Abstract
Mice produce ultrasonic vocalizations (USVs) in different social contexts across lifespan. There is ethological evidence that pup USVs elicit maternal retrieval and adult USVs facilitate social interaction with a conspecific. Analysis of mouse vocal and social repertoire across strains, sex and contexts remains not well explored. To address these issues, in inbred (C57BL/6, FVB) and outbred (CD‐1) mouse strains, we recorded and evaluated USVs as neonates and during adult social encounters (male–female and female–female social interaction). We showed significant strain differences in the quantitative (call rate and duration of USVs) and qualitative vocal analysis (spectrographic characterization) from early stage to adulthood, in line with specific patterns of social behaviors. Inbred C57BL/6 mice produced a lower number of calls with less internal changes and shorter duration; inbred FVB mice displayed more social behaviors and produced more syllables with repeated internal changes; outbred CD‐1 mice had an intermediate profile. Our results suggest specific vocal signatures in each mouse strain, thus helping to better define socio‐communicative profiles of mouse strains and to guide the choice of an appropriate strain according to the experimental settings.
Keywords: development, mouse communication, neonatal behavior, social interaction, vocal repertoire
Inbred C57BL/6 mice produced a lower number of calls with less internal changes and shorter duration; inbred FVB mice displayed more social behaviors and produced more syllables with repeated internal changes; outbred CD‐1 mice had an intermediate profile. Our results suggest specific vocal signatures in each mouse strain, thus helping to better define socio‐communicative profiles of mouse strains and to guide the choice of an appropriate strain according to the experimental settings.
1. INTRODUCTION
Ultrasonic vocalizations (USVs) by laboratory mice have been collected and deeply analyzed in different contexts during the early phases of postnatal development and at adulthood.
During the neonatal stages, USVs are emitted when pups are isolated from the nest to gain their mother's attention. 1 These calls are defined “isolation‐induced USVs” and have been extensively characterized. The rate of emission follows a clear ontogenetic profile, peaking during the first postnatal week and then decreasing to zero when pups are 2‐week old. 2 , 3 Since their first description it was suggested that neonatal USVs played an important role in vocal communication. 4 Although functional significance of such vocalizations has been debated, 5 , 6 there is sound ethological evidence that pup USVs elicit maternal orientation/approach and retrieval. 3 , 7 , 8 , 9 Previous data suggested that mother's genotype or strain and maternal responsiveness (an index of mother's solicitude towards pups in a potentially dangerous situation) can affect neonatal USV emission. 10 It is likely that pups' behavioral changes are expressed in parallel to mother's behavior.
USVs have been also detected in adolescent mice of both sexes after weaning, during a social interaction paradigm (consisting of 5‐days social housing and one‐day social isolation between behavioral tests 11 ), as well as in mice exposed to an anesthetized female. 12
In adult mice, emission of USVs has been primarily reported in reproductive contexts, with males being responsible of most of the calls, 13 , 14 although females have been recently shown to actively participate to the vocal interaction, but with a lower number of USVs when compared with males' vocalization rate. 15 , 16 , 17 A short or long exposure to either a female partner (previous socio‐sexual or reproductive experience) or to female urine induces a clear USV response in adult male mice. 13 , 14 , 18 , 19 , 20 , 21 , 22 , 23 The quantitative analysis performed by Holy and Guo in 2005 illustrated for the first time that the male USVs are characterized by temporal sequences and that they are specific for each individual. 19 More recent studies based on analyses of spectral parameters and temporal sequences on USVs highlighted the individual signatures in both pup and adult mice. 24 , 25 , 26 , 27 The production of USVs during adult female–female mouse encounters is also a sound phenomenon 28 : resident female mice during encounters with a female intruder emit a large number of USVs, at rates comparable to those of the male–female interaction. 29 These female calls, that only occur during resident‐intruder interactions in laboratory conditions, contribute to the establishment of female social dominance hierarchies, 28 but may also serve to enhance physical proximity and enable social information gathering. 17 , 28 , 30 , 31 Both pup and adult mice emit USVs to communicate with each other and to convey their emotional state. 1
Whereas the earlier studies initially provided only quantitative data (primarily rate of USV emission and duration), in the last years qualitative analyses had been also carried out. Several categorizations of the spectrographic appearances of the calls have been proposed, some of which share some basic principles. 11 , 32 , 33 Following pivotal works, USV categorization has been used as a biomarker to identify qualitative alterations of the vocal repertoire in different mouse models of neurodevelopmental disorders. USV categorizations have been explored in both neonates and adult subjects modeling socio‐communicative deficits, including autism spectrum disorders. 1 , 34 Recent USV data from different mouse strains are also available, so far limited to early phases of postnatal development (first 2–3 weeks of postnatal life). 11 , 35 , 36 Together with previous data on cross fostering at birth 37 or embryo transfer procedure, 38 these data indicate that USV production and their acoustic variations are subjected to genetic and background control. 39 , 40 Crucially, differences in USV emission have been detected even between mouse substrains, such as C57BL/6N and C57BL/6J. 38 The embryo transfer study showed that the difference between C57BL/6J OlaHsd and C57BL/6N Crl in USV rate was primarily dependent on the dyadic interaction between mother and pup. 38
Aim of our study was to evaluate vocal repertoire in three common mouse strains (C57BL/6, FVB and CD‐1) in both males and females, at two developmental stages (neonatal and adult), as for adult subjects in two different social contexts (male–female and female–female interaction) known to elicit maximal vocalization rates in laboratory settings. In adult testing, the social investigation was also recorded to obtain a more complete picture of the social responses in these three strains. We selected the inbred strain C57BL/6 since it is the commonest background for genetically modified lines and widely used in mouse phenotyping studies; the inbred strain FVB since it is considered highly social and some features (i.e., albinism, litter and body size) render it an ideal control for outbred strains; and CD1 as the commonest mouse outbred strain, extensively used in neuroscience, neuropharmacology and neurotoxicology studies. The choice of C57BL/6 substrains is a critical issue in experimental designs dealing with genetically modified mouse lines, since several behavioral phenotypic differences have been reported among mouse substrains. 38 , 41 , 42 We selected the C57BL/6N substrain that has been less characterized in previous studies and it is becoming increasingly popular because of large initiatives like the International Knockout Mouse Consortium (IKMC, https://www.mousephenotype.org/).
We hypothesized differences between inbred and outbred mouse in: 1) USV emission in both pups and adults; 2) social responses in adults; 3) qualitative USV patterns with the effect of age and social context.
2. MATERIALS AND METHODS
2.1. Animals and housing
C57BL/6N (inbred, hereinafter B6), FVB/NHan™Hsd (inbred, hereinafter FVB) and CD‐1 (outbred) breeding pairs were purchased from Harlan Laboratories (S. Pietro al Natisone, Italy) and bred in our mouse facility. Mice were housed on a reversed 12:12 h light: dark cycle (lights on at 19:00 h) in standard wire‐topped polycarbonate cages ages (33 cm × 13 cm × 14 cm) with sawdust bedding and water and food (DP/1000, Altromin‐Rieper, Vandoies‐BZ, Italy) ad libitum. Temperature was maintained at 21 ± 1°C, and relative humidity at 60 ± 10%. Females were individually housed and subsequently daily inspected for pregnancy and delivery 10 days after mating. The day of birth was considered as postnatal day (pnd) 0. Pups were tattooed on the paw with animal tattoo ink (Ketchum permanent Tattoo Inks green paste, Ketchum Manufacturing Inc., Brockville ON Canada) by subcutaneous injection (30G needle) into paw plantar surface. The procedure was performed at 2 days of age, immediately after behavioral testing.
Subject mice for adult social interaction tests were weaned into cages of same sex pairs. After weaning on postnatal day 21, each animal was socially housed with two same‐sex partners per cage. Mice were 2‐month‐old B6 (N = 12 males and 12 females), FVB (N = 12 males and 12 females) and CD1 (N = 12 males and 12 females) when tested for social interaction tests. Behavioral testing was always conducted between 9.30 and 13.30 h, during the dark phase of the circadian cycle, under red light.
All procedures were conducted in compliance with the European Communities guidelines (EC Council Directive 63/2010), Italian legislation on animal experimentation (DL 26/2014).
2.2. Ultrasonic vocalizations in pups
Tested litters contained more than seven pups. Within each litter, one male and one female underwent behavioral testing: B6 (N = 8 males and 8 females), FVB (N = 10 males and 10 females) and CD‐1 (N = 10 males and 10 females). The remaining pups (not tested as neonates) were pooled at weaning and assigned to adult social interaction tests (described in the following sections), as well as to other experimental designs (i.e., USV playback studies). Ultrasonic vocalization, body weight, and body temperature of pups were measured at pnd 2, 4, 6, 8, and 12. These pnds were chosen to be in accordance with previous studies focused on the ontogenetic profile of USV emission in inbred and outbred mouse strains. 36 , 43 , 44 On each day of testing, the pup was placed into an empty glass container (diameter, 5 cm; height 10 cm), located inside a sound‐attenuating styrofoam box, in a room under red light, and assessed for ultrasonic vocalizations during a 3‐min test. At the end of the recording session, each pup was weighed, and its axillary temperature measured by gentle insertion of the thermal probe in the skin pocket between upper foreleg and chest of the animal for about 30 s (Microprobe digital thermometer with mouse probe, Stoelting Co., IL). When the pup was returned to the nest, the mother and littermates were present.
