Abstract
The development and function of sensory systems require intact glutamatergic neurotransmission. Changes in touch sensation and vision are common symptoms in autism spectrum disorders, where altered glutamatergic neurotransmission is strongly implicated. Further, cortical visual impairment is a frequent symptom of GRIN disorder, a rare genetic neurodevelopmental disorder caused by pathogenic variants of GRIN genes that encode NMDA receptors. We asked if Grin1 knockdown mice (Grin1KD), as a model of GRIN disorder, had visual impairments resulting from NMDA receptor deficiency. We discovered that Grin1KD mice had deficient visual depth perception in the visual cliff test. Since Grin1KD mice are known to display robust changes in measures of learning, memory, and emotionality, we asked whether deficits in these higher‐level processes could be partly explained by their visual impairment. By changing the experimental conditions to improve visual signals, we observed significant improvements in the performance of Grin1KD mice in tests that measure spatial memory, executive function, and anxiety. We went further and found destabilization of the outer segment of retina together with the deficient number and size of Meissner corpuscles (mechanical sensor) in the hind paw of Grin1KD mice. Overall, our findings suggest that abnormal sensory perception can mask the expression of emotional, motivational and cognitive behavior of Grin1KD mice. This study demonstrates new methods to adapt routine behavioral paradigms to reveal the contribution of vision and other sensory modalities in cognitive performance.
Keywords: anxiety, autism, elevated plus maze, GRIN1, learning & memory, mice, Morris water maze, puzzle box, schizophrenia, tactile function, vision
Grin1 deficiency impairs visual and tactile perception in a mouse. Adapted behavioral procedures can reveal true cognitive ability.

1. INTRODUCTION
Behavior reflects a myriad of brain processes, including the integration of multiple sensory systems that map precise representations of the world. For instance, the combined senses of touch and vision generate a topographic reflection of the spatial coordinates of the body and the field of view. Visual‐tactile integration mediates the orientation of spatial attention and generates flexible and efficient navigation through the environment. The abnormal function of sensory systems significantly changes behavioral repertoire and, hence could significantly contribute to mechanisms underlying psychopathologies. 1
Despite autism spectrum disorder (ASD) and schizophrenia being brain disorders with different clinical presentations, there are some overlapping symptoms between them. 2 , 3 For example, sensory processing abnormalities are core phenotypes of both disorders. 4 Atypical sensory experience is observed in ~90% of ASD individuals, 5 affecting taste, 6 touch, 7 , 8 audition, 9 smell, 10 and vision. 11 Sensory disturbances in schizophrenia are often described as an inability to filter innocuous sensory stimuli 12 that manifests as hypervigilance and impaired attention. Clinical studies demonstrate that patients with schizophrenia have enhanced tactile sensation, 13 impaired contrast sensitivity, 14 olfactory sensory disturbance, 15 or auditory dysfunctions. 16 Notably, robust impairment in audiovisual temporal integration is detected in patients with ASD and schizophrenia, represented by an enlarged temporal binding window that is associated with positive symptoms of schizophrenia (hallucinations, delusions) 17 and severity of social deficits in ASD. 18 , 19
Hypofunction of glutamatergic signaling mediated by the N‐methyl‐D‐aspartate receptor (NMDAR) is strongly linked to the pathophysiology of schizophrenia 20 and idiopathic ASD. 21 , 22 , 23 NMDAR are heterotetramers, composed of two glycine‐binding GluN1 subunits encoded by the GRIN1 gene and two glutamate‐binding subunits GluN2A/2B encoded by GRIN2A and GRIN2B genes, respectively. Additionally, GluN2C, GluN2D, GluN3A/3B subunits can be present at certain neurodevelopment stages and cellular subpopulations.
Genetic studies have found the association between de novo mutations of GRIN genes and neurological disorders. 23 , 24 Accumulated genetic deep sequencing has identified 4413 GRIN variants in patients with various mental disorders including ASD and schizophrenia. 25 These findings led to the delineation of a clinical spectrum genetically defined as GRIN disorder. 26 , 27 , 28 , 29 GRIN disorder is a group of rare neurodevelopmental disorders 30 , 31 , 32 , 33 caused by a de novo or transmitted pathogenic variant in a GRIN gene. Pathogenic GRIN1 variants cause intellectual disability (100% of patients), muscular hypotonia (66% of patients), epilepsy (65% of patients), motor dysfunction (48% of patients), cortical visual impairment (CVI; 34% of patients), autism spectrum disorder (ASD; 22% of patients), and sleep problems (15% of patients). 33 Given that CVI, a clinical sensory phenotype of GRIN disorders, was identified in ~1/3 of Grin1 carriers, 32 ophthalmologic assessment is recommended for GRIN1 patients. Although CVI is caused by damage to the parts of the brain that process vision, the pathological role of GRIN1 cannot be excluded from the modulation of the retinal function. Indeed, the retinal Mϋller glial cells contain a GluN1 subunit and may promote the proliferation of retinal progenitor, the expression of the glutamate transporter, and the survival of ganglia cells, 34 , 35 , 36 directly affecting retinal morphology and its functionality.
Several animal models have been generated to study interconnections between NMDAR, schizophrenia and ASD including pharmacological inhibition of NMDAR by its antagonists, 37 or generation of Grin1 knockdown with ~85% reduced expression of NR1 subunit of the NMDAR complex—Grin1KD mouse line, 38 but the impact of sensory systems in modulating their behavioral impairment has not been fully recognized. Grin1KD mice showed a mixture of phenotypes related to schizophrenia: lack of habituation to a novelty, hyperactivity with stereotypic behavior, deficient sensorimotor gating, working memory, executive function, cognitive flexibility 39 and ASD: deficient sociability, communication, and repeated behavior. 40 , 41 , 42
The visual sensory system plays a role to define the mouse's behavior and therefore, its abnormal functioning affects multiple behavioral domains assessed in well‐accepted standardized behavioral tests designed for phenotyping of mice. 43 Thus, we hypothesized that re‐adjusting experimental conditions by facilitating the processing of visual information may unmask the true expression of emotional or cognitive behavioral performance in Grin1KD mice.
Therefore, we compared the behavior of Grin1KD mice assessed under standard and modified experimental conditions, designed to facilitate perception of visual signals. Firstly, we detected a deficient visual depth perception in Grin1KD mice, assessed in the “Cliff” test. The placement of proximate visual cues (as opposed to far cues in the “standard” design) or daily training mice for a visual platform in Morris's water‐maze task remarkably improved behavioral performance in Grin1KD mice. Further, the exposure of Grin1KD mice to a bigger size of the elevated plus‐maze (EPM) or a wider entrance into the goal box of the Puzzle box also ameliorated their emotional and cognitive behavior.
Altogether, our experiments demonstrated that re‐designing of experimental conditions unmasked expression of emotional and cognitive behavior in Grin1KD mice. Animal modeling of psychiatric disorders, including ASD and schizophrenia, is based on behavioral phenotyping as the main read‐out of genetic, pharmacological, or neuronal manipulations. Thus, re‐adjusting the standard experimental conditions is highly desirable to address the affected sensory perceptions in studied animals.
2. MATERIALS AND METHODS
2.1. Animals
Knockdown mice for the NR1 subunit of the NMDA receptor (GluN1 knockdown; Grin1KD) were generated in the animal facility of the University of Toronto as previously described. 44 The GRIN1 gene was modified via homologous recombination with an intervening sequence (neomycin cassette), and targeted the intron 10, flanked by loxP sites. Grin1+/flneo C57Bl/6J congenics and Grin1+/flneo 129 × 1/SvlmJ congenic mice were intercrossed to produce experimental mice (Grin1+/+ [wild‐type; WT]; and Grin1KD) mice as recommended and based on other studies 45 to minimize the confound of homozygous mutations on each parental strain. Grin1KD mice express only 5%–10% of normal levels of NR1 subunit of the NMDAR complex. Two to four mice of the same genotype were housed per cage with a 12 h light–dark cycle (7:00 a.m.–7:00 p.m.) with ad libitum access to food and water.
All experimental procedures were performed on naïve (i.e. without previous exposure to any behavioral test) male and female WT and Grin1KD mice between 12–14 weeks of age during the light cycle (9:00 a.m.–5:00 p.m.). Mice were transported from the mouse colony room to the experimental room for 30‐min of habituation before any behavioral procedure. All behavior was assessed by a skilled experimenter blind to genotype. All procedures were conducted following the University of Toronto Faculty of Medicine and Pharmacy Animal Care Committee in compliance with the Animals Research Act of Ontario and the Guidelines of the Canadian Council on Animal Care.
2.2. Behavioral tests
Behavioral results were collected using video‐tracking software (Biobserve Viewer2 tracking software (St. Augustin, Germany) to automatically generate data, which were validated by comparing manual and automated scoring of behavior (Data S1, Table S2).
2.2.1. Visual cliff
This test was designed to measure the visual perception of depth in mice and was performed based on study, 46 where half of a transparent arena is suspended over the edge of the table (visual cliff) (Figure 1B) and the time spent in the safe or cliff area of the arena was measured. A transparent plastic box (60 × 60 × 45 cm3) was positioned on the edge of a laboratory bench so that half of the base covered the bench (“safe side”) while the other half was suspended over the edge of the bench 90 cm above the floor (“cliff side”), creating the appearance of a cliff. A checkerboard pattern (5 cm × 5 cm) was placed under the “safe side” of the box as well as on the floor underneath the box to emphasize the cliff drop‐off. Mice were individually placed in the middle of the base at the edge of the cliff (on the “safe” side), allowing the mice to survey their surroundings. Their activity was recorded by video‐tracking software for 2 min. The time spent in each zone and the number of crossings between zones were recorded. All measurements were taken in the experimental room with regular light.
