot. 2019;70(16):4287‐4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eziz A, Yan Z, Tian D, Han W, Tang Z, Fang J. Drought effect on plant biomass allocation: a meta‐analysis. Ecol Evol. 2017;7(24):11002‐11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305(5686):994‐997. [DOI] [PubMed] [Google Scholar]
- 80. Huot B, Castroverde CDM, Velásquez AC, et al. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat Commun. 2017;8(1):1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xin XF, Nomura K, Aung K, et al. Bacteria establish an aqueous living space in plants crucial for virulence. Nature. 2016;539(7630):524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sehgal A, Sita K, Siddique KHM, et al. Drought or/and heat‐stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front Plant Sci. 2018;9:1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dornbos DL, Mullen RE. Influence of stress during soybean seed fill on seed weight, germination, and seedling growth rate. Can J Plant Sci. 1991;71:373‐383. [Google Scholar]
- 84. Karasov TL, Chae E, Herman JJ, Bergelson J. Mechanisms to mitigate the trade‐off between growth and defense. Plant Cell. 2017;29:666‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Deryng D, Elliot J, Folverth C, et al. Regional disparities in the beneficial effects of rising CO2 concentrations on crop water productivity. Nat Clim Change. 2016;6:786‐790. 10.1038/nclimate2995. [DOI] [Google Scholar]
- 86. Keenan TF, Hollinger DY, Bohrer G, et al. Increase in forest water‐use efficiency as atmospheric carbon dioxide concentrations rise. Nature. 2013;499:324‐327. [DOI] [PubMed] [Google Scholar]
- 87. Mhamdi A, Noctor G. High CO2 primes plant biotic stress Defences through redox‐linked pathways. Plant Physiol. 2016;172(2):929‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smith MR, Myers SS. Impact of anthropogenic CO2 emissions on global human nutrition. Nat Clim Change. 2018;8:834‐839. 10.1038/s41558-018-0253-3. [DOI] [Google Scholar]
- 89. Beach RH, Sulser TB, Crimmins A, et al. Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: a modelling study. Lancet Planet Health. 2019;3(7):e3017‐e3017, e317. 10.1016/s2542-5196(19)30094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. De Bruyne L, Höfte M, De Vleesschauwer D. Connecting growth and defense: the emerging roles of brassinosteroids and gibberellins in plant innate immunity. Mol Plant. 2014;7(6):943‐959. [DOI] [PubMed] [Google Scholar]
- 91. Robert‐Seilaniantz A, Grant M, Jones JDG. Hormone crosstalk in plant disease and defense: more than just jasmonate‐salicylate antagonism. Annu Rev Phyotpathol. 2011;49:317‐343. [DOI] [PubMed] [Google Scholar]
- 92. Chater C, Peng K, Movahedi M, et al. Elevated CO2‐induced responses in stomata require ABA and ABA signaling. Curr Biol. 2015;25(20):2709‐2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Major IT, Yoshida Y, Campos ML, et al. Regulation of growth‐defense balance by the JASMONATE ZIM‐DOMAIN (JAZ)‐MYC transcriptional module. New Phytol. 2017;215(4):1533‐1547. 10.1111/nph.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Guo Q, Yoshida Y, Major IT, et al. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc Natl Acad Sci U S A. 2018;115(45):E10768‐E10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Qureshi R, Raza BG. Ethnobotany of plants used by the Thari people of Nara Desert, Pakistan. Fitoterapia. 2008;79(6):469‐473. [DOI] [PubMed] [Google Scholar]
- 96. Ballabh B, Chaurasia OP, Ahmed Z, Singh SB. Traditional medicinal plants of cold desert Ladakh—used against kidney and urinary disorders. J Ethnopharmacol. 2008;118(2):331‐339. [DOI] [PubMed] [Google Scholar]
- 97. Khanum F, Bawa AS, Singh B. Rhodiola rosea: a versatile Adaptogen. Compr Rev Food Sci Food Saf. 2006;4:55‐62. [DOI] [PubMed] [Google Scholar]
- 98. Bravo LA, Griffith M. Characterization of antifreeze activity in Antarctic plants. J Exp Bot. 2005;56(414):1189‐1196. [DOI] [PubMed] [Google Scholar]
