Abstract
Background
Sickle cell disease (SCD) includes a group of inherited haemoglobinopathies affecting multiple organs including the eyes. Some people with SCD develop ocular manifestations. Vision‐threatening complications are mainly due to proliferative sickle retinopathy, which is characterised by proliferation of new blood vessels. Laser photocoagulation is widely applicable in proliferative retinopathies. It is important to evaluate the efficacy and safety of laser photocoagulation in the treatment of proliferative sickle retinopathy (PSR) to prevent sight‐threatening complications.
Objectives
To evaluate the effectiveness of various techniques of laser photocoagulation therapy in SCD‐related proliferative retinopathy.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group’s Haemoglobinopathies Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. Date of last search: 4 July 2022.
We also searched the following resources (26 June 2022): Latin American and Caribbean Health Science Literature Database (LILACS); WHO International Clinical Trials Registry Platforms (ICTRP); and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials comparing laser photocoagulation to no treatment in children and adults with SCD.
Data collection and analysis
Two review authors independently assessed eligibility and risk of bias of the included trials; we extracted and analysed data, contacting trial authors for additional information. We assessed the certainty of the evidence using the GRADE criteria.
Main results
We included three trials (414 eyes of 339 children and adults) comparing the efficacy and safety of laser photocoagulation to no therapy in people with PSR. There were 160 males and 179 females ranging in age from 13 to 67 years. The trials used different laser photocoagulation techniques; one single‐centre trial employed sectoral scatter laser photocoagulation using an argon laser; a two‐centre trial employed feeder vessel coagulation using argon laser in one centre and xenon arc in the second centre; while a third trial employed focal scatter laser photocoagulation using argon laser. The mean follow‐up periods were 21 to 32 months in one trial, 42 to 47 months in a second, and 48 months in the third. Two trials had a high risk of allocation bias due to the randomisation method for participants with bilateral disease; the third trial had an unclear risk of selection bias. One trial was at risk of reporting bias. Given the unit of analysis is the eye rather than the individual, we chose to report the data narratively.
Using sectoral scatter laser photocoagulation, one trial (174 eyes) reported no difference between groups for complete regression of PSR: 30.2% in the laser group and 22.4% in the control group. The same trial also reported no difference between groups in the development of new PSR: 34.3% of lasered eyes and 41.3% of control eyes (very low‐certainty evidence). The two‐centre trial using feeder vessel coagulation, only presented data at follow‐up for one centre (mean period of nine years) and reported the development of new sea fan in 48.0% in the treated and 45.0% in the control group; no statistical significance (P = 0.64). A third trial reported regression in 55% of the laser group versus 28.6% of controls and progression of PSR in 10.5% of treated versus 25.7% of control eyes. We graded the evidence for these two primary outcomes as very low‐certainty evidence.
The sectoral scatter laser photocoagulation trial reported visual loss in 3.0% of treated eyes (mean follow‐up 47 months) versus 12.0% of controlled eyes (mean follow‐up 42 months) (P = 0.019). The feeder vessel coagulation trial reported visual loss in 1.14% of the laser group and 7.5% of the control group (mean follow‐up 26 months at one site and 32 months in another) (P = 0.07). The focal scatter laser photocoagulation trial (mean follow‐up of four years) reported that 72/73 eyes had the same visual acuity, while visual loss was seen in only one eye from the control group. We graded the certainty of the evidence as very low.
The sectoral scatter laser trial detected vitreous haemorrhage in 12.0% of the laser group and 25.3% of control with a mean follow‐up of 42 (control) to 47 months (treated) (P ≤ 0.5). The two‐centre feeder vessel coagulation trial observed vitreous haemorrhage in 3.4% treated eyes (mean follow‐up 26 months) versus 27.5% control eyes (mean follow‐up 32 months); one centre (mean follow‐up nine years) reported vitreous haemorrhage in 1/25 eyes (4.0%) in the treatment group and 9/20 eyes (45.0%) in the control group (P = 0.002). The scatter laser photocoagulation trial reported that vitreous haemorrhage was not seen in the treated group compared to 6/35 (17.1%) eyes in the control group and appeared only in the grades B and (PSR) stage III) (P < 0.05). We graded evidence for this outcome as low‐certainty.
Regarding adverse effects, only one occurrence of retinal tear was reported. All three trials reported on retinal detachment, with no significance across the treatment and control groups (low‐certainty evidence). One trial reported on choroidal neovascularization, with treatment with xenon arc found to be associated with a significantly higher risk, but visual loss related to this complication is uncommon with long‐term follow‐up of three years or more.
The included trials did not report on other adverse effects or quality of life.
Authors' conclusions
Our conclusions are based on the data from three trials (two of which were conducted over 30 years ago). Given the limited evidence available, which we assessed to be of low‐ or very low‐certainty, we are uncertain whether laser therapy for sickle cell retinopathy improves the outcomes measured in this review. This treatment does not appear to have an effect on clinical outcomes such as regression of PSR and development of new incidences. No evidence is available assessing efficacy in relation to patient‐important outcomes (such as quality of life or the loss of a driving licence). Further research is needed to examine the safety of laser treatment compared to other interventions such as intravitreal injection of anti‐vascular endothelial growth factors (VEGFs) . Patient‐important outcomes as well as cost‐effectiveness should be addressed.
Keywords: Adolescent; Adult; Aged; Child; Female; Humans; Male; Middle Aged; Young Adult; Anemia, Sickle Cell; Anemia, Sickle Cell/complications; Choroidal Neovascularization; Choroidal Neovascularization/etiology; Intravitreal Injections; Laser Therapy; Laser Therapy/adverse effects; Quality of Life; Vision Disorders
Plain language summary
Laser therapy for retinopathy in sickle cell disease
Review question How effective are the various techniques of laser photocoagulation in sickle cell disease‐related proliferative retinopathy (development of sight‐threatening complications due to excessive growth of blood vessels in the back of the eye)?
Background Sickle cell disease is a genetic disorder affecting many organs including the eyes. The back of the eye (retina) can develop problems due to sickle cell disease. A certain number of people with sickle cell disease develop sight‐threatening complications due to excessive blood vessel growth in the retina which is known as proliferative sickle retinopathy. Laser therapy is used to control the growth of new blood vessels in affected eyes. There are different types and techniques of laser used in treatment. However, we do not know whether these various laser treatments offer advantages compared to no treatment or other interventions with regards to effectiveness and safety.
Search date The evidence is current to: 26 June 2022.
Trial characteristics We included three randomised trials (414 eyes, 399 participants), comparing laser treatment to no intervention. There were 160 males and 179 females ranging in age from 13 to 67 years. The trials used different types of laser treatment. One trial applied lasers to the retina near the new blood vessels (sectoral scatter laser treatment) using an argon laser. Another applied lasers directly to feeding blood vessels (feeder vessel laser coagulation) using either xenon arc or argon laser. The third trial used focal scatter laser photocoagulation with an argon laser. Participants were followed up for an average of 21 to 48 months.
Key results There is low‐ to very low‐certainty evidence on the effects of using laser therapy in people with retinopathy related to sickle cell disease. In one trial, the effect of laser therapy on stopping the progression of new blood vessels and the development of new lesions did not differ greatly between the groups (very low‐certainty evidence). From the evidence found, we are not sure if laser therapy can prevent loss of vision (very low‐certainty evidence), but it may prevent sight‐threatening complications (low‐certainty evidence). The trials did not report on patient‐important outcomes, such as quality of life.
Evidence from the three trials showed that the safety of laser treatment is acceptable (few adverse effects), particularly scatter laser treatment using an argon laser. Although xenon arc lasers are linked to a higher number of complications, a loss of vision is not common. However, given that there are few trials with relatively low‐certainty evidence, results should be treated with caution. Further research is needed to examine the safety of laser treatment compared to other interventions. Trials should also measure patient‐important outcomes (such as quality of life and loss of driving licence) as well as cost‐effectiveness.
Certainty of the evidence We thought there was a risk of bias due to the way participants were selected for groups in two trials, especially since treatment may be required for both eyes. We thought there was a risk of bias in one trial which only presented some results for one of the two treatment groups; and we thought a third trial had a risk of bias as it did not clearly state in the Methods section of the paper which outcomes they intended to report.
Summary of findings
Summary of findings 1. Summary of findings ‐ laser therapy compared with no laser therapy for retinopathy in sickle cell disease (SCD).
| Laser therapy compared with no laser therapy for retinopathy in SCD | ||||||
|
Patient or population: children and adults with SCD and PSR Settings: outpatients Intervention: laser therapy (photocoagulation) Comparison: control (defined as no laser photocoagulation) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI)1 | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Laser therapy | |||||
|
Regression of PSR: change in number and size (in clock hours or in degrees of retinal circumference) of new blood vessels Follow‐up (mean): Study 1: 42 ‐ 47 months Study 2: 4 years |
In 1 study, complete regression of PSR was seen in 30/99 eyes (30.2%) in the laser therapy group and in 17/75 eyes (22.4%) in the control group. Partial regression was seen in 51/99 eyes (51%) in the laser therapy group and 18/75 eyes (23.7%) in the control group. A 2nd study reported that regression was seen in 21/38 eyes (55%) in the laser group and 10/35 eyes (28.6%) in the control group . |
NA | 247 eyes / 217 participants (2 studies) | ⊕⊝⊝⊝
very low
a,b,c |
Another study of 167 eyes in 122 participants reported this outcome for the laser therapy group only (the data for the control group were not provided). 78/87 eyes (89.6%) in the laser therapy group showed complete closure of neovascularization (Jampol 1983). |
|
|
Development of new PSR: proliferation of blood vessels at a new area/progression of existing PSR after treatment Follow‐up (mean): 42 ‐ 48 months |
One study reported new PSR developed in 34/99 eyes in the laser therapy group (34.3%) and in 31/75 eyes (41.3%) in the control group. One study reported on progression of existing PSR and it was seen in 4/38 treated eyes (10.5%) and 9/35 control eyes (25.7%) (Sayag 2008). |
NA | 247 eyes / 217 participants (2 study) | ⊕⊝⊝⊝
very lowa,b,c |
Another study reported only the long‐term follow‐up of 45 eyes for between 5.75 to 12 years. Development of new sea fan was reported in 12/25 eyes (48.0%) in the laser therapy group and 9/20 eyes (45.0%) in the control group (Jampol 1983). |
|
|
Quality of life Follow‐up: NA |
Outcome not reported | NA | ||||
|
Change in visual loss associated with PSR: visual loss is defined by the deterioration of visual acuity of 3 lines or more with the Snellen chart Follow‐up (mean): 21 ‐ 48months |
One study reported visual loss in 3/99 eyes (3.0%) in the laser therapy group compared to 9/75 eyes (12.0%) from the control group at a mean follow‐up of up to 47 months One study reported visual loss in 1/87 eyes (1.14%) in the laser therapy group compared to 6/80 eyes (7.5%) in the control group at a mean follow‐up of up to 32 months. A third study reported that at a mean follow up of 4 years 72/73 eyes showed no change in visual acuity. Visual loss was seen in only 1 eye from the control group (Sayag 2008). |
NA | 414 eyes / 339 participants (3 studies) | ⊕⊝⊝⊝
very lowa,b,d |
||
|
Occurence of vitreous haemorrhage Follow‐up (mean): 21 ‐ 48 months; |
In 1 study, vitreous haemorrhage was detected in 12/99 eyes (12.0%) in the laser therapy group compared to 19/75 (25.3%) from the control group at a mean follow‐up of up to 47 months. In 1 study, vitreous haemorrhage was detected in 3/87 eyes (3.4%) in the laser therapy group compared to 22/80 eyes (27.5%) in the control group at a mean follow up of up to 32 months. A 3rd study reported that vitreous haemorrhage was not seen in the treated group compared to 6/35 (17.1%) in the control group up to 4 years. |
NA | 414 eyes / 339 participants (3 studies) | ⊕⊕⊕⊝a,b low |
Long‐term follow‐up of 45 eyes in 1 study between 5.75 to 12 years showed that vitreous haemorrhage occurred in 1/25 eyes (4.0%) in the laser therapy group and 9/20 eyes (45.0%) in the control group. |
|
|
Adverse effects Follow‐up (mean): 21 ‐ 48 months |
In 1 study, at a mean follow‐up of up to 32 months, 41/87 eyes (47.12%) in the developed choroidal neovascularization and retinal detachment was reported in 5/87 eyes (5.74%) in the laser therapy group. Neither of these events occurred in the control group. In 1 study, at a mean follow‐up of up to 47 months, retinal detachment was reported in 3/99 eyes (3.0%) eyes in the laser therapy group and 8/75 eyes (10.6%) in the control group. The third study there were 3/35 (8.6%) retinal detachments in the control eyes compared to none in the treated eyes. |
NA | 414 eyes / 339 participants (3 studies) | ⊕⊕⊕⊝a,b low |
Long‐term follow‐up of 45 eyes in 1 study between 5.75 to 12 years showed 1/25 eyes (4.0%) in the laser therapy group developed a retinal tear (Fox 1993). No retinal haemorrhage or choroidal haemorrhage were reported in any of the studies. |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not applicable; PSR: proliferative sickle retinopathy; SCD: sickle cell disease. | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a The unit of allocation and analysis in all 3 studies was the eye (rather than the individual), therefore the treatment groups are not independent. For this reason, narrative results within the treatment groups are presented, but comparative statistics (e.g. P values) which are not adjusted for non‐independence are not presented.