An Ultrasound Microphone (Avisoft Ultra Sound Gate condenser microphone capsule CM16, Avisoft Bioacoustics, Berlin, Germany) sensitive to frequencies of 10–180 kHz was placed through a hole in the middle of the cover of the styrofoam sound‐attenuating box, about 20 cm above the pup in its plastic container. Room temperature was maintained at 22 ± 1°C. Vocalizations were recorded using Avisoft Recorder software (Version 3.2). Settings included sampling rate at 250 kHz; format 16 bit.
2.3. Adult social interaction tests
Within each strain, 2‐month‐old mice (not previously tested as neonates, to exclude any potential confounders on adult behavior) were evaluated in two different social interactions: 1) male–female (N = 12); 2) female–female (N = 12). Male and female mice were weighed the same day of the test (mean ± SD of B6, FVB and CD‐1 males are respectively: 21.93 ± 1.44; 25.58 ± 1.39; 33.51 ± 1.64; mean ± SD of B6, FVB and CD‐1 females are respectively: 19.25 ± 0.62, 20.0 ± 0.98, 24.55 ± 1.43). Behavioral tests were conducted under red light, videotaped using a Panasonic monochrome CCD camera and subsequently analyzed with Observer 10XT software (Noldus Information Technology, NL). The cage contained sawdust (1.5‐cm deep) and the lid was removed during the test.
For a 3‐min session of female–female interaction test, an unfamiliar stimulus mouse was placed into the home cage of a subject mouse who had resided in the cage for the previous 5 days without enrichment materials. In the male–female interaction test, a group‐housed male was used as subject mouse and the 3‐minute test session was conducted in a clean cage with clean bedding (1.5‐cm deep sawdust layer), representing a novel situation for both male subject and female partner. The videocamera was mounted facing the side of the cage to record the session for subsequent scoring of social investigation parameters. The ultrasonic microphone (same as in pup vocalization experiment) was mounted 20 cm above the floor of the cage to record the session.
Stimulus mice were matched to the subject mice by strain, sex, age, and body weight. Stimulus mice were bred in our colony as described above, and maintained in social groups of three per cage. On the day of male–female testing, the vaginal estrous condition of each stimulus female was assessed as previously described. 45 Only females in estrus were selected for the test. A total of 72 stimulus mice (N = 24 for each strain) were employed.
Social interactions were scored from the videotapes for the frequencies and durations of the following behavioral responses performed by the subject mouse: anogenital sniffing (direct contact with the anogenital area), body sniffing (sniffing or snout contact with the flank area), nose to nose sniffing (sniffing or snout contact with the head/neck/mouth area), locomotor activity by line crossings, rearing up against the wall of the home cage, digging in the bedding, and grooming (self‐cleaning, licking any part of its own body). Vocalizations were recorded using Avisoft Recorder software version 3.2. Settings included sampling rate at 250 kHz; format 16 bit.
2.4. Ultrasonic vocalization analysis
For acoustical analysis, recordings collected from pups and adults were transferred to Avisoft SASLab Pro (Version 4.40) and a fast Fourier transformation (FFT) was conducted as previously described. 46 Spectrograms from pup vocalizations were generated with an FFT‐length of 1024 points, while adult vocalizations requested spectrograms with an FFT‐length of 512 points and a time window overlap of 75% (100% Frame, Hamming window). The spectrogram was produced at a frequency resolution of 488 Hz and a time resolution of 1 ms. A lower cut‐off frequency of 20 kHz was used to reduce background noise outside the relevant frequency band to 0 dB. Parameters analyzed for each test day included number of calls, duration of calls, qualitative and quantitative analyses of sound frequencies measured in terms of frequency and amplitude at the maximum of the spectrum.
Start times for the video and audio files were synchronized during social encounters. However, it was not possible to synchronize scoring of behaviors with calls using the currently available recording technology. The software used for the behavioral (Noldus, Observer X) and spectrographic (Avisoft Bioacoustics, Avisoft SASLabPro version 4.40) analyses cannot be combined on the same screen because they proceed with different speeds: behavioral events occurred in a time frame of seconds whereas vocalizations occurred in a time frame of milliseconds.
Waveform patterns of calls for pups and adults were examined in depth in the sonograms collected from each subject tested. Each call was identified as one of nine distinct categories, based on internal pitch changes, lengths, and shapes, as described below: 1) Complex calls displayed one component containing two or more directional changes in pitch, each ≥6.25 kHz; 2) Two‐component calls consisted of two components: a main call (flat or downward) with an additional punctuated component towards the end; 3) Upward‐modulated calls exhibited a continuous increase in pitch that was ≥12.5 kHz, with a terminal dominant frequency at least 6.25 kHz more than the pitch at the beginning of the vocalization; 4) Downward‐modulated calls exhibited a continuous decrease in pitch that was ≥12.5 kHz, with a terminal dominant frequency at least 6.25 kHz less than the pitch at the beginning of the vocalization; 5) Chevron calls resembled an “inverted‐U,” which was identified by a continuous increase in pitch ≥12.5 kHz followed by a decrease that was ≥6.25 kHz; 6) Short calls were punctuated and shorter than 5 ms; 7) Composite calls were formed by two harmonically independent components, emitted simultaneously; 8) Frequency steps were instantaneous frequency changes appearing as a vertically discontinuous “step” on a spectrogram, but with no interruption in time; 9) Flat calls displayed a constant beginning and the ending of the pitch frequency remained constant (≤3 kHz of each other). 46 , 47
We classified pup vocalizations according to strain and sex: 1820 B6 calls (N = 1070 emitted by males and N = 750 by females); 3416 FVB calls (N = 1490 emitted by males and N = 1926 by females) and 8501 CD‐1 calls (N = 4744 emitted by males and N = 3757 by females). All pups except 6 mice (N = 2 B6, N = 2 FVB, N = 2 CD‐1) vocalized at least at one time point (pnd). Data related to pup vocalizations were subjected to three different analyses: a) strain‐dependent effects on the frequency and duration of the vocalizations emitted by each subject at pnd 2, 4, 6, 8, 12; b) strain‐dependent effects on the probability of producing calls (proportion of calls in each category for each subject) from each of the nine categories of USVs; c) a descriptive analysis which included strain‐dependent effects on the percentage of calls emitted by each subject in each of the nine categories of USVs within and between postnatal days.
Waveform patterns of adult calls were examined in depth in the sonograms collected from each subject tested, using the classification based on nine call categories (see above pup analysis). In the female–female encounter, we classified: 2418 B6 calls (N = 11 subjects), 12,891 FVB calls (N = 12 subjects) and 8707 CD‐1 calls (N = 12 subjects); in the male–female encounter, we classified: 4442 B6 calls (n = 10), 8667 FVB calls (n = 12) and 5343 CD‐1 calls (N = 12). The rest of the subjects were not analyzed because they did not emit vocalizations. Inter‐rater reliability was 98% between the two investigators who scored the call categories. Call category data were subjected to three different analyses: a) strain‐dependent effects on the frequency and duration of the vocalizations emitted by each adult subject; b) strain‐dependent effects on the probability of producing calls (proportion of calls in each category for each subject) from each of the nine categories of USVs; c) a descriptive analysis which included strain‐dependent effects on the percentage of calls emitted by each subject in each of the nine categories of USVs within and between social encounters.
2.5. Statistical analysis
A mixed‐model Analysis of Variance (ANOVA) with Repeated Measures was used to analyze 1) body weight and body temperature of pups with the strain as factor and postnatal days as the repeated measures; 2) neonatal USV quantitative parameters with the strain as factor and postnatal days as the repeated measures; 3) adult social behaviors with the strain as factor and sniffing of different body areas as the repeated measures; 4) probability of vocalizations with the strain as factor and social context as the repeated measures. Probability of vocalizations within each strain was calculated as number of calls in each category for each subject/total number of calls analyzed in each subject and standardized by angular transformation. As the analysis of sonographic patterns is of an exploratory nature (and not confirmatory), we did not adjust the results for multiple testing. A one‐way analysis of variance (ANOVA) was used to analyze adult USV quantitative parameters in each social context. An Analysis of Covariance (ANCOVA) on USV rate and duration with body weight and temperature as covariates were performed to investigate more deeply differences among strains.
To compare variability in inbred and outbred strains, both neonatal (pnd 8, paralleling with previous data) and adult USV data (rate and duration) were also analyzed by a nonparametric analysis of variance (Kruskal–Wallis), considering as variable of interest not measurements per se but their individual deviation from the average within each group [i.e. individual difference (in absolute value) between individual value and mean value of USV rate or duration within the experimental group considered]. 48
Post‐hoc comparisons were performed using Tukey's HSD test when a significant F value was determined. For all comparisons, significance was set at p < 0.05.
3. RESULTS
3.1. Pups
B6 pups had lower body weight than FVB and CD1 pups (p = 0.01 and p < 0.01, respectively). All pups showed an increased body weight from pnd 2 to 12 (p < 0.001) (Figure 1A).
FIGURE 1.