FIGURE 1.

(A–D) The behavior of Grin1KD and WT mice in the “Visual Cliff” test to assess a visual depth perception. (A–B) The experimental installation, side (A) and top (B) views. (C) The percentage of time spent in the “safe” and “cliff” areas; (D) The number of entries into the “cliff” area. ***p < 0.001—in comparison with WT mice; ### p < 0.001—in comparison with the “safe” area within genotype. N = 8–9 per genotype
2.2.2. The Morris' water‐maze (MWM)
Given our finding that Grin1KD mice show impaired visual perception, we asked whether this visual abnormality affected their performance in cognitive tasks. We compared performance of Grin1KD mice in a standard Morris's water maze (MWM) installation (“Protocol 1”) to the modified MWM configurations where additional visual cues were provided (“Protocol 2”) or visual cues were moved to a more proximate location on the walls of the pool (“Protocol 3”) (Figure 2C). Morris's water maze (MWM) was performed based on study 47 with modifications. The procedure included: 1) acquisition and 2) probe sessions. The opaque‐white Plexiglas, cylindrical pool (80 cm: inner diameter; 1 m: outer diameter) was filled with opaque water (40 cm height; 24°C ± 1°C). The pool was divided into four equal quadrants: northeast (NE), northwest (NW), southeast (SE), and southwest (SW). The circular escape platform (15‐cm diameter) was made of clear Plexiglas. Four distal visual cues (posters' size: 35 × 45 cm with black‐white stripes [5 cm width] oriented diagonally left; right; horizontally and vertically) were fixed on each wall ∼1 m from the pool edge and kept for all experiments, except the 3d experiment, marking four main sides of the world.
FIGURE 2.

(A–I) Spatial learning and memory was measured in the standard and modified Morris's water‐maze (MWM). (A–C). Schematic illustrations (images were generated via BioRender.com) of three protocols of MWM: (A) Standard protocol #1 (WT: N = 6; Grin1KD = 8), where the visual cues were distantly located on walls of the experimental room to mark south, north, east and west sides; the visual escape platform (V) was used on the 1st day of training and the hidden escape platform was used for the rest of the acquisition; (B). Modified protocol #2, where a visual platform was used during the entire acquisition session (WT; N = 9; Grin1KD = 11); (C) Modified protocol #3 was similar to the Standard protocol #1, but proximate visual cues were attached to walls of the pool (WT: N = 9; Grin1KD: N = 11). (D–F) Latency (seconds) to reach the escape platform during the acquisition session (D—standard protocol #1; E—modified protocol #2, F—modified protocol #3), where visual (V) platform was used on Day 0; ***p < 0.001—in comparison with WT mice; # p < 0.05; ## p < 0.01—in comparison with protocol #1. (G–I) The percentage of time spent in the targeted quadrant of the pool or non‐targeted area (the averaged percentage of time spent in other three quadrants) during the probe day ((G)—standard protocol #1; (H)—modified protocol #2, (I)—modified protocol #3). ***p < 0.001—in comparison with WT mice; # p < 0.05; ### p < 0.001—in comparison with non‐target area within each genotype
Activity in the water maze was recorded using a video camera on the ceiling above the center of the pool attached to an automated video‐tracking system to establish experimental parameters and analyze the following behavioral parameters: 1) latency to reach the escape platform; 2) traveled distance (cm); 3) velocity (cm/s) (for the acquisition session) and 4) duration of time spent in each quadrant of the pool (s) (for the probe session). Behavioral parameters recorded during the acquisition were averaged across 4 daily trials for the statistical analyses. The duration of time spent in three non‐targeted quadrants was averaged and the percentage of time spent in targeted and non‐targeted area during the probe session was analyzed.
The acquisition session began from the training of mice to escape water into the visible platform which was raised 0.5 cm above the water surface and demarcated with a vertical “tower” (10 cm × 1.5 cm). Mice were given 4 trials with a ∼40 min inter‐trial interval (ITI). In each trial, mice were released facing the pool wall from one of four pseudorandomized locations (N, S, W, E) at the pool periphery. The platform was at the center of the target quadrant (SE; T) and 20 cm from the pool wall. The maximum duration for a platform' search was 60 s. Animals that found the platform remained on it for an additional 15 s, whereas unsuccessful animals were assigned a 60‐s latency and gently placed onto the platform for 15 s. The acquisition session continued on the next day for four consecutive days (4 trials per day; 1 h ITI). Each day was performed similarly to the visible platform task, except the platform was now submerged ∼1 cm below the surface of the water (hidden) in the targeted quadrant.
Retention of acquired spatial memory was assessed in a 60‐s probe trial (“probe session”) that occurred 18 h after the last acquisition trial. In the probe trial, the platform was removed, and mice were released from the point furthest (NW) from the previous location of the platform. Performance in the probe trial was quantified by examining the percentage of time spent and the number of crosses over an area that was twice the platform diameter, centered over its former location.
Three independent cohorts of mice were used in each MWM experiment. The 1st experiment was done using the standard MWM protocol as described above (“protocol 1”). The 2nd experiment was done using the pedestal‐like object placed on the escape platform during the acquisition session. Four wooden cubes (3 cm3) have been arranged together to make the inverted T‐shape object, which was fixed on the top of the escape platform and was used throughout the entire experiment (“protocol 2”).
The 3rd experiment was performed following the “protocol 1” except that visible cues (posters [A4 size] with printed black‐white stripes positioned horizontally, vertically; black‐white circles and diamonds) were fixed on the wall of the pool (80 cm—the inner diameter; 1 m—the outer diameter) labelling east, west, south and north sides of the pool (“protocol 3”).
2.2.3. Puzzle box (PB)
“Protocol 1”: The protocol was performed based on study 48 with modifications. The puzzle box consisted of a white box was divided by a removable barrier into two zones: a bright zone without a roof (58 cm × 28 cm), and a dark zone (“goal box”) with an openable roof (15 cm × 28 cm) (Lantz Enterprises Inc., Hamilton, Canada). A narrow pathway (4 cm wide) was located under the barrier. During the experiment, the goal box was covered, with some beddings and a shelter inside. The protocol consisted of 3 sessions × 3 trials per session with inter‐session interval (ISI) 24 h and with ~10–15 min as the Inter Trial Interval (ITI). Introduced into the start zone, mice were trained to move into the goal zone through a narrow underpass. In session 1 (training), the underpass was open, and the barrier had an open door (4 cm × 4 cm) over the location of the underpass during trial 1 (T1). On T2 and T3 the barrier had no doorway and mice entered via the small underpass. On session 2 (burrowing puzzle), T4 was identical to T2 and T3, but on T5, T6 and T7 the underpass was filled with sawdust and mice had to dig their way through. This sequence of trials allowed assessing problem‐solving ability (T2 and T5), and short‐term memory (T3 and T6), while the repetition after 24 h provided a measure of long‐term memory (T4 and T7). Each trial started by placing the mouse in the start zone and ended when the mouse entered the goal box with all four paws, or after a maximum of 3 min. The performance of mice in the PB was assessed by recording the latency to enter the goal box.
“Protocol 2”: This experiment aimed to assess whether a more visible doorway could impact the performance of the Grin1KD mice in the PB. The box, light condition and experimental protocol were the same as those of the standard protocol. However, during "T1, a divider with a larger doorway (12 cm × 12 cm) was used. During "T2, a divider with a doorway (12 cm × 2 cm) was used with an open underpass. "T3 and "T4 were a repetition of "T2. The door was completely closed on "T5 with a still open underpass. "T6 and "T7 were identical to "T5. Lastly, the underpass was filled by bedding on "T8 and "T9, identical to T5‐T7 of the standard PB protocol.
2.2.4. Digging test
The reduced digging capacity observed in Grin1KD mice was assessed in the digging test. 49 Mice were placed individually in a Plexiglas box (20 × 20 × 45 cm3) filled with bedding (thickness is 5 cm) and observed for 3 min. The total duration of the digging behavior was manually scored by skilled observers.
2.2.5. Elevated plus‐maze (EPM)
The elevated plus‐maze was performed based on the study. 50 The mouse' size of EPM (mEPM) consisted of two open arms (25 × 5 cm), two enclosed arms (25 × 5 × 30 cm), arranged so that the two arms of each type were opposite each other and extended from a central platform (5 × 5 cm). The rat's size of EPM (rEPM) consisted of two open arms (50 cm × 10 cm), two enclosed arms (50 × 10 × 40 cm), arranged so that the two arms of each type were opposite each other and extended from a central platform (10 × 10 cm). The mEPM and rEPM were elevated to a height of 50 cm. All measurements were taken in a dimly lit experimental room: 110 Lux in the open arms; 10 Lux in the enclosed arms. The central platform of the EPM was illuminated by a lamp heightened for 1.5 m above the central platform. Each mouse was placed into the center of the EPM facing the enclosed arms. Over a 5‐min test period, the following parameters were recorded: 1) open area time (time spent on open arms and center); 2) enclosed arms time expressed as a percentage of total testing time; 3) open arm entries, closed arm entries and central platform entries to calculate the total number of entries; 4) the number of defecations; 5) the number of passes between closed arms without stopping in the center; 6) the number of passes between open arms without stopping in the center; 7)the number of head‐dips and 8) duration of time spent at the “end” of the open arms (the last 1/3 of the open arm' length).