b Downgraded twice due to risk of bias particularly across the domains of randomisation and allocation concealment. 2 studies were at high risk of selection bias as participants with bilateral disease had their right eye randomised and the left eye received the other intervention. In the 3rd study the risk of selection bias was unclear. Also, none of the studies described the method of allocation concealment and are likely to have been underpowered to determine non‐inferiority of laser photocoagulation for the primary outcomes.
c Downgraded once due to applicability: the included studies recruited adults aged 16 ‐ 63 years. The Sayag study includes 59.4% HbSS therefore the results of the study may not be generalisable to all individuals with SCD.
d Downgraded once due to imprecision: there were very low event rates for this outcome.
Background
See: appendices for glossary (Appendix 1).
Description of the condition
Sickle cell disease (SCD) is common genetic disorder affecting millions of people worldwide. It is characterised by the presence of haemoglobin S in which the glutamic acid in position 6 of the beta (β) chain of adult haemoglobin is replaced by valine. It is most endemic in tropical regions, mainly sub‐Saharan Africa, India and the Middle East (Weatherall 2001). It has become a global issue due to the migration of population from these areas to Europe and other parts of the world, particularly over the last few decades (Roberts 2007). Sickle cell disease includes homozygous SCDs, also known as sickle cell anaemia (Hb SS), sickle cell‐haemoglobin C disease (Hb SC), sickle cell‐β thalassaemia (Sβº Thal and Sβ⁺ Thal) and other less prevalent double heterozygous conditions (Serjeant 2001). It is a systemic disease that affects almost all the organs and leads to neurological, cardiac, pulmonary, hepatic, renal, ophthalmic, musculoskeletal and dermatological manifestations (Ballas 2010).
The main pathophysiology associated with ophthalmic manifestations in SCD is vaso‐occlusion that occurs in any vascular bed of ocular structures including conjunctiva, anterior segment, choroid, retina and optic nerve with potential visual impairment (Emerson 2005). Sight‐threatening problems in SCD are mainly due to proliferative sickle retinopathy (PSR), which is secondary to occlusion of the peripheral retinal vasculature, which in turn leads to retinal ischaemia and proliferation of new blood vessels with characteristic sea fans appearance. The incidence of PSR is more common in Hb SC disease and Sβ⁺ Thal, being approximately 33% and 14%, respectively, compared to 3% in Hb SS (Lutty 1994). The incidence of PSR increases with age, it is relatively common between 15 and 29 years of age (Condon 1972), but there have been reported studies in which PSR was detected in children as young as seven to 13 years (Abiose 1978; Condon 1974a; Erachulu 2006). The peak prevalence of PSR in people with Hb SS occurs between 25 and 39 years in both men and women, whereas in the Hb SC genotype it occurs earlier, from 15 to 24 years in men and 20 to 39 years in women (Elagouz 2010).
Goldberg developed a classification of PSR according to the severity of fundus changes (Table 2) (Goldberg 1971). Subsequently, researchers from a Jamaican sickle cohort study proposed a new classification for early peripheral retinal vascular changes in SCD, based on fundus fluorescein angiographic changes (Table 3) (Penman 1994).
1. Clinical classification of PSR.
| Stage | Staging of proliferative sickle retinopathy (PSR) |
| Stage I | Peripheral arteriolar occlusion |
| Stage II | Vascular remodelling, formation of arteriovenous anastomoses |
| Stage III | Peripheral retinal neovascularization |
| Stage IV | Vitreous haemorrhage |
| Stage V | Retinal detachment |
PSR: proliferative sickle retinopathy
2. Angiographic classification of PSR.
| Types | Angiographic appearances of peripheral retinal capillary beds |
| Type 1 | Qualitatively similar to normal, may be displace posteriorly with loss of capillary beds. |
| Type 2 ‐ Type 2A ‐ Type 2B |
Qualitatively abnormal, with abrupt termination of small‐ or medium‐sized vessels. ‐ presence of unstable border with capillary buds or stumps extending into non perfused retina. ‐ absence of capillary buds or stumps. |
| Type 3 | Indeterminate because recent acute arteriolar occlusion involving the vascular border gave rise to a type II pattern that reverted to normal following subsequent reperfusion of the vascular bed. |
PSR: proliferative sickle retinopathy
Early stages of PSR (stage I and II) may not need any intervention, as these early changes are asymptomatic or may even resolve due to auto‐infarction. Spontaneous regression is seen in 32% of eyes with PSR without any blinding complications (Downes 2005). Regression of PSR is more common in the eyes of people with Hb SS disease, seen in 40% compared to 20% of Hb SC; and complete non‐perfusion of PSR lesion is observed in 20% of SS and 7% of Hb SC (Fox 1991). Although permanent visual loss is rare, incidence of visual loss among people with Hb SS and Hb SC has been reported as 31 per 1000 eyes affected by PSR compared to 1.4 per 1000 eyes without PSR over a mean follow‐up period of 6.9 years (Moriaty 1988). Visual loss in PSR is commonly due to vitreous haemorrhage (stage IV) and tractional retinal detachment (stage V) (Moriaty 1988), and affects relatively younger people, indicating that early detection with timely effective treatment of stage III PSR is necessary to prevent such visual loss.
Description of the intervention
Various treatment options, such as diathermy, cryotherapy and transpupillary or transscleral diode laser photocoagulation, have been proven to be effective treatments of PSR (Condon 1974b; Goldbaum 1979; Seiberth 2001). Transpupillary laser photocoagulation is the safest and the preferred method among the available techniques, as cryotherapy is associated with adverse effects like retinal detachment (Goldbaum 1979). Transscleral diode laser photocoagulation is considered as an alternative in cases only when transpupillary laser coagulation is not applicable due to media opacities (Seiberth 2001).
Given the favourable chances of spontaneous regression, indication for the treatment of PSR varies among clinicians. Treatment is usually indicated in cases with peripheral neovascularization of more than 60° of circumference. This is particularly the case in eyes with bilateral involvement, spontaneous vitreous haemorrhage, large and elevated sea fans, rapid progression of new blood vessels, or precious eye in which the fellow eye has been lost due to PSR (Emerson 2006). The aim of treatment is to induce regression in stage III PSR prior to complications to prevent visual loss (Goldberg 1983). The different types of laser mainly used to achieve these goals are white xenon arc or blue/green argon.
The specific methods of laser in PSR include feeder vessel coagulation and scatter laser coagulation, either localised or 360° peripheral scatter coagulation (Ballas 2012). Scatter laser photocoagulation is considered to be the preferred method for PSR due to low rate of complications (Castro 1999). There are two types of scatter laser photocoagulation, the first being sectoral or localised and the second being 360° or circumferential laser treatment. In sectoral ablation, laser burns are applied only to the localised area around new blood vessels whereas in circumferential or 360° scatter laser, burns are applied circumferentially to entire peripheral retina (Cruess 1983; Kimmel 1986). The latter is usually indicated in those who are non‐compliant (Ballas 2012). Laser therapy is most effective when peripheral lesions are diagnosed early before involving the central retina (Castro 1999).
How the intervention might work
Laser photocoagulation has been considered safe as well as effective in the treatment of PSR, as it maintains quality of life and preserves the vision by preventing vision‐threatening complications in affected population (Goldbaum 1979; Goldberg 1983).
The mechanism of laser treatment in feeder vessel coagulation is to occlude the feeding vessels by applying direct, heavy laser burns to feeding arterioles leading to closure of neovascular fronds. Ocular media should be clear enough over the feeder vessels for successful photocoagulation (Goldbaum 1979). Both xenon arc and argon laser photocoagulation are used for feeder vessel coagulation; however, currently argon is more commonly used by clinicians as xenon has a higher complication rate compared to argon (Emerson 2005). Scatter laser coagulation has an indirect effect, as it destroys the ischaemic retina responsible for production of vascular endothelial growth factor (VEGF) that triggers the proliferation of new blood vessels (Ballas 2012). This technique is primarily used to treat proliferative diabetic retinopathy. The fact that laser photocoagulation to ischaemic retina results in regression of new blood vessels in eyes with proliferative diabetic retinopathy has led to this technique being adapted for treatment of PSR. To achieve this goal, blue/green argon laser burns are applied to the retina with laser setting of 500 micrometre (µm) spot size and 0.1 second duration.
Studies have demonstrated that laser treatment for PSR has been accepted for several decades (Cruess 1983; Kimmel 1986; Rednam 1982). Timely, successful treatment avoids the need for surgical interventions with their potential complications and morbidities (Cohen 1986; Goldberg 1983).
Why it is important to do this review
Proliferative sickle retinopathy is a leading cause of visual impairment in people with SCD. Cochrane Reviews of randomised controlled trials (RCTs) have been published for prophylaxis and treatment in other organs affected by SCD (Hirst 2012; Marti‐Carvajal 2012), but none to date for ocular involvement. Cochrane Reviews evaluating the effects of laser photocoagulation in other proliferative retinopathies, such as proliferative diabetic retinopathy and neovascular age‐related macular degeneration, have found that laser treatment has beneficial effects in preventing visual loss (Evans 2014; Virgili 2009). Despite the well‐known clinical applications of laser photocoagulation in PSR, it is imperative to identify the treatment effect in people with SCD, given the potentially complication of blinding due to PSR if treatment is delayed.