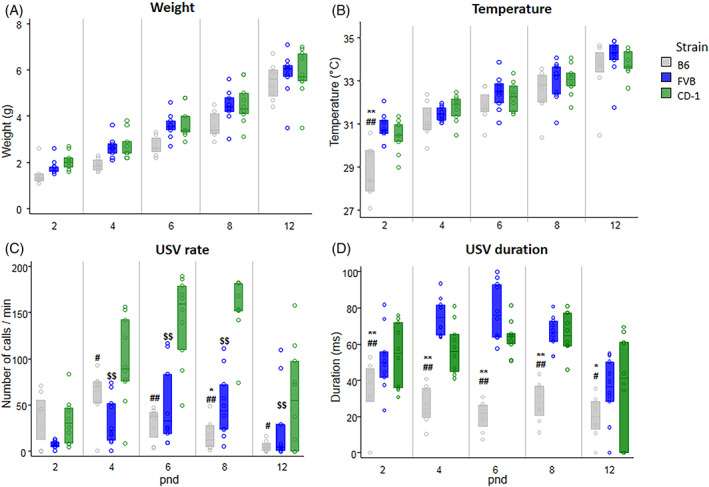
Neonatal body weight (A), body temperature (B), ultrasonic vocalization (USV) rate (C) and duration (D) in B6 (n = 16), FVB (n = 20), and CD‐1 pups (n = 20). Neonatal USVs at postnatal day (pnd) 2, 4, 6, 8, and 12 are emitted by pups in response to social isolation (3‐minute session). Significant difference between B6 and FVB (p < 0.05*, p < 0.01**); B6 and CD‐1 (p < 0.05#, p < 0.01##); FVB and CD‐1 (p < 0.01$$). Data are expressed as median ± 1st and 3rd interquartile.
B6 pups had lower body temperature than FVB and CD‐1 pups (p < 0.01). All pups increased body temperature from pnd 2 to pnd 12 (p < 0.001). Only B6 pups had a lower temperature at pnd 2 in comparison to other two strain pups (p < 0.01) (Figure 1B).
USV rate changed from pnd 2 to 12 (p < 0.001). B6 pups emitted a lower USV rate than FVB and CD‐1 pups (p = 0.05 and p < 0.01, respectively). Posthoc comparisons (performed on the significant interaction strain × pnd) confirmed that B6 pups vocalized less than CD‐1 pups at pnd 4 (p < 0.05), 6, 8 (p < 0.01) and 12 (p < 0.05), and less than FVB pups only at pnd 8 (p < 0.05). Also, FVB pups vocalized less than CD‐1 pups at pnd 4, 6, 8, and 12 (p < 0.01) (Figure 1C). No significant strain differences were detected on peak frequency and peak amplitude of USVs.
USV duration changed from pnd 2 to 12 (p < 0.001). FVB and CD‐1 pups emitted calls longer than B6 pups (p < 0.01). Posthoc comparisons (performed on the significant interaction strain x pnd) revealed that both FVB and CD‐1 calls were longer than B6 calls at pnd 2, 4, 6, 8 (p < 0.01), and 12 (p < 0.05) (Figure 1D).
Analysis of covariance ruled out the possibility that the strain effects found for USV rate and duration were because of the differences in body weight or body temperature (main effect of strain and strain × pnd interaction were still significant when body weight and body temperature were used as covariates in the repeated measure design). Detailed statistical analysis are reported in Table 1.
TABLE 1.
Statistical analysis of neonatal body weight, body temperature and USV data (from pnd 2 to 12)
| Main effect of strain | Main effect of pnd | Interaction of strain × pnd | |
|---|---|---|---|
| Body weight | F(2, 25) = 5.26, p = 0.01 | F(2, 4) = 333.58, p < 0.001 | F(8, 100) = 0.473, NS |
| Body temperature | F(2, 25) = 7.33, p < 0.01 | F(2, 4) = 107.99, p < 0.001 | F(8, 100) = 3.76, p < 0.01 |
| USV rate | F(2, 25) = 52.47, p < 0.01 | F(2, 4) = 14.47, p < 0.001 | F(8, 100) = 7.76, p < 0.01 |
| USV duration | F(2, 25) = 109.96, p < 0.01 | F(2, 4) = 17.35, p < 0.001 | F(8, 100) = 8.76, p < 0.01 |
| Peak frequency of USVs | F(2, 25) = 42.47, NS | F(2, 4) = 4.53, NS | F(8, 100) = 12.23, NS |
| Peak amplitude of USVs | F(2, 25) = 19.86, NS | F(2, 4) = 18.35, NS | F(8, 100) = 8.35, NS |
Abbreviation: NS, not statistically significant.
3.2. Adult social interaction tests
3.2.1. Male–female
During the 3‐min interaction of a male with a sexually receptive female, FVB males emitted a higher USV rate than B6 and CD‐1 males (p < 0.01 and p < 0.05, respectively). FVB calls were also longer than B6 ones (p < 0.05) (Figure 2A,B). No significant strain differences were detected on peak frequency and peak amplitude of USVs.
FIGURE 2.
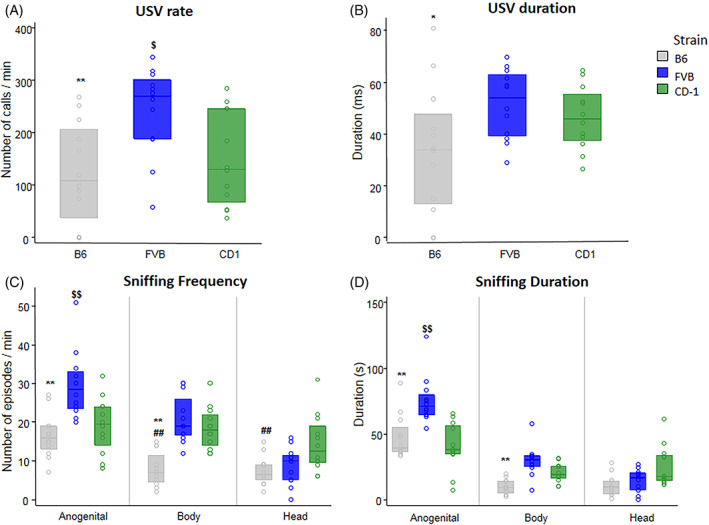
Male–female social interaction. A 3‐min session measured parameters of direct interaction of a male (n = 12 for each strain) with a sexually receptive female of the same strain. USV rate (A) and duration (B), sniffing frequency (C), and sniffing duration (D). Significant differences between B6 and FVB (p < 0.05*, p < 0.01**); B6 and CD‐1 (p < 0.01##); FVB and CD‐1 (p < 0.05$, p < 0.01$$). Data are expressed as median ± 1st and 3rd interquartile.
Analysis of the frequency of social sniffing showed that FVB and CD‐1 males had higher values than B6 males (p < 0.01). Posthoc comparisons (performed on the significant interaction strain × body area) confirmed that B6 and FVB males sniffed the corresponding female partner more frequently in the anogenital area versus body and head area (p < 0.01), whereas in CD‐1 males frequency of sniffing did not vary according to the different body areas. Moreover, FVB males sniffed the anogenital area of the partner more frequently than B6 and CD‐1 males (p < 0.01). FVB and CD‐1 males sniffed the body of the partner more than B6 males (p < 0.01), whereas CD‐1 males sniffed the head of the partner more frequently than B6 males (p < 0.01) (Figure 2C).
Analysis of the duration of social sniffing provided a similar picture. FVB sniffing response was longer than the one of B6 and CD‐1 (p < 0.01). Also sniffing duration was longer for anogenital area versus body and head area within each strain (p < 0.01). Posthoc comparisons (performed on the significant interaction strain x body area) reported that FVB males sniffed the anogenital area of the partner longer than B6 and CD‐1 (p < 0.01) and the body area of the partner longer than B6 males (p < 0.01) (Figure 2D). Detailed statistical analysis are reported in Table 2.
TABLE 2.
Statistical analysis of adult data (USVs and social response) during social interaction tests
| Main effect of strain | Main effect of body area | Interaction of strain x body area | |
|---|---|---|---|
| Male–Female | |||
| USV rate | F(2, 33) = 5.46, p < 0.01 | ||
| USV duration | F(2, 33) = 3.17, p = 0.05 | ||
| Peak frequency of USVs | F(2, 33) = 0.46, NS | ||
| Peak amplitude of USVs | F(2, 33) = 2.54, NS | ||
| Frequency of social sniffing | F(2, 33) = 15.52, p < 0.01 | F(2, 66) = 44.37, NS | F(4, 66) = 8.15, p < 0.01 |
| Duration of social sniffing | F(2, 33) = 17.08, p < 0.01 | F(2, 66) = 88.46, p < 0.01 | F(4, 66) = 9.41, p < 0.01 |
| Female–Female | |||
| USV rate | F(2, 33) = 45.51, p < 0.01 | ||
| USV duration | F(2, 33) = 2.78, NS | ||
| Peak frequency of USVs | F(2, 33) = 2.15, NS | ||
| Peak amplitude of USVs | F(2, 33) = 3.43, NS | ||
| Frequency of social sniffing | F(2, 33) = 8.61, p < 0.01 | F(2, 66) = 91.88, p < 0.01 | F(4, 66) = 9.19, p < 0.01 |
| Duration of social sniffing | F(2, 33) = 24.27, p < 0.01 | F(2, 66) = 81.13, p < 0.01 | F(4, 66) = 11.67, p < 0.01 |
Abbreviation: NS, not statistically significant.
3.2.2. Female–Female
During the 3‐min interaction of a female with a same sex conspecific, FVB females emitted a higher USV rate than B6 and CD‐1 females, and CD‐1 females emitted a higher USV rate than B6 ones (p < 0.01) (Figure 3). No significant strain differences were detected on USV duration (Figure 3B), peak frequency and peak amplitude of USVs.