2.3. Histology
2.3.1. Hematoxylin and eosin staining of retina
After anesthesia (CO2 asphyxiation), the eyes was removed and fixed in 10% buffered formalin (Fisher Scientific, Cat# 245–685) for 48 hours, then processed and embedded into paraffin. Sections were cut at 5 mkm thickness and stained with hematoxylin and eosin. The retinal' layers and total thickness was imaged under light microscopy at magnification ×40, obtaining 4 images for each eye in total, on the right and left side of the optic nerve. The retinal frailty was imaged under light microscopy at magnification ×10, where the length of the non‐attached retinal pigmented epithelium (RPE) to the outer segment (OS) and the total length of RPE were assessed. In addition, we measured thickness of optic nerve (x10), axial length and lens width under magnification ×2. All images were analyzed using ZenCore v3.4 software (CarlZeissMicroscopy GmbH).
2.3.2. Hematoxylin and eosin staining of the hind paw
Intact right hind paws were removed, cleaned with chemical depilator NairTM and water and fixed in 4% paraformaldehyde for 5 days with follow up demineralization in Cal‐ExII (Thermo Fisher Scientific, Canada) for 10 days. The paraffin‐embedded hind paws (1st digit [thumb]) were sectioned 5 μm thickness with 60 μm between levels and stained with hematoxylin and eosin as described. 51 Slides were imaged under magnification ×20 using light microscopy to quantify the number of Meissner's corpuscles in 3 regions of interest (ROI): superficial to each end of the metatarsal, and superficial to the distal end of the distal phalange. The high magnification (x63 oil immersion objective) was taken with a Zeiss Axio Observer Z1 microscope equipped with the Zeiss Axio Cam IC color camera. Corpuscles were identified according to the following criteria: clear invagination in the dermis, presence of dark‐staining nuclei, and presence of light‐staining nerve fibers with a “stacked” appearance based on the study. 51 The total number of fully developed Meissner's corpuscles, and their size (width of the “head‐like” structure and width of the “leg‐like” part of the corpuscle), length (from the top of the “head‐like” of the corpuscle to the end of the straight part of the corpuscle/end of the papillae) were analyzed using Zen Blue 2.3 software.
2.4. Statistical analysis
Statistical analysis was performed using the SPSS (version 22; IBM, New York, NY) and Prism GraphPad (La Jolla, CA) software. Raw data have been checked for homoscedasticity before the statistical analysis. Behavioral data were analyzed using one‐way, two‐way ANOVA and two‐way ANOVA with repeated measures (RM ANOVA), with further post‐hoc analysis (Tukey's test) or unpaired Student t‐test. Pearson product–moment correlations were used to probe the relationships between behavioral performance in the “Cliff” test, MWM, PB and EPM, using TIBCO software (StatSoft, Dell).
3. RESULTS
ANOVA did not reveal a sex effect within each genotype on any studied behavioral parameter (all p's > 0.05) due to the low number of male and female mice within each genotype. The total number of experimental mice is presented in Table S1. Therefore, all data from males and females were combined and analyzed together.
3.1. Grin1KD mice showed deficient visual depth perception the Cliff test
Repeated measures one‐way ANOVA detected a main effect of type of area (cliff; safe) [F 1,30 = 26.9; p < 0.001] and an interaction of genotype × type of area [F 1,30 = 30.9; p < 0.001] on the percentage of time spent in each type of area. Post‐hoc analysis found that WT mice spent significantly more time on the safe area rather than on the cliff compartment (p < 0.001), whereas Grin1KD mice did not differentiate safe from cliff and spent an equal amount of time on both types of area (p > 0.05) (Figure 1C). Unpaired t‐test detected a main effect of genotype [t 1,15 = 8.3; p < 0.001] on the number of entries into the cliff area. Grin1KD mice more often visited the cliff area than their WT littermates (Figure 1D).
3.1.1. Grin1KD mice show deficient spatial learning and memory in the standard Morris's water‐maze (“Protocol 1”)
In the standard MWM set up, there were main effects of daily training [F 4,48 = 4.7; p < 0.0103] and genotype [F 1,12 = 70.9; p < 0.0001] on the latency to reach the platform of the MWM (repeated measures one‐way ANOVA). The latency to reach the escape platform was higher in Grin1KD mice than in WT littermates during all days of training (Figure 2D), whereas WT animals gradually learned the location of the escape platform. In addition, although Grin1KD mice expressed lower velocity in the water‐maze than WT mice (Figure S1B), they expressed thigmotaxis and circling behavior (Figure S4B–D), which significantly increased total traveled distance (Figure S1A).
After training, mice were probed for spatial memory by removing the platform and measuring the amount of time spent in each quadrant of the pool. Repeated measures one‐way ANOVA detected a main effect of the targeted quadrant [F 1,12 = 200.0; p < 0.001], genotype [F 1,12 = 23.3; p < 0.001] and their interactions [F 1,12 = 92.9; p < 0.001] on the percentage of time spent in target vs non‐target area of the water‐maze. WT mice remembered the location of the platform and spent more time in the targeted area rather than in the non‐targeted areas (p < 0.001), whereas Grin1KD mice did not distinguish the targeted quadrant from the non‐targeted area (p > 0.05) (Figure 2G).
3.1.2. A platform visual cue improves Grin1KD training performance but not memory retrieval (“Protocol 2”)
During training sessions with the standard MWM arena, Grin1KD mice displayed thigmotaxis, circling the perimeter of the pool instead of searching for the platform (Figure S4B,D). Moreover, trial‐by‐trial analysis of the latency to reach the escape platform during the 1st day of the acquisition with visible platform revealed that despite Grin1KD mice showing similar performance with their WT littermates during trial 1 (Data S1, Figure S2), they do not remain on the platform during the rest of trials. Hence, we hypothesized that Grin1KD mice could be less motivated to escape the water than WT mice. To test this hypothesis, we made the platform more visible by adding an object on top of the platform for all training sessions. Repeated measures ANOVA found a main effect of daily training [F 4,68 = 15.21; p < 0.001], genotype [F 1,17 = 28.8; p < 0.001] and training × genotype interaction [F 4,68 = 14.24; p < 0.001] on the latency to reach the platform. Grin1KD mice took significantly more time than WT littermates to reach the platform during the first 3 days of the training (p's < 0.001), but their latencies were comparable with WT mice during the last 2 days of spatial learning (Figure 2E). Exposure of mice to the visible platform with the object during the entire acquisition session significantly improved performance of Grin1KD mice, decreasing their latency to reach the escape platform (p < 0.05 on Day 2; p < 0.01 on Day 3; p's < 0.001 on Days 4–5 in comparison with their performance on Day1) (Figure 2E). Notably, the velocity of Grin1KD mice was comparable with WT littermates (Figure S1D). This reduced the distance traveled by Grin1KD mice during training on all days but the first day of training (Figure S1C).
On the probe trial, where the platform is removed, repeated measures one‐way ANOVA found a main effect of type of area (targeted vs non‐targeted) [F 1,17 = 123.4; p < 0.001], genotype [F 1,17 = 8.1; p < 0.01] and their interaction [F 1,17 = 32.4; p < 0.001] on the percentage of time that mice spent in each area. Both genotypes of mice spent more time in the target quadrant than in the non‐target area (p < 0.001 for WT; p < 0.05 for Grin1KD mice); however, Grin1KD mice still spent less time in the target area than WT mice (p < 0.001) (Figure 2H).
3.1.3. Proximal visual cues improve both learning and memory of Grin1KD mice (“Protocol 3”)
To determine whether visual impairments contribute to the poor cognitive performance of Grin1KD mice in the MWM, we repositioned the visual cues to be located on the walls of the pool rather than on the walls of the room. The platform was only visible on the first day and was submerged for all subsequent training days. There was a main effect of daily training [F 4,72 = 9.1; p < 0.001] and genotype [F 1,18 = 254.1; p < 0.0001] on the latency to reach the platform. Although the proximate visual cues did not normalize Grin1KD performance to WT levels during training, the change in latency between Day 1 and Day 4 indicated that Grin1KD mice were steadily learning the platform location (Figure 2F) in comparison with Grin1KD trained under the standard protocol (Figure 2D). The traveled distance and velocity of Grin1KD mice (Figure S1E–F) in Protocol #3 were comparable to those in Protocol #1 (Figure S1A–B).
In the test of spatial memory, repeated measures one‐way ANOVA found a main effect of the targeted area [F 1,18 = 64.3; p < 0.001] on the percentage of time spent in each area. Both WT and Grin1KD mice spent more time in the targeted quadrant than in the non‐targeted area (p's < 0.001) (Figure 2F).