Even though laser photocoagulation in PSR is a relatively simple and safe treatment, there is a lack of summarised safety and efficacy data comparing this treatment to no treatment or to other treatment options in people with PSR. Furthermore, various techniques of laser photocoagulation have been practised among clinicians based on preference and facilities. It is therefore essential to perform a systematic review to evaluate the evidence for the effectiveness and potential adverse effects of different laser photocoagulation therapies in people with PSR for preventing visual loss and ocular morbidity.
Objectives
To evaluate the effectiveness of various techniques of laser photocoagulation therapy in sickle cell disease (SCD)‐related proliferative retinopathy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We also planned to include quasi‐RCTs if there had been sufficient evidence that the intervention and control groups were similar at baseline.
Types of participants
Children and adults diagnosed with SCD and proliferative sickle retinopathy (PSR), irrespective of phenotype, age, gender, race, ethnic origin and setting.
Types of interventions
All types of laser photocoagulation therapy to the retina compared to no intervention or to other forms of treatment such as cryotherapy or intravitreal anti‐vascular endothelial growth factor (VEGF) injection.
Types of outcome measures
We planned to assess the following outcomes at up to one month, over one month to six months, over six months to one year and over one year.
Primary outcomes
Regression of PSR (change in number and size (in clock hours or in degrees of retinal circumference) of new blood vessels)
Development of new PSR (proliferation of blood vessels at a new area after treatment) or progression of existing PSR (defined by increased size of existing lesions associated to leakage) (post hoc)
Secondary outcomes
Quality of life (using any validated measures)
Change in visual loss associated with PSR (visual loss is defined by the deterioration of visual acuity of three lines (post hoc) or more with the Snellen chart)
Occurence of vitreous haemorrhage (post hoc)
-
Adverse effects, such as:
retinal breaks or tears;
retinal detachment;
retinal haemorrhage;
choroidal haemorrhage;
choroidal neovascularization.
We planned to tabulate all adverse effects related to laser photocoagulation for the treatment of PSR that are reported in the included studies.
Search methods for identification of studies
There were no restrictions regarding language or publication status.
Electronic searches
We identified relevant studies from the Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND retinopathy.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of last search of Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register: 4 July 2022.
We also searched the following resources: Latin American and Caribbean Health Science Literature Database (LILACS) (lilacs.bvsalud.org/en/); WHO International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/) and ClinicalTrials.gov (www.clinicaltrials.gov). We did not restrict the electronic searches for trials by date or language.
See: appendices for details of search strategy for LILACS, the WHO ICTRP and ClinicalTrials.gov (Appendix 2).
Date of last search of LILACS, ICTRP, ClinicalTrials.gov: 26 June 2022.
Searching other resources
We also searched the reference lists of review articles for details regarding the relevant publication. We contacted laser manufacturers by email for information on ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (KTM, SS) independently assessed trial eligibility by screening the titles and abstracts of all RCTs identified during the search process. We contacted the trial authors for missing information from the trials published as full‐text papers and those published in abstract form only. The same two review authors independently reviewed full texts of all potentially relevant trials and assessed the eligibility according to the specific criteria for the inclusion of trials stated above. We tried to resolve any disagreements by discussion and we requested opinion of third review author (HN) if necessary.
We recorded the excluded studies in the 'Characteristics of excluded studies' table in Review Manager Web with reasons for exclusion (RevMan Web 2022).
Data extraction and management
Two review authors (KTM, HN) independently extracted data from eligible trials using standard data collection forms for optimal reliability. We checked for any errors and inconsistencies. We tried to resolve any disagreements by discussion and consensus. We maintained a record regarding any disagreement related with the extracted data. One review author (KTM) entered data into Review Manager Web (RevMan Web 2022) and a second review author (AWT) checked for any errors or discrepancies.
We extracted the following data.
Participants' characteristics: demographic data (age, sex, race); eligibility (inclusion and exclusion criteria); total number in comparison groups; sickle cell types (SS, SC, Sβ‐thalassemia); withdrawals or dropouts and losses to follow‐up with reasons.
Methods: trial design; time and duration of trial; randomisation; allocation concealment method, blinding of participants.
Characteristics of PSR: location in retinal quadrants (superotemporal, superonasal, inferotemporal, inferonasal); extent in number of clock hours or degree in circumference of the retina; surface (raised or flat).
Interventions: method of laser (feeder vessel coagulation, generalised scattered or sectoral scattered coagulation); types of laser (argon, xenon or other); laser setting (laser power or intensity, spot size, duration of laser photocoagulation; number of laser sessions.
Outcomes: outcomes mentioned above with time of assessment and length of follow‐up.
Although we planned to report our outcomes at up to one month, over one month to six month, over six months to one year and over one year in our protocol (Myint 2013), we were only able to report over one year, according to the data reported in the included trials.
Assessment of risk of bias in included studies
Two review authors (KT, SM) independently assessed the risk of bias in the included trials and followed the domain‐based evaluation according to the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We evaluated the following six domains as 'low risk'; 'unclear risk'; or 'high risk' of bias.
Random sequence generation
Concealment of allocation
Blinding of participants, personnel and outcome assessors
Incomplete outcome data
Selective outcome reporting
Other sources of bias
We evaluated the assessments and discussed any inconsistencies between the review authors in the interpretation of risk of bias. We resolved any disagreement by discussion with a third review author (HN). We recorded results on the above six domains in the relevant risk of bias tables in Review Manager Web (RevMan Web 2022).
Measures of treatment effect
In this version of the review, we have been unable to enter data into the 'Data and analyses' section, given the unit of analysis issue referred to below. For future updates, when possible, we plan to analyse extracted data using Review Manager Web (RevMan Web 2022). Specifically, for future updates, if we identify new eligible trials, we will assess the treatment effect as detailed below.
For dichotomous data (regression of PSR, development of new PSR, changes in visual loss associated with PSR, occurrence of vitreous haemorrhage and adverse reactions) we will calculate the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome.
For continuous outcome data (quality of life), if the outcomes are measured by the same scale within the trials, we will use the mean difference (MD) and corresponding CIs. If different scales are used to measure the same outcome we will use the standardised mean difference (SMD) and corresponding 95% CIs.
Unit of analysis issues
We assessed the included trials to determine the unit of analysis reported, which may be the eye or the participant. The unit of analysis reported in all of the included trials was the eye (rather than the individual), making standard data analysis not possible given that these data were not independent; therefore we presented these results narratively.
Dealing with missing data
We requested any missing data from the original investigators of the included trials. For each selected trial, we assessed the number of dropouts, withdrawals or losses to follow‐up. The included trials documented the reasons for missing data and we conducted the analysis based on participants with complete data. We contacted authors for any missing information.
Assessment of heterogeneity
We intended to use Chi² test and I² statistic to evaluate statistical heterogeneity between the trials. For future updates, when more trials are included, we will assess statistical heterogeneity between trials using the Chi² test. We will consider results to be statistically significant if the P value is less than 0.1. We will use the I² statistic to quantify heterogeneity and interpret the values of this as follows (Deeks 2021):
0% to 40% as not significant heterogeneity;
30% to 60% as moderate heterogeneity;
50% to 90% as substantial heterogeneity; and
75% to 100% as considerable heterogeneity.
Assessment of reporting biases
We performed comprehensive searches including search of abstracts and contacting manufacturer of laser machines to minimise publication and reporting bias. Within the trials, we considered selective outcome reporting as part of the risk of bias assessment. For those trials with a full published paper, we compared the 'Methods' section to the 'Results' section to ensure that all the outcomes which were measured, were reported. We did not use funnel plots to assess publication bias as there were insufficient number of trials (i.e. less than 10) and we only included three trials in this review.
Data synthesis
In all of the included trials, the main comparison was between laser photocoagulation and no intervention. We did not perform meta‐analysis for this review given the unit of analysis issue referred to above.
For future updates, if there are eligible trials we will perform meta‐analysis using fixed‐effect model for combining data if there is an absence of significant heterogeneity, both statistical and clinical, amongst included studies. We will use a random‐effects model if we identify substantial or considerable heterogeneity (I² value of 50% or more).
Subgroup analysis and investigation of heterogeneity
For future updates of the review, if we identify statistically significant heterogeneity for the primary outcomes, we plan to conduct subgroup analyses as follows:
different types of laser photocoagulation (argon, xenon);
different methods of laser photocoagulation (feeder vessel coagulation, sectoral scattered coagulation, circumferential scattered coagulation);
types of sickle cell disease (Hb SS, Hb SC disease, SβThal).
Sensitivity analysis
We planned to perform a sensitivity analysis to determine the robustness of results regarding the risk of bias. However, we were not able to do so since there were only three trials included in this version of the review.
Summary of findings and assessment of the certainty of the evidence
In a post hoc change in line with current Cochrane guidance, at the 2022 update we added a summary of findings table, presenting all outcomes of the review (Table 1).
Regression of PSR
Development of new PSR
Quality of life
Change in visual loss associated with PSR
Occurence of vitreous haemorrhage
Adverse effects
We determined the certainty of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if they considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
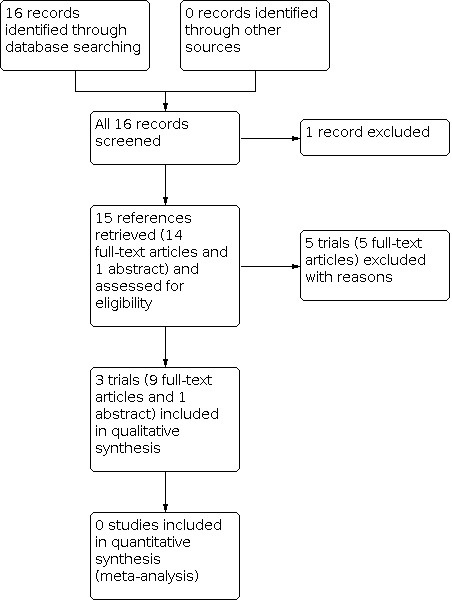
The searches of registers and databases identified 16 references. We discarded one reference and retrieved the full text of 15 references. We included three trials (10 references) assessing the effects of laser treatment versus no intervention (Farber 1991; Jampol 1983; Sayag 2008). We did not identify any RCTs reporting other interventions, such as cryotherapy and anti‐VEGF. We excluded five trials (one reference each) (Acheson 1991; Berman 1989; Condon 1974; Lemaire 2013; Osuji 2003). See the PRISMA diagram for details of the screening and selection process (Figure 1).
1.
Included studies
Methods and participants
All three trials used a parallel group design. One trial was conducted at single centre in Jamaica (Farber 1991), while the second trial was conducted at two centres, one in the USA (Chicago) and the other in Jamaica (Kingston) (Jampol 1983). The third trial was conducted in single centre in Sickle Cell Disease Centre and University Eye Clinic of Créteil, France (Sayag 2008). The Farber and Jampol trials were conducted by the same researcher group. The Farber trial was funded by a Comprehensive Sickle Cell Centre Grant from the National Heart, Lung, Blood Institute and National Eye Institute, National Institutes of Health, Bethesda, Maryland and by Research to Prevent Blindness Inc, New York, NY (Farber 1991). The Jampol trial was funded by a Comprehensive Sickle Cell Center grant from the National Heart, Lung, and Blood Institute, National Institute of Health, Bethesda, Maryland (Jampol 1983). The Sayag trial did not state the funding sources (Sayag 2008).