FIGURE 3.
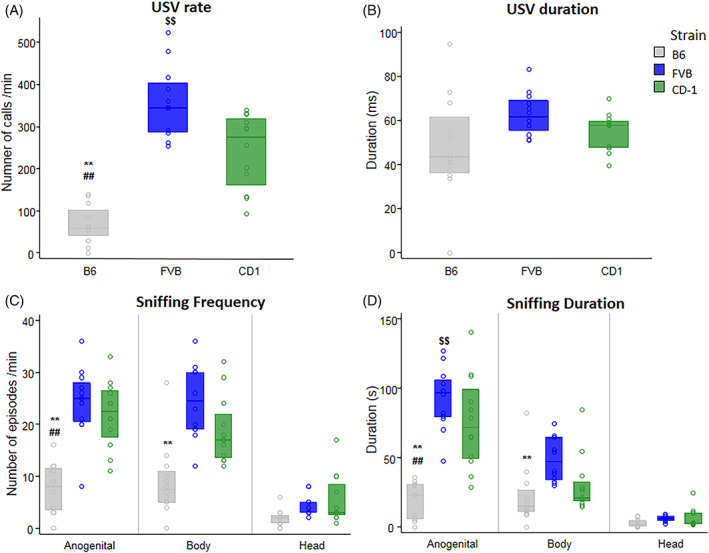
Female–female social interaction. A 3‐min session measured parameters of interaction of a resident female (n = 12 for each strain) with an unfamiliar female of the same strain. USV rate (A) and duration (B), sniffing frequency (C), and sniffing duration (D). Significant differences between B6 and FVB (p < 0.05*, p < 0.01**); B6 and CD‐1 (p < 0.05#, p < 0.01##); FVB and CD‐1 (p < 0.05$, p < 0.01$$). Data are expressed as median ± 1st and 3rd interquartile.
Analysis of frequency of social sniffing revealed significant differences among strains and body areas. B6 females investigated the female partner less frequently than CD‐1 and FVB females (p < 0.01). Females sniffed more frequently the anogenital area of the female partner than the head area within each strain (p < 0.01). Posthoc analysis (performed on the significant interaction strain x body area) revealed that only CD‐1 females spent more time sniffing the anogenital than the body area (p < 0.01). FVB and CD‐1 females sniffed the anogenital area of their partner more than B6 (p < 0.01); FVB females also sniffed the body of their partner more frequently than B6 females (p < 0.01) (Figure 3C).
Analysis of duration of social sniffing response revealed significant differences among strains and body areas. B6 females spent less time investigating the female partner than CD‐1 and FVB females, and CD‐1 females spent less time in social investigation than FVB females (p < 0.01). Post hoc analysis (performed on the significant interaction strain × body area) revealed that CD‐1 and FVB females spent more time sniffing the anogenital area of the female partner than the body and the head, whereas only FVB females spent more time sniffing the body area than the head (p < 0.01). FVB and CD‐1 females also sniffed the anogenital area of their partner longer than B6 females, and FVB also longer than CD‐1 females (p < 0.01); FVB sniffed the body of their partner longer than B6 females (p < 0.01) (Figure 3D). Detailed statistical analysis are reported in Table 2.
Data concerning comparison of variability of USV rate and USV duration of inbred (B6 and FVB) versus outbred (CD‐1) strains were analyzed using nonparametric Kruskal–Wallis test. The variable considered for each individual data was the difference (absolute value) between individual datum and mean value for the group (e.g., for each B6 male pup, the 8/12 dots correspond to individual USV rate minus the mean value of USV rate of male B6 pups). As shown in Figure S1, only in the female–female social interaction test, variability of CD‐1 females resulted significantly higher (p < 0.01) than B6 females; no other difference in variability emerged in the other comparisons.
3.3. Pattern of sonographic structure among strains in different social contexts
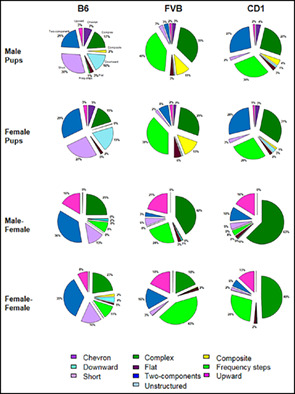
Figure 4 describes the percentages of different call categories emitted by male and female pups and adults for each strain. B6, FVB and CD‐1 mice emitted a different spectrum of call categories.
FIGURE 4.
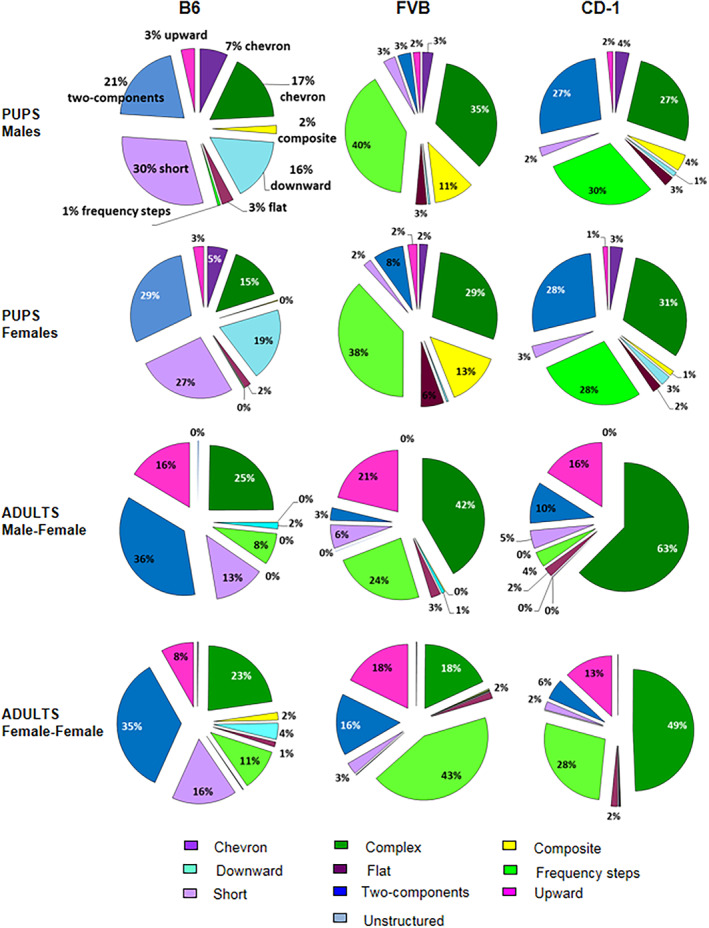
Distributions of call categories in pup B6, FVB and CD‐1 (at pnd 8) and adult B6, FVB and CD‐1 during each type of social encounter. Pie graphs display the percentages of the different call categories within each strain for male pups at pnd 8, female pups at pnd 8, adult male–female, and female–female social interactions. Percentages were calculated in each strain as number of calls in each category for each subject/total number of calls analyzed in each subject.
At postnatal day 8, B6 pups preferred emitting short (males 30%, females 27%) and two‐component (males 21%, females 29%) calls, with a reduced proportion of complex and downward calls. By contrast, FVB displayed high prevalence in production of complex (males 35%, females 29%), frequency steps (males 40%, females 38%), and composite (males 11%, females 13%) calls. Similarly, CD‐1 displayed high prevalence in production of complex (males 27%, females 31%), frequency steps (males 30%, females 28%), along with two‐component (males 27%, females 28%) calls.
During adult male–female interaction, B6 preferred emitting two‐component (36%) and complex (25%) calls, with a reduced proportion of upward (16%) and short (13%) calls. FVB produced a consistent number of complex (42%), frequency steps (24%), and upward calls (21%). CD‐1 emitted predominantly a type of nine call categories, showing 63% of complex calls, with a small number of upward (16%) and two‐component (10%) calls.
During adult female–female interaction, B6 preferred emitting two‐component (35%) and complex (23%) calls, with a reduced proportion of short (16%), frequency steps (11%) and upward (8%) calls. FVB produced a consistent number of frequency steps (43%), along with a similar proportion of complex (18%), two‐component (16%) and upward (18%) calls. CD‐1 emitted predominantly two types of call categories: 49% of complex and 28% of frequency‐steps calls.
When analyzing each USV category separately (see Figure 5), the probability of producing defined call categories differed across strains and social contexts (neonatal stage, adult male–female and female–female interaction). Analysis revealed: 1) a main effect of strain on the proportion of eight call categories (complex, two‐components, downward, chevron, short, composite, frequency steps, and flat); 2) a main effect of social context on the proportion of seven call categories (complex, upward, downward, chevron, composite, frequency steps, and flat; 3) a significant strain × social context interaction on the proportion of eight call categories (complex, two‐components, downward, chevron, short, composite, frequency steps, and flat).
FIGURE 5.