3.1.4. Pearson correlations between “Cliff” test and MWM behavior
To assess the potential interactions between impaired vision and spatial learning & memory deficits in MWM, we correlated percentage of time spent on the cliff area with the latency to escape water maze during daily training as well as with the percentage of time spent in each quadrant during the probe session. The time spent in the cliff area significantly correlated with the latency to detect visible platform during the 1st trial of Day 0 (“Protocol #1”) in WT mice (r = 0.9; p < 0.01) (Figure S3A), whereas such relationship was lost in Grin1KD animals (r = 0.07; p > 0.05) (Figure S3B). There was no other significant correlations between time spent on the cliff area and parameters on MWM (all p's > 0.05; Table S3).
3.1.5. Performance of Grin1KD mice is influenced by visual (size of the door) and tactile (size of the door, bedding obstacle) aspects of the puzzle box test
The puzzle box test is used to measure higher level aspects of cognition that include executive function, cognitive flexibility, and memory. In this test mice are placed in an open arena and timed for their latency to overcome different barriers to reach a goal arena (Figure 3C). Grin1KD mice perform poorly in this task, taking longer to reach the goal box through an open doorway [F 1,10 = 6.8; p < 0.05] (Figure 3B).
FIGURE 3.

(A–E) Executive function, working memory and long‐term memory were assessed in the standard (A–B) and modified (C–D) “Puzzle Box” test. (A) Illustrations of all trials (T1–T7) used in the standard procedure; (C)—illustrations of all trials ("T1–"T9) used in the modified procedure. Latency (seconds) to enter the goal box assessed in the standard (B) and modified (D) Puzzle Box is presented. (E) The duration of digging behavior (seconds) was assessed in an independent “Digging” test. *p < 0.05; **p < 0.01; ***p < 0.001—in comparison with WT mice; # p < 0.05—in comparison with the standard protocol within genotype. N = 6/WT; 6/Grin1KD (standard protocol); N = 7/WT; 6/Grin1KD (modified protocol); N = 13/WT; 17/Grin1KD (“Digging” test)
The session with closed door/open underpass (T2–T4): One‐way ANOVA with repeated measures found a main effect of genotype [F 1,10 = 3.8; p < 0.05], and trial × genotype interaction [F 2,20 = 3.4; p < 0.05] on the latency to enter the goal box. WT and Grin1KD mice were able to solve obstacle given on trials 2 and 3 to enter the goal box (Figure 3B). Grin1KD mice showed deficient long‐term memory on trial 4 (p < 0.01).
The session with closed door/underpass is filled with bedding (T5‐T7): One‐way ANOVA with repeated measures found a main effect of genotype [F 1,10 = 311.8; p < 0.001] on the latency to enter the goal box. Grin1KD mice did not enter the goal box once the underpass was filled by bedding in comparison with WT littermates (Figure 3B).
3.1.6. Exposure of Grin1KD mice to a bigger entrance into the goal box improved their performance in the puzzle box
To compensate for the visual and whisker‐related tactile impairments observed in Grin1KD mice, the size of the entrance of the puzzle box was increased during the training session (trial 1) (Figure 3C) following by the trials (trial 2–trial 4), where the doorway was not completely closed as in the standard protocol. We hypothesized that the goal box, still visually perceivable under such conditions, would provide a visual cue to Grin1KD mice and facilitate their performance in the puzzle box. Indeed, two‐way ANOVA detected a main effect of PB modification [F 1,21 = 5.1; p < 0.05], and genotype [F 1,21 = 14.9; p < 0.001] on the latency to enter the goal box on trial1. Grin1KD mice were able to reduce their latency to enter the goal box as compare with their performance in the standard protocol (p < 0.05).
The session with a doorway (12 × 2 cm)/open underpass (T2–T4): ANOVA with repeated measures did not reveal a main effect of genotype [F 1,11 = 3.9; p > 0.05], trial [F 2,22 = 1.7; p > 0.05] or their interactions [F 2,22 = 0.9; p > 0.05] on the latency to enter the goal box. Grin1KD mice showed comparable behavior with WT animals on T2–T4 trials (Figure 3D).
The session with closed door/open underpass (T5–T7): ANOVA did not reveal significant effect of genotype [F 1,11 = 2.7; p > 0.05], trial [F 2,22 = 0.7; p > 0.05] or their interaction [F 2,22 = 0.9; p > 0.05] on the latency to enter the goal box.
The session with closed door/underpass is filled with bedding (T8–T9): ANOVA with repeated measures revealed a main effect of genotype [F 1,11 = 45.6; p < 0.001] on the latency to enter the goal box. Grin1KD mice were still unable to overcome the “bedding” obstacle given on trials 8 and 9 (Figure 3D).
Given that Grin1KD mice were unable to overcome the “bedding” obstacle in two independent experiments, we directly probed their digging activity in a mouse cage filled with 5 cm of bedding. Unpaired t‐test found that the duration of the digging was significantly lower in Grin1KD mice as compared with WT animals (t 1,28 = 14.92; p < 0.001) (Figure 3E). Thus, cognitive performance in this last phase of the puzzle box is masked by the potentially affected tactile function in Grin1KD mice.
3.1.7. Opposite elevated plus maze preference in an arena configuration with widened arms
It has been shown that Grin1KD 52 , 53 or other Grin1 deficient mutant lines 54 , 55 express a high preference for the open arms in the elevated plus maze (EPM). Given that exposure of Grin1KD mice to a bigger entrance to the goal box improved their performance in the puzzle box, we asked if increasing the width of the arms in the EPM could also modify their behavior in this test. We hypothesized that wider arms would facilitate visual and somatosensory perception of the enclosed arms and hence, alter preference of the open and enclosed compartments in the EPM arena. Therefore, we compared behavior of the mice in arenas of two sizes—EPMs designed for testing mice (mEPM) or rats (rEPM).
Open arms: Two‐way ANOVA detected a main effect of genotype [F 1,22 = 235.7; p < 0.0001], size (mEPM; rEPM) [F 1,22 = 46.2; p < 0.0001] and genotype × size interactions [F 1,22 = 123.9; p < 0.0001] on the percentage of time spent in open arms. As expected, WT mice avoided open arms of both the mEPM and rEPM (Figure 4C,D), spending ~80% of their time in the enclosed arms (Table S4). In contrast, Grin1KD mice spent ~90% of the testing period on the open arms of the mEPM and spent nearly half of the time at the ends of open arms (45.8% at the ends of open arms and 54.2% on the rest of the open arms, respectively; Table S4; Figure 4C). Consequently, Grin1KD animals moved more often between open arms than WT littermates (Table S4; p < 0.01). However, when Grin1KD mice were assessed in the rEPM, they instead spent most of the time in the enclosed arms (p's < 0.001) (Table S4), dramatically reducing their preference for the open arms (Figure 4D). In addition, Grin1KD mice also decreased their number of passes between open arms (p < 0.05; Table S4) and head‐dips (p < 0.001; Table S4). The total number of entries into each type of the EPM' area was similar in Grin1KD mice regardless of the EPM' size (Data S1; Table S4).
FIGURE 4.

(A–H) The behavior of Grin1KD and WT mice in the mouse‐sized elevated plus‐maze (mEPM) (A) and rat‐sized elevated plus‐maze (rEPM) (B). (C–D) The percentage of time spent in the open arms of the mEPM (C) and rEPM (D); (E–F) The number of defecations in mEPM (E) and rEPM (F). (G–H) The correlation analysis between behavior in the “Cliff” test and behavior in the mEPM for WT (G) and Grin1KD (H) mice. ## p < 0.01—in comparison with mEPM within each genotype; *p < 0.05; **p < 0.01; ***p < 0.001—in comparison with WT mice; mEPM: N = 7/WT; 7/Grin1KD; rEPM: N = 6/WT; 6/Grin1KD
Defecations: Defecation can be used as an indicator of fear and anxiety in mice. 56 Since Grin1KD mice preferred the open arms of the mEPM and the closed arms of the rEPM, we studied defecation behavior (fecal bolus number) as an indicator of their anxiety in the two maze configurations. Two‐way ANOVA detected a main effect of genotype [F 1,22 = 30.1; p < 0.001] and interaction between genotype × EPM size [F 1,22 = 8.8; p < 0.01] on the number of fecal boli. Grin1KD mice defecated more often than WT mice regardless of the EPM size (p < 0.001; p < 0.05—for mEPM and rEPM, respectively) (Figure 4E,F). WT mice showed an increased number of defecations when exposed to the rEPM in comparison with mEPM (p < 0.01), whereas Grin1KD mice had a tendency to decrease number of defecations when exposed to the rEPM compared with the mEPM (p = 0.062) (Figure 4F).
Additional manipulations with lighting conditions or re‐testing of mice in the mEPM were not able to change the behavioral repertoire of Grin1KD mice (Figure S5A–B).
Lastly, to rule out the impact of potentially affected vision in Grin1KD mice as detected by the “Cliff” test, we conducted the Pearson product–moment correlations between the behavior in the “Cliff” and mEPM tests. There was a significant relationship between the percentage of time spent on the OA of the mEPM and percentage of time spent on the Cliff area for WT mice (r = 0.56; p < 0.05) (Figure 4G), but this interaction was disrupted in Grin1KD mice (r = −0.24; p > 0.05) (Figure 4H).