The trials included 339 participants with PSR (aged 13 years to 67 years, 160 males and 179 females). The unit of randomisation in all trials were the eyes. Farber randomised 174 eyes of 116 participants in Jamaica (Farber 1991); Jampol randomised a total of 167 eyes of 122 participants in Chicago and Kingston (Jampol 1983). Sayag recruited 202 eyes of 101 participants in Créteil, but randomised a total of 73 eyes of 67 participants with stage III PSR (Sayag 2008).
In two trials, participants with bilateral disease had their right eye randomised to either treatment or no treatment, with the other eye receiving the opposite modality (Farber 1991; Jampol 1983). Participants with only one eye eligible for the trial were randomised to treatment or control for that eye. If the second eye became eligible later that eye received the opposite modality of treatment from the first eye. In the Sayag trial, three participants had bilateral disease and 67 had unilateral disease, but the randomisation method was not stated (Sayag 2008).
In the Farber trial, there were 93 participants with Hb SC, 21 with Hb SS and two with Sβ Thal (Farber 1991); Jampol recruited people with Hb SS, Hb SC and Sβ Thal, but did not provide details on the proportions with each type (Jampol 1983). Both trials reported the extent of PSR in four groups according to circumferential extent of neovascularization; 1° to 30°, 31° to 60°, 61° to 90° and more than 91° (Farber 1991; Jampol 1983). In the Sayag trial, there were 33 participants with HbSC, 63 with Hb SS and five with Sβ Thal but stage III PSR was seen in 35 eyes of Hb SC and 38 eyes of Hb SS (Sayag 2008). Sayag reported the new classification for stage III PSR depend on the size, haemorrhage, fibrosis and visible vessels (Table 4).
3. New Grading of Satge III PSR by Sayag 2008.
| Grade | Description |
| A | Sea fan flat with leakage < 1 MPS disc area |
| B | Elevated sea fan with haemorrhage |
| C | Elevated sea fan with partial fibrosis |
| D | Complete sea fan fibrosis without well demarcated vessels |
| E | Complete sea fan fibrosis with well demarcated vessels |
MPS: Macular Photocoagulation Study; PSR: proliferative sickle retinopathy
Interventions
All trials compared the effects of laser with no intervention. An additional table gives details of the laser treatment employed in the trials (Table 5). Farber employed sectoral scatter laser photocoagulation using argon laser in 99 eyes, with 75 eyes assigned into the control group (Farber 1991). Jampol employed feeder vessel coagulation using argon in Chicago, USA and xenon arc in Kingston, Jamaica (Jampol 1983). Among the 87 eyes assigned to the feeder vessel coagulation group, 34 eyes from the Chicago centre received argon laser photocoagulation, whereas the 53 eyes from the Kingston centre received xenon arc coagulation (Jampol 1983). Sayag employed focal scatter laser photocoagulation using argon green laser in 38 eyes in Créteil, France with 35 eyes assigned to the control group (Sayag 2008).
4. Characteristics of intervention and control in each study.
| Study ID | Intervention (Laser type) | Parameters | Control |
| Farber 1991 | Sectoral scatter laser photocoagulation by Argon blue/green (Britt 3250) in Kingston, Jamaica |
500 µm spot size, 0.1 second duration | Observation |
| Jampol 1983 | Feeder vessel photocoagulation Chicago ‐ Argon laser Kingston, Jamaica ‐ Xenon arc |
500 µm spot size, 0.2 second duration, 300 ‐ 600 mW power Intensity 5 ‐ 10, Size 3 ‐ 6 and 0.5 ‐ 2 second duration |
Observation |
| Sayag 2008 | Focal scatter photocoagulation using Argon green laser in Créteil, France |
Spot size 300 ‐ 500 m, 0.1 second duration with a mild whitening of the retina |
Observation |
m:metre; µm: micrometre
Outcomes
Farber reported data for this review's primary outcomes (regression of PSR and development of new PSR) (Farber 1991). The time point reported in the trial was a mean follow‐up of 47 months for treated eyes and 42 months for the control eyes.
Jampol only reported data for the regression of PSR in the laser group (mean follow‐up of 21 months for Chicago and 32 months for Jamaica); investigators did not report data for the control group (Jampol 1983). We contacted the original investigators, but data were not available. Over the long‐term follow‐up period of nine years, 29 participants from the Chicago centre in Jampol trial reported the development of new PSR.
Sayag reported data for the regression of PSR for both the laser and control groups (Sayag 2008). Athough the trial did not report this review's original second primary outcome (development of new PSR), it did report the progression of existing PSR associated with leakage which has been added as a primary outcome in a post hoc change. The time point reported was a mean follow‐up of four years.
Regarding the secondary outcomes for this review, all three trials reported changes in visual loss associated with PSR, the incidence of vitreous haemorrhage, and the adverse event of retinal detachment (Farber 1991; Jampol 1983; Sayag 2008). Jampol also reported the incidence of choroidal neovascularization (Jampol 1983).
The trials did not assess quality of life or some adverse effects such as retinal haemorrhage or choroidal haemorrhage (Farber 1991; Jampol 1983; Sayag 2008).
We planned to report results at one month, over one month to six months, over six months to one year and over one year, but in this review we reported outcomes according to the mean and median duration as reported by original investigators.
Excluded studies
See: Characteristics of excluded studies.
We excluded a total of five trials which did not meet our inclusion criteria; none of these were RCTs of a relevant intervention (Acheson 1991; Berman 1989; Condon 1974; Lemaire 2013; Osuji 2003). One trial assessed the effect of scatter laser in iatrogenic choriovitreal neovascularization (Acheson 1991), the second assessed the treatment of proliferative vitreoretinopathy (Berman 1989), the third assessed the effect of photocoagulation and diathermy in eyes with PSR (Condon 1974), the fourth assessed the blood hyperviscosity in people with severe PSR (Lemaire 2013), and the last assessed the screening methods using panoramic fundus camera (Osuji 2003).
Risk of bias in included studies
We assessed the risk of bias of the included trials according to the six domains outlined in the Assessment of risk of bias in included studies section of the review. Two figures demonstrate the overall assessment of risk of bias in the included trials (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Sequence generation
Two trials reported the use of computer‐generated randomisation; however, due to the randomisation method in participants with bilateral disease where one eye was randomised to either treatment or control and the second eye was allocated to the alternative group, we have assessed both of these trials as having a high risk of bias for this domain (Farber 1991; Jampol 1983). The third trial reported that "patients were randomised to treatment or no treatment group", but the paper did not provide further information (Sayag 2008). We contacted the trial authors for additional information, but did not receive any response. We assessed this trial as having unclear risk of bias.
Allocation concealment
None of the three trials described the method of allocation concealment and we have therefore assessed these as having an unclear risk of bias (Farber 1991; Jampol 1983; Sayag 2008).
Blinding
None of the included trials mentioned blinding, but performance bias is unlikely with this type of intervention and the reported outcomes were objective outcomes which were not likely to be influenced by assessor bias. Therefore, we rated all trials as having a low risk of bias (Farber 1991; Jampol 1983; Sayag 2008).
Incomplete outcome data
Two trials had incomplete outcome data, but adequately described the number of withdrawals or dropouts and gave the reasons for these (Farber 1991; Jampol 1983). Farber reported "11 patients moved after average of 24 months (range 11 to 60 months) and one patient died after 15 months" (Farber 1991). Jampol reported "Chicago: Two patients lost to follow‐up after 8 and 44 months and one refuse to cooperate. Kingston: eight patients lost to follow‐up due to emigration after average 12 months of follow‐up and two refuse to cooperate" (Jampol 1983). Investigators analysed all eyes as randomised (Farber 1991; Jampol 1983). We judged the risk of bias as low for both trials.
The third trial reported "patients missing one visit were excluded from the study", but did not provide the numbers of participants or the reasons for missing visits so we assessed this trial as having an unclear risk of bias (Sayag 2008).
Selective reporting
The trial protocols were not available (although we did not expect this given the age of the trials) and we were not able to establish whether they had been prospectively registered in a publicly accessible database. The methods section of the three included trials did not mention pre‐stated outcomes but the expected outcomes were reported in the results section of the Farber trial and the Sayag trial, therefore we considered these as having a low risk of bias (Farber 1991; Sayag 2008). In the Jampol trial, the outcome 'regression of new blood vessel' was reported only for the treatment group, not for the control group, we therefore assessed this trial as having a high risk of bias for this domain (Jampol 1983).
Other potential sources of bias
The trials were possibly underpowered to be able to demonstrate non‐inferiority of laser photocoagulation for primary outcomes such as complete regression of PSR and development of new PSR or progression of existing PSR with reasonable follow‐up duration of 32 to 48 months. All three trials were assessed as unclear risk of bias for this domain.
Effects of interventions
See: Table 1
Given the unit of analysis issue referred to above, we were not able to perform meta‐analysis for this version of review. We have therefore presented the data in the additional tables (Table 6) and provided a summary of the main findings of the review in Table 1.
5. Effects of intervention.
| Outcome |
Farber 1991 (randomised 174 eyes) Mean follow‐up: 42 ‐ 47 months |
Jampol 1983 (randomised 167 eyes) Mean follow‐up: 21 ‐ 32 months |
Sayag 2008 (randomised 73 eyes) Mean follow‐up: 4 years |
||||
|
Laser (Argon Scatter) |
Control |
Laser (Feeder Vessel) |
Control |
Laser (Argon Focal) |
Control | ||
| 1 | Complete regression of PSR |
30/99 (30.2%) | 17/75 (22.4%) | 78/87 (89.6%) | Not reported | 21/38 (55.3%) | 10/35 (28.6%) |
| Partial regression of PSR | 51/99 (51.0%) | 18/75 (23.7%) | |||||
| 2 | Development of new PSR/ increased size of pre‐existing lesions |
34/99 (34.3%) | 31/75 (41.3%) | Not reported | 4/38 (10.5%) | 9/35 (25.7%) | |
| 3 | Visual loss | 3/99 (3.0%) | 9/75 (8.3%) | 1/87 (1.14%) | 4/80 (5.0 %) | 0/38 (0%) | 1/35 (2.8%) |
| 4 | Occurrence of vitreous haemorrhage | 12/99 (12.0%) | 19/75 (25.3%) | 3/87 (3.4%) | 22/80 (27.5%) | 0/38 (0%) | 6/35 (17.1%) |
| 5 | Adverse effect: retinal tear | Not reported | Not reported | Not reported | |||
| 6 | Adverse effect: retinal detachment | 3/99 (3.0%) | 8/75 (10.6%) | 5/87 (5.74%) | No event | 0/38 (0%) | 3/35 ( 8.5%) |
| 7 | Adverse effect: choroidal neovascularisation | Not reported | 41/87 (47.12%) | No event | Not reported | ||
Unit of analysis is the eye.