- 99. Singh J, Singh RP, Khare R. Influence of climate change on Antarctic flora. Polar Sci. 2018;18:94‐101. 10.1016/j.polar.2018.05.006. [DOI] [Google Scholar]
- 100. Della Rocca G, Hernando C, Madrigal J, et al. Possible land management uses of common cypress to reduce wildfire initiation risk: a laboratory study. J Environ Manage. 2015;159:68‐77. 10.1016/j.jenvman.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 101. Rashford J. The use of baobab leaves (Adansonia digitata L.) for food in Africa: a review. Econ Bot. 2018;72(4):478‐495. 10.1007/s12231-018-9438-y. [DOI] [Google Scholar]
- 102. Lachenmeier DW, Sohnius EM, Attig R, López MG. Quantification of selected volatile constituents and anions in Mexican Agave spirits (Tequila, Mezcal, Sotol, Bacanora). J Agric Food Chem. 2006;54(11):3911‐3915. [DOI] [PubMed] [Google Scholar]
- 103. Takshak S, Agrawal SB. Defense potential of secondary metabolites in medicinal plants under UV‐B stress. J Photochem Photobiol B. 2019;193:51‐88. [DOI] [PubMed] [Google Scholar]
- 104. Colinas M, Goossens A. Combinatorial transcriptional control of plant specialized metabolism. Trends Plant Sci. 2018;23(4):324‐336. [DOI] [PubMed] [Google Scholar]
- 105. Schweizer F, Colinas M, Pollier J, et al. An engineered combinatorial module of transcription factors boosts production of monoterpenoid indole alkaloids in Catharanthus roseus . Metab Eng. 2018;48:150‐162. [DOI] [PubMed] [Google Scholar]
- 106. Legris M, Klose C, Burgie ES, et al. Phytochrome B integrates light and temperature signals in Arabidopsis. Science. 2016;354(6314):897‐900. [DOI] [PubMed] [Google Scholar]
- 107. Jung JH, Domijan M, Klose C, et al. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354(6314):886‐889. [DOI] [PubMed] [Google Scholar]
- 108. Qiu Y, Li M, Kim RJ‐A, Moore CM, Chen M. Daytime temperature is sensed by phytochrome B in Arabidopsis through a transcriptional activator HEMERA. Nat Commun. 2019;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bredow M, Walker VK. Ice‐binding proteins in plants. Front Plant Sci. 2017;8:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Strimbeck GR, Shaberg PG, Fossdal CG, Schröder WP, Kjellsen TD. Extreme low temperature tolerance in woody plants. Front Plant Sci. 2015;6:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Adams RP, Kendall E, Kartha KK. Comparison of free sugars in growing and desiccated plants of Selaginella lepidophylla . Biochem Syst Ecol. 1990;18(2‐3):107‐110. 10.1016/0305-1978(90)90044-g. [DOI] [Google Scholar]
- 112. Yobi A, Wone BW, Xu W, et al. Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol Plant. 2013;6(2):369‐385. [DOI] [PubMed] [Google Scholar]
- 113. Guillén S, Terrazas T, Casas A. Effects of natural and artificial selection on survival of columnar cacti seedlings: the role of adaptation to xeric and mesic environments. Ecol Evol. 2015;5(9):1759‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ahrar M, Doneva D, Tattini M, et al. Phenotypic differences determine drought stress responses in ecotypes of Arundo donax adapted to different environments. J Exp Bot. 2017;68(9):2439‐2451. [DOI] [PubMed] [Google Scholar]
- 115. Zhang H, Mittal N, Leamy LJ, Barazani O, Song B‐H. Back into the wild—apply untapped genetic diversity of wild relatives for crop improvement. Evol Appl. 2016;10:5‐24. 10.1111/eva.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yu G, Nguyen TT, Guo Y, et al. Enzymatic functions of wild tomato methylketone synthases 1 and 2. Plant Physiol. 2010;154(1):67‐77. 10.1104/pp.110.157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Thiebaut F, Hemerly AS, Ferreira PCG. A role for epigenetic regulation in the adaptation and stress responses of non‐model plants. Front Plant Sci. 2019;10:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lämke J, Bäurle I. Epigenetic and chromatin‐based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017;18(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Begcy K, Dresselhaus T. Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod. 2018;31(4):343‐355. 