Retinal morphology in Grin1KD and WT mice
Based on our behavioral observations, we aimed to directly quantify potential alterations of visual and somatosensory systems. Retinal morphology is a well‐accepted approach to probe structural changes of the mouse' eye. Hence, we performed microscopic assessment of retinal cross sections from each genotype. There was no gross abnormalities between genotypes as evident by the comparable axial length of the eyeball, lens, thickness of optic nerve, total length of retina' pigmented epithelium (Table S5). Two‐way ANOVA found no effect of sex or genotype × sex interaction on any measured parameters (all p's > 0.05), but detected a main effect of genotype on thickness of ONL [F 1,15 = 7.2; p < 0.05], OS [F 1,15 = 9.2; p < 0.01] and trend to be significant for the total thickness of retina [F 1,15 = 3.9; p = 0.06]. Grin1KD mice showed significantly thicker ONL and OS retina' layers as well as the total thickness than their WT littermates (Table S5; Figure 5C–D,F). The length of the non‐attached RPE to OS was significantly longer in Grin1KD mice than in WT animals [Student's t‐test1,4 = 50.8; p < 0.01] (Figure 5A–B,E).
FIGURE 5.
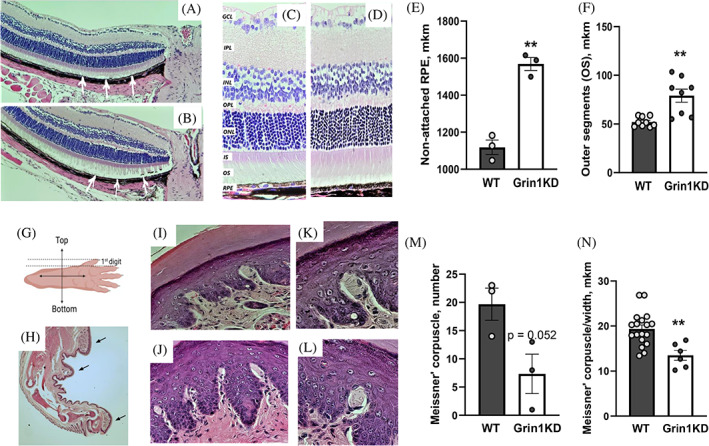
(A–N) The histological changes in retina' stabilization and mechanical sensors of the paw' skin (Meissner's corpuscles), in Grin1KD and WT mice. (A–B) Illustration of the central retina near the head of the optic nerve with the attached outer segment (OS) to the retina pigmented epithelium (RPE) in WT mice (A; white arrows), and less stable connections of OS to the RPE were detected in Grin1KD mice (B); magnification ×10. (C–D) Thickness of retina' layers in WT (C) and Grin1KD (D) mice. The retina of Grin1KD mice (D) is characterized by thicker outer segment (OS) layer (F) and longer non‐attached retinal pigmented epithelium (EPL) (E) than in WT mice. N = 3 mice per genotype; regions of interest (ROI): n = 9 (WT)/n = 8 (Grin1KD). (G) Schematic orientation of the hind paw for the preparation of the paraffinized samples. The analysis of Meissner corpuscles was performed on sections of the 1st digit. (H) Three ROIs are indicated by arrows on image of the hind paw (magnification ×2). Images of the glaborous footpad of WT (I) and Grin1KD (J) mice containing Meissner corpuscles. High magnification (×63) light microscopy images of Meissner corpuscles in WT (K) and Grin1KD (L) animals. N = 3 mice per genotype; 3 ROI per mouse. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segments; RPE, retinal pigmented epithelium
Meissner' corpuscles morphology in Grin1KD and WT mice
To address deficient performance in the digging test, paw morphology was performed. Of the four mechanoreceptor types, only Meissner corpuscles were found in hind paw skin identifiable with hematoxylin and eosin staining alone. The number of Meissner' corpuscles was lower in Grin1KD mice [Student's t‐test1,4 = 7.5; p = 0.052] (Figure 5K). Further, width of the “leg‐like” part of Meissner' corpuscles was thinner in Grin1KD mice [F 1,22 = 12.4; p < 0.01] than in WT littermates (Figure 5J,L). The length (WT: 213.0 ± 12.3 μm; Grin1KD: 209.2 ± 21.6 μm) and width of the “head‐like” part of the Meissner' corpuscles (WT: 34.1 ± 1.7 μm; Grin1KD: 32.7 ± 2.9 μm) were comparable between genotypes.
4. DISCUSSION
Behavior is affected by multiple factors, including sensory functions and, hence conclusions must be carefully generated, avoiding reductionism, which is currently dominating in the field of mouse behavioral neuroscience during the last decades due to the progress of the molecular‐cellular approaches. 57 The current findings consistently demonstrated that “standard” behavioral protocols must be adapted for mice with impaired sensory processing. By changing the dimensions of an arena or improving visual cues, the “cognitive” deficits of Grin1KD mice were revealed to be at least in part sensory deficits. Although Grin1KD mice have been used by numerous labs for over 20 years, this was the first study to demonstrate deficient visual depth perception using the visual cliff test, and the first to document that their open‐arm preference reflects an aversion to small enclosures rather than a lack of fear. Furthermore, to our surprise, Grin1KD mice were able to learn and recall the location of the escape platform in the MWM if they were either trained with a visible platform or if proximate rather than distant visual cues were used. This finding reveals the impact of their abnormal vision on their performance in this spatial learning and memory task. In the puzzle box test, enlarging the size of the doorway, which enhances the visual–spatial perception of the goal box, significantly reduced the latency of Grin1KD mice to complete the test. Interestingly, we also observed an inability of Grin1KD mice to dig in bedding, which could reflect abnormal tactile perception in these animals and explains their poor performance in the “bedding” obstacle of the puzzle box. Lastly, the exposure of Grin1KD mice to a larger EPM arena (rat‐sized EPM vs mouse‐sized) dramatically changed their behavioral repertoire in ways that were not seen in wildtype mice. The inclusion of defecation measures to the EPM study demonstrated that Grin1KD mice were more fearful than wildtype mice, even though they spent more time in the open arms of the mouse‐sized plus maze. We went further and found destabilization of the retina' outer segment layer in Grin1KD mice likely affecting their vision‐dependent behavior. Moreover, correlation analysis found impaired relationship between behavioral performance in the “Cliff” test and water maze or EPM in Grin1KD but not in wild type mice, supporting the affected visual functions in Grin1 deficient mice. In addition, deficiency of the mechanical sensors (Meissner's corpuscles) was revealed in hind paws of Grin1KD mice, likely contributing to their abnormal digging behavior. Altogether, these experiments demonstrate that Grin1KD mice require modified visual‐ and tactile conditions to properly assess their learning, memory, motivation, and emotionality. It is essential to adjust experimental conditions to accommodate any abnormal sensory functions of experimental animals, since these confounds can affect behavioral outputs under standard protocols.
We initiated this study because the Grin1KD mouse line has been used to model a syndromic form of autism caused by loss‐of‐function variants in the human GRIN1 gene. 58 A substantial percentage of GRIN1 patients have cortical visual impairment, and we hypothesized that Grin1KD mice might be similarly impaired. Not only are NMDARs expressed in the retina, they are required for the retinotopic organization of the lateral geniculate nucleus and visual cortex. 59 , 60 , 61 , 62 , 63 Thus, it is not surprising that GRIN disorder patients experience CVI or that mouse models of GRIN disorder would have abnormal vision. We assessed visual depth perception with the visual cliff test developed to detect aberrant vision in mice. 46 Grin1KD mice spent an equal amount of time suspended on the visual cliff as they did on the safe area of the arena, suggesting impaired visual acuity or depth perception. Other mouse models of autism have shown deficits in this task, including Rbfox2 knockout mice, 64 Fmr1−/y mice 65 or miR‐132/miR‐212 null mice. 66 Notably, the correlation analysis found relationship between time spent on the cliff area, open arms of the EPM (Figure 4G–H) and latency to escape the water maze on the 1st trial with visual platform in wild type mice, but not in Grin1KD animals (Figure S3A–B), supporting the “Cliff” test as a sensitive assessment of vision, which should be employed before interpreting the results from behavioral paradigms that employ visual cues.
As is the case with the visual system, the role of NMDA receptors in hippocampal spatial learning and memory is well‐established. 67 It was not surprising then, that in the standard Morris' water maze configuration, Grin1KD animals did not learn the location of the hidden platform during training and could not recall its location during a probe test. However, when visual cues were placed closer to the mouse, on the walls of the tank, Grin1KD mice did show learning (albeit delayed) and more importantly showed intact memory during the probe test. Such changes in learning strategy in MWM protocol‐dependent manner were demonstrated by other studies. 68 , 69 So, Grin1KD mice daily exposed to a visible escape platform significantly dropped their traveled distance, reflecting their “spatial” strategy in contrast to the “procedural” learning expressed in the standard MWM conditions with a hidden escape platform. Moreover, such enhancement of vision‐based stimulation likely increased the motivation of Grin1KD mice based on their significant reduction of the latency to reach the visual platform. In addition, Grin1KD mice did not express stereotypic circling behavior under this modified protocol, opposite to the standard MWM conditions, which is in agreement with the observation that floating significantly affects MWM behavior in mice. 70 Thus, our observations suggest that environmental conditions that provoke exploration can ameliorate the goal‐oriented deficit in Grin1KD mice.