PSR: proliferative sickle retinopathy.
Farber 1991: mean follow‐up of 42 months for control group and 47 months for treatment group.
Jampol 1983: mean follow‐up of 21 months for Chicago centre and 32 months for Kingston, Jamaica (overall participants).
Sayag 2008: mean follow‐up of 4 years.
Three trials (414 eyes of 339 participants (children and adults)) were included comparing efficacy and safety of laser photocoagulation to no therapy in participants with proliferative sickle retinopathy (Farber 1991; Jampol 1983; Sayag 2008).
Primary outcomes
1. Regression of PSR
All trials reported on this outcome. Faber reported that with a mean follow‐up of 47 months for the treatment group and 42 months for the control group, the complete regression of PSR was seen in 30 our of 99 eyes (30.2%) in laser group and in 17 out of 75 eyes (22.4%) in the control group (Farber 1991). Partial regression was seen in 51 out of 99 eyes (51%) in the laser group and 18 out of 75 eyes (23.7%) in the control group. Data for long‐term follow‐up of participants with Hb SC disease from the Farber trial were presented in a separate paper to avoid the differences in behaviour between genotypes; in these participants seven out of 74 (9.45%) treated eyes and two out of 60 (3.33%) control eyes had complete infarction of PSR over the median follow‐up of 2.9 years (P = 0.3) (Fox 1993).
Farber also reported that treated eyes had more regression than control eyes, but it was only significant in participants younger than 25 years at enrolment (P < 0.001), not in older participants (P = 0.6) and in small (less than 15°) PSR lesions, as well as flat rather than elevated PSR (Fox 1993).
Jampol only reported the regression of PSR for the treatment group (Jampol 1983). At mean follow‐up of 26 (Chicago group) to 32 (Kingston group) months, using feeder vessel coagulation, 78 out of 87 eyes (89.6%) showed complete closure of neovascularization. The data for the control group were not provided; we contacted the trial author who was not able to provide relevant data due to the time elapsed since the trial was conducted.
Sayag reported that regression of PSR was seen in 21 out of 38 eyes (55%) in laser group and in 10 out of 35 eyes (28.6%) in control group at mean follow‐up of four years (Sayag 2008). Sayag also reported the regression was seen in treated more than the control eyes, but it was only significant in eyes with grade B (P = 0.02), not in the grade A and C of new classification of stage III PSR (Table 4). Grade D and E showed no changes (Sayag 2008).
We judged the certainty of the evidence for this outcome as very low.
2. Development of new PSR or progression of PSR
All trials reported on this outcome. Farber reported that new PSR developed in 34 out of 99 laser‐treated eyes (34.3%) and in 31 out of 75 eyes (41.3%) in the control group at mean follow‐up of 42 to 47 months (P = 0.3) (Farber 1991).
Jampol did not report this outcome in the initial publication, but did follow up the participants for a mean period of nine years (range 5.75 to 12 years) in the Chicago centre; 29 participants (45 eyes, with 25 eyes in laser group and 20 eyes in the control group) completed this follow‐up (Jampol 1983). The development of new sea fan was reported in 12 out of 25 eyes (48.0%) in the treated group and nine out of 20 eyes (45.0%) in the control group. There was no statistical significance (P = 0.64).
Sayag reported the progression of PSR, defined as increased size of existing lesion associated to leakage, was seen in four out of 38 treated eyes (10.5%) and nine out of 35 controlled eyes (25.7%) at mean follow‐up of four years (Sayag 2008). It was also reported that progression was seen in grade A, B and C of stage III PSR. This was only statistically significant for grade B (P <0.05), whereas no significance in grade A and C (P > 0.05). No case of progression was reported in grade D and E (Sayag 2008).
We judged the certainty of the evidence for this outcome as very low.
Secondary outcomes
1. Quality of life
This outcome was not assessed in the included trials (Farber 1991; Jampol 1983; Sayag 2008).
2. Change in visual loss associated with PSR
Visual loss, defined as deterioration of visual acuity three lines or more from the Snellen chart, was reported in all three trials.
Farber reported that, at a mean follow‐up of 42 and 47 months for the control and treatment group, respectively, visual loss is seen in three out of 99 eyes (3.0%) treated by scatter laser photocoagulation compared to nine out of 75 eyes (12.0%) from control group (Farber 1991). Kaplan‐Meier survival analysis (two‐year survival curves for visual loss) showed a significant difference between the treated and control groups (P = 0.019).
Jampol reported that visual loss was seen in one out of 87 eyes (1.14%) treated by feeder vessel coagulation group and six out of 80 eyes (7.5%) in the control group at a mean follow‐up of 26 (in Chicago) to 32 months (in Kingston) (P = 0.07) (Jampol 1983).
Sayag reported that at mean follow‐up of four years, 72 out of 73 eyes included in the trial had the same visual acuity and visual loss is seen in only one eye from the control group (Sayag 2008).
We judged the certainty of the evidence for this outcome as very low.
3. Occurence of vitreous haemorrhage
All three trials reported this outcome. In the Farber trial (sectoral scatter laser photocoagulation), vitreous haemorrhage was detected in 12 out of 99 (12.0%) laser‐treated eyes and in 19 out of 75 (25.3%) control eyes in mean follow‐up of 42 months (treatment group) to 47 months (control group) (Farber 1991). Investigators performed an analysis controlling vitreous haemorrhage and the amount of neovascularization at entry which showed a significant difference between the treated and control eyes (P ≤ 0.5).
Jampol reported that three out of 87 (3.4%) eyes treated with feeder vessel coagulation, compared to 22 out of 80 (27.5%) controlled eyes, were complicated by vitreous haemorrhage at mean follow‐up of 26 (treatment group) to 32 months (control group). With long‐term mean follow‐up of nine years in the Chicago centre, vitreous haemorrhage occurred in one out of 25 eyes (4.0%) in the treatment group and nine out of 20 eyes (45.0%) in the control group (P = 0.002) (Jampol 1983).
Sayag (scatter laser photocoagulation) reported that vitreous haemorrhage was not seen in the treated group compared to six out of 35 (17.1%) eyes in the control group and appeared only in the grade B and E PSR stage III (P <0.05) (Sayag 2008).
We judged the certainty of the evidence for this outcome as low.
4. Adverse effects
a. Retinal tear
Two trials did not report this adverse effect (Farber 1991; Sayag 2008). There was no report of retinal tear at initial follow‐up of the Jampol trial. However, with long‐term follow‐up of 5.75 to 12 years, it was reported that in one of the 25 eyes that completed the follow‐up (Chicago centre), retinal tear developed at the base of the treated sea fan in the feeder vessel coagulation group (Jampol 1983).
b. Retinal detachment
All three trials reported retinal detachment (Farber 1991; Jampol 1983; Sayag 2008).
At mean follow‐up of 42 (treatment group) to 47 months (control group), retinal detachment was reported in three out of 99 (3.0%) eyes in the scatter laser group and eight out of 75 (10.6%) in the control group (Farber 1991).
At mean follow‐up of 26 (in Chicago) to 32 months (in Kingston), retinal detachment was seen in five out of 87 (5.74%) eyes treated by feeder vessel coagulation (confined to argon laser group) and not at all in the control group (Fisher's exact test, P = 0.07; Pearson's Chi2 , P = 0.03) (Jampol 1983).
In Sayag trial, there was no event of retinal detachment in the treated eyes, whereas there were three out of 35 (8.6%) controlled eyes at mean follow‐up of four years (P = 0.0526) (Sayag 2008).
We judged the certainty of the evidence for this outcome as low.
c. Choroidal neovascularization
Choroidal neovascularization, which is solely an adverse effect of laser therapy, particularly with xenon arc, was reported in the Jampol trial (Jampol 1983).
At follow‐up of 21 to 32 months, 41 out of 87 (47.12%) treated eyes were reported to have developed this adverse effect, which was seen in 38 out of 53 xenon‐treated eyes and three out of 34 argon lasered‐eyes. There was no such event in the control group (Jampol 1983). The incidence of choroidal neovascularization was significantly higher in the xenon group (P < 0.0001).
We judged the certainty of the evidence for this outcome as low.
Other complications, such as retinal haemorrhage and choroidal haemorrhage, were not reported in any of the three trials (Farber 1991; Jampol 1983; Sayag 2008).
Discussion
Summary of main results
Only three trials contributed data to this systematic review (Farber 1991; Jampol 1983; Sayag 2008).
All three trials reported on our primary outcome of regression, but one trial only reported data for the intervention group precluding any comparison (Jampol 1983). One trial, involving 174 eyes with proliferative sickle retinopathy (PSR), compared the effect of scatter argon laser photocoagulation and no treatment over a follow‐up of 42 to 47 months; it revealed that there was a higher rate of regression of PSR in the laser group but no significant difference in complete regression (Farber 1991). Spontaneous regression was also noted without treatment. Treated eyes had more regression than control eyes, but it was only significant in people younger than 25 years at enrolment, in small (less than 15°) PSR lesions as well as flat rather than elevated PSR (Fox 1993). The third trial, involving 73 eyes with PSR stage III, compared the effect of focal scatter argon laser photocoagulation and no treatment over the follow‐up of four years; also revealed that there were higher rate of regression in laser group, but it was significant only in the PSR grade B (elevated sea fan with haemorrhage) and no significant differences in other grades of sea fans (Sayag 2008).
Neither sectoral scatter argon laser photocoagulation nor feeder vessel coagulation exhibited a significant protective effect against the development of new vessels or the progression of existing sea fans (Farber 1991; Jampol 1983; Sayag 2008).
None of the trials reported on the outcome of quality of life.
All three trials (414 eyes) reported changes in visual loss measured by the deterioration of three lines or more from the Snellen chart (Farber 1991; Jampol 1983; Sayag 2008). Data from these trials suggested that laser photocoagulation probably prevented visual loss at mean follow‐up of over one year. It also reported that laser therapy had a protective effect for the occurrence of vitreous haemorrhage. Data from all trials indicated that laser treatment prevented the occurrence of vitreous haemorrhage with both argon and xenon laser; with the protective effect not significantly different between feeder vessel and scatter laser photocoagulation (Farber 1991; Jampol 1983; Sayag 2008).
As for adverse effects, the incidence of retinal tear was very minimal with only one event reported from the long‐term follow‐up of the Jampol trial (Jampol 1983); the remaining two trials did not report this adverse effect (Farber 1991; Sayag 2008). There was no statistical difference in retinal detachment between the laser and the control arms in two trials (Farber 1991; Sayag 2008), and only borderline statistical significance in the Jampol trial (Jampol 1983); however, overall the result was not statistically significant. As for choroidal neovascularization, xenon arc treatment was found to be associated with a significantly higher risk, but visual loss related to this complication is uncommon with long term‐follow‐up of three years or more (Jampol 1983).
The results of this review suggest that laser photocoagulation therapy for eyes with PSR may prevent visual loss and occurrence of vitreous haemorrhage. It also shows a positive effect in relation to the regression of PSR, but it may not be sufficient to prevent progression or development of new lesions.
Overall completeness and applicability of evidence
We did not perform meta‐analyses because the units of analysis in the included trials were not independent (i.e. eyes, not participants). Moreover, different time points were used to present data and different methods of laser therapy were employed in the trials.