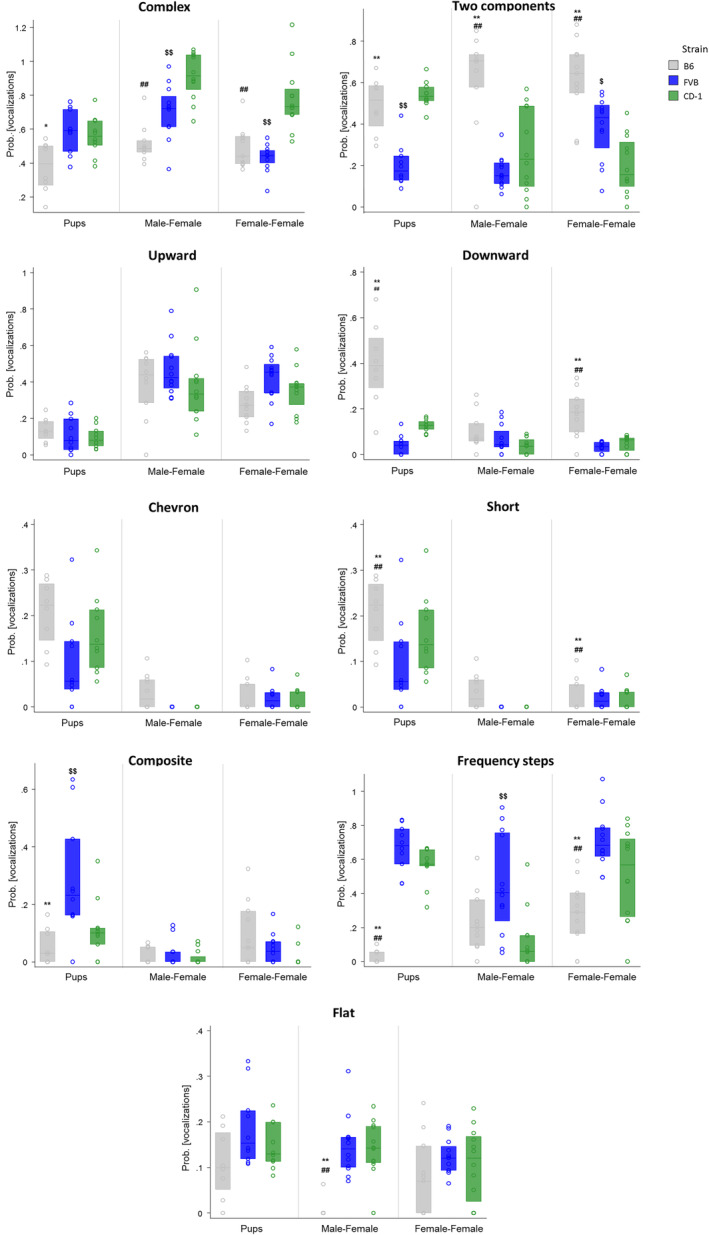
Production of calls across strains. Probability of producing calls from each of the nine categories of USVs at pnd 8 (pups), during adult male–female and female–female social interactions. Data are expressed by angular transformation (y‐axes are different for each graph). Significant differences between B6 and FVB (p < 0.05*, p < 0.01**); B6 and CD‐1 (p < 0.01##); FVB and CD‐1 (p < 0.05$, p < 0.01$$)
Detailed statistical analysis are reported in Table 3. Call differences between strains are also described in the Supporting Information, Table S1.
TABLE 3.
Statistical analysis of call categories across strains and social contexts (neonatal stage, adult male–female and female–female interaction)
| Main effect of strain | Main effect of social context | Interaction of strain x social context | |
|---|---|---|---|
| Complex | F(2, 88) = 36.48, p < 0.01 | F(2, 88) = 17.81, p < 0.01 | F(4, 88) = 6.58, p < 0.01 |
| Two‐components | F(2, 88) = 35.35, p < 0.01 | F(2, 88) = 45.70, NS | F(4, 88) = 10.40, p < 0.01 |
| Upward | F(2, 88) = 45.11, NS | F(2, 88) = 40.67, p < 0.01 | F(4, 88) = 11.74, p < 0.01 |
| Downward | F(2, 88) = 52.21, p < 0.01 | F(2, 88) = 22.70, p < 0.01 | F(4, 88) = 13.22, NS |
| Chevron | F(2, 88) = 6.46, p < 0.01 | F(2, 88) = 73.20, p < 0.01 | F(4, 88) = 3.12, p < 0.05 |
| Short | F(2, 88) = 39.40, p < 0.01 | F(2, 88) = 86.55, NS | F(4, 88) = 4.67, p < 0.01 |
| Composite | F(2, 88) = 6.16, p < 0.01 | F(2, 88) = 17.65, p < 0.01 | F(4, 88) = 7.17, p < 0.01 |
| Frequency steps | F(2, 88) = 37.49, p < 0.01 | F(2, 88) = 12.35, p < 0.01 | F(4, 88) = 6.92, p < 0.01 |
| Flat | F(2, 88) = 15.34, p < 0.01 | F(2, 88) = 5.09, p < 0.01 | F(4, 88) = 2.37, p = 0.05 |
Abbreviation: NS, not statistically significant.
As for individual data variability across inbred and outbred strains, our analyses of absolute values of differences from mean group values identify significant differences only within the female–female social interaction test (Kruskal–Wallis test: H = 6.164, p < 0.0459), namely CD‐1 female data show higher variability than B6 data (Mann–Whitney test: U = 16, p = 0.0037) whereas no evidence of significant difference in variability was detected within the other groups (Figure S1).
4. DISCUSSION
Our findings corroborated results from previous studies showing that strain or genetic background, age, sex, as well as responsiveness to the environmental stimuli (i.e., body temperature) influence the social mouse behaviors, specifically how mice communicate and socially interact with conspecifics. 28 , 46 , 49 , 50 , 51 In the current study, differences among three strains emerged both in the USV rate and vocal repertoire (according to spectrographic features of each call), as well as in social performances.
We initially focused on vocal differences among strains at neonatal stages. This assessment is crucial considering that USV analysis is one of the few assays that can be performed during the first postnatal weeks of life to define developmental trajectories. It also has high value from a translational perspective, since developmental trajectories are investigated in several mouse models of neurodevelopmental disorders, often generated on different genetic background. 1 , 52 , 53 We chose to analyze five time points rather than one single postnatal day, to verify potential strain differences in USV profile across the entire neonatal period. Testing a pup for a brief period (3 min session at each time point) could not be considered a stressful event, if compared with prolonged periods of maternal isolation (from 15 to 45 min/day). 54 , 55 , 56 Thus, in the absence of stressful conditions, it can be assumed that repeated assessments of pups did not substantially affect their USV performance during development. Our analyses detected differences already at early stages for both USV rate and duration: CD‐1 pups emitted a higher number, while B6 pups a lower number of calls compared with the other two strains, in line with previous studies. 10 , 43 , 57 , 58 , 59 , 60 When looking at the mean duration of calls, both FVB and CD‐1 calls were longer than B6. Thus, B6 pups significantly produced a reduced number of calls and those emitted were also shorter than FVB and CD‐1, 43 , 44 as also confirmed by the analysis of sonographic structure (see below). In comparison to B6, FVB pups produced longer USVs, in line with previous data collected on those strains. 36 The ontogenetic profile of USV rate also showed strain differences: B6 pups had a peak in the calling rate earlier, around postnatal day 4, while CD‐1 and FVB pups around pnd 6–8. 2 , 32 , 57 , 58 , 59 , 61 As Figure 1C depicted, the large degree of differences in USV rate emission at pnd 4, 6, and 8 is because of the outbred CD‐1 pups. In response to social isolation and with the aim to activate maternal care, the increased USV emission of CD‐1 pups could be considered as an index of more anxiety‐like behavior in comparison to “low anxiety” profile of inbred B6 and FVB pups. 62 Several factors could be seen as “confounders” in ultrasonic emission, such as body temperature and weight. 63 In our analysis, strain differences in rate and duration of vocalizations were somehow paralleled by differences in temperature and weight. However, analyses of co‐variance, with either temperature or body weight as covariates, indicated that strain differences were still well detectable, ruling out the possibility that the different patterns shown by B6 pups was substantially because of lower values of body weight and temperatures.
Our second investigation targeted the adult vocal profile, in association to social behavior. We found that FVB males vocalized more and for longer time than B6 in presence of a sexually receptive female, as pups did at neonatal age for activating maternal care. The increased USV emission of FVB males was associated with increased social investigation (both frequency and duration) of the female partner, focusing on the anogenital area. In a similar manner, FVB females expressed high levels of social behavioral responses, since they vocalized more than B6 and CD‐1, displayed more frequent and longer anogenital and body area sniffing than B6, as well as longer anogenital sniffing than CD‐1. As for B6 strain, both males and females, as compared with FVB and CD‐1 mice, conserved a low vocal and social profile, differently from previous data found in adult male–male interactions. 64 Noteworthy, B6 has been extensively applied as genetic background to generate mutant mouse lines and used as “control” strain in several experiments, for example, in comparison with inbred strains with low social responsiveness. 65 , 66 It has been also considered as a suitable candidate for behavioral studies since episodes of aggressive attacks rarely occur during social interaction. 28 , 47 , 64 , 67 Present data indicate that B6 is the strain that has lower USV emission rate as neonate and adults (both sexes), and emit simpler and shorter calls. Thus, it might not be the preferential strain to choose in experimental settings and conditions that are hypothesized to reduce USVs and social responses. Our data also evidenced that CD‐1 males had a lower production of USVs and a reduced interest to investigate the partner than FVB, and CD‐1 females showed an intermediate profile for levels of USV emission and social investigation of the female partner when compared with both B6 and FVB strains.