Despite the beneficial effects of daily training of the Grin1KD mice to the visible platform with a pedestal‐like object on spatial learning, the expression of long‐term spatial memory was still not comparable with controls under these experimental conditions. One explanation could be related to the potentially poor vision, affecting ability to associate distant visual cues with the location of the escape platform and supporting the idea that Grin1KD mice likely used the “procedural” strategy during the acquisition. Proximately‐located visual cues in the water maze significantly improved their spatial long‐term memory, providing evidence that Grin1KD animals can build associations between the proximate visual cues and the escape platform and supporting the impact of poor distant vision on spatial memory in Grin1 deficient mice. Indeed, the lack of GluN1 in all hippocampal dental gyrus and dorsal CA1 neurons did not disrupt long‐term spatial memory in GluN1ΔGCA1 mice in the water maze, 71 suggesting that hippocampal NMDARs are not critical for long‐term associative spatial memories.
Nevertheless, with the adapted MWM arena, we now know that memory encoding is possible, but is delayed and requires additional training in Grin1KD mice.
Multiple studies 72 , 73 , 74 demonstrated that mouse behavior in the Morris water maze is vision‐dependent. It is highly desirable to directly measure the functional capacity of the retina in Grin1KD mice using such behavioral tests as an optokinetic drum 75 or visual water box 76 in parallel with an electroretinogram as a physiological assay to probe retinal responses in mice. 77 Nevertheless, the histological assessment of the retina in Grin1KD mice suggests destabilization of the outer segment of the retina, decreasing its attachment to the retinal pigmented epithelium. Interestingly, a similar retinal phenotype was reported for the Fragile X Syndrome mouse model of autism 78 with a significant reduction of rhodopsin, affecting synaptic destabilization of retinal neurons and retinal immaturity. Fmr1 knockout mice expressed hyperactivity, impaired social behavior and cognitive capacities, including water maze 79 similarly to Grin1KD phenotypes. A recent imaging study 80 demonstrated a significant role of GRIN1 in synaptic connections between retinal ganglion cells and bipolar cells using CreER‐J‐RGC:FRT‐EGFP:Grin1flox/flox (J‐RGC‐Grin1‐FRT) triple transgenic mice to conditionally delete GRIN1 in retinal ganglion cells. This “Grin1‐KO” mice expressed a reduced proportion of subtype 2 and 4 cone bipolar cells together with the increased rod bipolar cells. Hence, the identified destabilization of the outer segment (OS) of the retina in Grin1KD mice may suggest the impaired functions of these retinal cells based on the study. 80 There is an urgent need to selectively alter the GRIN1 expression in these retinal cellular subpopulations to probe the implication of the retinal GRIN1 in the regulation of behavioral phenotypes in future studies.
Besides the impaired vision, the altered somatosensory function also could contribute to the Grin1KD behavior in MWM. It could be suggested that the exposure of Grin1KD mice to water might be considered as a gentle tactile stimulation rather than anxiogenic, which could affect their goal‐oriented behavior to reach the escape platform. Interestingly, chronic training in the water maze for 3 weeks reduced tactile allodynia in experimental mice, 81 supporting potential relationship between the tactile sensory system and performance in a water maze.
Overall, adaptations to the standard water maze arena were able to reveal the true expression of spatial learning and memory in Grin1KD mice, which were likely masked by their phenotypes in the domains of vision and motivation.
We used the same strategy of arena modification to determine whether sensory phenotypes masked performance in other tests where Grin1KD mice show robust “cognitive” deficits. The puzzle box test was originally developed to assess executive function, which is affected in schizophrenia 82 and autism. 83 Mice need to solve the obstacle to access the safer dark compartment—the goal box. Firstly, the impaired performance of Grin1KD mice in the puzzle box was confirmed as reported earlier. 84 , 85 Grin1KD mice spent a longer time in the bright area than WT littermates before reaching the goal box during the 1st trial, supporting their high emotionality, low motivation and/or altered sensory functions as hypothesized above. We hypothesized that a bigger entrance to the goal box could facilitate their performance in the first trial of puzzle box test, making the doorway and the goal box more visible. Indeed, this modification improved their performance, normalizing their latency to reach the goal box and suggesting that poor visual or spatial perception could mask the expression of cognitive and motivational behavior in Grin1KD mice. Similarly, enlarging the size of the underpass in trials two through four improved their latency, providing further support that visual or tactile phenotypes influence the performance of this task. Interestingly, Grin1KD mice failed to complete the “burrowing” obstacle assessed in the current study, replicating previous reports. 84 , 85 The inability of Grin1KD animals to dig the standard bedding, confirming observations in the “burrowing” test with food pellets, 50 also suggests abnormal tactile perception. Indeed, histological assessment of hind paws revealed deficient number of Meissner' corpuscles and impaired size in Grin1KD mice, suggesting that affected somatosensory function is an important factor affecting digging behavior among others. 49 , 86
Interpretation of the EPM behavior is complex since it reflects multiple factors, including 3 main factors: anxiety (% of time spent in open arms and enclosed arms), locomotion (total number of entries into each arm or total raveled distance) and decision making (% of the central time), in addition to such factors as risk assessment (stretched attend posture), vertical activity (rearings), and exploration (number of head‐dips). 87 Several studies consistently reported that Grin1 loss‐of‐function mutant mice showed an “anxiolytic‐like” behavior in the open field test, in elevated plus maze, or in light–dark box. This was observed in Grin1 knockdown mice in this study and previous studies, 50 , 53 in Grin1 D481N homozygous mice, 54 and in Grin1 R844C mice. 55 However, in our study we also observed that Grin1KD mice expressed higher emotionality than WT littermates, evidenced by an increased number of defecations in the elevated plus maze. Moreover, we observed an increased number of passes between the open arms by Grin1KD mice together with increased number of head‐dips, potentially reflecting an active avoidance of aversive conditions and high stereotypy rather than anxiolytic‐like behavior.
Manipulations with lighting conditions and repeated exposure of Grin1KD mice to the EPM did not change their preference for the open arms (Figure S5A–B). Interestingly, the arm preferences in the EPM were studied in two inbred strains with impaired vision—A/J (with the albino mutation) and CBA/J (with the retinal degeneration mutation) under varying light. 88 That study suggested that other factors than light sensitivity contribute to the arm preference in the elevated plus‐maze test. A more recent study explored the possibility that the tactile system can compensate for deficient vision in C3H inbred strain. 89 There the authors demonstrated that whiskers are an essential tactile sense that contributes to motor coordination, gait control, spontaneous ambulation and anxiety in blind C3H mice.
Strikingly, the exposure of Grin1KD mice to the rat‐sized EPM, which is twice larger than the mouse‐sized EPM, increased their duration of time spent in the enclosed arms, but reduced stereotypic behavior and did not affect emotionality (number of defecations), hence, excluding the anxiogenic effect of a bigger‐sized plus maze on Grin1KD mice. In opposite, the rat‐sized EPM elicited a threatening effect on WT mice, increasing this index of emotionality and reducing their ambulation, supporting other studies. 90 One explanation for such observations is that Grin1KD mice may avoid the enclosed arms of the mouse EPM due to their altered spatial/somatosensory perception. Indeed, laboratory mice use their whiskers as the primary sense for exploring surroundings to navigate through the environment. 91 The whisker‐mediated touch system has its anatomical and functional brain system: “barrelettes” in the brainstem nuclei, “barreloids” in the sensory thalamus, and “barrels” in the cortex. 91 GRIN1 plays a major role in the patterning of topographic sensory maps in the brain. For instance, thalamus‐specific deletion of the GRIN1 gene in mice caused a lack of barrel formation which affected a range of behavioral domains 92 or disruption of the GRIN1 in L4 cortical neurons reduced neuronal activity in the neonatal barrel cortex. 93 Lee with co‐authors demonstrated that the reduction of GRIN1 expression in mice by 70%–80% prevented trigeminal afferent terminals and postsynaptic cells to form discrete modules (“barrelettes”) in the trigeminal principal nucleus (PrV) in a mouse brain. 94 Further, trigeminal neurons show patchiness and small terminal branches in control mice, whereas a highly branched arbor field was observed in GRIN1 deficient mice, 94 despite the reduced NMDA currents recorded from the barrelette neurons. 95 Hence, the dedicated characterization of whisking behavior in our Grin1KD mouse line in future is needed, which requires the specialized expertise as described. 96 It will help to identify its impact on the abnormal motor functions, emotional, and cognitive behavior in Grin1 deficient mice.
Altogether, our results demonstrated that abnormal visual–spatial sensory perception can mask behavioral phenotypes of Grin1KD mice assessed in a battery of widely‐used behavioral tests. The selective correction of vision or/and somatosensory function in Grin1KD mice as a next step will accurately determine the impact of this physiological system on abnormal behavior, including hyperactivity, stereotypy, social, emotional and cognitive performance. For instance, the elegant study of Orefiece with colleagues 97 demonstrated that genetic lack of Shank3 or Mecp2 in peripheral mechanosensory neurons reduced the density of the inhibitory interneurons in the somatosensory cortex (S1) and basolateral amygdala, causing autism‐related behavior in mice, which was corrected by peripherally restricted GABAa receptor agonist. Given that animal modeling of psychiatric disorders, including autism and schizophrenia, is based on standardized behavioral phenotyping 43 as a major read‐out of genetic, pharmacological or neuronal manipulations, a re‐design of standard experimental conditions suitable for abnormal sensory functions is highly desirable in preclinical neuroscience.