10.1007/s00497-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kooke R, Morgado L, Becker F, et al. Epigenetic mapping of the Arabidopsis metabolome reveals mediators of the epigenotype‐phenotype map. Genome Res. 2019;29(1):96‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schmid MW, Heichinger C, Coman Schmid D, et al. Controbution of epigenetic variation to adaptation in Arabidopsis. Nat Commun. 2018;9(1):4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. González APR, Preite V, Verhoeven KJF, Latzel V. Transgenerational effects and epigenetic memory in the clonal plant Trifolium repens . Front Plant Sci. 2018;9:1677. 10.3389/fpls.2018.01677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Verhoeven KJF, Jansen JJ, van Dijk PJ, Biere A. Stress‐induced DNA methylation changes and their heritability in asexual dandelions. New Phytol. 2010;185(4):1108‐1118. [DOI] [PubMed] [Google Scholar]
- 124. Gao L, Geng Y, Li B, Chen J, Yang J. Genome‐wide DNA methylation alterations of Alternanthera philoxeroides in natural and manipulated habitats: implications for epigenetic regulation of rapid responses to environmental fluctuation and phenotypic variation. Plant Cell Environ. 2010;33(11):1820‐1827. [DOI] [PubMed] [Google Scholar]
- 125. Ganguly DR, Crisp PA, Eichten SR, Pogson BJ. The Arabidopsis DNA methylome is stable under transgenerational drought stress. Plant Physiol. 2017;175:1893‐1912. 10.1104/pp.17.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Luna E, Bruce TJA, Roberts MR, Flors V, Ton J. Next‐generation systemic acquired resistance. Plant Physiol. 2012;158(2):844‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu XS, Wu H, Ji X, et al. Editing DNA methylation in the mammalian genome. Cell. 2016;167(1):233‐47.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang X, Yang M, Deng H, Ding Y. New era of studying RNA secondary structure and its influence on gene regulation in plants. Front Plant Sci. 2018;22(9):671. 10.3389/fpls.2018.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rouskin S, Zubradt M, Washietl S, Kellis M, Weissman JS. Genome‐wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature. 2014;505:701‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pai AA, Luca F. Environmental influences on RNA processing: biochemical, molecular and genetic regulators of cellular response. Wiley Interdiscip Rev RNA. 2019;10(1):e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang Y, Burkhardt DH, Rouskin S, Li GW, Weissman JS, Gross CA. A stress response that monitors and regulates mRNA structure is central to cold shock adaptation. Mol Cell. 2018;70(2):274‐86.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang L, Wessler SR. Role of mRNA secondary structure in translational repression of the maize transcriptional activator Lc. Plant Physiol. 2001;125(3):1380‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ding Y, Tang Y, Kwok CK, Zhang Y, Bevilacqua PC, Assmann SM. In vivo genome‐wide profiling of RNA secondary structure reveals novel regulatory features. Nature. 2014;505(7485):696‐700. [DOI] [PubMed] [Google Scholar]
- 134. Zubradt M, Gupta P, Persad S, Lambowitz AM, Weissman JS, Rouskin S. DMS‐MaPseq for genome‐wide or targeted RNA structure probing in vivo. Nat Methods. 2017;14(1):75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Koeberl C, MacLeod KG. Catastrophic events and mass extinctions: impacts and beyond. GSA Spec Pap. 2002;1:356. 10.1130/spe356. [DOI] [Google Scholar]
- 136. Ryu HK, Huang L, Kang HM, Schiefelbein J. Single‐cell RNA sequencing resolves molecular relationships among individual plant cells. Plant Physiol. 2019;179(4):1444‐1456. 10.1104/pp.18.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wu H‐YL, Song G, Walley JW, Hsu PY. The tomato translational landscape revealed by transcriptome assembly and ribosome profiling. Plant Physiol. 2019;181:367‐380. 10.1104/pp.19.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zenobi R. Single‐cell metabolomics: analytical and biological perspectives. Science. 2013;342(6163):1243259. 10.1126/science.1243259. [DOI] [PubMed] [Google Scholar]