The analysis of variability in the number of calls emitted by the three strains showed comparable levels of variability around mean values for each strain. This result substantially confirms and extends recent data showing that outbred CD‐1 calls are not more variable than inbred FVB ones. 35 Such a convergence on comparable degree of variability in inbred and outbred USVs, using different methodological approaches and targeting different USV parameters (fairly counterintuitive on the basis of heterogeneity of genetic background), is worth of further comparisons of behavioral traits, not necessarily limited to vocalization patterns. 68 Nevertheless, present CD‐1 USV profile suggests that this outbred strain can be suitable for both neonatal and adult studies focused on modulation (by different conditions or agents) of the vocal repertoire.
Further, we investigated more in detail both neonatal and adult vocal repertoires, classifying them into nine categories based on spectrographic appearance. For neonatal repertoire, we focused on postnatal day 8 to be consistent with previous data. 32 , 43 , 44 Differently from the other two strains, B6 pups (both sexes) emitted a significant portion of calls with a simple sonographic structure (short and downward) and also as adults maintained the highest percentage of short calls among the three strains. In addition, B6 pups (both sexes) also showed a remarkable percentage of two components (as CD‐1 pups), and maintain this feature also in the adult vocal repertoire, whereas the same call category is no longer so prevalent in adult FVB and CD‐1 mice. These features are in full agreement with our previous data. 43 , 46 , 47 The vocal repertoire of FVB mice depicted a different sonographic pattern: they emitted more composite calls (on postnatal day 8) and frequency steps calls (across social contexts) than the other strains, as also previously observed during both infancy and adolescence. 36 , 43 Similarly to FVB, CD‐1 strain displayed a complex and modulated vocal repertoire in both neonatal and adult social contexts: CD‐1 mice produced more two‐component and frequency‐step calls during neonatal age, while more complex and fewer frequency‐step calls during adult social encounters. Branchi and colleagues already reported that 8‐day‐old CD‐1 mice emitted a higher percentage of frequency steps and complex calls, along with low numbers of flat and short calls, although the spectrum of USVs was classified into five categories only (flat, complex, frequency steps, short, and composite). 32 In the current study, upward call represented the only call category that did not vary among strains at adulthood: B6, FVB and CD‐1 mice emitted this type of call in a similar manner in both male–female and female–female social interaction, suggesting that upward calls production is a stable element of adult social interaction. These results are supported by a similar acoustic signature with a high proportion of upward calls in B6 adult mice, 69 and in wild‐derived male house mice following urine exposure, 25 during male–female direct social interaction, 21 and after sexual priming. 23 All together, these findings highlight that inbred B6 mice produce simpler syllables with fewer internal changes and shorter duration, inbred FVB mice produce syllables with repeated internal changes (i.e.: more frequency‐step and complex calls), while outbred CD‐1 mice seem to have an intermediate profile.
Following mice from early age to the adulthood, it can be detected that pups displayed a wider vocal profile, based on six or more types of calls (i.e.: complex, two‐component, frequency steps, composite, downward and short), while adults preferred to emit primarily four types of call categories (i.e., upward, complex, two‐component and frequency steps). Mouse pups thus have a less defined vocal signature during early development and tend to define it with age, supporting the idea of a progressive change towards adult acoustic features and syllable composition. 52 , 70 At adulthood, a different prevalence of call categories is observed in each strain. Such strain‐dependent patterns of call categories could affect mate‐choice and/or probability to interact with a conspecific. Additional studies are needed to better understand the role and characteristics of vocal communication during social interactions (e.g., differences between courtship and mating or USV production) across strains, since few studies have detailed this aspect so far. 71 , 72
It is worth highlighting that USVs are a useful tool for evaluating emotion and motivation in rodents. 73 , 74 In juvenile and adult rats, call categories have been extensively used as measures of emotional/affective state. Flat 22‐kHz USVs indicate a negative affective state, while frequency 50‐kHz USVs indicate a positive affective state. 75 , 76 To our knowledge, the meaning of each call category is not well‐established in mice, although a main hypothesis is that the spectrotemporal call complexity may reflect motivational and emotional states. For example, the presence of complex and harmonic calls has been considered as a valuable index of positive emotions in mice. 77 Crucially, emotional and motivational aspects play a role in modulating the number of USVs and the type of call in several mouse models of psychiatric disorders, as autism spectrum disorders, schizophrenia, and stress‐related disorders. 78 Comparing the use of call types by different strains, B6 mice disproportionally emitted higher pitch and more downward modulated calls than BALB mice during vigorous social approaches. 11 In our study, we supposed that more complex and modulated vocal repertoires depicted in FVB and CD‐1 mice, in comparison to B6 ones, were functionally related to the strain‐dependent differences in behavioral responses to social stimuli and environmental factors, as well as it appeared in other emotional behaviors (i.e., anxiety, stress). 79 , 80
A first limitation to the current study is the difficulty to distinguish which mouse of the dyad vocalized during the adult social interaction with current available protocols and most common analysis tools. Previous investigations dealt with the issue to determine whether the source of call production is exclusively the mouse test. 16 , 81 We suppose that in our experimental setting, given the protocol we applied to record USVs, the test mouse (male, in the male–female interaction; resident female in the female–female interaction) is the primary vocal emitter. However, we cannot rule out that also the female partner vocalized during the social encounter. Indeed, it has been recently demonstrated, through a multiple‐microphone array system that allows to identify the vocalizer of a group of mice, that during male–female interactions also female mice vocalize, to interact with the male and transfer social and receptive information 16 ; even in these more naturalistic settings, however, greater proportion of vocalizations are produced by males. During our qualitative analysis carried out by visual inspection of spectrograms, we did not detect overlapping of signals; although we cannot exclude that it sporadically occurred, we can rule out that it systematically affected our measurements. A novel insight comes from a recent deep learning approach evidencing the differences between male and female USVs through the investigation of spectrotemporal properties. The full spectrogram characteristics were informative about the emitter's sex, at least during analysis of female–male social interaction. 82 Future and in‐depth investigations including the use of multiple‐microphones, source localization methods and machine learning approaches are needed to determine the location of the sound and identify the mice vocalizing in the dyadic social interaction.
A second limitation consists in the limited number (three) of strains and types of social contexts/age (pups during maternal isolation, adult males and females during social encounters) used. Future studies are needed to expand such specific vocal evaluation to other mouse strains and additional settings, possibly synchronizing USV recordings and social behavior in adult mouse. 83
As a third limitation, we did not evaluate potential strain‐related differences in mother's behavior after reunion and their effects on subsequent USV neonatal emission. Data from literature indicated that increased maternal responsiveness may lead to a reduction in “isolation‐induced USVs.” 10 C57BL/6 mothers exhibited higher levels of maternal responsiveness in comparison to BALB/c ones, which in turn were associated to a lower number of neonatal USVs. 10 Thus, in our study it is possible the dams belonging to different strains recognized distinct signals (i.e., olfactory) from a pup placed in the nest following the experimental session and they activated differently maternal care and maternal responsiveness.
5. CONCLUSION
Our data fit with other investigations emphasizing that USVs carry relevant social information about species, strain, sex and individuals, and potentially vary in response to mouse internal state, social experience and behavioral interactions with conspecifics. 19 , 52 , 81 , 84 , 85
Present USV quantitative and qualitative results demonstrate that there is a large degree of variance among B6, FVB and CD‐1 mouse strains across age and social contexts. We conclude that the number of USVs, as well as their acoustic features and sound shapes, may be influenced by strain, age and social context of assessment. In association with USV detection, analyzing behavioral social responses during two types of adult encounters represent an additional item that capture more defined mouse social profiles.
Our findings illustrate the importance of considering strain, as well as age and social/environmental conditions of mice, prior to set up USV experimental paradigms. Also background strain of genetically modified lines should be taken into account when dealing with previous findings or planning pharmacological/toxicological experiments. The intermediate profile of the outbred CD‐1 strain, compared with inbred B6 and FVB strains, should be considered when dealing with the choice of inbred and outbred strains appropriate to different contexts or experimental settings.
AUTHOR CONTRIBUTIONS
Laura Ricceri and Maria Luisa Scattoni conceived the hypothesis and designed the study; Laura Ricceri, Maria Luisa Scattoni, and Maria Adelaide Marconi performed experiments; Angela Caruso, Maria Adelaide Marconi, and Laura Ricceri analyzed and plotted the data; Angela Caruso, Laura Ricceri, and Maria Luisa Scattoni wrote the manuscript. All authors read and edited the manuscript.
Supporting information
Figure S1 Individual variability for USV rate (upper panel) and duration (lower panel). The individual variability is reported as the absolute difference of each value from the mean for each group. The x‐axes show the individual variability for pups (pnd 8, males and females), and adults during male–female (M–F) and female–female (F–F) social interaction. USV rate values from CD‐1 adult females show greater variability than B6 values during the female–female social interaction, whereas no difference was evident in the other groups. Data are box plots; n = 8/12 in each group; **p < 0.01.
ACKNOWLEDGMENTS
The authors thank Tommaso Salvitti for editing graphs; Luigia Cancemi for helping with animal maintenance and care. The authors declare no conflict of interest.