CONFLICT OF INTEREST
A.J.R. is a paid consultant to CureGRIN Research Foundation. The authors declare no potential conflict of interest.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
This work was supported by grants from CIHR (A.J.R. and A.S.), NSERC (A.J.R.), Simons Foundation for Autism Research Initiative (A.J.R.), and CureGRIN Research Foundation (A.J.R.). The authors wish to acknowledge the contribution of Nathaniel Winsor (Department of Immunology, University of Toronto), Kyle Roberton, Xueyuan Shang and Milan Ganguly in Department of Pathology at the TCP for fixation, sectioning and H&E stating of tissues.
Lipina T, Men X, Blundell M, Salahpour A, Ramsey AJ. Abnormal sensory perception masks behavioral performance of Grin1 knockdown mice. Genes, Brain and Behavior. 2022;21(6):e12825. doi: 10.1111/gbb.12825
Funding information Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada; CureGRIN Research Foundation; Institute of Neurosciences, Mental Health and Addiction; Simons Foundation for Autism Research Initiative; CIHR
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Levit‐Binnun N, Davidovitch M, Golland Y. Sensory and motor secondary symptoms as indicators of brain vulnerability. J Neurodev Disord. 2013;5(1):26‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15(2):146‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chisholm K, Lin A, Abu‐Akel A, Wood SJ. The association between autism and schizophrenia spectrum disorders: a review of eight alternative models of co‐occurrence. Neurosci Biobehav Rev. 2015;55:173‐183. [DOI] [PubMed] [Google Scholar]
- 4. Zhou H‐Y, Cai X‐L, Weigl M, Bang P, Cheung EFC, Chan RCK. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: a systematic review and meta‐analysis. Neurosci Biobehav Rev. 2018;86:66‐76. [DOI] [PubMed] [Google Scholar]
- 5. Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparable study using the short sensory profile. Am J Occup Ther. 2007;61:190‐200. [DOI] [PubMed] [Google Scholar]
- 6. Tavassoli T, Baron‐Cohen S. Taste identification in adults with autism spectrum conditions. J Autism Dev Disord. 2012;42:1419‐1424. [DOI] [PubMed] [Google Scholar]
- 7. Marco EJ, Khatibi K, Hill SS, et al. Children with autism show reduced somatosensory response: an MEG study. Autism Res. 2012;5:340‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puts NAJ, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RAE. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol. 2014;111:1803‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonnel A, Mottron L, Peretz I, et al. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J Cogn Neurosci. 2003;15:226‐235. [DOI] [PubMed] [Google Scholar]
- 10. Rozenkrantz L, Zachor D, Heller I, et al. A mechanistic link between olfaction and autism spectrum disorder. Curr Biol. 2015;25:1904‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simmons DR, Robertson AE, McKay LS, et al. Vision in autism spectrum disorders. Vision Res. 2009;49:2705‐2739. [DOI] [PubMed] [Google Scholar]
- 12. Freedman R, Adler LE, Gerhardt GA, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669‐678. [DOI] [PubMed] [Google Scholar]
- 13. Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I. Touch feel illusion in schizophrenic patients. Biol Psychiatry. 2000;48(11):1105‐1108. [DOI] [PubMed] [Google Scholar]
- 14. Bernardin F, Schwan R, Lalanne L, et al. The role of the retina in visual hallucinations: a review of the literature and implications for psychosis. Neuropsychologia. 2017;99:128‐138. [DOI] [PubMed] [Google Scholar]
- 15. Turetsky BI, Hahn C‐G, Borgmann‐Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull. 2009;35(6):117‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donde C, Mondino M, Brunelin J, Haesebaert F. Sensory‐targeted cognitive training for schizophrenia. Expert Rev Neurother. 2019;19(3):211‐225. [DOI] [PubMed] [Google Scholar]
- 17. Stevenson RA, Park S, Cochran C, et al. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr Res. 2017;179:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woynaroski TG, Kwakye LD, Foss‐Feig JH, Stevenson RA, Stone WL, Wallace MT. Multisensory speech perception in children with autism spectrum disorders. J Autism Dev Disord. 2013;43:2891‐2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldman JL, Dunham K, Cassidy M, Wallace MT, Liu Y, Woynarski TG. Audiovisual multisensory integration in individuals with autism spectrum disorder: a systematic review and meta‐analysis. Neurosci Biobehav Rev. 2018;95:220‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165‐179. [DOI] [PubMed] [Google Scholar]
- 21. Carlson GC. Glutamate receptor dysfunction and drug targets across models of autism spectrum disorders. Pharmacol Biochem Behav. 2012;100(4):850‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myers RA, Casals F, Gauthier J, et al. A population genetic approach to mapping neurological disorder genes using deep resequencing. PLoS Genet. 2011;7(2):e1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Roak BJ, Deriziotis P, Lee C, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43(6):585‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Ligt J, Willemsen MH, van Bon BWM, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367(20):1921‐1929. [DOI] [PubMed] [Google Scholar]
- 25. Garcia‐Recio A, Santos‐Gomez A, Soto D, et al. GRIN database: a unified and manually curated repertoire of GRIN variants. Hum Mutat. 2021;42:8‐18. [DOI] [PubMed] [Google Scholar]
- 26. Burnashev N, Szepetowski P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol. 2015;20:73‐82. [DOI] [PubMed] [Google Scholar]
- 27. Lemke JR, Geider K, Helbig KL, et al. Delineating the GRIN1 phenotypic spectrum: a distinct genetic NMDA receptor encephalopathy. Neurology. 2016;86(23):2171‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemke JR, Hendrick R, Geider K, et al. GRIN2B mutations in west syndrome and intellectual disability with focal epilepsy. Ann Neurol. 2014;75(1):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Platzer K, Yuan H, Schütz H, et al. GRIN2B encephalopathy: novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J Med Genet. 2017;54(7):460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemke JR. Predicting incidences of neurodevelopmental disorders. Brain. 2020;143(4):1046‐1048. [DOI] [PubMed] [Google Scholar]
- 31. Robertson CE, Baron‐Cohen S. Sensory perception in autism. Nat Rev Neurosci. 2017;18:671‐684. [DOI] [PubMed] [Google Scholar]
- 32. Platzer K, Lemke JR. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GRIN1‐Related Neurodevelopmental Disorder. GeneReviews® [Internet]. University of Washington, 1993–2021. Bookshelf; 2019. https://www.ncbi.nlm.nih.gov/books [PubMed] [Google Scholar]
- 33. Benke TA, Park K, Krey I, et al. Clinical and therapeutic significance of genetic variation in the GRIN gene family encoding NMDARs. Neuropharmacology. 2021;199:108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramírez M, Lamas M. NMDA receptor mediates proliferation and CREB phosphorylation in postnatal Müller glia‐derived retinal progenitors. Mol Vis. 2009;15:713‐721. [PMC free article] [PubMed] [Google Scholar]
- 36. Furuya T, Pan Z, Kashiwagi K. Role of retinal glial cell glutamate transporters in retinal ganglion cell survival following stimulation of NMDA receptor. Curr Eye Res. 2012;15:713‐721. [DOI] [PubMed] [Google Scholar]
- 37. Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201‐205. [DOI] [PubMed] [Google Scholar]
- 38. Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427‐436. [DOI] [PubMed] [Google Scholar]
- 39. Ramsey AJ. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Prog Brain Res. 2009;179:51‐58. [DOI] [PubMed] [Google Scholar]
- 40. Moy SS, Nadler JJ, Poe MD, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duncan GE, Moy SS, Perez A, et al. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153(2):507‐519. [DOI] [PubMed] [Google Scholar]
- 42. Gandal MJ, Anderson RL, Billingslea EN, Carlson GC, Roberts TPL, Siegel SJ. Mice with reduced NMDA receptor expression: more consistent with autism than schizophrenia? Genes Brain Behav. 2012;11(6):740‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sukoff Rizzo SJ, Crawley JN. Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annu Rev Anim Biosci. 2017;5:371‐389. [DOI] [PubMed] [Google Scholar]
- 44. Mielnik CA, Binko MA, Chen Y, et al. Consequences of NMDA receptor deficiency can be rescued in the adult brain. Mol Psychiatry. 2021;26(7):2929‐2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silva AJ, Simpson EM, Takahashi JS, et al. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury conference on genetic background in mice. Neuron. 1997;19:755‐759. [DOI] [PubMed] [Google Scholar]
- 46. Glynn D, Bortnick RA, Morton AJ. Complexin II is essential for normal neurological function in mice. Hum Mol Genet. 2003;12(19):2431‐2448. [DOI] [PubMed] [Google Scholar]
- 47. Duffy S, Labrie V, Roder JC. D‐serine auguments NMDA‐NR2B receptor‐dependent hippocamapal long‐term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33(5):10004‐11018. [DOI] [PubMed] [Google Scholar]
- 48. Ben Abdallah NM‐B, Fuss J, Trusel M, et al. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol. 2011;227(1):42‐52. [DOI] [PubMed] [Google Scholar]
- 49. Deacon RMJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1(1):122‐124. [DOI] [PubMed] [Google Scholar]