Few data contributed to the primary outcomes of this review, only two trials (247 eyes of 217 participants with PSR receiving either laser treatment or no treatment) out of the three included trials assessed regression of PSR and progression of PSR or development of new PSR (Farber 1991; Sayag 2008). A clinically meaningful difference for the complete regression of PSR and the development of new PSR, has not yet been demonstrated in this population. As mentioned above, different types and techniques of laser photocoagulation were used to induce the regression of new blood vessel in eyes with PSR (Background). The regression is largely dependent on the size, extent and surface of the PSR lesions, therefore, drawing any conclusion about this outcome would be difficult.
Three trials provided evidence on the effect of laser photocoagulation in preventing visual loss and vitreous haemorrhage in 414 eyes of 339 participants with PSR (Farber 1991; Jampol 1983; Sayag 2008). Two trials reported no significant differences between lasered and control eyes for retinal detachment (Farber 1991; Sayag 2008); whereas the other trial reported borderline statistical significance for retinal detachment in the laser group treated by argon feeder vessel coagulation (Jampol 1983). Choroidal neovascularization is a major complication of xenon arc and occurred in 80% of participants treated by xenon over the follow‐up of three years or more (Jampol 1983). It is also seen in eyes treated by argon, but the incidence was significantly higher with xenon‐treated eyes. None of the trials reported quality of life and other adverse effects, such as retinal haemorrhage or choroidal haemorrhage.
Overall, the evidence is applicable to individuals presenting with stage III PSR. However, the evidence is of limited quality, due to the fact that only three trials were included, and these were conducted several years ago.
Currently, argon laser scatter photocoagulation is commonly used in practice in most countries, whereas feeder vessel coagulation is of limited use due to a higher rate of complications. Feeder vessels coagulation by argon laser was associated with retinal detachment while xenon arc is almost obsolete due to the higher rate of complications, particularly choroidal neovascularization.
Quality of the evidence
We found only thee trials eligible for our review; two were conducted over 30 years ago (Farber 1991; Jampol 1983) and one almost 15 years ago (Sayag 2008), when standards of reporting and trial conduct were not high. The trials were of open‐label, parallel design and aimed to demonstrate the effectiveness of laser therapy in preventing sight‐threatening complications in eyes with PSR. The overall risk of bias in the included trials was fairly unclear due to the inadequate reporting of methods and results of the included trials. Risk of bias related to sequence generation was high due to the randomisation method of participants with bilateral disease. The risk of bias related to incomplete outcome data was considered low in two trials (Farber 1991; Jampol 1983), but unclear in one trial (Sayag 2008). There is an unclear risk of bias in relation to allocation concealment and blinding, as none of the trials described these adequately. However, we judged both performance and detection bias to be low, given that the type of intervention and measurement of objective outcomes were unlikely to be influenced by blinding. One trial did not report the event data in the control group for the primary outcome 'regression of PSR' which we regard as a high risk of bias for selective reporting (Jampol 1983). We contacted the original investigators of this trial but were unable to retrieve the appropriate data, which is likely due to the time length since trial completion. Although there is evidence that laser photocoagulation therapy is effective in preventing visual loss and vitreous haemorrhage, the sample size was small. It also induced regression of new blood vessels, but it is significant only in younger participants with small flat lesions. The trials were possibly under‐powered to be able to demonstrate non‐inferiority of laser photocoagulation for primary outcomes, such as complete regression of PSR and development of new PSR with reasonable follow‐up duration of 32 to 47 months.
The certainty of the evidence for primary outcomes was very low, as assessed using the GRADE criteria, and for the secondary outcomes (that were reported on in the three included trials) varied very low to low, see summary of findings tables (Table 1).
Potential biases in the review process
Despite retinopathy being a sight‐threatening condition in a substantial population affected by Sickle cell disease (SCD), there are few randomised controlled trials (RCTs) eligible for inclusion in our review. This may suggest that we were unable to identify and retrieve small trials, particularly those with inconclusive results, indicating the possibility of publication or retrieval bias. However, we believe this to be unlikely, given we searched multiple sources with no date or language restrictions. The small numbers of trials included in this review may introduce publication bias, but we were unable to perform funnel plot assessment for which more trials are needed. We were also unable to identify ongoing trials that met our inclusion criteria for SCD‐related retinopathy. A more credible explanation is that argon laser photocoagulation has become standard treatment for stage III PSR in many countries and RCTs, especially as a trial with no intervention or a placebo used as a control, may be unethical.
Agreements and disagreements with other studies or reviews
We are unaware of any similar reviews on this topic. Available evidence comes from small prospective non‐randomised studies (Cruess 1983; Kimmel 1986; Rednam 1982). One prospective non‐randomised study presented the effect of scatter circumferential argon laser photocoagulation in 40 eyes with PSR and showed that 26% of eyes were associated with complete regression; there were no complications reported related to treatment (Cruess 1983). Three years later, Kimmel reported the effect of laser treatment in 70 eyes of 44 participants having a total of 220 sea fans. At average follow‐ up of 3.3 years, 33% of pre‐existing sea fans were associated with complete regression, 46% with partial regression, 19% with no change and 2% with progression. Vitreous haemorrhage developed in only one participant (2%) over the period of follow‐up (Kimmel 1986). In one small prospective study, Rednam and colleagues investigated the effect of localised scatter photocoagulation in 21 eyes of 19 individuals with PSR lesions (Rednam 1982). They reported that flat sea fans responded dramatically with complete regression in 24 of 28 (85.7%) PSR lesions. Elevated sea fans responded less rapidly with complete regression seen only in four out of 17 (23.5%) lesions (Rednam 1982).
Authors' conclusions
Implications for practice.
In the absence of further evidence, laser treatment for sickle cell disease (SCD)‐related retinopathy should be considered as a current therapeutic option for preventing visual loss and vitreous haemorrhage. However, it does not appear to have a significantly different effect on other clinical outcomes, such as the regression of existing instances of proliferative sickle retinopathy (PSR) and the development of new ones. Overall, scatter argon laser photocoagulation therapy is superior in terms of adverse effects, although feeder vessel coagulation has a better effect in preventing vitreous haemorrhage. We judged the evidence to be of low certainty due to the method of sequence generation and the reporting of trials which were performed over 20 years ago, after which scatter argon laser photocoagulation has become the mainstay of treatment for stage III PSR to prevent blinding complications.
Implications for research.
Sickle cell disease‐related retinopathy has vision threatening complications in certain populations and scatter argon laser photocoagulation is standard practice for stage III PSR in many countries, following recommendations by Farber (Farber 1991). Under these circumstances, it is unethical to conduct randomised, placebo‐controlled trials to answer the efficacy and safety of laser treatment. Since intravitreal anti‐vascular endothelial growth factor (VEGF) is becoming well known to control the proliferation of new blood vessels in the treatment of various proliferative retinopathies, trials involving comparisons between laser and intravitreal injection of anti‐VEGF are needed to inform clinical decisions. There have been case reports of effectiveness of anti‐VEGF in treatment of PSR in recent decades (Shaikh 2008; Siqueira 2006). However, uncertainties in the progression and variation in PSR lesions in these individuals and problems with identifying stage III PSR may pose logistic problems in the implementation and interpretation of such trials.
What's new
| Date | Event | Description |
|---|---|---|
| 12 December 2022 | New search has been performed | Searches of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register identified three potentially eligible references. One was an additional reference to an already included trial, but there was no additional information to be included in the review (Farber 1991). We discarded the remaining two references as one was not an RCT (Goldbaum 1979) and the second assessed the treatment for proliferative vitro retinopathy (Berman 1989). One trial (two references), previously listed in 'Studies awaiting classification', has now been included in the review (Sayag 2008). |
| 12 December 2022 | New citation required but conclusions have not changed | Two references representing one trial previously under awaiting classification in previous version was included in the review and the amendments were made accordingly (Sayag 2008). The conclusions of the review have not changed. |
History
Protocol first published: Issue 10, 2013 Review first published: Issue 10, 2015
Acknowledgements
We would like to acknowledge Mrs. Nikki Jahnke, and Miss Tracey Remmington, Managing Editors for Cochrane Cystic Fibrosis and Genetic Disorder Group (CFGD) for their advice and support throughout the development and update of this review. We thank Mrs. Natalie Hall, Information Specialist for CFGD for running electronic searches and Heather Maxwell, Copy Editor for copy‐editing the review. We are grateful to the Editors of the CFGD group and peer reviewers for improving the quality of this review. We also like to express our gratitude to authorities of respective institutions and of the Julius Centre, University of Malaya, for their support and encouragement. Last but not the least, our thanks go to Professor Prathap Tharyan, Director of South Asian Cochrane Center and Network for his invaluable guidance in this review.
Appendices
Appendix 1. Glossary
| Term | Explanation |
| Anti‐ Vascular endothelial growth factor (Anti‐VEGF) | The drug that suppress or inhibit the effect of VEGF. |
| Cryotherapy | Cryotherapy, also called cryoablation is a minimally invasive treatment that uses extreme cold to freeze and destroy diseased tissue. During cryotherapy, liquid nitrogen or argon gas flows into a needle‐like applicator (a cryoprobe) creating intense cold that is placed in contact to diseased tissue. |
| Diathermy | Using high‐frequency electrical current to produce deep heating of tissue. |
| Intravitreal anti‐VEGF | Injection of anti‐VEGF into vitreous cavity. |
| Laser photocoagulation | Using laser light to treat certain disorders at the back of the eye. |
| New vessels | This term is used to signify the abnormal growth of vessels in the eye in response to a need for more oxygen. On the optic disc ‐ new vessels disc 'NVD', on the retina ‐ new vessels elsewhere 'NVE'. Generally, these new blood vessels do not have the normal integrity of blood vessel and tends to bleed. |
| Regression | New vessels stop growing or obliterated. |
| Retinal detachment | The retina has fallen away from its correct position at the back of the eye, which leads to a defect in the field of vision and ultimately loss of vision. |
| Retinopathy | Disease of the retina, for example, diabetic retinopathy is disease of the retina secondary to diabetes, sickle retinopathy is disease of retina secondary to sickle cell disease. |
| Vitrectomy | Surgical removal of the vitreous. |
| Vitreous | Soft gelatinous material that fills the back of the eye and sits behind the lens. |
| Vitreous haemorrhage | Bleeding into the vitreous cavity. |
| Vascular endothelial growth factor (VEGF) | A substance produced from retina particularly when the oxygen supply is insufficient. It causes growth of new blood vessels in the retina. |
Appendix 2. Additional electronic search strategies
| Database | Search strategy | Date last searched |
| LILACS (lilacs.bvsalud.org/en/) |
(sickle cell) AND (haemoglobinopathies) AND (retinopathy) AND (laser or photocoagulation) | 26 June 2022 |
| WHO International Clinical Trials Registry Platform (ICTRP) |
sickle cell OR haemoglobinopathies AND retinopathy AND laser or photocoagulation | 26 June 2022 |
| US National Institute of Health Ongoing Trials Register (ClinicalTrials.gov) | sickle cell OR haemoglobinopathies AND retinopathy | 26 June 2022 |
[Enter text here]
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Farber 1991.