Caruso A, Marconi MA, Scattoni ML, Ricceri L. Ultrasonic vocalizations in laboratory mice: strain, age, and sex differences. Genes, Brain and Behavior. 2022;21(5):e12815. doi: 10.1111/gbb.12815
Funding information 2020‐2022 Ricerca Corrente ISS, Centro per le Scienze Comportamentali e la salute mentale "Modelli sperimentali di disturbi del neurosviluppo e neuropsichiatrici”
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Caruso A, Ricceri L, Scattoni ML. Ultrasonic vocalizations as a fundamental tool for early and adult behavioral phenotyping of autism Spectrum disorder rodent models. Neurosci Biobehav Rev. 2020;116:31‐43. doi: 10.1016/j.neubiorev.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 2. Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol. 1982;15(3):221‐227. doi: 10.1002/dev.420150306 [DOI] [PubMed] [Google Scholar]
- 3. Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5(4):371‐387. doi: 10.1002/dev.420050410 [DOI] [PubMed] [Google Scholar]
- 4. Zippelius HM, Schleidt WM. Ultraschall‐Laute bei jungen Mausen. Naturwissenschaften. 1956;21:1‐2. [Google Scholar]
- 5. Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: an acoustic by‐product of laryngeal braking? Behav Neurosci. 1990;104(5):808‐817. doi: 10.1037//0735-7044.104.5.808 [DOI] [PubMed] [Google Scholar]
- 6. Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev. 2001;108(1):83‐95. doi: 10.1037/0033-295x.108.1.83 [DOI] [PubMed] [Google Scholar]
- 7. Cohen‐Salmon C, Carlier M, Roubertoux P, Jouhaneau J, Semal C, Paillette M. Differences in patterns of pup care in mice. V‐‐pup ultrasonic emissions and pup care behavior. Physiol Behav. 1985;35(2):167‐174. doi: 10.1016/0031-9384(85)90331-2 [DOI] [PubMed] [Google Scholar]
- 8. Ehret G. Categorical perception of mouse‐pup ultrasounds in the temporal domain. Anim Behav. 1992;43(3):409‐416. [Google Scholar]
- 9. Tasaka GI, Feigin L, Maor I, et al. The temporal association cortex plays a key role in auditory‐driven maternal plasticity. Neuron. 2020;107(3):566‐579 e7. doi: 10.1016/j.neuron.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35(1):103‐112. [DOI] [PubMed] [Google Scholar]
- 11. Panksepp JB, Jochman KA, Kim JU, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PloS One. 2007;2(4):e351. doi: 10.1371/journal.pone.0000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ju A, Hammerschmidt K, Tantra M, Krueger D, Brose N, Ehrenreich H. Juvenile manifestation of ultrasound communication deficits in the neuroligin‐4 null mutant mouse model of autism. Behav Brain Res. 2014;270:159‐164. doi: 10.1016/j.bbr.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 13. Maggio JC, Maggio JH, Whitney G. Experience‐based vocalization of male mice to female chemosignals. Physiol Behav. 1983;31(3):269‐272. doi: 10.1016/0031-9384(83)90186-5 [DOI] [PubMed] [Google Scholar]
- 14. Whitney G, Nyby J. Cues that elicit ultrasounds from adult male mice. Am Zool. 1979;19(2):457‐463. [Google Scholar]
- 15. Heckman JJ, Proville R, Heckman GJ, Azarfar A, Celikel T, Englitz B. High‐precision spatial localization of mouse vocalizations during social interaction. Scientific Reports. 2017;7(1):3017. doi: 10.1038/s41598-017-02954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neunuebel JP, Taylor AL, Arthur BJ, Egnor SE. Female mice ultrasonically interact with males during courtship displays. eLife. 2015;4:e06203. doi: 10.7554/eLife.06203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warren MR, Spurrier MS, Roth ED, Neunuebel JP. Sex differences in vocal communication of freely interacting adult mice depend upon behavioral context. PloS One. 2018;13(9):e0204527. doi: 10.1371/journal.pone.0204527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burke K, Screven LA, Dent ML. CBA/CaJ mouse ultrasonic vocalizations depend on prior social experience. PloS One. 2018;13(6):e0197774. doi: 10.1371/journal.pone.0197774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3(12):e386. doi: 10.1371/journal.pbio.0030386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanno K, Kikusui T. Effect of Sociosexual experience and aging on number of courtship ultrasonic vocalizations in male mice. Zoolog Sci. 2018;35(3):208‐214. doi: 10.2108/zs170175 [DOI] [PubMed] [Google Scholar]
- 21. Nicolakis D, Marconi MA, Zala SM, Penn DJ. Ultrasonic vocalizations in house mice depend upon genetic relatedness of mating partners and correlate with subsequent reproductive success. Front Zool. 2020;17:10. doi: 10.1186/s12983-020-00353-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nyby JG. Auditory communication among adults. Handbook of Mouse Auditory Research. CRC Press; 2001:16. [Google Scholar]
- 23. Zala SM, Nicolakis D, Marconi MA, et al. Primed to vocalize: wild‐derived male house mice increase vocalization rate and diversity after a previous encounter with a female. PloS One. 2020;15(12):e0242959. doi: 10.1371/journal.pone.0242959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes TD, Rieger MA, Dougherty JD, Holy TE. Group and individual variability in mouse pup isolation calls recorded on the same day show stability. Frontiers in Behavioral Neuroscience. 2017;11:243. doi: 10.3389/fnbeh.2017.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marconi MA, Nicolakis D, Abbasi R, Penna DJ, Zala SM. Ultrasonic courtship vocalizations of male house mice contain distinct individual signatures. Anim Behav. 2020;169:169‐197. [Google Scholar]
- 26. Melotti L, Siestrup S, Peng P, et al. Individuality, as well as genetic background, affects syntactical features of courtship songs in male mice. Anim Behav. 2021;180:179‐196. [Google Scholar]
- 27. Verjat A, Rodel HG, Feron C. Isolation calls in house mouse pups: individual consistency across time and situations. Dev Psychobiol. 2019;61(8):1135‐1145. doi: 10.1002/dev.21884 [DOI] [PubMed] [Google Scholar]
- 28. Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus). J Comp Psychol. 1985;99(4):420‐436. [PubMed] [Google Scholar]
- 29. Zala SM, Reitschmidt D, Noll A, Balazs P, Penn DJ. Sex‐dependent modulation of ultrasonic vocalizations in house mice (Mus musculus musculus). PloS One. 2017;12(12):e0188647. doi: 10.1371/journal.pone.0188647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoier S, Pfeifle C, von Merten S, Linnenbrink M. Communication at the garden fence‐‐context dependent vocalization in female house mice. PloS One. 2016;11(3):e0152255. doi: 10.1371/journal.pone.0152255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moles A, Costantini F, Garbugino L, Zanettini C, D'Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behavioural Brain Research. 2007;182(2):223‐230. doi: 10.1016/j.bbr.2007.01.020 [DOI] [PubMed] [Google Scholar]
- 32. Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33(3):249‐256. doi: [DOI] [PubMed] [Google Scholar]
- 33. Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation‐induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34(3):195‐204. [DOI] [PubMed] [Google Scholar]
- 34. Scattoni ML, Michetti C, Ricceri L. Chapter 42 ‐ rodent vocalization studies in animal models of the autism Spectrum disorder. In: Brudzynski SM, ed. Handbook of Behavioral Neuroscience. Elsevier; 2018:445‐456. [Google Scholar]
- 35. Binder MS, Shi HD, Bordey A. CD‐1 outbred mice produce less variable ultrasonic vocalizations than FVB inbred mice, while displaying a similar developmental trajectory. Front Psych. 2021;12:687060. doi: 10.3389/fpsyt.2021.687060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peleh T, Eltokhi A, Pitzer C. Longitudinal analysis of ultrasonic vocalizations in mice from infancy to adolescence: insights into the vocal repertoire of three wild‐type strains in two different social contexts. PloS One. 2019;14(7):e0220238. doi: 10.1371/journal.pone.0220238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kikusui T, Nakanishi K, Nakagawa R, Nagasawa M, Mogi K, Okanoya K. Cross fostering experiments suggest that mice songs are innate. PloS One. 2011;6(3):e17721. doi: 10.1371/journal.pone.0017721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wohr M, Dahlhoff M, Wolf E, Holsboer F, Schwarting RK, Wotjak CT. Effects of genetic background, gender, and early environmental factors on isolation‐induced ultrasonic calling in mouse pups: an embryo‐transfer study. Behav Genet. 2008;38(6):579‐595. doi: 10.1007/s10519-008-9221-4 [DOI] [PubMed] [Google Scholar]
- 39. Arriaga G, Jarvis ED. Mouse vocal communication system: are ultrasounds learned or innate? Brain Lang. 2013;124(1):96‐116. doi: 10.1016/j.bandl.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashbrook DG, Roy S, Clifford BG, et al. Born to cry: a genetic dissection of infant vocalization. Frontiers in Behavioral Neuroscience. 2018;12:250. doi: 10.3389/fnbeh.2018.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bryant CD, Zhang NN, Sokoloff G, et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008;22(4):315‐331. doi: 10.1080/01677060802357388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Frontiers in Behavioral Neuroscience. 2010;4:29. doi: 10.3389/fnbeh.2010.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T + tf/J mouse model of autism. PloS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiaderkiewicz J, Glowacka M, Grabowska M, Jaroslaw‐Jerzy B. Ultrasonic vocalizations (USV) in the three standard laboratory mouse strains: developmental analysis. Acta Neurobiol Exp. 2013;73(4):557‐563. [DOI] [PubMed] [Google Scholar]