- 139. Dou M, Clair G, Tsai CF, et al. High‐throughput single cell proteomics enabled by multiplex isobaric labeling in a nanodroplet sample preparation platform. Anal Chem. 2019;91(20):13119‐13127. 10.1021/acs.analchem.9b03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Abu‐Remaileh M, Wyant GA, Kim C, et al. Lysosomal metabolomics reveals V‐ATPase‐ and mTOR‐dependent regulation of amino acid efflux from lysosomes. Science. 2017;358(6364):807‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chen WW, Freinkman E, Sabatini DM. Rapid immunopurification of mitochondria for metabolite profiling and absolute quantification of matrix metabolites. Nat Protoc. 2017;12(10):2215‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell. 2016;166(5):1324‐37.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang Y, Malzahn AA, Sretenovic S, Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat Plants. 2019;5:778‐794. [DOI] [PubMed] [Google Scholar]
- 144. Shih PM. Towards a sustainable bio‐based economy: redirecting primary metabolism to new products with plant synthetic biology. Plant Sci. 2018;273:84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Huang S, Weigel D, Beachy RN, Li J. A proposed regulatory framework for genome‐edited crops. Nat Genet. 2016;48:109‐111. 10.1038/ng.3484. [DOI] [PubMed] [Google Scholar]
- 146. Long SP, Ort DR. More than taking the heat: crops and global change. Curr Opin Plant Biol. 2010;13(3):241‐248. [DOI] [PubMed] [Google Scholar]
- 147. Fergus G. File: All palaeotemps.svg, c 2014. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:All_palaeotemps.svg. Accessed October 18, 2019.
- 148. Royer DL, Berner RA, Montañez IP, Tabor NJ, Beerling DJ. CO2 as a primary driver of phanerozoic climate. GSA Today. 2004;14(3):4‐10. [Google Scholar]
- 149. Zachos JC, Dickens GR, Zeebe RE. An early Cenozoic perspective on greenhouse warming and carbon‐cycle dynamics. Nature. 2008;451(7176):279‐283. [DOI] [PubMed] [Google Scholar]
- 150. Hansen J, Sato M, Russel G, Kharecha P. Climate sensitivity, sea level and atmospheric carbon dioxide. Philos Trans A Math Phys Eng Sci. 2013;371:20120294. 10.1098/rsta.2012.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lisiecki LE, Raymo ME. A Pliocene‐Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography. 2005;20(1):PA1003‐PA1019. 10.1029/2004pa001071. [DOI] [Google Scholar]
- 152. Jouzel J, Masson‐Delmotte V, Cattani O, et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science. 2007;317(5839):793‐796. [DOI] [PubMed] [Google Scholar]
- 153. Foster GL, Royer DL, Lunt DJ. Future climate forcing potentially without precedent in the last 420 million years. Nat Commun. 2017;8:14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Siegenthaler U, Stocker TF, Monnin E, et al. Stable carbon cycle‐climate relationship during the late Pleistocene. Science. 2005;310(5752):1313‐1317. [DOI] [PubMed] [Google Scholar]
- 155. Pépin L, Raynaud D, Barnola J‐M, Loutre MF. Hemispheric roles of climate forcings during glacial‐interglacial transitions as deduced from the Vostok record and LLN‐2D model experiments. J Geophys Res. 2001;106(D23):31885‐31892. [Google Scholar]
- 156. Monnin E, Indermühle A, Dällenbach A, et al. Atmospheric CO2 concentrations over the last glacial termination. Science. 2001;291(5501):112‐114. [DOI] [PubMed] [Google Scholar]
- 157. Petit JR, Jouzel J, Raynaud D, et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429‐436. [Google Scholar]
- 158. Keeling CD, Bacastow RB, Bainbridge AE, et al. Atmospheric carbon dioxide variations at Mauna Loa observatory. Hawaii Tellus. 1976;28(6):538‐551. [Google Scholar]
- 159. Thoning KW, Tans PP, Komhyr WD. Atmospheric carbon dioxide at Mauna Loa observatory: 2. Analysis of the NOAA GMCC data, 1974‐1985. J Geophys Res. 1989;94(D6):8549‐8565. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparent‐Peer‐Review‐Record