| Study characteristics | ||
| Methods | RCT, single‐centre, parallel, open‐label design. | |
| Participants | 174 eyes of 116 participants. Males: 61 (52.6%); females: 55 (47.4%). Hb SC = 93 (80.2%), Hb SS = 21 (18.1%), Sβ thalassaemia = 2 (1.7%). Age: 16 to 60 years. |
|
| Interventions | Unit of allocation was the eye. Sectoral scatter laser photocoagulation (n = 99) using Argon blue‐green laser (Britt model 3250) through 3 mirror lenses. Spot size 500 µm, 0.1 second duration. Burns were placed approximately one burn diameter apart, placed from 1 disc diameter anterior and posterior to sea fan, 1 clock hour to each sides without treating sea fans or feeder vessels directly. Additional laser administered one week later if necessary. Control group: (n = 75) no laser photocoagulation. |
|
| Outcomes | 1. Regression of neovascularisation. 2. Development of new sea fan. 3. Visual acuity decreased by 3 lines of the Snellen chart 4. Vitreous haemorrhage 5. Retinal detachment Trial period: February 1982 to January 1989, follow‐up; mean 47.4 months (5 ‐ 99 months), for treatment group, 42.4 months (5 ‐ 75 months) for control group. |
|
| Notes | This trial was funded by Comprehensive Sickle Cell Center grant and by training grant and core grant from National Eye Institute, National Institute of Health, Bethesda and by unrestricted grants from Research to Prevent Blindness Inc, New York, NY. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " patients with bilateral disease had their right eye randomized to either scatter photocoagulation or no treatment, with other eye receiving opposite modality". " ..we randomized three of every four eyes in unilateral cases to treatment group". "computer‐ generated randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "..174 eyes were included in the study (99 for treatment and 75 for control) and 11 patients moved after average of 24 months (range 11‐60 months) and one patient died after 15 months". Trial analysed all eyes as randomised although 11 participants moved out and 1 died. |
| Selective reporting (reporting bias) | Low risk | Trial did not clearly state the pre‐specified outcomes under the methods section, but all the expected outcomes were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned in the methods section, it is an open‐label trial but performance bias is unlikely with this type of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the outcomes reported were objective. |
| Other bias | Unclear risk | The trial was possibly under powered to be able demonstrate non‐inferiority of laser photocoagulation for the primary outcomes. |
Jampol 1983.
| Study characteristics | ||
| Methods | Randomised, multicentre, parallel, open‐labelled design. | |
| Participants | 167 eyes of 122 participants with Hb SC, Hb SS and Sβ thalassaemia. Chicago: 64 eyes. Kingston: 103 eyes. Males = 60, females = 62. Age 13 to 67 years. |
|
| Interventions | Unit of allocation was the eye. Chicago Feeder vessel photocoagulation (n = 34) using Coherent Radiation Model 800 Argon laser through 3 mirror lens. 500 µm spot size, 0.2 second duration and 300‐800 mW power. Control group (n = 30): no photocoagulation. Kingston Feeder vessel photocoagulation (n = 53) using O'Malley Log 2 xenon arc photocoagulator with a direct ophthalmoscope delivery system without contact lens. intensity 5 ‐ 10, size 3 ‐ 6, duration 0.5 ‐ 2 seconds. Control group (n = 50): no photocoagulation. Between 1 to 5 laser sessions given in both centres. |
|
| Outcomes | 1. Complete closure of neovascularisation 2. Visual acuity decreased by 3 lines of the Snellen chart 3. Vitreous haemorrhage 4. Retinal detachment 5. Choroidal neovascularisation Chicago: trial period ‐ October 1977 to January 1982. Follow up ‐ mean 21.3 months (range 0 ‐ 52 months) Kingston: trial period ‐ April 1978 to September 1980. Follow up ‐ mean 32.0 months (range 0 ‐ 46 months) |
|
| Notes | Supported partly by Comprehensive Sickle Cell Center grant from National Heart, Lung and Blood institute and National Institute of Health, Bethesda, Maryland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " patient with unilateral neovascularization ... were randomly assigned (by computer randomisation) to either photocoagulation or the control group." "patient with bilateral neovascularization, right eye was randomized and left eye was given alternate modality". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Investigators reported "Chicago: Two patients lost to follow‐up after 8 and 44 months and one refuse to cooperate. Kingston: eight patients lost to follow‐up due to emigration after average 12 months of follow‐up and two refuse to cooperate" |
| Selective reporting (reporting bias) | High risk | The trial did not clearly state the pre‐specified outcomes in methods section, and the outcome 'regression of new blood vessel' was reported only for the treatment group, not for the control group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned in the methods section, it is an open‐labelled trial but performance bias is unlikely with an intervention of this nature. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the outcomes reported were objective. |
| Other bias | Unclear risk | The trial was possibly under‐powered to demonstrate non‐inferiority of laser photocoagulation for primary outcomes. |
Sayag 2008.
| Study characteristics | ||
| Methods | RCT, single centre, parallel, open‐label design. | |
| Participants | 202 eyes of 101 participants were analysed and 73 eyes with PSR Stage III randomised. Male: 39 (38.6%); Female: 62 (61.4%). Hb SC = 33 (32.7 %), Hb SS = 60 (59.4%), Sβ thalassaemia = 5 (4.9%). Age: 18 to 63 years. |
|
| Interventions | Unit of allocation was the eye. Focal scatter photocoagulation (n = 38) using Argon green laser through a 3‐mirror lens. Burns were placed around sea fans without treating the sea fans or feeder vessels directly. Burns were spaced approximately one burn diameter apart and usually placed from 2 disc diameters anterior to the sea fans and 2 clock hours to each of its sides. Spot size 300 to 500 m, 0.1 second duration with a mild whitening of the retina. A first follow‐up visit was scheduled 6 weeks later and additional laser was administered if necessary. An average of 2 photocoagulation procedures (range 1‐3) per eye were performed. Control group: (n = 35) no laser photocoagulation. |
|
| Outcomes | 1. Disease regression ‐ identified by reduction of sea fan size, no perfusion or no leakage or auto infarction 2. Disease progression ‐ defined by increased size of existing lesions associated to leakage 3. Visual acuity 4. Vitreous haemorrhage 5. Retinal detachment Trial period ‐ January 1991 to January 2004. Follow‐up ‐ mean 4 years (range 1 ‐13 years). |
|
| Notes | Did not mention any support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " Each patient was randomized to treatment or no treatment (treated or untreated group)" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Low risk | Trial did not clearly state the pre‐specified outcomes under the methods section but all the expected outcomes were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the outcomes reported were objective. |
| Other bias | Unclear risk | The trial was possibly underpowered to be able demonstrate non‐inferiority of laser photocoagulation for the primary outcomes. |
Hb SC: sickle cell haemoglobin C; Hb SS: homozygous sickle cell disease; RCT: randomised controlled trial; Sβ thalassaemia: sickle cell‐β thalassaemia; µm: micrometre.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acheson 1991 | Not an RCT or CCT . |
| Berman 1989 | Assessed the treatment for proliferative vitreoretinopathy. |
| Condon 1974 | Not an RCT or CCT. |
| Lemaire 2013 | Not an RCT or CCT. |
| Osuji 2003 | Not an RCTor CCT. |
CCT: controlled clinical trial; RCT: randomised controlled trial.
Differences between protocol and review
Differences between protocol and review
We changed the definition of visual loss. In the protocol version of this review, we defined visual loss as the deterioration of visual acuity two lines or more from the Snellen chart. However, trials report this outcome using a definition of visual loss as three lines or more (Farber 1991; Jampol 1983).
We removed the outcome 'Change in leakage in FFA (fundus fluorescein angiogram) after three months of treatment', and we added 'Occurrence of vitreous haemorrhage' as a secondary outcome. The primary outcome of regression of proliferative sickle retinopathy (PSR) is basically assessed by reviewing FFA, hence we did not report this as a separate outcome. All three trials eligible for our review reported the outcome 'vitreous haemorrhage'. We considered this as an important outcome since it is a complication of PSR itself and is associated with visual loss in the affected eye.
Differences between original review and update
The review’s original second primary outcome was defined as development of new PSR. Sayag trial did not report the development of new PSR but it reported the progression of existing PSR associated with leakage which we added as another criterion for this primary outcome (Sayag 2008).