- 50. McGirr A, Lipina TV, Mun HS, et al. Specific inhibition of phosphodiesterase‐4B results in anxiolysis and facilitates memory acquisition. Neuropsychopharmacology. 2016;41(4):1080‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wai V, Roberts L, Michaud J, Bent LR, Clark AL. The anatomical distribution of mechanoreceptors in mouse hind paw skin and the influence of integrin α1β1 on meissner‐like corpuscle density in the footpads. Front Neuroanat. 2021;15:62871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barkus C, Dawson LA, Sharp T, Bannerman DM. GluN1 hypomorph mice exhibit wide‐ranging behavioral alterations. Genes Brain Behav. 2012;11:342‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Halene TB, Ehrlichman RS, Liang Y, et al. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model of schizophrenia. Genes Brain Behav. 2009;8(7):661‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Labrie V, Clapcote SJ, Roder JC. Mutant mice with reduced NMDA‐NR1 glycine affinity or lack of D‐amino acid oxidase function exhibit altered anxiety‐like behaviors. Pharm Biochem Behav. 2009;91(4):610‐620. [DOI] [PubMed] [Google Scholar]
- 55. Umemori J, Takao K, Koshimizu H, et al. ENU‐mutagenesis mice with a non‐synonymous mutation in Grin1 exhibit abnormal anxiety‐like behaviors, impaired fear memory, and decreased acoustic startle response. BMC Res Notes. 2013;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ramos A, Mormede P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22(1):33‐57. [DOI] [PubMed] [Google Scholar]
- 57. Cahill L, McGaugh JL, Weinberger NM. The neurobiology of learning and memory: some reminders to remember. Trends Neurosci. 2001;24(10):578‐581. [DOI] [PubMed] [Google Scholar]
- 58. Intson K, van Eede MC, Islam R, et al. Progressive neuroanatomical changes caused by Grin1 loss‐of‐function mutation. Neurobiol Dis. 2019;132:104527. [DOI] [PubMed] [Google Scholar]
- 59. Hogan‐Cann AD, Anderson CM. Physiological roles of non‐neuronal NMDA receptors. Trends Pharmacol Sci. 2016;37(9):750‐767. [DOI] [PubMed] [Google Scholar]
- 60. Sikora M, Tokarski K, Bobula B, et al. NMDA receptors on dopaminoceptive neurons are essential for drug‐induced conditioned place preference. eNeuro. 2016;3(3):ENEURO.0084‐15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baltan S. Age‐specific localization of NMDA receptors on oligodendrocytes dictates axon function recovery after ischemia. Neuropharmacology. 2016;110(Pt B):626‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schulz DC, Pandey SK, Bursztyn LLCD. Optic nerve atrophy in N‐methyl‐D‐aspartate (NMDA) encephalitis. Can J Neurol Sci. 2020;47(1):139‐141. [DOI] [PubMed] [Google Scholar]
- 63. McCoy PA, Huang H‐S, Philpot BD. Advances in understanding visual cortex plasticity. Curr Opin Neurobiol. 2009;19(3):298‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gu L, Kawaguchi R, Capriol J, Piri N. The effect of Rbfox2 modulation on retinal transcriptome and visual function. Sci Rep. 2020;10(1):19683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Felgerolle C, Hebert B, Ardourel M, et al. Visual behavior impairments as an aberrant sensory processing in the mouse model of fragile X syndrome. Front Behav Neurosci. 2019;13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mazziotti R, Baroncelli L, Ceglia N, et al. Mir‐132/212 is required for maturation of binocular matching of orientation preference and depth perception. Nat Commun. 2017;8:15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sengar AS, Li H, Zhang W, et al. Control of long‐term synaptic potentiation and learning by alternative splicing of the NMDA receptor subunit GluN1. Cell Rep. 2019;29(13):4285‐4294.e5. [DOI] [PubMed] [Google Scholar]
- 68. Cho W‐H, Park J‐C, Chung C, Jeon WK, Han JS. Learning strategy preference of 5XFAD transgenic mice depends on the sequence of place/spatial and cued training in the water maze task. Behav Brain Res. 2014;273:116‐122. [DOI] [PubMed] [Google Scholar]
- 69. De Coninck M, Dam DV, Ginneken CV, Deyn PPD. Adapted Morris water maze protocol to prevent interference from confounding motor deficits on cognitive functioning. Somatosens Mot Res. 2017;34(3):172‐178. [DOI] [PubMed] [Google Scholar]
- 70. Vorhees CV, Williams M. Assessing spatial learning and memory in rodents. ILAR. 2014;55(2):310‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bannerman DM, Bus T, Taylor A, et al. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2013;15(8):1153‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007;14(3):134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wong AA, Brown RE. A neurobehavioral analysis of the prevention of visual impairment in the DBA/2J mouse model of glaucoma. Invest Ophthamol Vis Sci. 2012;53(9):5956‐5966. [DOI] [PubMed] [Google Scholar]
- 74. Wong AA, Brown RE. Prevention of vision loss protects against age‐related impairment in learning and memory performance in DBA/2J mice. Front Aging Neurosci. 2013;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611‐4616. [DOI] [PubMed] [Google Scholar]
- 76. Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389‐403. [DOI] [PubMed] [Google Scholar]
- 77. Leinonen H, Tanila H. Vision in laboratory rodents—tools to measure it and implications for behavioral research. Behav Brain Res. 2018;352:172‐182. [DOI] [PubMed] [Google Scholar]
- 78. Rossignol R, Ranchon‐Cole I, Paris A, et al. Visual sensorial impairments in neurodevelopmental disorders: evidence for a retinal phenotype in fragile X syndrome. PLoS One. 2014;9(8):e105996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bontekoe CJ, Mcllwain KL, Nieuwenhuizen IM, et al. Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet. 2002;11(5):487‐498. [DOI] [PubMed] [Google Scholar]
- 80. Young B, Sanchez CM, Ramakrishnan C, Wang P, Deisseroth K, Tian N. NMDA receptor activity regulates synaptic connections between retinal ganglion and bipolar cells. Invest Ophthalmol Vis Sci. 2018;59(9):1864. [Google Scholar]
- 81. Santos RSD, Sorgi CA, Peti APF, et al. Involvement of spinal cannabinoid CB2 receptors in exercise‐induced antinociception. Neuroscience. 2019;418:177‐188. [DOI] [PubMed] [Google Scholar]
- 82. Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35(1):258‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Demetriou EA, DeMayo MM, Guastella AJ. Executive function in autism spectrum disorder: history, theoretical models, empirical findings, and potential as an endophenotype. Front Psych. 2019;10:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Milenkovic M, Mielnik CA, Ramsey AJ. NMDA receptor‐deficient mice display sexual dimorphism in the onset and severity of behavioral abnormalities. Genes Brain Behav. 2014;13:850‐862. [DOI] [PubMed] [Google Scholar]
- 85. Islam R, Trepanier M‐O, Milekovic M, et al. Vulnerability to omega‐3 deprivation in a mouse model of NMDA receptor hypofunction. NPJ Schizophr. 2017;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pond HL, Heller AT, Gural BM, McKissick OP, Wilkinson MK, Manzini MC. Digging behavior discrimination test to probe burrowing and exploratory digging in male and female mice. J Neurosci Res. 2021;99(9):2046‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus‐maze test of anxiety. Pharmacol Biochem Behav. 1995;52(2):297‐303. [DOI] [PubMed] [Google Scholar]
- 88. Cohen RM, Kang A, Gulick C. Quantitative trait loci affecting the behavior of a/J and CBA/J intercross mice in the elevated plus maze. Mamm Genome. 2001;12:501‐507. [DOI] [PubMed] [Google Scholar]
- 89. Voller J, Potuzakova B, Simecek V, Vozeh F. The role of whiskers in compensation of visual deficit in a mouse model of retinal degeneration. Neurosci Lett. 2014;558:149‐153. [DOI] [PubMed] [Google Scholar]
- 90. Hogg S. A review of the validity and variability of the elevated plus‐maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54(1):21‐30. [DOI] [PubMed] [Google Scholar]
- 91. Adibi M. Whisker‐mediated touch system in rodents: from neuron to behavior. Front Syst Neurosci. 2019;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arakawa H, Suzuki A, Zhao S, et al. Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors. J Neurosci. 2014;34(36):12001‐12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mizuno H, Rao MS, Mizuno H, Sato T, Nakazawa S, Iwasato T. NMDA receptor enhances correlation of spontaneous activity in neonatal barrel cortex. J Neurosci. 2021;41(6):1207‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee L‐J, Lo F‐S, Erzurumlu RS. NMDA receptor‐dependent regulation of axonal and dendritic branching. J Neurosci. 2005;25(9):2304‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lee L‐J, Erzurumlu RS. Altered parcellation of neocortical somatosensory maps in N‐methyl‐D‐aspartate receptor‐deficient mice. J Comp Neurol. 2005b;485(1):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Simanaviciute U, Ahmed J, Brown RE, et al. Recommendations for measuring whisker meovements and locomotion in mice with sensory, motor and cognitive deficits. J Neurosci Methods. 2020;331:108532. [DOI] [PubMed] [Google Scholar]
- 97. Orefice LL, Mosko JR, Morency DT, et al. Targeting peripheral somatosensory neurons to improve tactile‐related phenotypes in ASD models. Cell. 2019;178(4):867‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