- 45. Rugh R. The Mouse: its Reproduction and Development. Oxford University Press; 1990. [Google Scholar]
- 46. Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33(4):508‐515. doi: 10.1016/j.neubiorev.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T + tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10(1):44‐56. doi: 10.1111/j.1601-183X.2010.00623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vigli D, Palombelli G, Fanelli S, et al. Maternal immune activation in mice only partially recapitulates the autism Spectrum disorders symptomatology. Neuroscience. 2020;445:109‐119. doi: 10.1016/j.neuroscience.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 49. Gourbal BE, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91(8):381‐385. doi: 10.1007/s00114-004-0543-7 [DOI] [PubMed] [Google Scholar]
- 50. Heckman J, McGuinness B, Celikel T, Englitz B. Determinants of the mouse ultrasonic vocal structure and repertoire. Neurosci Biobehav Rev. 2016;65:313‐325. doi: 10.1016/j.neubiorev.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 51. Sasaki E, Tomita Y, Kanno K. Sex differences in vocalizations to familiar or unfamiliar females in mice. R Soc Open Sci. 2020;7(12):201529. doi: 10.1098/rsos.201529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PloS One. 2011;6(3):e17460. doi: 10.1371/journal.pone.0017460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeskind PS, McMurray MS, Garber KA, et al. Development of translational methods in spectral analysis of human infant crying and rat pup ultrasonic vocalizations for early neurobehavioral assessment. Front Psych. 2011;2:56. doi: 10.3389/fpsyt.2011.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burenkova OV, Averkina AA, Aleksandrova EA, Zarayskaya IY. Brief but enough: 45‐min maternal separation elicits behavioral and physiological responses in neonatal mice and changes in dam maternal behavior. Physiology & Behavior. 2020;222:112877. doi: 10.1016/j.physbeh.2020.112877 [DOI] [PubMed] [Google Scholar]
- 55. D'Amato FR, Cabib S. Chronic exposure to a novel odor increases pups' vocalizations, maternal care, and alters dopaminergic functioning in developing mice. Behav Neural Biol. 1987;48(2):197‐205. doi: 10.1016/s0163-1047(87)90738-2 [DOI] [PubMed] [Google Scholar]
- 56. D'Amato FR, Cabib S, Puglisi‐Allegra S, et al. Effects of acute and repeated exposure to stress on the hypothalamo‐pituitary‐adrenocortical activity in mice during postnatal development. Horm Behav. 1992;26(4):474‐485. doi: 10.1016/0018-506x(92)90015-n [DOI] [PubMed] [Google Scholar]
- 57. Bell RW, Nitschke W, Zachman TA. Ultra‐sounds in three inbred strains of young mice. Behav Biol. 1972;7(6):805‐814. doi: 10.1016/s0091-6773(72)80172-x [DOI] [PubMed] [Google Scholar]
- 58. Hahn ME, Hewitt JK, Schanz N, Weinreb L, Henry A. Genetic and developmental influences on infant mouse ultrasonic calling. I. a diallel analysis of the calls of 3‐day olds. Behav Genet. 1997;27(2):133‐143. doi: 10.1023/a:1025637408900 [DOI] [PubMed] [Google Scholar]
- 59. Hennessy MB, Li J, Lowe EL, Levine S. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol. 1980;13(6):573‐584. doi: 10.1002/dev.420130603 [DOI] [PubMed] [Google Scholar]
- 60. Thornton LM, Hahn ME, Schanz N. Genetic and developmental influences on infant mouse ultrasonic calling. III. Patterns of inheritance in the calls of mice 3‐9 days of age. Behav Genet. 2005;35(1):73‐83. doi: 10.1007/s10519-004-0857-4 [DOI] [PubMed] [Google Scholar]
- 61. Robinson DJ, D'Udine B. Ultrasonic calls produced by three laboratory strains of Mus musculus. J Biol. 1982;197:383‐389. [Google Scholar]
- 62. Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behavioural Brain Research. 2002;136(2):489‐501. doi: 10.1016/s0166-4328(02)00200-0 [DOI] [PubMed] [Google Scholar]
- 63. Caruso A, Sabbioni M, Scattoni ML, Branchi I. Quantitative and qualitative features of neonatal vocalizations in mice. In: Brudzynski SM, ed. Handbook of Ultrasonic Vocalization Elsevier; 2018:139‐145:chap 13. [Google Scholar]
- 64. Faure A, Pittaras E, Nosjean A, Chabout J, Cressant A, Granon S. Social behaviors and acoustic vocalizations in different strains of mice. Behav Brain Res. 2017;320:383‐390. doi: 10.1016/j.bbr.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 65. Crawley JN. What's Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley‐Liss Publication; 2000. [Google Scholar]
- 66. Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13(1):4‐26. doi: 10.1038/sj.mp.4002082 [DOI] [PubMed] [Google Scholar]
- 67. Canastar A, Maxson SC. Sexual aggression in mice: effects of male strain and of female estrous state. Behav Genet. 2003;33(5):521‐528. doi: 10.1023/a:1025722700138 [DOI] [PubMed] [Google Scholar]
- 68. Tuttle AH, Philip VM, Chesler EJ, Mogil JS. Comparing phenotypic variation between inbred and outbred mice. Nat Methods. 2018;15(12):994‐996. doi: 10.1038/s41592-018-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Matsumoto YK, Okanoya K. Mice modulate ultrasonic calling bouts according to sociosexual context. R Soc Open Sci. 2018;5(6):180378. doi: 10.1098/rsos.180378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114(6 Pt 1):3412‐3422. doi: 10.1121/1.1623787 [DOI] [PubMed] [Google Scholar]
- 71. Nomoto K, Hashiguchi A, Asaba A, et al. Female C57BL/6 and BALB/c mice differently use the acoustic features of male ultrasonic vocalizations for social preferences. Experimental Animals. 2020;69(3):319‐325. doi: 10.1538/expanim.19-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sugimoto H, Okabe S, Kato M, et al. A role for strain differences in waveforms of ultrasonic vocalizations during male‐female interaction. PloS One. 2011;6(7):e22093. doi: 10.1371/journal.pone.0022093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes Brain Behav. 2011;10(1):4‐16. doi: 10.1111/j.1601-183X.2010.00603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wohr M, Schwarting RK. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013;354(1):81‐97. doi: 10.1007/s00441-013-1607-9 [DOI] [PubMed] [Google Scholar]
- 75. Burgdorf JS, Brudzynski SM, Moskal JR. Using rat ultrasonic vocalization to study the neurobiology of emotion: from basic science to the development of novel therapeutics for affective disorders. Curr Opin Neurobiol. 2020;60:192‐200. doi: 10.1016/j.conb.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 76. Simola N, Granon S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology. 2019;159:107420. doi: 10.1016/j.neuropharm.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 77. Gaub S, Fisher SE, Ehret G. Ultrasonic vocalizations of adult male Foxp2‐mutant mice: behavioral contexts of arousal and emotion. Genes Brain Behav. 2016;15(2):243‐259. doi: 10.1111/gbb.12274 [DOI] [PubMed] [Google Scholar]
- 78. Granon S, Faure A, Chauveau F, Cressant A, Ey E. Why should my mouse call me? Acoustic communication in mouse models of social disorders: ultrasonic vocalizations as an index of emotional and motivational states. In: Brudzynski SM, ed. Handbook of Behavioral Neuroscience. Elsevier; 2018:423‐431:chap 40. [Google Scholar]
- 79. Eltokhi A, Kurpiers B, Pitzer C. Behavioral tests assessing neuropsychiatric phenotypes in adolescent mice reveal strain‐ and sex‐specific effects. Scientific Reports. 2020;10(1):11263. doi: 10.1038/s41598-020-67758-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kopp C, Vogel E, Misslin R. Comparative study of emotional behaviour in three inbred strains of mice. Behav Processes. 1999;47(3):161‐174. doi: 10.1016/s0376-6357(99)00057-1 [DOI] [PubMed] [Google Scholar]
- 81. Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PloS One. 2012;7(7):e40782. doi: 10.1371/journal.pone.0040782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ivanenko A, Watkins P, van Gerven MAJ, Hammerschmidt K, Englitz B. Classifying sex and strain from mouse ultrasonic vocalizations using deep learning. PLoS Comput Biol. 2020;16(6):e1007918. doi: 10.1371/journal.pcbi.1007918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sangiamo DT, Warren MR, Neunuebel JP. Ultrasonic signals associated with different types of social behavior of mice. Nat Neurosci. 2020;23(3):411‐422. doi: 10.1038/s41593-020-0584-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chabout J, Serreau P, Ey E, et al. Adult male mice emit context‐specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PloS One. 2012;7(1):e29401. doi: 10.1371/journal.pone.0029401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hoffmann F, Musolf K, Penn DJ. Spectrographic analyses reveal signals of individuality and kinship in the ultrasonic courtship vocalizations of wild house mice. Physiology & behavior. 2012;105(3):766‐771. doi: 10.1016/j.physbeh.2011.10.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Individual variability for USV rate (upper panel) and duration (lower panel). The individual variability is reported as the absolute difference of each value from the mean for each group. The x‐axes show the individual variability for pups (pnd 8, males and females), and adults during male–female (M–F) and female–female (F–F) social interaction. USV rate values from CD‐1 adult females show greater variability than B6 values during the female–female social interaction, whereas no difference was evident in the other groups. Data are box plots; n = 8/12 in each group; **p < 0.01.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