Contributions of authors
| Roles and responsibilities | Authors undertook the task |
| Protocol stage: draft the protocol | KTM, HN |
| Review stage: select which trials to include (2 + 1 arbiter) | KTM, SS |
| Review stage: extract data from trials (2 people) | KTM, HN |
| Review stage: enter data into RevMan | KTM, AWT |
| Review stage: carry out the analysis | AWT, SM |
| Review stage: interpret the analysis | KTM, AWT |
| Review stage: draft the final review | KTM, SM |
| Update stage: update the review | KTM, HN |
Sources of support
Internal sources
-
Melaka Manipal Medical College, Malaysia
Salary and logistic support for SS, AWT and SM
-
SEGi University, Malaysia
Salary and logistic support for KTM
-
Newcastle University Medicine , Malaysia
Salary and logistic support for HN
External sources
-
Julius Center, University of Malaya, Malaysia
Cochrane workshop for review completion
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
All authors: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Farber 1991 {published data only}
- Farber MD, Jampol LM, Fox P, Moriarty BJ, Acheson RW, Rabb MF, et al. A randomized clinical trial of scatter photocoagulation of proliferative sickle cell retinopathy. Archives of Ophthalmology 1991;109(3):363-7. 5500100000000467 [DOI] [PubMed] [Google Scholar]
- Fox PD, Minninger K, Forshaw ML, Vessey SJ, Morris JS, Serjeant GR. Laser photocoagulation for proliferative retinopathy in sickle haemoglobin C disease. Eye (London, England) 1993;7(Pt 5):703-6. 5500100000000604 [DOI] [PubMed] [Google Scholar]
- Jampol LM, Farber M, Fox P, Moriarty B, Acheson R, Rabb M, et al. Photocoagulation of proliferative sickle cell retinopathy. The Macula Society 1991:26. [DOI] [PubMed]
- Jampol LM, Farber M, Rabb MF, Serjeant G. An update on techniques of photocoagulation treatment of proliferative sickle cell retinopathy. Eye (London, England) 1991;5:260-3. 5500100000000478 [DOI] [PubMed] [Google Scholar]
Jampol 1983 {published data only}
- Condon P, Jampol LM, Farber MD, Rabb M, Serjeant G. A randomized clinical trial of feeder vessel photocoagulation of proliferative sickle cell retinopathy. II. Update and analysis of risk factors. Ophthalmology 1984;91(12):1496-8. 5500100000000214 [DOI] [PubMed] [Google Scholar]
- Jacobson MS, Gagliano DA, Cohen SB, Rabb MF, Jampol LM, Farber MD, et al. A randomized clinical trial of feeder vessel photocoagulation of sickle cell retinopathy. A long-term follow-up. Ophthalmology 1991;98(5):581-5. 5500100000000475 [DOI] [PubMed] [Google Scholar]
- Jampol LM, Condon P, Farber M, Rabb M, Ford S, Serjeant G. A randomized clinical trial of feeder vessel photocoagulation of proliferative sickle cell retinopathy. I. Preliminary results. Ophthalmology 1983;90(5):540-5. 5500100000000178 [DOI] [PubMed] [Google Scholar]
- Jampol LM, Farber M, Rabb MF, Serjeant G. An update on techniques of photocoagulation treatment of proliferative sickle cell retinopathy. Eye (London, England) 1991;5:260-3. 5500100000000478 [DOI] [PubMed] [Google Scholar]
Sayag 2008 {published data only}
- Sayag D, Binaghi M, Souied E, Pawlak D, Galacteros F, Coscas G, et al. Peripheral retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy: a prospective clinical trial with new sea fan classification. European Journal of Ophthalmology 2008;18(2):248-54. [CENTRAL: 638176;CRS:5500050000000040;CRSREF:2762960] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sayag D, Binaghi M, Souied E, Pawlak D, Galacteros F, Coscas G, et al. Peripheral retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy: is it always indispensable? In: IOVS. Vol. AVO E-Abstract:5266. 2004. [CENTRAL: 598655:CRS:5500050000000039;CRSREF:2672961]
References to studies excluded from this review
Acheson 1991 {published data only}
- Acheson RW, Fox PD, Chuang EL, Serjeant GR. Treatment of iatrogenic choriovitreal neovascularisation in sickle cell disease. British Journal of Ophthalmology 1991;75(12):729-30. 5500100000000502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 1989 {published data only}
- Berman DH, Gombos GM. Proliferative vitreoretinopathy: does oral low-dose colchicine have an inhibitory effect? A controlled study in humans. Ophthalmic Surgery 1989;20(4):268-72. [CENTRAL: CN-00060553] [PMID: ] [PubMed] [Google Scholar]
Condon 1974 {published data only}
- Condon PI, Serjeant GR. Photocoagulation in sickle cell retinopathy [abstract]. In: Commonwealth Caribbean Medical Research Council, 19th Scientific Meeting; 1974 April 26-29; Mona, Jamaica. 1974:1-2. 5500100000001736
Lemaire 2013 {published data only}
- Lemaire C, Lamarre Y, Lemonne M, Waltz X, Chahed S, Cabot F, et al. Severe proliferative retinopathy is associated with blood hyperviscosity in sickle cellhemoglobin-C disease but not in sickle cell anemia. Clinical Hemorheology and Microcirculation 2013;55:205-11. [DOI: 10.3233/CH-2012-1622] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osuji 2003 {published data only}
- Osuji NC, Majone B, Lumley H, Day A, O'Sullivan E, De Alwis D. Screening for sickle retinopathy: a new, rapid and effective approach using the panoramic 200 panretinal camera [abstract]. British Journal of Haematology 2003;121 Suppl 1:43-4. 5500100000002620 [Google Scholar]
Additional references
Abiose 1978
- Abiose A, Lesi FE. Ocular findings in children with homozygous sickle cell anaemia in Nigeria. Journal of Paediatric Ophthalmology and Strabismus 1978;15(2):92-5. [DOI] [PubMed] [Google Scholar]
Ballas 2010
- Ballas SK, Leiff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, et al. Definitions of phenotypic manifestations of sickle cell disease. American Journal of Haematology 2010;85(1):6-13. [DOI: 10.1002/ajh.21550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballas 2012
- Ballas SK, Kesen MR, Goldberg MF, Lutty GA, Dampier K, Osunkwo I, et al. Beyond the definitions of the phenotypic complications of sickle cell disease: an update on management. Scientific World Journal http://www.hindawi.com/journals/tswj/2012/949535 (accessed 15 April 2013). [DOI: 10.1100/2012/949535] [DOI] [PMC free article] [PubMed]
Castro 1999
- Castro O. Management of sickle cell disease: recent advances and controversies. British Journal of Haematology 1999;107(1):2-11. [DOI] [PubMed] [Google Scholar]
Cohen 1986
- Cohen SB, Fletcher ME, Goldberg MF, Jednock NJ. Diagnosis and management of ocular complications of sickle haemoglobinopathies: part IV. Ophthalmic Surgery 1986;17(5):312-5. [PubMed] [Google Scholar]
Condon 1972
- Condon PI, Serjeant GR. Ocular findings in homozygous sickle cell anaemia in Jamaica. American Journal of Ophthalmology 1972;73(4):533-4. [DOI] [PubMed] [Google Scholar]
Condon 1974a
- Condon PI, Gray R, Serjeant GR. Ocular findings in children with sickle cell haemoglobin C disease in Jamaica. British Journal of Ophthalmology 1974;58(7):644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Condon 1974b
- Condon PI, Serjeant GR. Photocoagulation and diathermy in the treatment of proliferative sickle retinopathy. British Journal of Ophthalmology 1974;58(7):650-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruess 1983
- Cruess AF, Stephens RF, Magargal LE, Brown GC. Peripheral circumferential retinal scatter photocoagulation for treatment of proliferative sickle retinopathy. Ophthalmology 1983;90(3):272-8. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks J, Higgins JP, Altman D (editors). Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 [updated February 2021]. Cochrane, 2021.. Available fromtraining.cochrane.org/handbook/archive/v6.2..
Downes 2005
- Downes SM, Hambleton IR, Chuang EL, Lois N, Serjeant GR, Bird AC. Incidence and natural history of proliferative sickle cell retinopathy: observation from a cohort study. Ophthalmology 2005;112(11):1869-75. [DOI] [PubMed] [Google Scholar]
Elagouz 2010
- Elagouz M, Jyothi S, Gupta B, Sivaprasad S. Sickle cell disease and the eye: old and new concepts. Survey of Ophthalmology 2010;55(4):359-77. [DOI] [PubMed] [Google Scholar]
Emerson 2005
- Emerson GG, Lutty GA. Effects of sickle cell disease on eye: clinical features and treatment. Hematology/Oncology Clinics of North America 2005;19(5):957-73. [DOI] [PubMed] [Google Scholar]
Emerson 2006
- Emerson GE, Harlan JB, Fekrat S, Lutty GA, Goldberg MF. Hemoglobinopathies. In: Ryan SJ, editors(s). Retina. 4th edition. Vol. 2. Edinburgh: Elsevier, 2006:1429-45. [Google Scholar]
Erachulu 2006
- Erachulu UV, Pam VA, Akuse RM. Ocular findings in children with severe clinical symptoms of homozygous sickle cell anaemia in Kaduna, Nigeria. West African Journal of Medicine 2006;25(2):88-91. [DOI] [PubMed] [Google Scholar]
Evans 2014
- Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD011234. [DOI: 10.1002/14651858.CD011234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 1991
- Fox PD, Vessey SJ, Forshaw ML, Serjearnt GR. Influence of genotype on the natural history of untreated proliferative sickle retinopathy. British Journal of Ophthalmology 1991;75(4):229-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 1993
- Fox PD, Minninger K, Forshaw ML, Vessey SJ, Morris JS, Serjeant GR. Laser photocoagulation for proliferative retinopathy in sickle haemoglobin C disease. Eye (London, England) 1993;7(Pt 5):703-6. 5500100000000604 [DOI] [PubMed] [Google Scholar]
Goldbaum 1979
- Goldbaum MH, Fletcher RC, Jampol LM, Goldberg MF. Cryotherapy for proliferative sickle retinopathy,II: a triple freeze-thaw cycle. British Journal of Ophthalmology 1979;63(2):97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 1971
- Goldberg MF. Natural history of untreated proliferative sickle retinopathy. Archives of Ophthalmology 1971;85(4):428-37. [DOI] [PubMed] [Google Scholar]
Goldberg 1983
- Goldberg MF, Jampol LM. Treatment of neovascularization, vitreous haemorrhage and retinal detachment in sickle cell retinopathy. Transactions of the New Orleans Academy of Ophthalmology 1983;31:58-81. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org.
Hirst 2012
- Hirst C, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD003427. [DOI: 10.1002/14651858.CD003427.pub2] [DOI] [PubMed] [Google Scholar]
Kimmel 1986
- Kimmel AS, Magagal LE, Stephens RS, Cruess AF. Peripheral circumferential retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy. An update. Ophthalmology 1986;93(11):1429-34. [DOI] [PubMed] [Google Scholar]
Lutty 1994
- Lutty GA, Goldberg MF. Ophthalmologic complications. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors(s). Sickle Cell Disease: Basic Principle and Clinical Practice. New York: Raven Press, 1994:703-24. [Google Scholar]
Marti‐Carvajal 2012
- Marti-Carvajal AJ, Knight-Madden JM, Martinez-Zapata MJ. Interventions for treating leg ulcers in people with sickle cell disease. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD008394. [DOI: 10.1002/14651858.CD008394.pub2] [DOI] [PubMed] [Google Scholar]
Moriaty 1988
- Moriaty BJ, Acheson RW, Condon PI, Serjeant GR. Patterns of visual loss in untreated sickle cell retinopathy. Eye 1988;2(Pt 3):330-5. [DOI] [PubMed] [Google Scholar]
Myint 2013
- Myint KT, Sahoo S, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD010790. [DOI: 10.1002/14651858.CD010790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penman 1994
- Penman AD, Talbot AF, Chuang EL, Thomas P, Serjeant GR, Bird AC. New classification of peripheral retinal vascular changes in sickle cell disease. Bnrtish Journal ofOphthalmology 1994;78:681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rednam 1982
- Rednam KR, Jampol IM, Goldberg MF. Scatter retinal photocoagulation for proliferative sickle cell retinopathy. American Journal of Ophthalmology 1982;93(5):594-9. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.14.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Roberts 2007
- Roberts I, Montalembert M. Sickle cell disease as a paradigm of immigration hematology: new challenges for haematologists in Europe. Haematologica 2007;92(07):865-92. [DOI] [PubMed] [Google Scholar]
Seiberth 2001
- Seiberth V. Transscleral and transpupillary laser coagulation in proliferative sickle-cell retinopathy. Ophthalmologe 2001;98(2):199-202. [DOI] [PubMed] [Google Scholar]
Serjeant 2001
- Serjeant GR. The emerging understanding of sickle cell disease. British Journal of Haematology 2001;112(1):3-18. [DOI] [PubMed] [Google Scholar]
Shaikh 2008
- Shaikh S. Intravitreal bevacizumab (Avastin) for the treatment of proliferative sickle retinopathy. Indian Journal of Ophthalmology 2008;56(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siqueira 2006
- Siqueira RC, Costa RA, Scott IU, Cintra LP, Jorge R. Intravitreal bevacizumab (Avastin) injection associated with regression of retinal neovascularization caused by sickle cell retinopathy. Acta Ophthalmologica Scandinavica 2006;84:834-5. [DOI: 10.1111/j.1600-0420.2006.00779.x] [DOI] [PubMed] [Google Scholar]
Virgili 2009
- Virgili G, Bini A. Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD004763. [DOI: 10.1002/14651858.CD004763.pub2] [DOI] [PubMed] [Google Scholar]
Weatherall 2001
- Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problems. Bulletin of the World Health Organization 2001;79(8):1-15. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
MYint 2013
- Myint KT, Sahoo S, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD010790. [DOI: 10.1002/14651858.CD010790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myint 2015
- Myint KT, Sahoo S, Thein AW, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD010790. [DOI: 10.1002/14651858.CD010790.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


